Abstract
Type 2 diabetes in East Asians is characterized primarily by β‐cell dysfunction, and with less adiposity and less insulin resistance compared with that in Caucasians. Such pathophysiological differences can determine the appropriate therapeutics for the disease. Incretins, glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐1, are secreted in response to meal ingestion, and enhance insulin secretion glucose‐dependently. Incretin‐based drugs, dipeptidyl peptidase‐4 inhibitors (DPP‐4i) and glucagon‐like peptide‐1 receptor agonists, that ameliorate β‐cell dysfunction with limited hypoglycemia risk are now widely used in type 2 diabetes management. Recent meta‐analyses of clinical trials on DPP‐4i and glucagon‐like peptide‐1 receptor agonists found that the drugs were more effective in Asians, most likely because of amelioration of β‐cell dysfunction. In addition, we found increased glycated hemoglobin‐lowering effects of DPP‐4i to be associated with intake of fish in type 2 diabetes, which suggests that dietary customs of East Asians might also underlie the greater efficacy of DPP‐4i. Despite the limited risk, cases of severe hypoglycemia were reported for DPP‐4i/sulfonylureas combinations. Importantly, hypoglycemia was more frequent in patients also receiving glibenclamide or glimepiride, which activate exchange protein directly activated by cyclic adenosine monophosphate 2, a critical mediator of incretin signaling, and was less frequent in patients receiving gliclazide, which does not activate exchange protein directly activated by cyclic adenosine monophosphate 2. Prevention of insulin‐associated hypoglycemia by DPP‐4i has gained attention with regard to the enhancement of hypoglycemia‐induced glucagon secretion by insulinotropic polypeptide, but remains to be investigated in East Asians. Despite the safety issues, which are paramount and must be carefully monitored, the incretin‐based drugs could have potential as a first choice therapy in East Asian type 2 diabetes patients.
Keywords: Dipeptidyl peptidase‐4 inhibitors, East Asian, Glucagon‐like peptide‐1 receptor agonists
Introduction
The rapid increase in type 2 diabetes is one of the most serious global health problems today. The number of patients with diabetes, estimated to be 415 million in 2015, is expected to rise to 642 million by 20401, partly as a result of a drastic increase in the number of patients in East Asian countries, which now comprise one‐quarter of the global diabetes population. The etiology of type 2 diabetes involves genetic predispositions and lifestyle factors, such as dietary habits and physical activity, as well as aging, all of which influence insulin secretion from the pancreatic β‐cells and/or reduce insulin sensitivity of target organs. It is becoming widely recognized that East Asian type 2 diabetes is characterized primarily by β‐cell dysfunction, which is evident immediately after glucose ingestion (Figure 1), and by generally lesser obesity and higher insulin sensitivity compared with that in Caucasians2. These pathophysiological differences have a crucial impact on the appropriate therapeutic approach. To ameliorate β‐cell dysfunction, insulin secretagogues, such as sulfonylureas (SU) and glinides, have been used as preferred drugs in East Asian countries; however, SU and glinides are associated with hypoglycemia and bodyweight gain, and better therapeutics have long been sought. Incretin‐based therapies, dipeptidyl peptidase‐4 inhibitors (DPP‐4i) and glucagon‐like peptide‐1 receptor agonists (GLP‐1RA), have been widely used in the management of type 2 diabetes. These drugs ameliorate β‐cell dysfunction with limited risk of hypoglycemia and bodyweight gain, and are widely used in East Asia. More than 70% of patients treated with antidiabetic drugs receive DPP‐4i or GLP‐1RA today; approximately 60% of these are drug‐naïve, indicating that DPP‐4i is rapidly becoming a first‐line antidiabetic drug in Japan (Figure 2). In the present article, we discuss recent findings regarding the efficacy and safety of DPP‐4i and GLP‐1RAs from an East Asian perspective. We also discuss the novel interaction of medical nutrition therapy with the glucose‐lowering effects of DPP‐4i.
Figure 1.
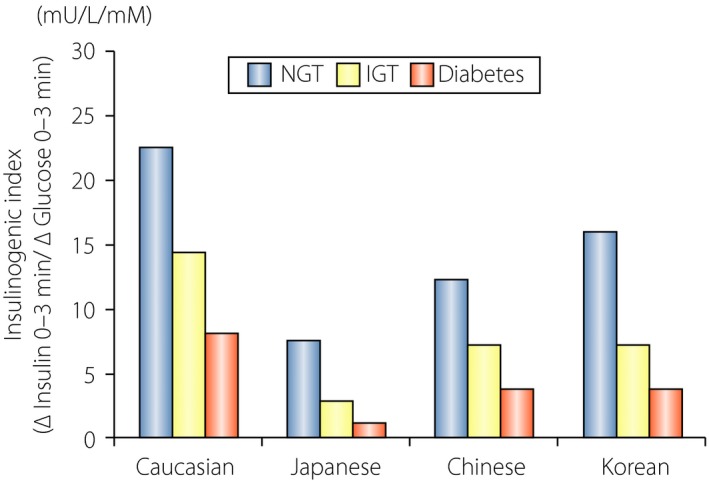
Reduced early‐phase insulin secretion in East Asian individuals compared with Caucasian individuals. Insulinogenic index (ΔInsulin 0–30 min/ΔGlucose 0–30 min) was indirectly compared between East Asians and Caucasians with or without type 2 diabetes. Blue bars, participants with normal glucose tolerance (NGT). Orange bars, participants with isolated impaired glucose tolerance (IGT). Red bars, participants with type 2 diabetes (Diabetes). Reproduced from Yabe et al.50 with permission.
Figure 2.
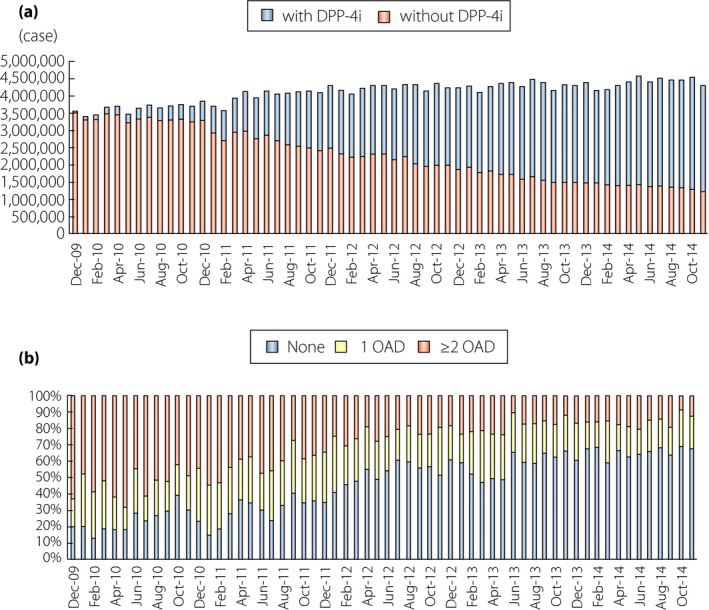
Wide use of dipeptidyl peptidase‐4 inhibitors (DPP‐4i) as a first choice therapy in Japan. (a) The number of individuals receiving DPP‐4i among those treated with oral antidiabetic drugs (OAD). Those treated with insulin and/or glucagon‐like peptide‐1 are not included. Note that more than 70% of individuals with OAD receive DPP‐4i in Japan today. (b) Profiles of antidiabetic drug use before initiating DPP‐4i. Note that approximately 60% of individuals receive DPP‐4i as a first choice therapy in Japan. Data were derived from the Japan Medical Data Centre Claims Database (Japan Medical Data Centre Co., Ltd, Tokyo, Japan), which contains the following information on individuals aged <75 years in employment‐based health insurance programs: age and sex of patient; diagnosis of disease using International Classification of Diseases‐10 code; and prescribed drugs. Reproduced from Yabe et al.29 with permission.
β‐Cell Dysfunction and East Asian Type 2 Diabetes
As early as the 1970s, our group and others found that the insulin response to glucose ingestion in Japanese people, those having both normal glucose tolerance and type 2 diabetes, is much lower than it is in Caucasian people3. Cross‐sectional studies in Japanese people with normal glucose tolerance, impaired glucose tolerance and type 2 diabetes confirmed reduced β‐cell function and higher insulin sensitivity in comparison with Caucasian people3. These findings are supported by recent important studies: (i) a systematic review and meta‐analysis of β‐cell function and insulin sensitivity in i.v. glucose tolerance test found reduced β‐cell function and higher insulin sensitivity of East Asian people compared with Caucasian people4; (ii) studies of Caucasian and Japanese matched individuals in oral glucose tolerance test and i.v. glucose tolerance test showed reduced β‐cell function and higher insulin sensitivity in Japanese people5, 6; and (iii) long‐term cohort studies investigating the trajectory of normal glucose tolerance in Korea and the UK showed that decreased β‐cell function and impaired β‐cell compensation for progressive decline in insulin sensitivity are central in deteriorating glucose intolerance in Koreans, whereas decreased insulin sensitivity is a prerequisite for type 2 diabetes development in Caucasian people7, 8. Considering such differences in β‐cell function and insulin sensitivity, it seems reasonable that East Asians might have reduced β‐cell reserve capacity that makes them readily susceptible to a minor decline of insulin sensitivity. Although this model needs to be tested in sufficiently powered multi‐ethnic group studies, accumulating evidence implicates β‐cell dysfunction as the primary defect of type 2 diabetes in non‐obese East Asians, and shows the need for antidiabetic drugs that target β‐cell dysfunction for management of the disease. Indeed, insulin secretagogues, such as SU and glinides, have been used as preferred drugs in East Asian countries; more recently, incretin‐based drugs, DPP‐4i and GLP‐1RA that ameliorate β‐cell dysfunction with limited risk of hypoglycemia and bodyweight gain have been added. Importantly, recent systematic review and meta‐analyses of clinical trials on DPP‐4i and GLP‐1RA found the drugs to be more effective in Asians people9, 10.
Incretins and Incretin‐Based Drugs
The incretin concept, which opened up the possibility of incretin‐based drugs as novel antidiabetic therapeutics, was reported more than 100 years ago11, 12, 13. Inspired by Bayliss and Starling's discovery of secretin, Moore et al. hypothesized in 1906 that gut extracts contain a hormone that regulates the endocrine pancreas, and showed that gut extracts reduce urinary glucose excretion in patients with diabetes, possibly by stimulating the endocrine pancreas. La Barre purified the glucose‐lowering element from gut extracts in 1929, and named it incretin (INtestine seCRETtion INsulin). The classical studies by Elrick et al. and McIntyre et al. showed the pivotal role of incretin in the enhancement of insulin secretion after oral glucose loading in men14, 15. Later studies confirmed that incretin comprises a pair of intestinal hormones, gastric inhibitory polypeptide (GIP) and GLP‐111, 12, 13, secreted by the K cells and the L cells of the duodenum, respectively.
GIP is a 42‐amino‐acid hormone secreted from K cells of the upper small intestine (Figure 2)11. GIP, originally isolated from porcine intestine by Brown et al. on the basis of its ability to inhibit gastric acid secretion, stimulates insulin secretion in a glucose‐dependent manner in non‐diabetic individuals, and acts directly on pancreatic islets to stimulate insulin secretion glucose‐dependently11. By studying the insulin response in gastrectomized patients, our group showed that endogenous GIP also stimulates insulin secretion glucose‐dependently16. These lines of evidence revealed GIP as the first of the incretins, which was then renamed glucose‐dependent insulinotropic polypeptide. Because immunological depletion of GIP did not abolish all insulin‐stimulating activity in gut extracts, the existence of another incretin was inferred; GLP‐1, a 31‐amino‐acid hormone produced from proglucagon and secreted from L cells of the lower intestine and colon, was later shown to be the second incretin12, 13. It has been also shown that both GIP and GLP‐1 exert their insulinotropic effects through their specific receptors, the GIP receptor (GIPR) and the GLP‐1 receptor (GLP‐1R); genetic ablation of GIPR and GLP‐1R separately or simultaneously in mice shows their critical roles in the potentiation of glucose‐induced insulin secretion11, 12. These lines of evidence confirmed the critical role of GIP and GLP‐1 as incretins.
To develop incretin‐based drugs, several issues had to be resolved. First, secreted incretins undergo rapid degradation catalyzed by DPP‐4, which diminishes the insulinotropic effects of GIP and GLP‐111, 13. Second, it was initially reported that the insulinotropic effects of GIP are attenuated in individuals with type 2 diabetes17, identifying GLP‐1 as a major target for drug development. The demonstration that GLP‐1 secretion is reduced in individuals with type 2 diabetes18 also suggested amelioration of β‐cell dysfunction through activation of GLP‐1R signaling by increasing the levels of biologically intact GLP‐1 through DPP‐4 inhibition or supplementation of DPP‐4‐resistant GLP‐1RA. Although a recent systematic review and meta‐analysis showed little reduction of GLP‐1 secretion in type 2 diabetes19, 20, long‐term i.v. infusion of GLP‐1 has been shown to improve glycemic control21, establishing GLP‐1 and GLP‐1R signaling as therapeutic targets for type 2 diabetes. However, recent studies showed that the insulin response to GIP is restored in individuals with near‐normalized glycemia22. This observation is especially important in the treatment of type 2 diabetes with DPP‐4i, because its effect is influenced by both GIP and GLP‐1. Indeed, a recent report studying the effects of endogenous incretins showed that GLP‐1R antagonist did not completely attenuate the glucose‐lowering effects of DPP‐4i in individuals with near‐normalized glycemia23. Furthermore, the meal‐induced GLP‐1 response is attenuated, whereas the GIP response is somewhat increased with long‐term treatment of DPP‐4i (DY and YS, unpubl. obs.). Taken together with similar observations on the GLP‐1 response in DPP‐4i‐treated patients24, these results emphasize the importance of GIP in DPP‐4i treatment.
Glucose‐Lowering Effects of DPP‐4I in East Asians
It has been found that DPP‐4i and GLP‐1RA have greater glucose‐lowering effects in Asian people9, 10, 25, which is consistent with β‐cell dysfunction as the primary defect of East Asian type 2 diabetes and incretin‐based drugs to target this defect. Although no head‐to‐head clinical trials comparing long‐term glucose‐lowering effects of incretin‐based drugs exist, accumulating clinical data from East Asian countries suggest that DPP‐4i shows sustained glucose‐lowering effects in the management of East Asian type 2 diabetes26, 27, 28. In addition, our analysis on duration before prescription changes after initiating oral antidiabetic drugs using a large Japanese medical claims database showed that DPP‐4i exerted longer durability as monotherapy or add‐on therapy when compared with oral antidiabetic drugs29, suggesting efficacy and safety of DPP‐4i that is superior to other oral antidiabetic drugs in Japanese people. Interestingly, we also found that GLP‐1 secretion, but not GIP secretion, after ingestion of glucose or meals in Japanese people is lower than that of Caucasian people when measured by the same immunoassay30, 31. Although proof of ethnic differences in the secretion of GLP‐1 and GIP requires sufficiently powered multi‐ethnic group studies, GLP‐1 deficiency in addition to β‐cell dysfunction might well be partly responsible for the superior efficacy of incretin‐based drugs in East Asian type 2 diabetes.
Because GLP‐1 and GIP are secreted in response to meals, dietary habits might well influence the efficacy of DPP‐4i. Indeed, the glycated hemoglobin (HbA1c)‐lowering effects of DPP‐4i in the relatively short‐term are enhanced by fish intake, as estimated by food records and serum levels of eicosapentaenoic acids and docosahexaenoic acids, in individuals with type 2 diabetes (Figure 3)32, 33. Milk products and meat show weaker associations with the HbA1c‐lowering effects of DPP‐4i in a relatively short observation period. These findings suggest that nutrients in fish, meat and milk products promote GLP‐1 and GIP secretion, and thereby enhance the HbA1c‐lowering effects of DPP‐4i. Previous studies showed that intake of whey protein, glutamine or olive oils before carbohydrates enhanced GLP‐1 secretion and ameliorated postprandial glucose excursions in individuals both with and without type 2 diabetes34, showing that preload of a small amount of protein or fats before meals might be effective in postprandial glucose excursion through GLP‐1. We recently showed that eating fish before rice enhances GLP‐1 secretion and ameliorates postprandial glucose excursion in comparison with eating fish after rice35. Eating meat before rice has similar results, except that it robustly enhances GIP secretion, possibly because of the saturated and mono‐unsaturated fats in meat, which are strong enhancers of GIP secretion. As GIP, in collaboration with these fats, facilitates fat accumulation36, eating meat before rice chronically might be linked to bodyweight gain and subsequent insulin resistance, negating any HbA1c‐lowering effects of DPP‐4i. Indeed, a small but significant bodyweight gain is associated with deterioration of the HbA1c‐lowering effects of DPP‐4i in Japanese individuals with type 2 diabetes37, 38. Together, these results suggest that the greater efficacy of DPP‐4i in East Asians might be partly due to dietary habits along with lesser adiposity and insulin resistance.
Figure 3.
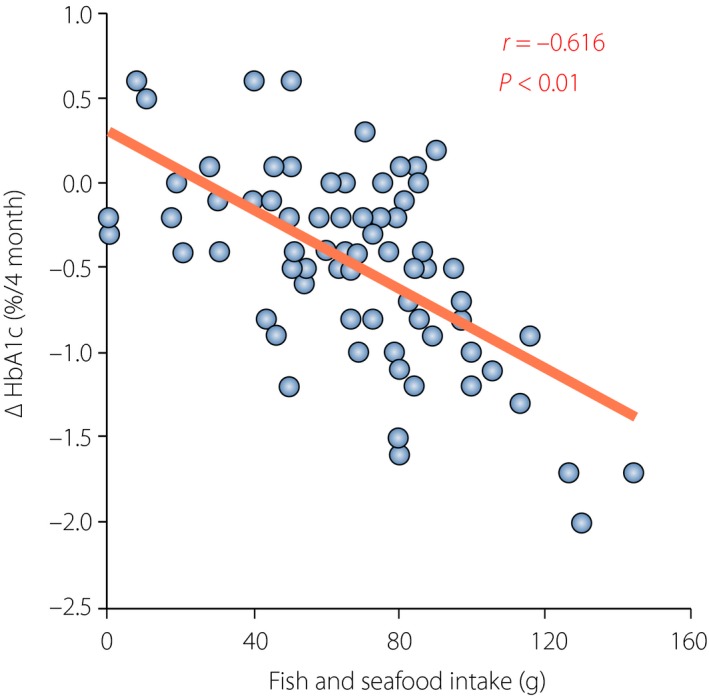
Association of glycated hemoglobin (HbA1c) reduction by dipeptidyl peptidase‐4 inhibitors and estimated intake of fish. Correlation between estimated intake of fish and seafood (fish intake) with HbA1c reduction (ΔHbA1c). Among fish and seafood (i.e., shellfish, squid and octopus, and crustaceans), only estimated intake of fish showed a significant association with HbA1c reduction by single regression analysis (r = −0.62, P < 0.01). Reproduced from Iwasaki et al.32 with permission.
Hypoglycemia and DPP‐4I in East Asians
Although DPP‐4i by itself is considered to have a very limited risk of hypoglycemia, cases of severe hypoglycemia were reported among individuals receiving DPP‐4i as add‐on to SU when the first DPP‐4i sitagliptin emerged in Japanese clinical practice (Figure 4). The estimated incidence of hypoglycemic coma was 16.3 per million patients who received sitagliptin during the first 6 months after its launch in Japan, and was approximately 6.4‐fold higher than that of the USA in the corresponding period39. The cases in Japan were mostly elderly, and were found to have renal insufficiency and high HbA1c even with use of high‐dose SU. Based on the characteristics of the cases with severe hypoglycemia by DPP‐4 inhibitor treatment, a committee of experts in the field (Chair, Y Seino of Kansai Electric Power Hospital; T Kadowaki of University of Tokyo; N Inagaki of Kyoto University; T Iwakura of Kobe City Hospital; Y Iwamoto of Tokyo Women's Medical University; S Seino of Kobe University) urged physicians to reduce the doses of preprescribed SU drugs, especially in the elderly and/or individuals with renal insufficiency, before co‐administration of DPP‐4i.
Figure 4.
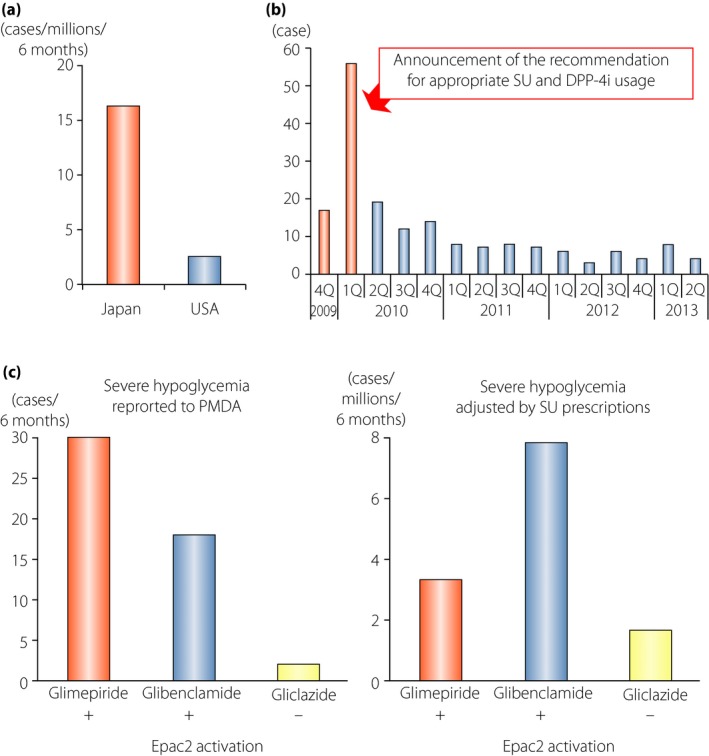
Severe hypoglycemia in individuals receiving dipeptidyl peptidase‐4 inhibitors (DPP‐4i) as add‐on to sulfonylureas (SUs). (a) Comparison of the incidence rate of severe hypoglycemia in individuals receiving the DPP‐4i, sitagliptin, in Japan and the USA. The incidence of hypoglycemic coma with sitagliptin was 16.3 per million patients who received sitagliptin during the first 6 months after its launch in Japan, and was approximately 6.4‐fold higher than that of USA in the corresponding period. (b) Transition in cases of severe hypoglycemia in individuals treated with sitagliptin in each quarter. The number was drastically reduced on announcement of the recommendation from the committee for appropriate use of incretin‐related drugs (glucagon‐like peptide‐1 receptor agonists and DPP‐4 inhibitors). (c) Comparison of the numbers of severe hypoglycemia cases in individuals receiving sitagliptin as add‐on to indicated SUs. Left, the numbers of cases reported to the Japanese Pharmaceuticals and Medical Devices Agency. Right, estimated incidence rates calculated by dividing the numbers of cases reported to the Japanese Pharmaceuticals and Medical Devices Agency by the numbers of individual prescriptions of indicated SUs combination with sitagliptin in the same period. EPAC2, exchange protein directly activated by cyclic adenosine monophosphate 2. Reproduced from Yabe and Seino39 with permission.
Two key investigations of incretin signaling in β‐cells provided clues to understand the mechanism of severe hypoglycemia on initiation of DPP‐4i in SU‐treated type 2 diabetes patients. The first study showed that GLP‐1R activation ameliorates glucose metabolism in β‐cells of non‐obese diabetic model Goto‐Kakizaki rats, thereby improving glucose‐induced insulin secretion40. Chronic hyperglycemia is known to enhance reactive oxygen species production, which then impairs glucose metabolism and reduces production of adenosine triphosphate (ATP) in β‐cells. As SU‐induced closure of KATP channels is known to be affected significantly by intracellular ATP levels, chronic hyperglycemia could make β‐cells less sensitive to SU, partly explaining “SU secondary failure.” The Goto‐Kakizaki rat study clearly showed that activation of GLP‐1R signaling reduces reactive oxygen species production and increases ATP, an exchange protein directly activated by cyclic adenosine monophosphate 2A (EPAC2A)‐dependently40. Thus, initiation of DPP‐4i in patients with “SU secondary failure” could result in hypoglycemia as a result of improved sensitivity of the pancreatic β‐cells to SU. Another clue came from a study revealing novel cross‐talk between SU and incretin signaling through EPAC2A41. It is known that activation of GIPR and GLP‐1R leads to an increase in intracellular cyclic adenosine monophosphate levels, which binds to and activates EPAC2A, thereby enhancing insulin secretion. In addition, SU such as glibenclamide and glimepiride but not gliclazide, bind to and activate EPAC2A, thereby enhancing insulin secretion. These results are suggestive of the cases in which SU is responsible for severe hypoglycemia. The estimated incidence rates of severe hypoglycemia in patients receiving sitagliptin with glimepiride (3.35 per 10,000) or glibenclamide (7.86 per 10,000) were more than twofold higher than in those receiving sitagliptin with gliclazide (1.66 per 10,000; Figure 4)39. Although numerous factors including reduced glucose counter‐regulation might affect the incidence rates of severe hypoglycemia by the combinations of sitagliptin and each SU, these data complement the original observations in clinical settings and provide insight on the suitability of the various SU to be used in combination with DPP‐4i. Taken together, these important findings explain why activation of incretin signaling by DPP‐4i enhances SU‐induced insulin secretion even in individuals with “SU secondary failure.” Therefore, with careful titration of SU doses and appropriate patient education on hypoglycemia, a combination of DPP‐4 inhibitors and SU drugs can be effective type 2 diabetes therapy.
Regarding hypoglycemia, GIP action on glucagon secretion has been gaining much attention recently, because DPP‐4i add‐on to insulin reduces hypoglycemia42, 43. As early as the 1970s, our group showed that GIP enhances glucagon secretion in rats and isolated rat islets44. Later, enhancement of glucagon secretion by GIP was confirmed in individuals with type 2 diabetes during insulin‐induced hypoglycemia45. It is also known that DPP‐4i vildagliptin enhances the glucagon response to insulin‐induced hypoglycemia46, suggesting that DPP‐4i reduces insulin‐induced hypoglycemia through GIP. However, our recent studies showed that DPP‐4i linagliptin did not enhance insulin‐induced glucagon secretion in Japanese type 2 diabetes patients (DY and YS, unpubl. data). Currently, it remains unknown whether differences in ethnicities and/or the DPP‐4i used could explain the differing results. Further studies are required to clarify the mechanisms of lower hypoglycemia risk using DPP4‐i.
Conclusion
The profound glucose‐lowering effects and low hypoglycemia risk of incretin‐based drugs have made them widely used in non‐obese type 2 diabetes across East Asian countries, especially in Japan. However, safety issues must always be kept in mind. As aforementioned, careful considerations are required to avoid severe hypoglycemia when DPP‐4i is co‐administrated with SU. It is also important to triage patients with risk of acute pancreatitis before prescribing incretin‐based drugs. Although the associations of incretin‐based drugs with acute pancreatitis in East Asians have been controversial47, 48, recent meta‐analysis of prospective, randomized controlled trials of DPP‐4i showed a small but significant increase of acute pancreatitis associated with DPP‐4i use49. Thus, adverse events, both known and unknown, must be carefully monitored for years. Nevertheless, given the pathophysiology of East Asian type 2 diabetes (insulin deficiency rather than insulin resistance), incretin‐based drugs, which primarily correct impaired early phase insulin secretion, might well be the more suitable treatment of disease in these patients, and has the potential to be a first choice therapy, as is presently the case for metformin in Caucasian type 2 diabetes patients.
Disclosure
D Yabe received consulting and/or speaker fees from Eli Lilly Japan K.K., MSD K.K., Sanofi K.K., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd. and Takeda Pharmaceutical Company Limited. D Yabe received clinical commissioned/joint research grants from Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly and Company, Taisho Toyama Pharmaceutical Co. Ltd., and MSD K.K. Y Seino received consulting and/or speaker fees from Eli Lilly Japan K.K., Sanofi K.K., Novo Nordisk Pharma Inc., Glaxo‐Smith‐Kline, Taisho Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Astellas Pharma Inc., BD, Nippon Boehringer Ingelheim Co., Ltd., Johnson & Johnson and Takeda Pharmaceutical Company Limited. Y Seino received clinical commissioned/joint research grants from Taisho Toyama Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly and MSD, K.K. H Kuwata declares no conflict of interest.
Acknowledgments
The authors thank current and former colleagues in the field, and apologize for citing only a part of the relevant work due to limited space, and are indebted to many authors for their contributions. The authors are especially grateful to Ms R Nishikino, Ms M Kaneko and Ms C Ito of Japan Medical Data Centre Co., Ltd for sharing data used in Figure 2. The authors also thank Ms Y Michiko of Kansai Electric Power Hospital and Ms Y Shigefuku of Kansai Electric Power Medical Research Institute for their secretarial support. The authors received Grants‐in‐Aid of Kansai Electric Power Medical Research Institute for Scientific Research from the Japan Society for Promotion of Science, Grants for Young Researchers from the Japan Association for Diabetes Education and Care, and Grants from the Japan Vascular Disease Research Foundation.
J Diabetes Investig 2016; 7: 102–109
This article is based on the presentations given by the authors at a symposium, Incretin 2015, July 29‐31, 2015, Vancouver, BC Canada.
References
- 1. IDF Diabetes Atlas, 6th edn Brussels, Belgium: IDF, 2015. [Google Scholar]
- 2. Yabe D, Seino Y, Fukushima M, et al Beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep 2015; 15: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seino Y, Kurahachi H, Goto Y, et al Comparative insulinogenic effects of glucose, arginine and glucagon in patients with diabetes mellitus, endocrine disorders and liver disease. Acta Diabetol Lat 1975; 12: 89–99. [DOI] [PubMed] [Google Scholar]
- 4. Kodama K, Tojjar D, Yamada S, et al Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta‐analysis. Diabetes Care 2013; 36: 1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moller JB, Dalla Man C, Overgaard RV, et al Ethnic differences in insulin sensitivity, beta‐cell function, and hepatic extraction between Japanese and Caucasians: a minimal model analysis. J Clin Endocrinol Metab 2014; 99: 4273–4280. [DOI] [PubMed] [Google Scholar]
- 6. Moller JB, Pedersen M, Tanaka H, et al Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care 2014; 37: 796–804. [DOI] [PubMed] [Google Scholar]
- 7. Tabak AG, Jokela M, Akbaraly TN, et al Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009; 373: 2215–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohn JH, Kwak SH, Cho YM, et al 10‐year trajectory of beta‐cell function and insulin sensitivity in the development of type 2 diabetes: a community‐based prospective cohort study. Lancet Diabet Endocrinol 2016; 4: 27–34. [DOI] [PubMed] [Google Scholar]
- 9. Kim YG, Hahn S, Oh TJ, et al Differences in the HbA1c‐lowering efficacy of glucagon‐like peptide‐1 analogues between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetes Obes Metab 2014; 16: 900–909. [DOI] [PubMed] [Google Scholar]
- 10. Kim YG, Hahn S, Oh TJ, et al Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and Non‐Asians: a systematic review and meta‐analysis. Diabetologia 2013; 56: 696–708. [DOI] [PubMed] [Google Scholar]
- 11. Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Investig 2010; 1: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes 2013; 62: 3316–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holst JJ. The physiology of glucagon‐like peptide 1. Physiol Rev 2007; 87: 1409–1439. [DOI] [PubMed] [Google Scholar]
- 14. Elrick H, Stimmler L, Hlad CJ Jr, et al Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 1964; 24: 1076–1082. [DOI] [PubMed] [Google Scholar]
- 15. McIntyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet 1964; 2: 20–21. [DOI] [PubMed] [Google Scholar]
- 16. Takemura J, Seino Y, Yamamura T, et al The role of endogenous gastric inhibitory polypeptide in the enteroinsular axis. J Clin Endocrinol Metab 1982; 54: 909–913. [DOI] [PubMed] [Google Scholar]
- 17. Vilsboll T, Krarup T, Madsbad S, et al Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia 2002; 45: 1111–1119. [DOI] [PubMed] [Google Scholar]
- 18. Vilsboll T, Krarup T, Deacon CF, et al Reduced postprandial concentrations of intact biologically active glucagon‐like peptide 1 in type 2 diabetic patients. Diabetes 2001; 50: 609–613. [DOI] [PubMed] [Google Scholar]
- 19. Calanna S, Christensen M, Holst JJ, et al Secretion of glucagon‐like peptide‐1 in patients with type 2 diabetes mellitus: systematic review and meta‐analyses of clinical studies. Diabetologia 2013; 56: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nauck MA, Vardarli I, Deacon CF, et al Secretion of glucagon‐like peptide‐1 (GLP‐1) in type 2 diabetes: what is up, what is down? Diabetologia 2011; 54: 10–18. [DOI] [PubMed] [Google Scholar]
- 21. Zander M, Madsbad S, Madsen JL, et al Effect of 6‐week course of glucagon‐like peptide 1 on glycaemic control, insulin sensitivity, and beta‐cell function in type 2 diabetes: a parallel‐group study. Lancet 2002; 359: 824–830. [DOI] [PubMed] [Google Scholar]
- 22. Hojberg PV, Vilsboll T, Rabol R, et al Four weeks of near‐normalisation of blood glucose improves the insulin response to glucagon‐like peptide‐1 and glucose‐dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2009; 52: 199–207. [DOI] [PubMed] [Google Scholar]
- 23. Aulinger BA, Bedorf A, Kutscherauer G, et al Defining the role of GLP‐1 in the enteroinsulinar axis in type 2 diabetes using DPP‐4 inhibition and GLP‐1 receptor blockade. Diabetes 2014; 63: 1079–1092. [DOI] [PubMed] [Google Scholar]
- 24. Aaboe K, Knop FK, Vilsboll T, et al Twelve weeks treatment with the DPP‐4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non‐glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2010; 12: 323–333. [DOI] [PubMed] [Google Scholar]
- 25. Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig 2015; 6: 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Umezawa S, Kubota A, Maeda H, et al Two‐year assessment of the efficacy and safety of sitagliptin in elderly patients with type 2 diabetes: post hoc analysis of the ASSET‐K study. BMC Endocr Disord 2015; 15: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen TY, Hsieh CJ. Real‐world effectiveness of sitagliptin as add‐on therapy in patients with type 2 diabetes mellitus. Postgrad Med 2014; 126: 205–215. [DOI] [PubMed] [Google Scholar]
- 28. Hsieh CJ, Shen FC. The durability of sitagliptin in elderly patients with type 2 diabetes. Clin Interv Aging 2014; 9: 1905–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yabe D, Kuwata H, Nishikino R, et al Use of the Japanese health insurance claims database to assess durability of DPP‐4 inhibitors in patients with diabetes: comparison with other anti‐diabetic drugs. Diabetologia 2015; 58(Suppl. 1): 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yabe D, Kuroe A, Watanabe K, et al Early phase glucagon and insulin secretory abnormalities, but not incretin secretion, are similarly responsible for hyperglycemia after ingestion of nutrients. J Diabetes Complications 2015; 29: 413–421. [DOI] [PubMed] [Google Scholar]
- 31. Vollmer K, Holst JJ, Baller B, et al Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008; 57: 678–687. [DOI] [PubMed] [Google Scholar]
- 32. Iwasaki M, Hoshian F, Tsuji T, et al Predicting efficacy of DPP‐4 inhibitors in patients with type 2 diabetes: association of HbA1c reduction with serum eicosapentaenoic acid and docosahexaenoic acid levels. J Diabetes Investig 2012; 3: 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Senmaru T, Fukui M, Kobayashi K, et al Dipeptidyl‐peptidase IV inhibitor is effective in patients with type 2 diabetes with high serum eicosapentaenoic acid concentrations. J Diabetes Investig 2012; 3: 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Phillips LK, Deane AM, Jones KL, et al Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol 2014; 11: 112–128. [DOI] [PubMed] [Google Scholar]
- 35. Kuwata H, Iwasaki M, Shimizu S, et al Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: a randomized, controlled cross‐over, exploratory trial. Diabetologia 2016; 59: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seino Y, Yabe D. Glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐1: incretin actions beyond the pancreas. J Diabetes Investig 2013; 4: 108–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kubota A, Yabe D, Kanamori A, et al Factors influencing the durability of the glucose‐lowering effect of sitagliptin combined with a sulfonylurea. J Diabetes Investig 2014; 5: 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanamori A, Matsuba I. Factors associated with reduced efficacy of sitagliptin therapy: analysis of 93 patients with type 2 diabetes treated for 1.5 years or longer. J Clin Med Res 2013; 5: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yabe D, Seino Y. Dipeptidyl peptidase‐4 inhibitors and sulfonylureas for type 2 diabetes: friend or foe? J Diabetes Investig 2014; 5: 475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mukai E, Fujimoto S, Sato H, et al Exendin‐4 suppresses SRC activation and reactive oxygen species production in diabetic Goto‐Kakizaki rat islets in an Epac‐dependent manner. Diabetes 2010; 60: 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang CL, Katoh M, Shibasaki T, et al The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 2009; 325: 607–610. [DOI] [PubMed] [Google Scholar]
- 42. Inzucchi SE, Nauck MA, Hehnke U, et al Improved glucose control with reduced hypoglycaemic risk when linagliptin is added to basal insulin in elderly patients with type 2 diabetes. Diabetes Obes Metab 2015; 17: 868–877. [DOI] [PubMed] [Google Scholar]
- 43. Hong ES, Khang AR, Yoon JW, et al Comparison between sitagliptin as add‐on therapy to insulin and insulin dose‐increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab 2012; 14: 795–802. [DOI] [PubMed] [Google Scholar]
- 44. Taminato T, Seino Y, Goto Y, et al Synthetic gastric inhibitory polypeptide. Stimulatory effect on insulin and glucagon secretion in the rat. Diabetes 1977; 26: 480–484. [DOI] [PubMed] [Google Scholar]
- 45. Christensen M, Vedtofte L, Holst JJ, et al Glucose‐dependent insulinotropic polypeptide: a bifunctional glucose‐dependent regulator of glucagon and insulin secretion in humans. Diabetes 2011; 60: 3103–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ahren B, Schweizer A, Dejager S, et al Vildagliptin enhances islet responsiveness to both hyper‐ and hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94: 1236–1243. [DOI] [PubMed] [Google Scholar]
- 47. Yabe D, Kuwata H, Kaneko M, et al Use of the Japanese health insurance claims database to assess the risk of acute pancreatitis in patients with diabetes: comparison of DPP‐4 inhibitors with other oral antidiabetic drugs. Diabetes Obes Metab 2015; 17: 430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lai YJ, Hu HY, Chen HH, et al Dipeptidyl peptidase‐4 inhibitors and the risk of acute pancreatitis in patients with type 2 diabetes in Taiwan: a Population‐Based Cohort Study. Medicine 2015; 94: e1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abbas AS, Dehbi HM, Ray KK. Cardiovascular and non‐cardiovascular safety of dipeptidyl peptidase‐4 inhibition: a meta‐analysis of randomised controlled cardiovascular outcome trials. Diabetes Obes Metab 2016; 18: 295–209. [DOI] [PubMed] [Google Scholar]
- 50. Yabe D, Kuwata H, Iwasaki M, et al Why are incretin‐based therapies more efficient in East Asians? Perspectives from the pathophysiology of type 2 diabetes and East Asian dietary habits. European Medical Journal 2015; 3: 57–65. [Google Scholar]


