Abstract
Little is known about variability in primary care providers’ (PCPs) adherence to opioid-monitoring guidelines for patients. We examined variability of adherence to monitoring guidelines among PCPs and ascertained the relationship between PCP adherence and opioid misuse by their patients. We included primary care patients receiving long-term opioids (≥3 prescriptions within 6 months) for chronic noncancer pain and PCPs with ≥4 eligible patients. We examined guideline adherence using: (1) electronic health record documentation of opioid treatment agreement, (2) past-year urine drug screen (UDS), and (3) evidence of misuse through early refills (≥2 opioid prescriptions written 7–25 days after the previous prescription). Covariates included morphine equivalent daily opioid medication dose (MED, >50 mg/d vs ≤50mg/d). Multilevel regression models assessed variability among PCPs, and odds ratios examined associations among patient-level binary outcomes. Sixty-seven PCPs prescribed opioids to 1546 patients. Significant variability was found between PCPs in use of agreement (variance = 1.27, P<0.001), UDS (variance = 1.75, P <0.001), and early refills (variance = 0.29, P = 0.002). Primary care providers had a mean of 48% of patients with agreement (range, 9%–84%), 56% with ≥1 UDS (range, 7%–91%) and 36% with early refills (range, 19%–60%). High MED among patients was associated with increased odds of agreement (1.93, confidence interval [CI], 1.53–2.44), UDS (2.65, CI: 2.06–3.41), and early refill (2.92, CI: 2.30–3.70). Primary care providers varied significantly in adherence to opioid prescription guidelines. Increased patient risk was associated with increased monitoring and with greater misuse. Future work should study system-level interventions to enable clinical monitoring and support opioid guideline adherence.
Keywords: Chronic pain, Substance abuse, Primary care
1. Introduction
Opioid misuse continues to be a major public health problem in the United States, causing 16,651 deaths in 2010.12 As the number of opioid prescriptions has increased since 1980,3,8,18 so have opioid misuse and opioid-related deaths.12,19,22 Physician prescriptions are among the most common sources of misused opioids.6,10,20
The American Pain Society and the American Academy of Pain Medicine have published guidelines with monitoring strategies for patients on chronic opioids for chronic noncancer pain.5 The guidelines recommend written documentation in the form of an “agreement” and periodic urine drug screens (UDS).5 The guidelines also recommend that level of patient risk for misuse, addiction, and diversion of opioids inform intensity of monitoring. For example, low-risk patients would require monitoring every 3 to 6 months, whereas the highest risk patients would require up to weekly monitoring.5
Despite these recommendations, current evidence indicates low prevalence of adherence to opioid prescribing guidelines even for high-risk patients.16,21 Little is known about differences in practice between individual primary care providers (PCPs) regarding opioid prescribing and monitoring of patients with chronic pain. Information about practice variability, along with information about the association between guideline adherence by a PCP and patient misuse of opioids, is crucial to understand how providers can decrease opioid misuse among primary care patients.
To inform future interventions, we examined variability among PCPs in adherence to opioid management guidelines in the form of agreements and UDS, to understand whether PCP-level or patient-level characteristics account for any observed variability. We hypothesized that higher use of UDS and agreements by PCPs would be associated with a lower level of opioid misuse by their patients.
2. Methods
2.1. Study design overview
We conducted a 12-month retrospective cohort study using electronic health record (EHR) data to estimate adherence to opioid prescribing guidelines among PCPs and potential medication misuse by their patients on long-term opioid therapy for chronic noncancer pain at 3 safety-net primary care clinics.
2.2. Patient population
We obtained clinical data from September 1, 2011 to August 31, 2012 from 2 federally qualified community health centers (Internal Medicine and Family Medicine) and 1 hospital-based primary care center (Internal Medicine) in Boston, MA. The PCPs studied included residents, attending physicians, and nurse practitioners.
2.3. Inclusion and exclusion criteria
We included patients if they were aged 18 to 89, had at least 1 visit to the practice during the study period, and were on long-term opioid therapy. We defined long-term opioid therapy as 3 or more opioid analgesic prescriptions written >21 days apart within a 6-month period. Opioid prescriptions included codeine, fentanyl, hydromorphone, meperidine, methadone, morphine, oxycodone, propoxyphene, codeine/acetaminophen, codeine/ aspirin, hydrocodone/acetaminophen, hydrocodone/ibuprofen, oxycodone/acetaminophen, oxycodone/ibuprofen, propoxyphene/ aspirin, and propoxyphene/acetaminophen. We included PCPs at the sites if they had at least 4 eligible patients.
We excluded patients if they were receiving active treatment for cancer, except nonmelanoma skin cancer. We identified cancer using ICD-9 codes. We excluded patients with any tumor (ICD 140–172.9, 174–195.8), leukemia (ICD 204–208.9), lymphoma (ICD 200–203.8), or metastatic solid tumors (ICD 196–199.1) and 3 or more visits to the hematology–oncology clinic at the affiliated hospital in the past year.
2.4. Data
We searched for EHR data from the practice sites stored in 2 clinical data warehouses. We extracted the data into de-identified files for statistical analysis. The Institutional Review Board at Boston Medical Center approved this study.
2.5. Outcome variables
We measured evidence of guideline adherence through: (1) EHR documentation of an opioid treatment agreement (agreement) ever (yes/no), and (2) UDS in the past 12 months (yes/no). We likely captured all agreements by this method because agreements became the standard of care after implementation of the EHR in 2000. For the analysis, 1 or more UDS within the same month beyond the index UDS were excluded as they were not likely to reflect periodic UDS as recommended in expert guidelines.4
Evidence of misuse was a binary outcome of 2 or more early opioid refills. We defined an early refill as a prescription written 7 to 25 days after the previous prescription for the same medication. Prescribers at the study sites generally write opioid prescriptions 28 to 30 days apart for stable refills. We assumed that a refill within 7 days was likely to be a reprint of the original prescription or an intentional change in therapy and not a true early refill. To verify that early refill was an accurate marker of misuse, we performed 200 total chart reviews of 100 individual patient records at the larger site and 50 at each of the 2 smaller sites. These chart reviews identified that 80% of the early refills did not have associated documentation indicating an intentional medical decision on the part of the prescriber explaining early refill (eg, the patient needed a vacation supply or the patient had an acute problem requiring additional medication). Thus, these unexplained early refills suggest opioid misuse, and a history of 2 or more early refills would increase the likelihood that the refills reflected opioid misuse. We counted early refills based on the date the prescription was written, and not on the date that it was filled.
2.6. Covariates
Covariates included patient demographics (patient age on September 1, 2011, gender, race, language, primary insurance type per EHR), number of primary care visits with PCPs at the study site in the past year, number of emergency department (ED) visits in the past year, number of patient risk factors for opioid misuse (age <45; drug use disorder, alcohol use disorder, tobacco use, and mental health disorder), PCP type (attending, resident, nurse practitioner), and site of care. Mental health disorders were identified by ICD-9 codes,13 as used in previous studies.21 We used an additional covariate, morphine equivalent daily opioid medication dose (MED), which we defined as follows: we divided the number of pills or patches in each prescription by the number of days from 1 prescription until the subsequent prescription, giving the number of pills per day.7 We then multiplied the daily pills by the milligrams of opioid in each pill or patch. We converted the total amount into the morphine equivalent daily opioid dose through an online conversion tool.14 We used the cutoff of greater than 50 mg MED to dichotomize this covariate (high MED vs low MED), with high MED indicating “high risk” since doses of 50 mg or more of MED are associated with an increased risk of intentional and unintentional overdose.7
2.7. Statistical analysis
To compare the 3 sites, we used χ2 tests for categorical variables and analysis of variance (F-statistics) for continuous variables. We calculated odds ratios to examine the associations among patient-level binary outcomes including agreement (yes vs no), UDS (yes vs no), and early refill (yes vs no). To ascertain whether high-risk patients, as designated by high MED, also received more intense monitoring, χ2 tests and adjusted odds ratios (aOR) were performed to determine the relationship between high-dose MED and 2 or more UDS, and between high-dose MED and early refills. We calculated aOR to control for patient sex, race, and risk factors. We used Pearson correlations to examine the relationships among PCP-level aggregates of patient outcomes within each PCP. To examine variability of opioid monitoring between individual PCPs, we used multilevel modeling to account for patient clustering within PCPs and examined variance attributable to PCP characteristics. We used a series of stepwise adjustment of covariates to evaluate the portions of outcome variance attributable to patient-level vs PCP-level characteristics. For hypothesis testing, we used a type I error rate of alpha = 0.05 and corresponding 95% confidence intervals (CIs). We performed all calculations using SAS software, version 9.3 (Cary, NC).
3. Results
Table 1 shows the characteristics of the 1546 patients who were cared for by 67 PCPs. The average age of the patients was 54.2 years, and there were more women than men (P < 0.01). Non- Hispanic white was the most common patient race (47%), although this rate varied significantly across the 3 sites from 95%, 63%, and 32% for sites 1 to 3, respectively (P < 0.001). At site 3, non-Hispanic black was the most common race (53%). The average number of patient risk factors for prescription opioid misuse was 1.7. Most patients had nonprivate insurance (84%), with little variation across the 3 sites. Medicare was the most common type of nonprivate insurance. Most patients had visited their PCP more than 4 times in the last year, and 47% had visited their PCP more than 7 times. Most patients had not visited the ED in the last year (60%), although 9% had 4 or more visits to the ED. Site 3 had more patients visit the ED than the other 2 sites, with 52% having visited the ED at least once, compared with 13% and 16% for sites 1 and 2, respectively. Seventy-five percent of patients were on less than 50 mg MED, whereas 11% of patients were prescribed more than 100 mg MED per day.
Table 1.
Patient and provider characteristics, overall and stratified by sites.
| Patients characteristics | Overall N = 1546 | Site 1 n = 271 | Site 2 n = 186 | Site 3 n = 1089 | P* |
|---|---|---|---|---|---|
| Average age | 54.2 | 51.8 | 55.3 | 54.7 | <0.01 |
| Sex, n (%) | |||||
| Female | 799 (52) | 151 (56) | 105 (56) | 543 (50) | 0.09 |
| Race, n (%) | |||||
| Non-Hispanic white | 728 (47) | 257 (95) | 118 (63) | 353 (32) | <0.001 |
| Non-Hispanic black | 624 (40) | 7 (3) | 43 (23) | 574 (53) | |
| Hispanic | 113 (7) | 0 (0) | 4 (2) | 109 (10) | |
| Other | 81 (5) | 7 (3) | 21 (11) | 53 (5) | |
| Average number of risk factors† | 1.7 | 1.2 | 1.4 | 1.9 | <0.001 |
| Language, n (%) | |||||
| English | 1423 (92) | 263 (97) | 165 (89) | 995 (92) | <0.001 |
| Non-English | 120 (8) | 8 (3) | 21 (11) | 91 (8) | |
| Insurance, n (%) | |||||
| Medicaid | 468 (30) | 69 (25) | 56 (30) | 343 (32) | <0.001 |
| Medicare | 309 (20) | 85 (31) | 73 (39) | 151 (14) | |
| Dual-eligible | 408 (26) | 12 (4) | 11 (6) | 385 (35) | |
| Private | 245 (16) | 71 (26) | 26 (14) | 148 (14) | |
| Others | 116 (8) | 34 (13) | 20 (11) | 62 (6) | |
| PCP visits, past year, n (%) | |||||
| 1–3 | 267 (17) | 59 (22) | 11 (6) | 197 (18) | <0.001 |
| 4–6 | 551 (36) | 89 (33) | 35 (19) | 427 (39) | |
| >7 | 728 (47) | 123 (45) | 140 (75) | 465 (43) | |
| ED visits, past year, n (%) | |||||
| 0 | 920 (60) | 235 (87) | 157 (84) | 528 (48) | <0.001 |
| 1–3 | 494 (32) | 32 (12) | 28 (15) | 434 (40) | |
| 4–6 | 87 (6) | 4 (1) | 1 (1) | 82 (8) | |
| >7 | 45 (3) | 0 (0) | 0 (0) | 45 (4) | |
| MED‡ | |||||
| <50 mg | 1165 (75) | 238 (88) | 152 (82) | 775 (71) | <0.0001 |
| 50–100 | 206 (13) | 23 (8) | 20 (11) | 163 (15) | |
| >100 | 175 (11) | 10 (4) | 14 (8) | 151 (14) | |
| Provider characteristics | Overall (N = 67) | Site 1 (n = 10) | Site 2 (n = 12) | Site 3 (n = 45) | |
|---|---|---|---|---|---|
| MD/physician | 53 (80) | 8 (80) | 10 (83) | 35 (78) | 0.25 |
| Nurse practitioner | 7 (10) | 2 (20) | 2 (17) | 3 (7) | |
| Resident | 7 (10) | 0 (0) | 0 (0) | 7 (16) |
χ2 test for categorical variables and analysis of variance for continuous variables across 3 sites.
Opioid misuse risk factors (age <45, drug use disorder, alcohol use disorder, tobacco use, mental health disorder as documented in the electronic medical record).
Average daily dose of morphine equivalent (mg).
ED, emergency department; MED, morphine equivalent daily opioid medication dose, calculated from pills per day and converted with an online conversion tool14; PCP, primary care providers.
Of the PCPs included in the analysis, 80% were attending physicians, 10% were nurse practitioners, and 10% were resident physicians. All resident physicians practiced at site 3, and sites 1 and 2 had similar proportions of attending physicians and nurse practitioners.
Tables 2 and 3 show prevalence of guideline adherence outcomes of agreements, UDS, and potential misuse outcome of early refill, based on patient-level (Table 2) and PCP-level (Table 3) analysis. Forty-four percent of patients had an agreement, with no difference across sites (P = 0.27). Thirty-six percent of patients had early refills, with no difference across sites (P = 0.51). Patient-level prevalence of UDS varied the most among the 3 sites, with an overall prevalence of 56% (P<0.0001) and a range of 24% to 67%.
Table 2.
Patient-level prevalence of guideline adherence and potential misuse outcomes.
| Patient adherence prevalence | Total (N = 1546), n (%) | Site 1 (n = 271), n (%) | Site 2 (n = 186), n (%) | Site 3 (n = 1089), n (%) | P* |
|---|---|---|---|---|---|
| Opioid treatment agreement† | 679 (44) | 122 (45) | 91 (49) | 466 (43) | 0.27 |
| Urine drug screen‡ | 861 (56) | 65 (24) | 68 (37) | 728 (67) | <0.0001 |
| Early refills§ | 555 (36) | 105 (39) | 63 (34) | 387 (36) | 0.51 |
χ2 test for categorical variables and analysis of variance for continuous variables across 3 sites.
Electronic health record documentation of an opioid treatment agreement ever.
Urine Drug Screen in the past 12 mo.
An opioid prescription written 7 to 25 d after the previous prescription for the same medication.
Table 3.
Proportions of patients within each primary care providers (PCP) with guideline adherence and potential misuse outcomes.
| PCP adherence proportions* | Total (N = 67) mean % ± SD (range) | Site 1 (n = 10) mean % ± SD (range) | Site 2 (n = 12) mean % ± SD (range) | Site 3 (n = 45) mean % ± SD (range) | P† |
|---|---|---|---|---|---|
| Opioid treatment agreement‡ | 48 ± 26 (0–100) | 59 ± 25 (20–83) | 51 ± 23 (0–75) | 45 ± 27 (0–100) | 0.30 |
| Urine drug screen§ | 56 ± 29 (0–100) | 31 ± 27 (4–92) | 35 ± 27 (0–71) | 67 ± 23 (0–100) | <0.0001 |
| Early refills|| | 36 ± 20 (0–75) | 41 ± 16 (10–67) | 25 ± 23 (0–57) | 38 ± 19 (5–75) | 0.09 |
Proportion of each individual PCP’s patients with each outcome.
χ2 test for categorical variables and analysis of variance for continuous variables across 3 sites.
Electronic health record documentation of an opioid treatment agreement ever.
Urine drug screen in the past 12 mo.
An opioid prescription written 7 to 25 d after the previous prescription for the same medication.
In Table 3, the mean PCP-level proportion of patient agreements was 48%, without significant site differences (P = 0.30). Each PCP had an average of 36% of patients with early refills, with marginally statistically significant difference across sites (P = 0.09). Each PCP had a mean proportion of 56% of patients with UDS, with significant variability between the 3 sites (P < 0.0001).
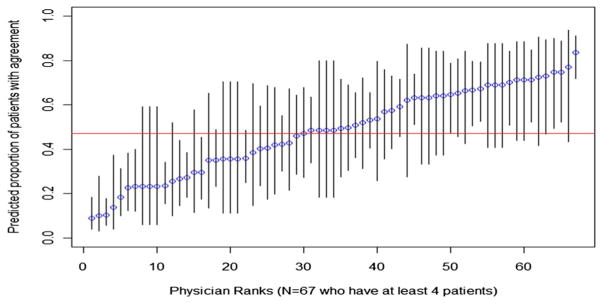
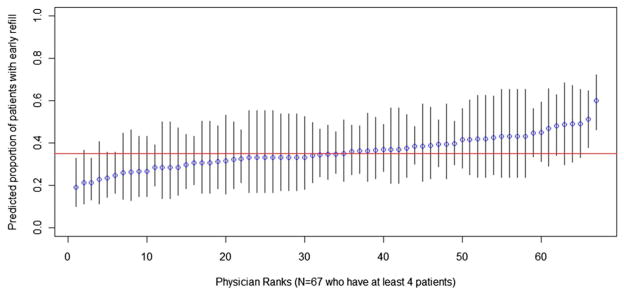
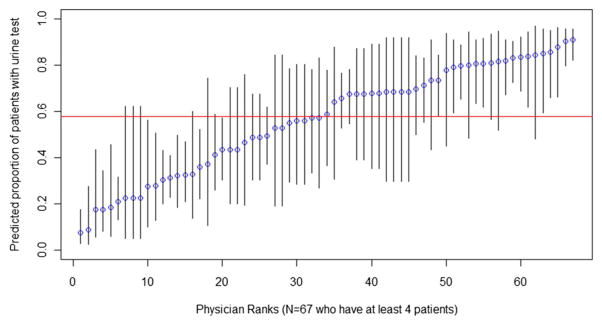
Figures 1–3 show the percentage of patients within each PCP with agreement (Fig. 1), UDS (Fig. 2), and early refill (Fig. 3), and the 95% CI for the estimates. In Figure 1, predicted proportions of agreement ranged from 9% to 84%. In Figure 2, predicted proportions of UDS ranged from 7% to 91%. In Figure 3, predicted proportions of early refill ranged from 19% to 60%.
Figure 1.
Primary care providers (PCP)-specific predicted proportion of patients with agreement. The percent of patients with agreement for each physician and 95% confidence intervals for that predicted proportion, arranged in order of physician rank. Variance between PCPs in use of agreement was 1.27, SE = 0.25, P < 0.001.
Figure 3.
Primary care providers (PCP)-specific predicted proportion of patients with early refill. The percent of patients with 2 or more early refills for each physician and 95% confidence intervals for that predicted proportion, arranged in order of physician rank are shown. Variance between PCPs in 2 or more early refills was 0.29, SE = 0.10, P = 0.002.
Figure 2.
Primary care providers (PCP)-specific predicted proportion of patients with urine drug screen (UDS). The percent of patients with at least 1 UDS for each physician and 95% confidence intervals for that predicted proportion, arranged in order of physician rank. Variance between PCPs in use of UDS was 1.75, SE = 0.35, P<0.001.
Based on the multilevel random intercepts model, we observed statistically significant variability among the 3 outcomes of agreement (variance = 1.27, SE = 0.25, P < 0.001) and UDS (variance = 1.75, SE = 0.35, P < 0.001), as well as early refills (variance = 0.29, SE = 0.10, P = 0.002). Primary care providers had a mean of 48% of patients with agreement (range, 9%–84%), and 56% with>1 UDS (range, 7%–91%) and 36% with early refills (range, 19%–60%). There were significantly greater variation of PCPs’ agreements (F66,66 = 4.38, P < 0.01) and UDS (F66,66 = 6.03, P<0.01) as compared with the variance of early refill. There was a marginal difference (F66,66 = 1.38, P = 0.10) comparing the variances of UDS and agreement. The variance estimate for UDS decreased from 1.75 to 0.87 (P < 0.001) after adjustment for patient-level covariates, PCP type, and site of care. Adjustment for patient-level covariates only decreased the variance from 1.75 (P < 0.001) to 1.14 (P < 0.001); further adjustment for PCP type did not decrease the variance. Adjustment by site decreased the variance to 0.88 (P < 0.001).
Among patient-level outcomes (N = 1546), high-dose MED was associated with increased odds of agreement (aOR = 1.57, 95% CI: 1.22, 2.02), UDS (aOR = 2.41, 95%CI: 1.79, 3.25), and early refill (aOR = 2.58, 95% CI: 2.01, 3.31). More patients on high-dose MED had early refills compared with those on low-dose MED (55% vs 30%, P<0.0001). Patients with agreements had increased odds of having an early refill (aOR = 1.40, 95% CI: 1.12, 1.75) and substantially higher odds of UDS (aOR = 8.29, 95% CI: 6.23, 11.03). The presence of early refills increased the odds of a patient having UDS (aOR = 1.88, 95% CI: 1.46, 2.43). Patients on high-dose MED had an increased odds of receiving >2 UDS, after adjustment for gender, race, and risk factors for opioid misuse (aOR = 2.05, 95% CI: 1.53, 2.75). Results from the χ2 test confirmed that patients on high-dose MED were more likely to have 2 or more UDS than those on low-dose MED, and approximately half of the patients on high-dose MED had 2 or more UDS (55% vs 35%, P < 0.0001).
Based on PCP-level aggregates of patient outcomes within each PCP (N = 67), early refills showed no correlation with agreements (r = −0.05, P = 0.71). Urine drug screen showed a moderate correlation with agreements (r = 0.34, P = 0.005) and a weaker correlation with early refills (r = 0.27, P = 0.03). High-dose MED showed a weak correlation with agreements (r = 0.18, P = 0.14), a moderate correlation with early refills (r = 0.38, P = 0.002), and a moderate correlation with UDS (r = 0.46, P < 0.0001).
4. Discussion
We found significant variability between PCPs in guideline adherence and in provisions of early refills to patients, which are suggestive of patient misuse. We also found that higher daily doses of opioids were associated with increased odds of guideline adherence by PCPs in the form of agreements and urine drug screening, as well as potential misuse by patients in the form of early refills. We found no evidence that PCP use of urine drug screening and agreement was associated with decreased markers for potential misuse.
Our findings are consistent with previous studies that have found low guideline adherence by PCPs even among high-risk patients.16,21 Starrels et al.21 reported a 24% prevalence of UDS in high-risk patients and Morasco et al.16 reported a 47% prevalence of UDS among patients with substance use disorder (SUD) and 18% among patients without SUD We report that 56% of patients received at least 1 UDS across the 3 sites, which is higher than that found in other studies. This finding may be explained in part by a higher number of opioid misuse risk factors (1.7), compared with previous studies in which 71.2% of patients had 1 or fewer risk factors.21 It is likely that temporal trends related to increased awareness of the opioid epidemic contributed to higher guideline adherence.11 Our findings add to the literature that there is substantial variability in practice patterns among individual PCPs and practice sites. Practice site patterns seem to influence the use of urine drug screening in particular. Additionally, we observed that although PCPs have better guideline adherence for high-risk patients relative to low-risk patients, there is also greater potential for misuse among such patients.
One notable finding is that site 3, despite having significantly more UDSs than the other sites, did not have a lower rate of early refills. Similarly, patients on higher doses of opioid as determined by morphine daily opioid dose equivalent, seem to have both more UDSs and early refills. This phenomenon suggests a complex relationship between urine drug screening and opioid prescribing. It would be important to understand how urine drug screening was used by examining the timing of early refills in relationship to urine screening. For example, we do not know whether early refills precede urine drug screening because the physician saw an early refill and then ordered a UDS, or vice versa. Because current guidelines for monitoring patients on opioids are based on expert opinion,17 developing empirical evidence for standard opioid monitoring and prescribing practices that reduce misuse is an essential next step. Although opioid prescribing guidelines are only one of the numerous initiatives addressing opioid overdose and misuse, 1,2,9 physician prescribing is one of the largest sources of opioid availability. The large-scale Risk Evaluation and Mitigation Strategy (REMS) program, which has been promulgated by the Food and Drug Administration to educate prescribers of long-acting opioids in an attempt to halt the opioid epidemic,23 is premised on the effectiveness of opioid-prescribing guidelines. If implementation of guidelines is not effective at curbing misuse of opioids, then efforts to decrease opioid misuse and overdose need to be re-engineered.
Our study has several limitations. First, all sites were safety-net clinics serving an urban population in 1 city, therefore results may not be generalizable to other settings. Second, this is a cross-sectional study, and observed associations do not imply causality. Third, we did not collect information on visits to outside facilities for early refills or UDSs, and consequently our results may be an underestimate of the prevalence of these outcomes among this population. Fourth, because a UDS can be ordered by any provider using the EHR, including inpatient providers, emergency room providers, or specialists, the UDS in our study may not represent only those ordered by PCPs and may not accurately characterize the practice of these clinicians. These UDS were immunoassays performed at safety-net hospital-affiliated community health centers or at a hospital-based primary care practice, and thus there was no financial incentive to test more frequently than necessary. In other settings where frequent UDS would result in more frequent reimbursement, increased monitoring with UDS may be a false measure of guideline adherence. Last, early refills are difficult to estimate, and they are an imperfect measure of patient opioid misuse. Early refills may overestimate opioid misuse in some cases because of provision of more frequent prescriptions related to more intensive monitoring. Additionally, there may have been a discrepancy between the date the prescription was written and the date the prescription was filled by the patient, and this study only captured the date prescriptions were written. It is also true that if a provider grants a request and the patient fills the prescription early, presumably there was medical decision making involved, and this was the appropriate course of action. However, we surmise there is a combination of appropriate medical decision making and patient misuse occurring, because in the 200 chart reviews done for this study, clinician documentation did not explain the majority of early refills. Of note, early refills are one of many aberrant behaviors that can indicate misuse or addiction,15 and using that as a proxy likely underestimates misuse.
In conclusion, our study found significant variability among PCPs in the use of opioid treatment agreements and UDSs, and a significant association between the use of agreements and early refills. We not only found that high-risk patients are more likely to receive intensive UDS monitoring but also more likely to demonstrate potential misuse. Future interventions should target system-level change to assist PCPs with opioid-monitoring guideline adherence. Understanding the timing of UDS and early refills would help determine the role and effectiveness of agreements and urine drug screening in the clinical practice of opioid monitoring.
Acknowledgments
Supported by a grant from the National Institute on Drug Abuse R01DA034252.
The authors acknowledge Linda Rosen, the clinical data warehouse research manager, for help with data extraction for this article. We also thank the participants in the study.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- 1.AMDG, editor. Interagency Guideline on Opioid Dosing for Chronic Non-cancer Pain. Washington State American Medical Directors; Olympia, WA: 2010. [Google Scholar]
- 2.Ballantyne JC, Sullivan MD, Kolodny A. Opioid Dependence vs Addiction: A Distinction Without a Difference? Arch Intern Med. 2012;172:1342–3. doi: 10.1001/archinternmed.2012.3212. [DOI] [PubMed] [Google Scholar]
- 3.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs 2000. PAIN. 2004;109:514–9. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miakowski C. Clinical guidelines for the use of chronic opioid therapy. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cicero TJ, Kurtz SP, Surratt HL, Ibanez GE, Ellis MS, Levi-Minzi MA, Inciardi JA. Multiple Determinants of Specific Modes of Prescription Opioid Diversion. J Drug Issues. 2011;41:283–304. doi: 10.1177/002204261104100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilson AM, Ryan KM, Joranson DE, Dahl JL. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997–2002. J Pain Symptom Manage. 2004;28:176–88. doi: 10.1016/j.jpainsymman.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Green TC, Mann MR, Bowman SE, Zaller N, Soto X, Gadea J, Cordy C, Kelly P, Friedmann PD. How does use of a prescription monitoring program change medical practice? Pain Med. 2012;13:1314–23. doi: 10.1111/j.1526-4637.2012.01452.x. [DOI] [PubMed] [Google Scholar]
- 10.Inciardi JA, Surratt HL, Cicero TJ, Beard RA. Prescription opioid abuse and diversion in an urban community: the results of an ultrarapid assessment. Pain Med. 2009;10:537–48. doi: 10.1111/j.1526-4637.2009.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson H, Paulozzi LJ, Porucznik C, Mack K, Herter B. Decline in drug overdose deaths after state policy changes- Florida, 2010–2012. MMWR Morb Mortal Wkly Rep. 2014;26:569–74. [PMC free article] [PubMed] [Google Scholar]
- 12.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–9. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 13.Liebschutz JM, Saitz R, Weiss RD, Averbuch T, Schwartz S, Meltzer EC, Claggett-Borne E, Cabral H, Samet JH. Clinical factors associated with prescription drug use disorder in urban primary care patients with chronic pain. J Pain. 2010;11:1047–55. doi: 10.1016/j.jpain.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAuley D. [Accessed September 9, 2014];Advanced Opioid Converter. Available at: http://www.globalrph.com/opioidconverter2.htm.
- 15.Meltzer EC, Rybin D, Meshesha LZ, Saitz R, Samet JH, Rubens SL, Liebschutz JM. Aberrant drug-related behaviors: unsystematic documentation does not identify prescription drug use disorder. Pain Med. 2012;13:1436–43. doi: 10.1111/j.1526-4637.2012.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J Gen Intern Med. 2011;26:965–71. doi: 10.1007/s11606-011-1734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuckols TK, Anderson L, Popescu I, Diamant AL, Doyle B, Di Capua P, Chou R. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47. doi: 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen Y, Daumit GL, Ford DE. Opioid prescriptions by U.S. primary care physicians from 1992 to 2001. J Pain. 2006;7:225–35. doi: 10.1016/j.jpain.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15:618–27. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 20.Rigg KK, Kurtz SP, Surratt HL. Patterns of prescription medication diversion among drug dealers. Drugs (Abingdon Engl) 2012;19:144–55. doi: 10.3109/09687637.2011.631197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011;26:958–64. doi: 10.1007/s11606-011-1648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner M, Chen LH, Makuc DM, Anderson RN, Minino AM. Drug poisoning deaths in the United States, 1980–2008. NCHS Data Brief. 2011;81:1–8. [PubMed] [Google Scholar]
- 23.Willy ME, Graham DJ, Racoosin JA, Gill R, Kropp GF, Young J, Yang J, Choi J, MaCurdy TE, Worrall C, Kelman JA. Candidate metrics for evaluating the impact of prescriber education on the safe use of extended-release/ long-acting (ER/LA) opioid analgesics. Pain Med. 2014;15:12459. doi: 10.1111/pme.12459. [DOI] [PubMed] [Google Scholar]