Abstract
Graphene oxide (GO) is the most common derivative of graphene and has been used in a large range of biomedical applications. Despite considerable progress in understanding its cytotoxicity, its potential inhalation toxicity is still largely unknown. As the pulmonary surfactant (PS) film is the first line of host defense, interaction with the PS film determines the fate of the inhaled nanomaterials and their potential toxicity. Using a coarse-grained molecular dynamics model, we reported, for the first time, a novel mechanism of toxicity caused by the inhaled GO nanosheets. Upon deposition, the GO nanosheets induce pores in the PS film and thus have adverse effects on the ultrastructure and biophysical properties of the PS film. Notably, the pores induced by GO nanosheets result in increasing the compressibility of the PS film, which is an important indication of surfactant inhibition. In vitro experiments have also been conducted to study the interactions between GO and animal-derived natural PS films, qualitatively confirming the simulation results.
Graphene oxide is a derivative of graphene containing different levels of reactive oxygen functional groups. With its water solubility, functionalizability, and fluorescence quenching ability,1–4 GO is a better candidate than the pristine graphene for many biological applications such as diagnostics, imaging, and drug delivery.5–10 Therefore, there is a general biosafety concern for human exposure to GO.11,12 Many in vitro studies have investigated the cytotoxicity of GO using different cell lines. It has been shown that the toxicity of GO depends not only on the dose,13–15 but also on its size, shape, number of layers, carbon/oxygen ratio, the density of oxide functional groups on the surface, and the tested cell lines.16–19 The respiratory tract is one of the most likely paths for GO to enter the body. In vivo studies showed that GO predominantly deposited in the lungs,20 and exposure to GO causes dose-dependent pulmonary inflammation,21,22 indicating that GO inhalation may have adverse health impacts.
Due to their small size, a large portion of the inhaled GO nanosheets can get through the airways and deposit in deep lung, where the GO nanosheets must first interact with the pulmonary surfactant (PS) film at the alveolar surface.23,24 The PS is a detergent-like substance composed of approximately 90% lipids, mostly phospholipids, and 10% proteins.25,26 It plays a dual role of host defense and biophysical surface tension reduction in the lungs.25 Studies have showed that interactions between nanoparticles (NPs) and the PS film inactivate the biophysical functions of PS.27–32 Our previous molecular dynamics simulations showed that depending on their physicochemical properties, the NPs may be trapped and wrapped by the PS film, or translocate across it.29 Regardless of the translocation status, the pristine NPs will be coated with a biomolecular corona and the biophysical properties of the PS film can be significantly compromised.29,30,33 It was also found that the shape of nanomaterials strongly impacts their interaction with biomembranes as fullerene, graphene and carbon nanotube show distinctly different translocation behaviors.30,34–38 Therefore, it is not unexpected that GO, being a 2D nanomaterial, interacts with the PS film differently from spherical NPs studied in our previous work.
Here, we studied the interaction mechanisms between PS films and GO nanosheets, using coarse-grained molecular dynamics (CGMD) simulations. We established a detailed model of the PS monolayer film containing phospholipids, cholesterol, and surfactant-associated proteins. As for the GO nanosheets, we considered mono- and trilayer square GO in different sizes. We also conducted in vitro experiments to examine interactions between GO and animal-derived natural PS films. Both in silico and in vitro results consistently showed that the GO nanosheets were stranded in the PS monolayer, thus having adverse effects on the structure and properties of the PS film, including increasing the compressibility of PS films, which is an important criterion for evaluating biophysical inhibition of PS.
In our CGMD simulations, the MARTINI force field39 was used. Four types of GO nanosheets were considered: 5 × 5 nm2 monolayer, 5 × 5 nm2 trilayer, 10 × 10 nm2 monolayer, and 10 × 10 nm2 trilayer. The PS monolayer in our simulation consists of dipalmitoyl phosphatidylcholine (DPPC) and palmitoyloleoyl phosphatidylglycerol (POPG) with a 7: 3 molar ratio, doped with 10 wt% cholesterol, 1.6 wt% surfactant protein (SP)-B, and 1.5 wt% SP-C. To simulate inhalation, we allowed the GO nanosheet to naturally deposit from air onto the pre-equilibrium PS monolayer. The water slab is 10 nm in thickness, containing Na+ ions to neutralize the system. The size of the simulation box is 49 × 49 × 50 nm3. Boundaries in the z-direction are set as wall, and periodic boundary conditions are used for the x and y directions. Fig. 1 illustrates the CGMD models and the simulation system setup. More details of the CG models and the simulation setup are included in the ESI,† along with Fig. S1 and S2.† The interactions were simulated for more than 300 ns. A Berendsen barostat was used for the semi-isotropic pressure coupling with a coupling constant τP = 4 ps. The system compressibility was set to be 5 × 10−5 bar−1 in the x–y plane and 0 in the z-direction. The temperature was maintained at 293 K by Berendsen temperature coupling with a coupling constant τT = 1 ps. The time step was 20 fs. The neighbour list was updated every 10 steps.
Fig. 1.
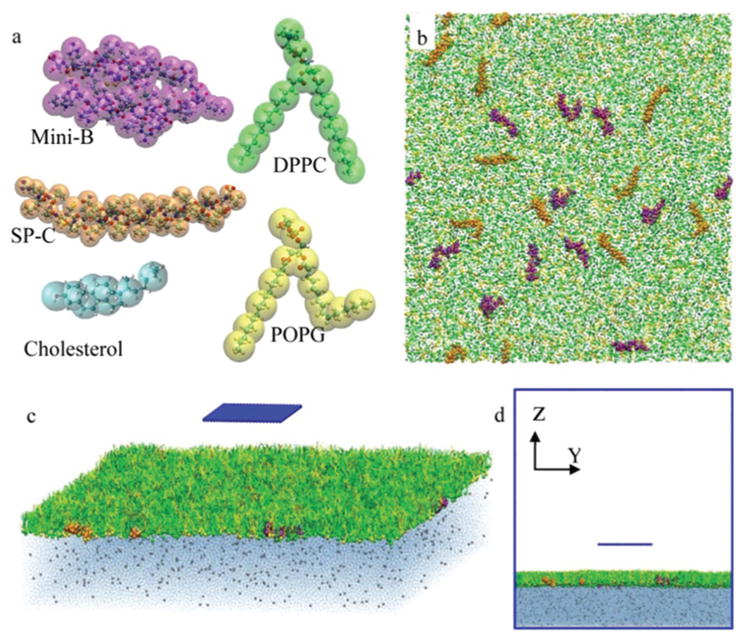
Coarse-grained molecular dynamics simulation setup. (a) Coarse-grained models of the 5 components of PS used in our simulation. The larger transparent spheres for the coarse-grained bead are colored by the type of the molecule and the smaller opaque spheres for the atoms are colored by the type of the atom. (b) Schematic of the PS membrane. (c, d) Schematic of the initial state of the system.
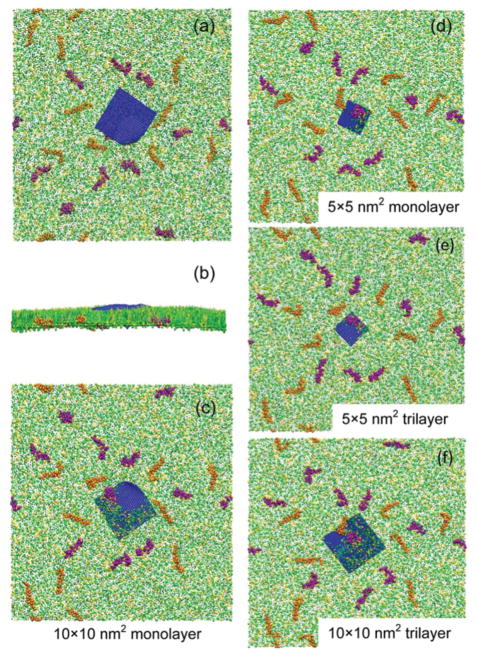
As shown in Fig. 2, all four types of GO nanosheets display similar behaviors on interacting with the PS film. The nanosheet lies down quickly after coming in contact with the film (Fig. S3 in the ESI† shows that this behaviour always happens despite the different initial orientations of the GO nanosheets). After the initial deposition, the GO nanosheet remains at the PS film without penetration. Most importantly, we found that the GO nanosheet induces a pore in the PS film. This pore-inducing process ends in just tens of nanoseconds, and after that the area and shape of the pore hardly change in the subsequent hundreds of nanoseconds. Fig. 3 shows the details of the pore formation in the GO–PS system. (Please also refer to the ESI Movie.†) The lipids near the original pore edge are pushed away rather than being squeezed to the water. This indicates that, except for the direct impact on the PS film by depositing and interacting with molecules around the contact point, the GO nanosheet alters the PS film locally as it compresses the monolayer to a certain extent. The lipid molecules around the GO nanosheet lie down with their hydrophilic head groups facing towards the pore edge of the GO nanosheet. This change of lipid orientation will be discussed later.
Fig. 2.
Interaction between GO nanosheets and the PS film. For the 10 × 10 nm2 monolayer GO, top (a), side (b) and bottom (c) views of the final states are shown. For the 5 × 5 nm2 monolayer (d), 5 × 5 nm2 trilayer (e), and 10 × 10 nm2 trilayer (f) GO, only the bottom views are shown. Each system is simulated for more than 300 ns. All GO nanosheets are found to lie on the PS film at equilibrium. A pore in the PS is induced by the GO nanosheet.
Fig. 3.
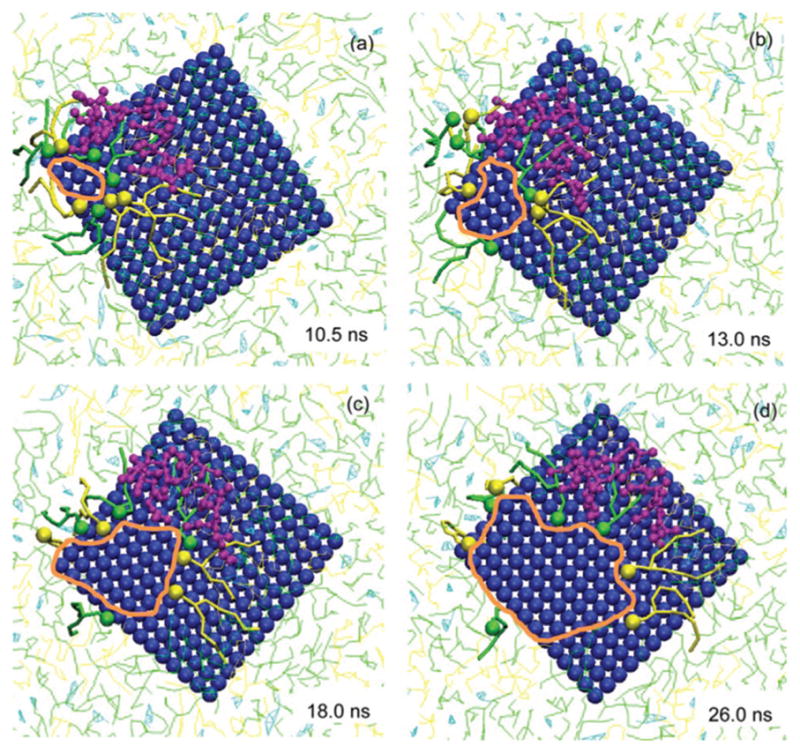
Progressive alternation of lipid molecules in the PS film during the GO-induced pore formation. Four moments of the pore formation process are shown, zooming in around the GO–PS contact area. The inserted time denotes the simulation time accounted for since the moment when all GO beads come in contact with the PS film. To track the movement of the lipid and protein molecules, 8 lipid molecules (4 DPPC and 4 POPG) closest to the pore at the initial moment and a SP-B molecule coming in contact with the GO nanosheet are clearly shown while others are blurred. The lipids are pushed backward by the GO nanosheet with the heads of lipids still facing the edge of the pore (denoted by the orange circle).
In our previous work29 we found that hydrophobicity of spherical NPs determines their translocation across the PS film: hydrophilic NPs can get through but hydrophobic NPs cannot. However, this conclusion does not hold true for GO nanosheets. With oxygen functional groups on it, the GO nanosheet is hydrophilic and thus tends to penetrate the PS membrane and contact water beneath. However, the unique 2D structure of the GO nanosheet makes it lie down quickly upon contacting the PS film. Hence, translocation across the PS monolayer film needs to overcome a very high energy barrier by pushing away a large amount of lipid molecules in the monolayer. The balance of the hydrophilic force and the energy barrier results in the final retention of the GO nanosheet at the PS with an induced pore, as shown in Fig. 3. This could be a reason why inhaled GO has a prolonged lifetime in the lungs as shown by in vivo studies.21,22
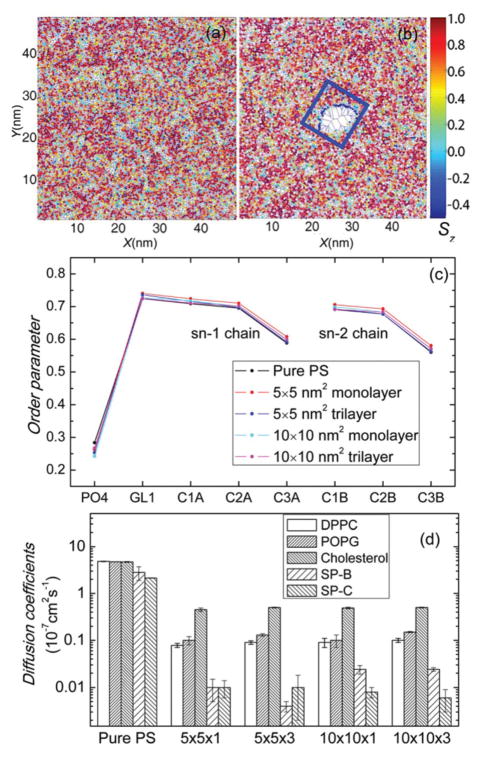
The order parameter that expresses the fatty acid tails’ consistency with the perpendicular is generally used to reflect the phase coexistence and transition of the PS film.40,41 As shown in Fig. 4(a and b), the order parameters are used to color the areas of corresponding molecules in a Voronoi diagram. The average data of different beads in DPPC are shown in Fig. 4(c). Details about the calculation of the order parameters are included in the ESI.† Lipids near the induced pore, as they lie down on the GO nanosheet (Fig. 3), exhibit very low order parameters. While in the area away from the GO nanosheet, the order parameters follow a normal composite distribution. GO nanosheets hardly change the overall order parameters. Therefore, it can be concluded that GO nanosheet affects the phase behavior of the PS film significantly only in the local range. The radial distribution function is analyzed in the ESI,† also showing that GO nanosheets have little impact on the overall phase behavior of the PS film. The PS should adsorb rapidly to expand the film at the air–water interface during inhalation.25,42 Diffusion coefficients are used to show the liquidity of the PS film quantitatively (Fig. 4(d)). For all five PS components, all types of GO nanosheets reduce the diffusion coefficients by at least one order of magnitude, which implies that the liquidity of PS is significantly limited by GO. Note that the diffusion coefficients show no obvious difference, regardless of the lateral size and the number of layers of these four types of GO nanosheets.
Fig. 4.
Impact of GO nanosheets on the order parameters and diffusion coefficients of the PS film. (a, b) The order parameters of lipid molecules’ hydrophobic tails are used to color the Voronoi lattices. Data of pure PS and PS affected by 10 × 10 nm2 monolayer GO are shown. The blue box represents the position of the GO nanosheet. Lipids near the pore in the GO system exhibit very low order parameters, indicating that they are largely disordered and are in a liquid expanded phase. (c) The order parameters of DPPC beads averaged over all the PS films. Data of pure PS and PS affected by all the four types of GO nanosheets are plotted. GO nanosheets hardly change the overall order parameters. (d) Impact of GO nanosheets on diffusion coefficients of PS. For pure PS and PS affected by 4 types of GO, diffusion coefficients of 5 components of the PS film are shown. An error estimate is given based on a block average over 5 blocks. In general, for all five PS components, all types of GO nanosheets reduce the diffusion coefficients by at least one order of magnitude.
To confirm the phenomena of GO-induced pores in the PS monolayer film, we have investigated the interactions between the PS film and reduced-GO. Reduced-GO with half oxide groups stays on the monolayer without the pore being induced. The order parameters and diffusion coefficients of the reduced-GO-affected PS film are much more similar to those of a pure PS film rather than those of a GO-affected PS as shown in Fig. S6 in the ESI.† Therefore, we believe that it is the pore and not the GO itself which affects the monolayer properties directly.
We have also conducted in vitro experiments to study the interactions between GO and animal-derived natural PS films. Experiments on the interaction between Infasurf (calfactant) and GO nanosheets were conducted using a Langmuir–Blodgett trough.27 Experimental details of the interactions between Infasurf (calfactant) and GO nanosheets are provided in the ESI.† It is found that the GO nanosheets adsorb onto the PS film, with the nanosheet partially covered by lipid molecules (Fig. 5(a–c), see Fig. S7† for more AFM images). This agrees with our in silico simulations. Furthermore, compared with the pure Infasurf, obvious reduction in domain formation can be found in the GO-containing Infasurf, indicating disruption of the monolayer ultrastructure. Typical compression–expansion cycles of pure Infasurf and Infasurf mixed with GO were compared using a constrained drop surfactometer30 (Fig. 5(d)). GO nanosheets significantly increased the hysteresis area, which indicates film instability and surfactant inhibition.43 The film compressibility is significantly increased, which is an important indication of surfactant inhibition.44 Upon film compression during exhalation, a good surfactant film should have a low compressibility, thus decreasing the surface tension to near-zero with less than 20% area compression.25 Higher compressibility means that more area compression is needed to achieve a low surface tension, leading to respiratory failure.
Fig. 5.
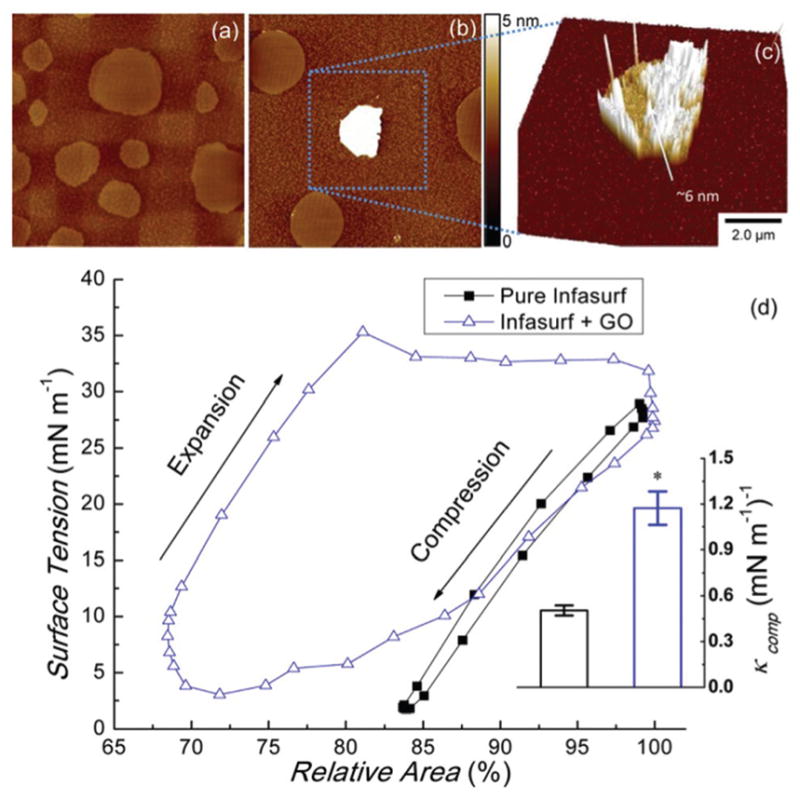
Experimental interaction between GO and PS. GO nanosheets were mixed and co-spread with Infasurf to a weight ratio of 1: 100. (a) Lateral structure of the pure PS film at a surface pressure of 30 mN m−1. (b) Lateral structure of the PS film at a surface pressure of 30 mN m−1 exposed to GO. Both are scanned by AFM at an area of 20 × 20 μm2. (c) Surface plot of a GO nanosheet adsorbed onto the PS film at a scan area of 10 × 10 μm2. (d) Biophysical simulations of Infasurf and Infasurf affected by GO nanosheets, studied using a constrained drop surfactometer. The film compressibility (κcomp) is shown in the subfigure. Tukey and Bonferroni mean comparison tests were used, and *p < 0.05 for comparison with pure Infasurf. GO nanosheets significantly increased the hysteresis area and the film compressibility.
Although having much smaller lengths and time scales, CGMD simulations show a qualitatively consistent result for the increasing compressibility after exposure to GO. PS films with and without a GO nanosheet were equilibrated at different membrane areas. It is found that when the side length of the membrane area is decreased from 493 Å to 488 Å, the surface tension of the pure PS decreases by 5.0 mN m−1, while the PS film affected by GO only decreases the surface tension by 4.1 mN m−1. This indicates that the compressibility of the GO-affected PS film is about 20% larger than that of the pure PS film. Besides, it is found that the pore induced by GO disappears when the membrane area is decreased, suggesting a likely correlation between the induced pore and the increasing compressibility of the PS film.
In summary, using CGMD simulation and experimental measurement, we illustrate the interactions between the PS monolayer films and the GO nanosheets. We report the retention and adverse biophysical impact of GO on the PS film. The GO nanosheets induce pores on the monolayer film, and affect its biophysical properties. Remarkably, GO nanosheets increase the compressibility of PS films, which indicates the biophysical inhibition of PS. We provide a new perspective to understand the inhalation toxicity of GO nanosheets by studying their interaction with the PS film.
Supplementary Material
Acknowledgments
This work was financially supported by MOST 2011CB707604, NSFC 11272321 (G.H.), NSF Grant No. CBET-1236596 (Y.Y.Z.), and LNM Open Fund. The MD simulations were performed on TianHe-1(A) at the National Supercomputing Center in Tianjin. We thank Walter Klein at ONY Inc. for donation of Infasurf samples.
Footnotes
Electronic supplementary information (ESI) available: Detailed description of simulation and experimental methods, additional simulation and experimental results, and a simulation video. See DOI: 10.1039/c5nr05401j
Contributor Information
Qinglin Hu, Email: guoqing.hu@imech.ac.cn.
Yi Y. Zuo, Email: yzuo@hawaii.edu.
Notes and references
- 1.Dreyer DR, Park S, Bielawski CW, Ruoff RS. Chem Soc Rev. 2010;39:228. doi: 10.1039/b917103g. [DOI] [PubMed] [Google Scholar]
- 2.Karim MR, Hatakeyama K, Matsui T, Takehira H, Taniguchi T, Koinuma M, Matsumoto Y, Akutagawa T, Nakamura T, Noro S, Yamada T, Kitagawa H, Hayami S. J Am Chem Soc. 2013;135:8097. doi: 10.1021/ja401060q. [DOI] [PubMed] [Google Scholar]
- 3.Hatakeyama K, Karim MR, Ogata C, Tateishi H, Funatsu A, Taniguchi T, Koinuma M, Hayami S, Matsumoto Y. Angew Chem, Int Ed. 2014;53:6997. doi: 10.1002/anie.201309931. [DOI] [PubMed] [Google Scholar]
- 4.Rao CN, Sood AK, Subrahmanyam KS, Govindaraj A. Angew Chem, Int Ed. 2009;48:7752. doi: 10.1002/anie.200901678. [DOI] [PubMed] [Google Scholar]
- 5.Chung C, Kim YK, Shin D, Ryoo SR, Hong BH, Min DH. Acc Chem Res. 2013;46:2211. doi: 10.1021/ar300159f. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Li Z, Wang J, Li J, Lin Y. Trends Biotechnol. 2011;29:205. doi: 10.1016/j.tibtech.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou SS, De M, Luo J, Rotello VM, Huang J, Dravid VP. J Am Chem Soc. 2012;134:16725. doi: 10.1021/ja306767y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian J, Wang D, Cai FH, Xi W, Peng L, Zhu ZF, He H, Hu ML, He S. Angew Chem, Int Ed. 2012;51:10570. doi: 10.1002/anie.201206107. [DOI] [PubMed] [Google Scholar]
- 9.Tang Q, Zhou Z, Chen Z. Nanoscale. 2013;5:4541. doi: 10.1039/c3nr33218g. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari AC, et al. Nanoscale. 2015;7:4598. doi: 10.1039/c4nr01600a. [DOI] [PubMed] [Google Scholar]
- 11.Singh V, Joung D, Zhai L, Das S, Khondaker SI, Seal S. Prog Mater Sci. 2011;56:1178. [Google Scholar]
- 12.Tay CY, Setyawati MI, Xie J, Parak WJ, Leong DT. Adv Funct Mater. 2014;24:5936. [Google Scholar]
- 13.Chang Y, Yang ST, Liu JH, Dong E, Wang Y, Cao A, Liu Y, Wang H. Toxicol Lett. 2011;200:201. doi: 10.1016/j.toxlet.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Sasidharan A, Panchakarla LS, Chandran P, Menon D, Nair S, Rao CN, Koyakutty M. Nanoscale. 2011;3:2461. doi: 10.1039/c1nr10172b. [DOI] [PubMed] [Google Scholar]
- 15.Liao KH, Lin YS, Macosko CW, Haynes CL. ACS Appl Mater Interfaces. 2011;3:2607. doi: 10.1021/am200428v. [DOI] [PubMed] [Google Scholar]
- 16.Seabra AB, Paula AJ, de Lima R, Alves OL, Duran N. Chem Res Toxicol. 2014;27:159. doi: 10.1021/tx400385x. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Liu R, Wang X, Liu Q, Chen Y, Valle RP, Zuo YY, Xia T, Liu S. ACS Nano. 2015 doi: 10.1021/acsnano.5b04751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chng EL, Pumera M. Chem – Eur J. 2013;19:8227. doi: 10.1002/chem.201300824. [DOI] [PubMed] [Google Scholar]
- 19.Chng EL, Chua CK, Pumera M. Nanoscale. 2014;6:10792. doi: 10.1039/c4nr03608e. [DOI] [PubMed] [Google Scholar]
- 20.Wang K, Ruan J, Song H, Zhang J, Wo Y, Guo S, Cui D. Nanoscale Res Lett. 2010;6:8. doi: 10.1007/s11671-010-9751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duch MC, Budinger GR, Liang YT, Soberanes S, Urich D, Chiarella SE, Campochiaro LA, Gonzalez A, Chandel NS, Hersam MC, Mutlu GM. Nano Lett. 2011;11:5201. doi: 10.1021/nl202515a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B, Yang J, Huang Q, Zhang Y, Peng C, Zhang Y, He Y, Shi J, Li W, Hu J, Fan C. NPG Asia Mater. 2013;5:e44. [Google Scholar]
- 23.Schinwald A, Murphy FA, Jones A, MacNee W, Donaldson K. ACS Nano. 2012;6:736. doi: 10.1021/nn204229f. [DOI] [PubMed] [Google Scholar]
- 24.Todoroffand J, Vanbever R. Curr Opin Colloid Interface Sci. 2011;16:246. [Google Scholar]
- 25.Zuo YY, Veldhuizen RAW, Neumann AW, Petersen NO, Possmayer F. Biochim Biophys Acta, Biomembr. 2008;1778:1947. doi: 10.1016/j.bbamem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Goerke J. Biochim Biophys Acta, Mol Basis Dis. 1998;1408:79. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 27.Fan Q, Wang YE, Zhao X, Loo JS, Zuo YY. ACS Nano. 2011;5:6410. doi: 10.1021/nn2015997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck-Broichsitter M, Ruppert C, Schmehl T, Günther A, Seeger W. Biochim Biophys Acta, Biomembr. 2014;1838:474. doi: 10.1016/j.bbamem.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Hu G, Jiao B, Shi X, Valle RP, Fan Q, Zuo YY. ACS Nano. 2013;7:10525. doi: 10.1021/nn4054683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valle RP, Wu T, Zuo YY. ACS Nano. 2015;9:5413. doi: 10.1021/acsnano.5b01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachan AK, Galla HJ. Small. 2014;10:1069. doi: 10.1002/smll.201300315. [DOI] [PubMed] [Google Scholar]
- 32.Lin X, Bai T, Zuo YY, Gu N. Nanoscale. 2014;6:2759. doi: 10.1039/c3nr04163h. [DOI] [PubMed] [Google Scholar]
- 33.Ge C, Du J, Zhao L, Wang L, Liu Y, Li D, Yang Y, Zhou R, Zhao Y, Chai Z, Chen C. Proc Natl Acad Sci U S A. 2011;108:16968. doi: 10.1073/pnas.1105270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong-Ekkabut J, Baoukina S, Triampo W, Tang IM, Tieleman DP, Monticelli L. Nat Nanotechnol. 2008;3:363. doi: 10.1038/nnano.2008.130. [DOI] [PubMed] [Google Scholar]
- 35.Qiao R, Roberts AP, Mount AS, Klaine SJ, Ke PC. Nano Lett. 2007;7:614. doi: 10.1021/nl062515f. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Yuan H, von dem Bussche A, Creighton M, Hurt RH, Kane AB, Gao H. Proc Natl Acad Sci U S A. 2013;110:12295. doi: 10.1073/pnas.1222276110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R, Wang X, Ji Z, Sun B, Zhang H, Chang CH, Lin S, Meng H, Liao YP, Wang M, Li Z, Hwang AA, Song TB, Xu R, Yang Y, Zink JI, Nel AE, Xia T. ACS Nano. 2013;7:2352. doi: 10.1021/nn305567s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin X, Zuo YY, Gu N. Sci China Mater. 2015;58:28–37. doi: 10.1007/s40843-014-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, de Vries AH. J Phys Chem B. 2007;111:7812. doi: 10.1021/jp071097f. [DOI] [PubMed] [Google Scholar]
- 40.Baoukina S, Mendez-Villuendas E, Tieleman DP. J Am Chem Soc. 2012;134:17543. doi: 10.1021/ja304792p. [DOI] [PubMed] [Google Scholar]
- 41.Lin X, Li Y, Gu N. Soft Matter. 2011;7:3882. [Google Scholar]
- 42.Serrano AG, Perez-Gil J. Chem Phys Lipids. 2006;141:105. doi: 10.1016/j.chemphyslip.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Schürch S, Green FHY, Bachofen H. Biochim Biophys Acta, Mol Basis Dis. 1998;1408:180. doi: 10.1016/s0925-4439(98)00067-2. [DOI] [PubMed] [Google Scholar]
- 44.Bachofen H, Schurch S, Urbinelli M, Weibel ER. J Appl Physiol. 1987;62:1878. doi: 10.1152/jappl.1987.62.5.1878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.