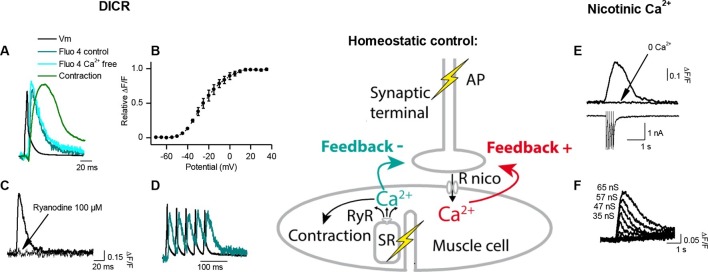
Figure 2. Calcium signaling in Xenopus muscle cell and synaptic homeostasis.
The middle scheme depicts our model of homeostatic control of the synaptic strength. The nicotinic Ca2+ influx elicits a positive retrograde feedback signal (red arrow) on the presynaptic neurotransmitter release. The positive feedback is balanced by a negative retrograde feedback signal (blue arrow) triggered by the muscle AP-induced DICR. (DICR = depolarization-induced calcium release, AP = action potential, RyR = ryanodine receptor, SR = sarcoplasmic reticulum, R nico = nicotinic receptors). (A) Independence of the DICR signal from external Ca2+. AP (black trace, induced with a brief postsynaptic current step), Ca2+ dye (Fluo4) relative fluorescence in standard external medium (dark blue trace) and in Ca2+ free medium (light blue trace), and qualitative measure of the cell contraction (green trace, see Materials and methods). (B) Voltage-dependence of the DICR signal. The Fluo4 fluorescence was measured at the steady-state of the Ca2+ signal during a classical voltage-step protocol from a holding potential of -80 mV and averaged (n = 3 cells). (C) Blockade of the DICR signal by loading the muscle cell with 100 µM ryanodine. (D) Representative example of the DICR signal (blue trace) during repetitive muscle APs (black trace). (E) Ca2+ build-up upon nicotinic receptor activation. Fluo4 signal (upper traces) in control and in Ca2+ free medium, during iontophoretic ACh applications (5 pulses) under postsynaptic voltage-clamp (-80 mV). The lower trace shows the nicotinic currents. (F) Dependence of the nicotinic Ca2+ build-up on the nicotinic conductance with increasing iontophoretic ACh applications at a constant membrane potential (holding potential –80 mV).