Abstract
The hormonal factors implicated as transmitters of signals from the gut to pancreatic β‐cells are referred to as incretins. Gastric inhibitory polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1) are incretins. In addition to the insulinotropic effects, we have shown, using the GIP receptor and GLP‐1 receptor‐deficient mice, that GIP and GLP‐1 have direct actions on adipocytes and the kidney, respectively. Because GIP receptors and GLP‐1 receptors are differentially expressed in a tissue‐specific manner, GIP and GLP‐1 have specific physiological activities, and further comprehensive characterization of the extrapancreatic actions of GIP and GLP‐1 is anticipated, as dipeptidyl peptidase IV inhibitors activate both GIP and GLP‐1 signaling.
Keywords: Gastric inhibitory polypeptide, Glucagon‐like peptide‐1, Incretin
Introduction
Incretins are hormones that stimulate insulin secretion from pancreatic β‐cells, which are responsive to the difference of insulin levels after oral and intravenous ingestion of glucose. Gastric inhibitory polypeptide (GIP) was originally isolated as a 42‐amino‐acid hormone from the duodenum, inhibiting gastric acid secretion and gastric motility1, 2. Subsequently, GIP was shown to be released from intestinal K cells and to stimulate insulin secretion from pancreatic β‐cells directly, and was renamed glucose‐dependent insulinotropic polypeptide3, 4. Glucagon‐like peptide‐1 (GLP‐1) was originally identified as a glucagon‐like polypepetide from the glucagon precursor5. GLP‐1(7‐37) and GLP‐1(7‐36) amide were shown to be released from intestinal L cells, and stimulate insulin secretion from pancreatic β‐cells directly6. Amino acid sequences of human GIP and GLP‐17, 8 were shown to be highly homologous, belonging to the glucagon/secretin family.
GLP‐1 and GIP have pancreatic effects
Receptors for GLP‐1 and GIP were cloned and shown to be coupled with the stimulatory G protein, belonging to the glucagon/secretin receptor family9, 10. Genetic deficiency of GLP‐1 receptor (GLP1R) and GIP receptor (GIPR) in mice was developed11, 12, 13. As shown in Figure 1, GLP1R‐deficent mice and GIPR‐deficient mice showed higher blood glucose levels with impaired initial insulin response after oral glucose loading. These results confirm that GLP‐1 and GIP act as incretin. Simultaneous ablation of GLP1R and GIPR further increased the blood glucose levels and decreased initial insulin response after glucose loading, showing that GLP‐1 and GIP cannot compensate adequately for each other.
Figure 1.
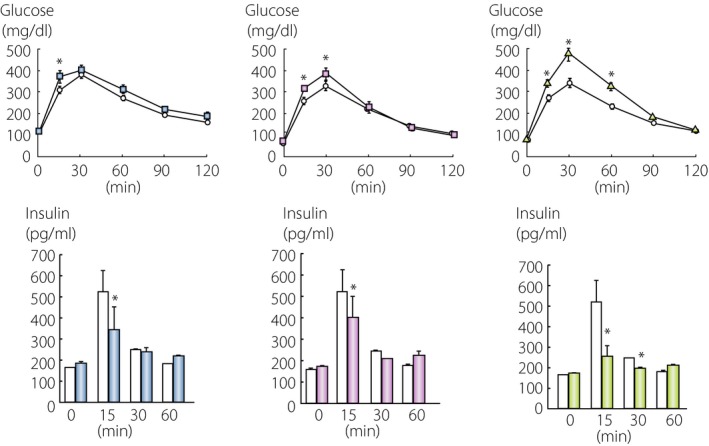
Incretin deficiency and glucose intolerance. Glucagon‐like peptide‐1 (GLP‐1) receptor‐deficient (left, blue), gastric inhibitory polypeptide (GIP) receptor‐deficient (middle, red) and double receptor‐deficient mice (right, green) in the C57BL/7 background were challenged with oral glucose, and levels of glucose and insulin levels were measured. *P = 0.05 versus wild‐type mice. Original mice are described in references11, 12, 13.
GLP‐1 and GIP have extra‐pancreatic effects
Receptors for GLP‐1 and GIP are expressed not only in pancreatic β‐cells, but also in several extrapancreatic tissues. Figure 2 shows the tissue‐specific expression patterns of GLP‐1 and GIP receptors in mice. GLP1R is highly expressed in the lung and duodenum of mice, and GIPR is highly expressed in the testis of mice. The expression patterns of GLP1R and GIPR are quite different, suggesting that GLP‐1 and GIP have their own physiological activities.
Figure 2.
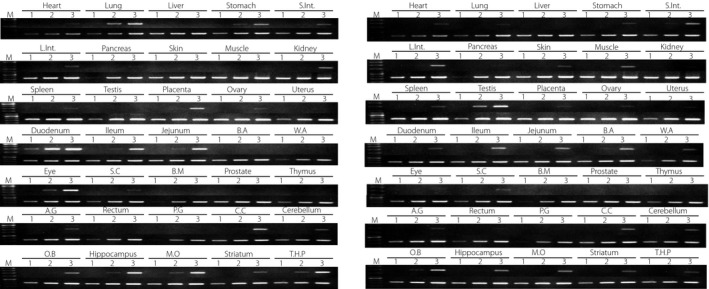
Expression patterns of incretin receptor. Gene expression of (a) glucagon‐like peptide‐1 receptor and (b) gastric inhibitory polypeptide receptor was examined using reverse transcription polymerase chain reaction. Complementary deoxyribonucleic acid templates from various mouse tissues (Genostaff, Tokyo, Japan) were amplified 25 cycles (lane 1), 30 cycles (lane 2) and 35 cycles, and fractionated on 1.5% agarose gels with polymerase chain reaction products of glyceraldehyde‐3‐phosphate dehydrogenase as controls. Heart, lung, liver, stomach, small intestine (S.Int.), large intestine (L.Int.), pancreas, skin, skeletal muscle, kidney, spleen, testis, placenta, ovary, uterus, duodenum, ileum, jejunum, brown adipose (B.A), white adipose (W.A), eye, spinal cord (S.C), bone marrow (B.M), prostate, thymus, adrenal gland (A.G), rectum, pituitary gland (P.G), cerebral cortex (C.C), cerebellum, olfactory bulb (O.B), hippocampus, medulla oblongate (M.O), striatum and thalamus + hypothalamus + pons (T.H.P) were examined.
The second amino acid from the NH3‐terminal of GLP‐1 and GIP is the alanine residue, and in vitro and in vivo studies showed that both GLP‐1 and GIP are substrates of dipeptidyl peptidase IV (DPP‐4), and that active peptides are degraded to inactive peptides14, 15. Mice separately deficient in GLP1R or GIPR could augment insulin secretion after treatment with DPP‐4 inhibitors, one class of antihyperglycemic agents widely used for treatment of diabetes, but the mice simultaneously deficient in GLP1R and GIPR could not respond to the treatment, showing that both GLP‐1 and GIP signaling can be augmented by treatment with DPP‐4 inhibitors. These results suggest that the extrapancreatic effects of GLP‐1 and GIP might be stimulated by treatment with DPP‐4 inhibitors, in addition to the increased pancreatic effects. Furthermore, DPP‐4‐resistant GLP1R agonists, another class of antihyperglycemic agents, stimulate the pancreatic and extrapancreatic effects of GLP‐1. Therefore, comprehensively understanding the pancreatic and extrapancreatic effects of GLP‐1 and GIP is essential.
GIP as gut‐derived satiation‐responsive polypeptide
As aforementioned, GIP was shown to have an activity to inhibit gastric acid secretion and was known as gastric inhibitory polypeptide. Subsequent studies showed that GIP has an activity to stimulate insulin secretion in a glucose‐dependent manner, thus it was renamed glucose‐dependent insulinotropic polypeptide. However, glucose‐dependent insulinotropic polypeptide might be an imprecise name, in two regards. First, GIP has several extrapancreatic effects in addition to stimulation of insulin secretion. Second, several peptides, including GLP‐1, can stimulate insulin secretion in a glucose‐dependent manner.
Our group has taken particular note of the extrapancreatic effects of GIP. As GIPR is expressed in white adipose tissues, and GIP increases glucose uptake and heparin‐releasable lipoprotein lipase activity of the differentiated 3T3‐L1 adipose cell line16, we hypothesized that diet‐induced GIP is responsive for promoting nutrient uptake into the adipose tissues. A high‐fat diet or overeating induces obesity in mice as well as humans, compared with the control diet (Figure 3). These diet styles increase blood GIP levels and insulin levels16. We bred GIP receptor‐deficient mice fed a high‐fat diet or induced by leptin deficiency to overeat, and found that mice lacking the GIPR were protected from obesity. The GIPR‐deficient mice had a lower respiratory quotient, suggesting that fat was used as the preferred energy substrate instead of storing it in the adipose tissues. Therefore, GIP directly links overnutrition to obesity. Next, we examined the effects of aging on obesity, as aging is associated with increased fat mass and decreased lean mass. Aged GIPR‐deficient mice on the normal diet showed significantly reduced fat mass with conserved lean mass. Furthermore, GIPR‐deficient mice showed higher heart rate, lower body temperature and increased physical activity17. These phenotypic characterizations of genetic inactivation of GIP signaling showed similarity with those of caloric‐restricted mice. Indeed, the GIP‐deficient mice showed lower adiposity18. From these results and other studies, we have proposed GIP as “gut‐derived satiation‐responsive polypeptide.”
Figure 3.
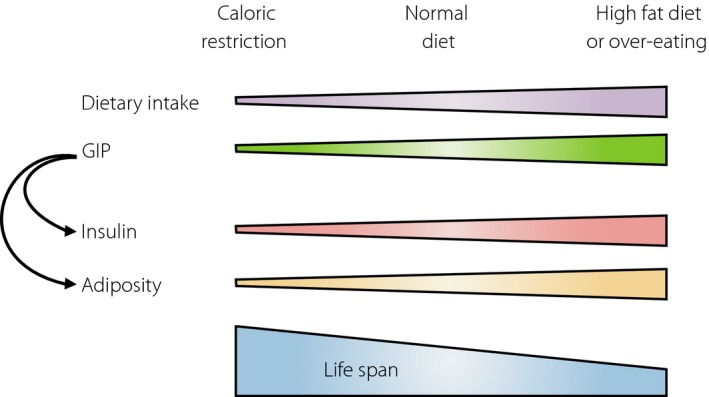
Gastric inhibitory polypeptide as a gut‐derived satiation responsive polypeptide.
GLP‐1 as a renoprotective factor
Diabetic patients might develop microvascular complications, such as retinopathy, nephropathy, and neuropathy; and macro‐vascular complications, such as cerebral infarction, myocardial infarction and peripheral arterial diseases, which are the major causes of morbidity and mortality. Because poor glucose control is the major risk factor for diabetic complications, antihyperglycemic agents, such as sulfonylureas, biguanides and insulin, have been given to diabetic patients.
Although GLP‐1 receptor messenger ribonucleic acid was detected in the kidney, its precise localization was controversial because of the poor specificity of anti‐GLP1R antibodies19. Using in situ hybridization, we have shown that GLP‐1 receptor messenger ribonucleic acid was expressed in glomerular capillary and vascular walls in the mouse kidney20. In vivo examination was carried out using Akita diabetic mice on KK/Ta or C57BL/6 background. Akita diabetic mice on C57BL/6 background are nephropathy‐resistant, and genetic ablation of GLP1R showed higher urinary albumin levels and more advanced mesangial expansion with increased glomerular superoxide and upregulated renal nicotinamide adenine dinucleotide phosphate oxidase, despite comparable levels of hyperglycemia. By contrast, nephropathy‐prone Akita diabetic mice on KK/Ta background treated with liraglutide, a GLP1R agonist, showed reduced albuminuria and mesangial expansion. These results showed that GLP‐1 has a crucial role in protection against increased renal oxidative stress under chronic hyperglycemia. Although it has not been defined as the primary end‐point of the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53 trial21, patients treated with saxagliptin, a DPP‐4 inhibitor, were significantly more likely to have an improved albumin‐to‐creatinine ratio, consistent with our animal studies.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors thank current and former members in the Department of Endocrinology, Diabetes and Geriatric Medicine, Akita University Graduate School of Medicine.
J Diabetes Investig 2016; 7: 76–79
This article is based on the presentations given by the authors at a symposium, Incretin 2015, July 29–31, 2015, Vancouver, BC Canada.
References
- 1. Brown JC, Mutt V, Pederson RA. Further purification of a polypeptide demonstrating enterogastrone activity. J Physiol 1970; 209: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown JC, Dryburgh JR. A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can J Biochem 1971; 49: 867–872. [DOI] [PubMed] [Google Scholar]
- 3. Pederson RA, Schubert HE, Brown JC. The insulinotropic action of gastric inhibitory polypeptide. Can J Physiol Pharmacol 1975; 53: 217–223. [DOI] [PubMed] [Google Scholar]
- 4. Taminato T, Seino Y, Goto Y, et al Synthetic gastric inhibitory polypeptide. Stimulatory effect on insulin and glucagon secretion in the rat. Diabetes 1977; 26: 480–484. [DOI] [PubMed] [Google Scholar]
- 5. Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 1983; 302: 716–718. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt WE, Siegel EG, Creutzfeldt W. Glucagon‐like peptide‐1 but not glucagon‐like peptide‐2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia 1985; 28: 704–707. [DOI] [PubMed] [Google Scholar]
- 7. Bell GI, Sanchez‐Pescador R, Laybourn PJ, et al Exon duplication and divergence in the human preproglucagon gene. Nature 1983; 304: 368–371. [DOI] [PubMed] [Google Scholar]
- 8. Takeda J, Seino Y, Tanaka K, et al Sequence of an intestinal cDNA encoding human gastric inhibitory polypeptide precursor. Proc Natl Acad Sci USA 1987; 84: 7005–7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco‐incretin hormone glucagon‐like peptide 1. Proc Natl Acad Sci USA 1992; 89: 8641–8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamada Y, Hayami T, Nakamura K, et al Human gastric inhibitory polypeptide receptor: cloning of the gene (GIPR) and cDNA. Genomics 1995; 29: 773–776. [DOI] [PubMed] [Google Scholar]
- 11. Scrocchi LA, Brown TJ, MaClusky N, et al Glucose intolerance but normal satiety in mice with a null mutation in the glucagon‐like peptide 1 receptor gene. Nat Med 1996; 2: 1254–1258. [DOI] [PubMed] [Google Scholar]
- 12. Miyawaki K, Yamada Y, Yano H, et al Glucose intolerance caused by a defect in the entero‐insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA 1999; 96: 14843–14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hansotia T, Baggio LL, Delmeire D, et al Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP‐IV inhibitors. Diabetes 2004; 53: 1326–1335. [DOI] [PubMed] [Google Scholar]
- 14. Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose‐dependent insulinotropic polypeptide and truncated glucagon‐like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995; 136: 3585–3596. [DOI] [PubMed] [Google Scholar]
- 15. Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl‐peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon‐like peptide‐1(7‐36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 1993; 214: 829–835. [DOI] [PubMed] [Google Scholar]
- 16. Miyawaki K, Yamada Y, Ban N, et al Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 2002; 8: 738–742. [DOI] [PubMed] [Google Scholar]
- 17. Yamada C, Yamada Y, Tsukiyama K, et al Genetic inactivation of GIP signaling reverses aging‐associated insulin resistance through body composition changes. Biochem Biophys Res Commun 2007; 364: 175–180. [DOI] [PubMed] [Google Scholar]
- 18. Nasteska D, Harada N, Suzuki K, et al Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high‐fat diet conditions. Diabetes 2014; 63: 2332–2343. [DOI] [PubMed] [Google Scholar]
- 19. Panjwani N, Mulvihill EE, Longuet C, et al GLP‐1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(‐/‐) mice. Endocrinology 2013; 154: 127–139. [DOI] [PubMed] [Google Scholar]
- 20. Fujita H, Morii T, Fujishima H, et al The protective roles of GLP‐1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int 2014; 85: 579–589. [DOI] [PubMed] [Google Scholar]
- 21. Scirica BM, Bhatt DL, Braunwald E, et al Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]


