Summary
Recently, radiation induced necrosis in the brain has been treated using bevacizumab, an anti-VEGF antibody. We validated the VEGF specificity by comparing the therapeutic efficacy of anti-VEGF with non-specific isotype control antibody. Additionally, we found that VEGF over-expression and RN developed simultaneously, which precludes preventative anti-VEGF treatment.
Keywords: Radiation Necrosis, Vascular Endothelial Growth Factor, Magnetic Resonance Imaging
Introduction
Upregulated vascular endothelial growth factor (VEGF) expression has been suggested as a key effector of radiation necrosis (RN) [1–3], a serious complication of radiation therapy, seen in up to 23% of patients [4], that can occur months to years after radiation. This suggestion has motivated the use of the anti-VEGF antibody, bevacizumab (Avastin), as a new approach for treating RN [5–7]. Similarly, an analogous anti-VEGF antibody (B20-4.1.1) has been shown to mitigate the development of radiation necrosis of the brain in a murine model [8]. However, proper control experiments have not been previously performed to ensure the therapeutic effect is exclusively due to the anti-VEGF specificity. It remains possible that a similar therapeutic effect could be achieved with non-specific antibody therapy.
While most studies looking at anti-VEGF therapy have focused on treating an apparent RN lesion, there is also interest in anti-VEGF therapy as a preventative therapy [9]. However, the timeline of VEGF-overexpression relative to RN lesion development is unknown. Prior evaluation of human RN samples have confirmed expression of VEGF in the lesion [2,10] but histological evaluation of VEGF before RN is impractical in humans.
Rodent models present a unique opportunity to study RN, independent of other obfuscating pathology, in ways that are not possible clinically. Our group has developed a robust and predictable model of RN, in which the lesion first appears circa 4 weeks post-irradiation (PIR) [11]. We have previously shown that, like in humans, an anti-VEGF antibody (B20-4.1.1) can mitigate the development of RN in this model [8]. In this manuscript, we validate that the treatment effect is exclusively due to the anti-VEGF properties of B20-4.1.1 by comparing it against an isotype-matched control antibody. Additionally, we performed immunohistochemistry (IHC) for VEGF and determined that VEGF expression does not precede RN lesion development. As such, anti-VEGF therapy should fail to prevent RN.
Materials and Methods
Mouse Models
All experiments were approved by the Washington University Division of Comparative Medicine and were performed on 8–9 week old female BALB/c mice (Harlan Laboratories, Indianapolis, IN). Mice received an intraperitoneal injection of ketamine/xylazine anesthetic and were then restrained in a custom-made holder that attaches to the stereotactic frame of the Leksell Gamma Knife® Perfexion™ (Elekta, Stockholm, Sweden). A single-fraction, 50 Gy radiation dose (50% isodose) was focused on the cortex of the left hemisphere ~ 3 mm posterior to bregma. Given the small size of the mouse brain, 4 mm collimator was used in all quadrants producing a 4mm sphere at the 50% isodose line and limiting the target volume to only the left hemisphere. At this dose, focal RN can be observed starting at approximately week 4 PIR [11].
Treatment was based on our previous work [8]. B20-4.1.1 (Genentech, San Francisco, CA) is a murine antibody that recognizes both human and mouse VEGF [12], while GP120:9239 (Genentech, San Francisco, CA) is a murine antibody of the same isotype that targets the HIV capsid protein. At week 4 PIR, mice underwent MRI examination to confirm the presence of RN. Mice were then randomly assigned to one of three treatment groups. The untreated-control group (n=5), as its name suggests, received no treatment, the isotype-control group (n=8) received 10 mg/kg of GP120:9239 given intraperitoneally twice weekly, and the anti-VEGF group (n=9) received 10 mg/kg of B20-4.1.1 given intraperitoneally twice weekly. All mice underwent MRI examination weekly from weeks 4 to 13 PIR. To minimize the acute effect of blocking VEGF activity on permeability and therefore contrast agent extravasation, all MRI studies were conducted two days after a treatment (i.e., inject Monday and Friday, image on Wednesday).
Magnetic Resonance Imaging
Mice were anesthetized with isoflurane and restrained in a 3-point head holder. Images were acquired with a 4.7 T small-animal Agilent/Varian DirectDrive1 scanner using an actively decoupled volume coil (transmit) and 1.5 cm surface coil (receive). Before loading into the magnet, mice were given an intraperitoneal injection of 0.5 mL MultiHance (gadobenate dimeglumine, Bracco Diagnostics Inc, Princeton, NJ) contrast agent diluted 1:10 in sterile saline. All data were collected with a field of view of 15 × 15 mm2, 21 contiguous slices with a thickness of 0.5 mm, imaging matrix of 128 by 128 and 4 averages. Spin-echo T2-weighted (TR = 2000 ms, TE = 50 ms), spin-echo T1-weighted (TR = 650 ms, TE =20 ms), and gradient-echo proton-density (PD) weighted (TR = 500 ms, TE = 5 ms, flip angle = 20) datasets were acquired, in this order, for each mouse at each time point.
Data Analysis and Statistics
Datasets were analyzed as previously described [11], using custom-written Matlab software (The Mathworks, Natick MA), with regions of interest (ROI) for the contralateral and ipsilateral hemispheres being drawn manually on the post-contrast T1-weighted image. The lesion was defined as the region of hyperintensity in T1 or T2-weighted images. The lesion T1 and T2-derived volumes were determined via a threshold segmentation algorithm within whole-brain (contralateral plus ipsilateral hemisphere) ROIs. To compensate for the inhomogeneity of the surface coil, the threshold segmentation was performed on a normalized T1 or T2-weighted image generated by dividing the T1 or T2-weighted image by the PD-weighted image. Since all images suffer equivalently from the surface coil inhomogeneity, this normalization cancels out the inhomogeneity and enhances the T1/T2 contrast. Areas of the brain brighter than the 95th percentile of the contralateral hemisphere were classified as the lesion. The lesion volume was then calculated as the sum of the lesion voxels multiplied by the voxel volume.
Graphs and statistical analyses were conducted on the region-based calculations with Prism version 5.01 (GraphPad Software, San Diego, CA). All graphs show the mean and standard deviation for each group. Statistical analysis was performed using Repeated Measures Two-Way ANOVA with Bonferroni post-tests.
IHC and histology
For mice treated with anti-VEGF antibodies, mice were intracardially perfused with 1x PBS and 10% formalin after the final imaging session at 13 weeks PIR. A separate set of untreated mice was similarly perfused at 2, 4, or 8 weeks PIR. Each mouse head was dissected and submerged in 10% formalin for 24 hours. The brain was then extracted from the skull, a 2 mm coronal section centered at ~3 mm behind bregma was obtained, processed through graded alcohols, embedded in paraffin, and sectioned at a thickness of eight microns. Tissue sections were stained with hematoxylin and eosin (H&E) per standard protocols. For immunohistochemistry (IHC), antigen retrieval was performed with the citrate buffer at 70°C overnight following non-specific blocking for one hour. Rabbit anti-VEGF (orb11553, Biorbyt, Cambridge, UK) at 1:500 was used as the primary antibody and DAB staining was performed by using Histostain plus Broad System kit (Invitrogen Life Technology, Frederick, MD). Briefly, sections were incubated with a broad spectrum secondary antibody for one hour and HRP-streptavidin for 30 minutes. Staining was visualized with a diaminobenzidine (DAB) precipitation reaction and photographed with a light microscope at 40x magnification.
Results
Non-specific antibody treatment is ineffective in the treatment of RN
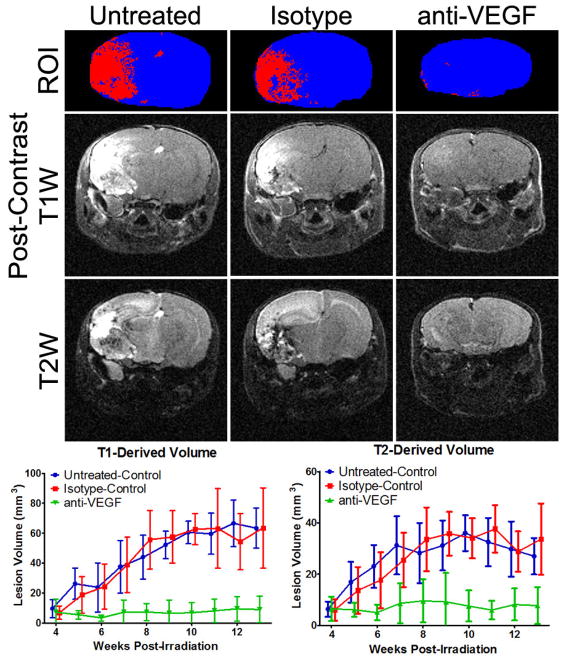
Mice were divided into treatment groups at 4 weeks PIR, the earliest time at which a lesion can be detected in either post-contrast T1 or T2-weighted images. As such, the T1 and T2-derived volumes measured at this time point constitute a pre-treatment baseline. Lesion volumes were measured for both modalities weekly for each mouse and the results are shown in Figure 1. For both T1 and T2-derived volumes, there is a significant difference between the anti-VEGF group compared with either control group, starting at week 7 PIR. There was no statistically significant difference between the isotype-control group and the untreated-control group at any time point for either measurement.
Figure 1.
Treatment with anti-VEGF antibodies is VEGF specific. At the top are representative T1-based segmentations (blue is brain, red is lesion), and post-contrast T1 and T2-weighted images at 13 weeks post irradiation for the three groups: untreated control, isotype-matched antibody treatment, and anti-VEGF antibody treatment. The lesion is bright in these images. Note the lack of obvious lesion and the reduced brain size (reduced swelling) in the anti-VEGF example. Below the images are the T1 and T2-derived volumes (mean ± SD) for the three groups. The anti-VEGF treated animals had significantly smaller lesions for both methodologies from week 7 to week 13 PIR as determined by 2-Way ANOVA with Bonferroni post tests (n = 5 – 9).
VEGF production is detected at 4 weeks PIR and progressively increases
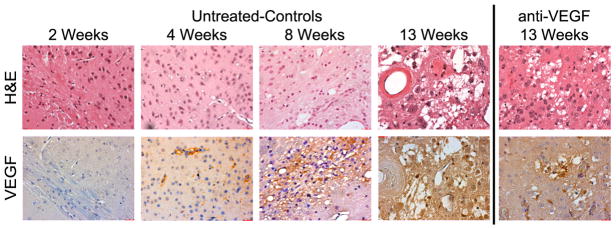
Vascular endothelial growth factor (VEGF) has been detected in clinical RN lesions [1,2]. We sought to identify the timeline of VEGF expression in our mouse model of RN. To do so, we performed IHC of the irradiation site at 1 (data not shown), 2, 4, 8, and 13 weeks PIR in untreated mice, as shown in Figure 2. VEGF was detected as early as 4 weeks PIR and the levels of VEGF increased over time. We had anticipated that VEGF upregulation would significantly precede MRI-detected permeability, but our data suggest a tight coupling of VEGF expression and permeability. We also evaluated VEGF expression in our anti-VEGF treated cohort and found similar levels of VEGF expression as in the untreated animals.
Figure 2.
VEGF is elevated as early as week 4 PIR and remains elevated after anti-VEGF treatment. H&E (top row) and VEGF staining (bottom row) of a corresponding region is presented at multiple time points for untreated mice receiving a 50 Gy (50% isodose) radiation dose. Brown indicates positive staining for VEGF. The staining is diffuse at later time points due to secreted VEGF. All images were acquired at 40x magnification. The 13 week samples are from the same mice whose imaging data are reported in Figure 1.
Discussion
Rodent models of RN present a unique opportunity to evaluate the evolution of the pathology over time independent of potentially obfuscating tumor pathology. In our model, we use a large single-fraction 50 Gy (50% isodose) radiation dose that is rarely used clinically to ensure that RN will occur in all mice in a reasonable time frame. A lower, more clinically relevant dose of 20 Gy (50% isodose) fails to produce any pathology up to 20 weeks post irradiation [13]. Nevertheless, the histological features of RN in these mice are consistent with those observed clinically including, but not limited to, neuronal necrosis, interstitial edema, hemorrhage, vascular telangectesia, cellular atypia, leukocyte infiltration, and fibrinoid necrosis. We have characterized this model with both post-contrast T1-weighted MRI, sensitive to the blood vessel permeability, and T2-weighted MRI, sensitive to edema [8,11]. The volumes derived from these measurements are correlated in this model [14], but may not be the same as they reflect different facets of the pathology and are based on images having different contrast-to-noise ratios. T2-weighted MRI can also detect perilesional edema of the white matter that leads to contralateral hyperintensity [13] though this is not included in our volume measurements. As these two modalities are sensitive to different components of RN pathology, monitoring response to therapy via both modalities should provide a more comprehensive assessment of response.
Elevated VEGF expression has been observed clinically [1,2] and in preclinical models of radiation injury to the CNS [15–17] and is believed to be a key effector of RN. VEGF expression could explain the permeability of the blood brain barrier and the vasogenic edema seen in these lesions [17]. It is, therefore, no surprise that anti-VEGF antibodies are currently being explored as a treatment option for RN of the brain [6–8]. However, given that inflammation and other immune processes could also be involved in RN [1,10], it was important to ensure that the therapeutic effect of anti-VEGF antibodies is solely due to their VEGF specificity. In our murine model of RN, mice treated with the isotype-matched control antibody had a response that was indistinguishable from untreated mice. As such, we conclude that generic antibody therapy does not have an impact on RN.
Another important detail regarding anti-VEGF therapy is the timing of expression of VEGF in RN. Our results indicate that VEGF expression is concurrent with increases in blood brain barrier permeability detected by post-contrast T1-weighted MRI [8,11]. It is therefore likely that VEGF regulates both permeability and vasogenic edema in RN. However, such timing would also preclude anti-VEGF therapies from being effective at preventing RN. Indeed, a recent clinical study seems to suggest that this is the case [9].
We also found that VEGF expression persisted after anti-VEGF therapy. There might be a risk that RN will recur once the treatment is stopped if VEGF levels remain elevated, as this could indicate that the underlying trigger might still be present. It is not surprising that VEGF expression is not abolished with anti-VEGF antibody treatment. VEGF is a secreted factor, and while the binding of the antibody should block its action, it should not directly affect its production or secretion. The factors that drive VEGF expression remain a critical unknown. Nonetheless, tracking VEGF expression (with positron emission tomography for example [18]) might be warranted in patients being treated with anti-VEGF therapies.
Acknowledgments
This project was supported by NIH grant R01 CA155365 (JRG), and funding from the Alvin J. Siteman Comprehensive Cancer Center (P30 CA091842), the Barnes-Jewish Hospital Foundation Cancer Frontier Fund, Mallinckrodt Institute of Radiology, and Elekta Instruments AB (Stockholm, Sweden). We gratefully acknowledge Genentech (South San Francisco, CA) for donation of the antibodies B20-4.1.1 and GP120:9239. We also wish to thank Messrs. John Engelbach and Jeremy Cates for their help with the irradiation of the animals.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yoritsune E, Furuse M, Kuwabara H, Miyata T, Nonoguchi N, Kawabata S, et al. Inflammation as well as angiogenesis may participate in the pathophysiology of brain radiation necrosis. J Radiat Res (Tokyo) 2014;55:803–11. doi: 10.1093/jrr/rru017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nonoguchi N, Miyatake S-I, Fukumoto M, Furuse M, Hiramatsu R, Kawabata S, et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol. 2011;105:423–31. doi: 10.1007/s11060-011-0610-9. [DOI] [PubMed] [Google Scholar]
- 3.Fink J, Born D, Chamberlain MC. Radiation Necrosis: Relevance with Respect to Treatment of Primary and Secondary Brain Tumors. Curr Neurol Neurosci Rep. 2012;12:276–85. doi: 10.1007/s11910-012-0258-7. [DOI] [PubMed] [Google Scholar]
- 4.Kumar AJ, Leeds NE, Fuller GN, Tassel PV, Maor MH, Sawaya RE, et al. Malignant Gliomas: MR Imaging Spectrum of Radiation Therapy- and Chemotherapy-induced Necrosis of the Brain after Treatment. Radiology. 2000;217:377–84. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol. 2007;67:323–6. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized Double-Blind Placebo-Controlled Trial of Bevacizumab Therapy for Radiation Necrosis of the Central Nervous System. Int J Radiat Oncol. 2011;79:1487–95. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tye K, Engelhard HH, Slavin KV, Nicholas MK, Chmura SJ, Kwok Y, et al. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. J Neurooncol. 2014;117:321–7. doi: 10.1007/s11060-014-1391-8. [DOI] [PubMed] [Google Scholar]
- 8.Jiang X, Engelbach JA, Yuan L, Cates J, Gao F, Drzymala RE, et al. Anti-VEGF Antibodies Mitigate the Development of Radiation Necrosis in Mouse Brain. Clin Cancer Res. 2014;20:2695–702. doi: 10.1158/1078-0432.CCR-13-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ney DE, Carlson JA, Damek DM, Gaspar LE, Kavanagh BD, Kleinschmidt-DeMasters BK, et al. Phase II trial of hypofractionated intensity-modulated radiation therapy combined with temozolomide and bevacizumab for patients with newly diagnosed glioblastoma. J Neurooncol. 2014:1–9. doi: 10.1007/s11060-014-1691-z. [DOI] [PubMed] [Google Scholar]
- 10.Miyatake S-I, Nonoguchi N, Furuse M, Yoritsune E, Miyata T, Kawabata S, et al. Pathophysiology, Diagnosis, and Treatment of Radiation Necrosis in the Brain. Neurol Med Chir (Tokyo) 2015;55:50–9. doi: 10.2176/nmc.ra.2014-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Torres CJ, Engelbach JA, Cates J, Thotala D, Yuan L, Schmidt RE, et al. Toward Distinguishing Recurrent Tumor From Radiation Necrosis: DWI and MTC in a Gamma Knife–Irradiated Mouse Glioma Model. Int J Radiat Oncol. 2014;90:446–53. doi: 10.1016/j.ijrobp.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang W-C, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, et al. Cross-species Vascular Endothelial Growth Factor (VEGF)-blocking Antibodies Completely Inhibit the Growth of Human Tumor Xenografts and Measure the Contribution of Stromal VEGF. J Biol Chem. 2006;281:951–61. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Torres CJ, Yuan L, Schmidt RE, Rich KM, Ackerman JJ, Garbow JR. Perilesional edema in radiation necrosis reflects axonal degeneration. Radiat Oncol. 2015;10:33. doi: 10.1186/s13014-015-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X, Perez-Torres CJ, Thotala D, Engelbach JA, Yuan L, Cates J, et al. A GSK-3β Inhibitor Protects Against Radiation Necrosis in Mouse Brain. Int J Radiat Oncol. 2014;89:714–21. doi: 10.1016/j.ijrobp.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y-Q, Ballinger JR, Nordal RA, Su Z-F, Wong CS. Hypoxia in Radiation-induced Blood-Spinal Cord Barrier Breakdown. Cancer Res. 2001;61:3348–54. [PubMed] [Google Scholar]
- 16.Nordal RA, Wong CS. Molecular targets in radiation-induced blood-brain barrier disruption. Int J Radiat Oncol. 2005;62:279–87. doi: 10.1016/j.ijrobp.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Tsao MN, Li YQ, Lu G, Xu Y, Wong CS. Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J Neuropathol Exp Neurol. 1999;58:1051–60. doi: 10.1097/00005072-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Marquez BV, Ikotun OF, Parry JJ, Rogers BE, Meares CF, Lapi SE. Development of a Radiolabeled Irreversible Peptide Ligand for PET Imaging of Vascular Endothelial Growth Factor. J Nucl Med Off Publ Soc Nucl Med. 2014;55:1029–34. doi: 10.2967/jnumed.113.130898. [DOI] [PubMed] [Google Scholar]