ABSTRACT
The ABC drug transporters, including ABCG2, are well known for their ability to efflux a wide spectrum of chemotherapeutic agents, thereby conferring a multidrug-resistant phenotype. However, studies over the past several years suggest that the ABC transporters may play additional role(s) in cell survival in the face of stress inducers that are not ABCG2 substrates (i.e., nutrient deprivation, ionizing radiation, rapamycin). The mechanism by which this occurs is largely unknown. In the present study, using several cancer cell lines and their ABCG2-overexpressing sublines, we show that cells overexpressing ABCG2 were more resistant to these stressors. This resistance was associated with an elevated level of autophagy flux, as measured by a higher rate of SQSTM1/p62 degradation and greater accumulation of LC3-II when compared to parental cells. Knockdown of ABCG2 reduced autophagic activity in resistant cells to a level similar to that observed in parental cells, confirming that the enhanced autophagy was ABCG2-dependent. Moreover, using cell viability, apoptosis, and clonogenic assays, we demonstrated that the ABCG2-expressing cells were more resistant to amino acid starvation and radiation-induced cell death. Importantly, knockdown of the critical autophagy factors ATG5 and ATG7 greatly reduced cell survival, verifying that enhanced autophagy was critical for this effect. Taken together, these data indicate that autophagy induced by various stressors is enhanced/accelerated in the presence of ABCG2, resulting in delayed cell death and enhanced cell survival. This defines a new role for this transporter, one with potential clinical significance.
KEYWORDS: ABC transporters, ABCG2, amino acid starvation, autophagy, BCRP, drug transporters, multidrug resistance, radiation, tumor cell survival
Introduction
The ATP-binding cassette (ABC) transporter family comprises more than 50 transmembrane proteins that function to translocate a wide variety of substrates across intra- and extracellular membranes. Family members are selectively expressed in many adult tissues, where they protect cells against environmental assault and play a role in the secretion of a wide variety of intracellular substrates. A subset of these transporters, including ABCG2, ABCB1/P-glycoprotein and ABCC1/MRP1, are often aberrantly overexpressed in human cancers, where their ability to recognize and transport multiple functionally unrelated drugs confers “multidrug resistance” to these chemotherapeutic agents.1,2 Indeed, ABCG2 overexpression has been observed in a number of cancer cell lines in vitro, as well as in hematological malignancies and in colon, esophageal, endometrial, pancreatic, breast, and lung tumors in patients.3-5 In addition, both ABCG2 and ABCB1 are also highly expressed and play a protective role in normal stem cells as well as in tumor initiating cells (cancer stem cells).6,7 Interestingly, while it appears that ABCB1 is not essential for the stem cell phenotype, some evidence suggests that ABCG2 may be required to maintain “stemness” and prevent differentiation.8
Although the best studied role for ABC drug transporters involves their ability to enhance cell survival by effluxing drug substrates from target cells, studies suggest that certain transporters, including ABCG2, can also protect normal, tumor and stem cells against apoptosis in the presence of stressors that are not substrates for the transporters.8-10 In normal placenta, inhibition of ABCG2 activity by Ko143 or knockdown of ABCG2 expression by siRNA significantly increases the sensitivity of placental trophoblasts to apoptotic injury in response to cytokines as well as C6 and C8 ceramides.9 Silencing of ABCG2 enhances apoptosis during forskolin-induced differentiation due to the reduced expression of the pregnancy hormones CGB/beta-hCG and ERVW-1/HERV-W.10 Human embryonic stem cells are better able to tolerate physical stress, drugs, and UV light when ABCG2 is present.11 Importantly, ABCG2 also confers a tumor survival advantage in the presence of nonsubstrate therapies in the clinic: its overexpression correlates with radiation resistance, and its expression is associated with worse clinical outcome in both lung cancer12 and medulloblastoma.13 Taken together, these studies strongly argue for a key role for ABCG2 in cell survival, much broader than its currently established role in drug efflux. However, the underlying mechanism by which ABCG2 protects cells from nonsubstrate induced cell death is not well understood.
The present study implicates ABCG2 in the regulation of autophagy. Autophagy is a conserved multistep cellular process that results in the breakdown of cellular components such as long-lived proteins and organelles, to maintain cytoplasmic homeostasis.14 In addition to this housekeeping function, autophagy also functions as a prosurvival mechanism in response to a variety of cellular stresses such as nutrient and growth factor deprivation, endoplasmic reticulum stress or microbial infection. It allows cells to defer apoptosis by catabolizing nonessential proteins to provide amino acids and energy, cleaning up dysfunctional organelles and misfolded protein aggregates, and attacking invading microorganisms. However, under certain conditions the uncontrolled upregulation of autophagy can lead to cell death, possibly by activating apoptosis or overwhelming cells with degraded cytoplasmic content.15 Therefore, the activity of autophagy is tightly regulated; it is induced when needed, but otherwise maintained at a basal level.
Autophagy participates in many physiological processes and disease states, and has recently emerged as an important determinant in cancer biology, as cancer cells often endure stress from a variety of sources and are still able to survive and proliferate. Since there is evidence of both tumor suppression and tumor promotion by autophagy, a context-dependent role of this cellular process in cancer has been proposed. A current hypothesis is that autophagy suppresses tumor initiation through the elimination of oncogenic protein substrates, toxic unfolded proteins and damaged organelles; alternatively, it can promote tumor growth in established cancers through intracellular recycling, thereby providing substrates for metabolism and maintaining a functional pool of mitochondria.16
In the course of our investigation of ABCG2 gene regulation, we noted that tumor cells overexpressing ABCG2, due to either drug selection or transfection, appeared more “resistant” to nonsubstrate stressors, including nutrient starvation and radiation, as compared to their non-ABCG2 expressing counterparts. In the present study, we show that this resistance is associated with an ABCG2-dependent increase in autophagy flux following exposure to environmental stress. This observation may explain previous studies that show a role of ABCG2 in protection against nonsubstrate stress inducers, defining a new role for this transporter and suggesting new targets for intervention.
Results
Cells expressing ABCG2 are less sensitive to nutrient deprivation
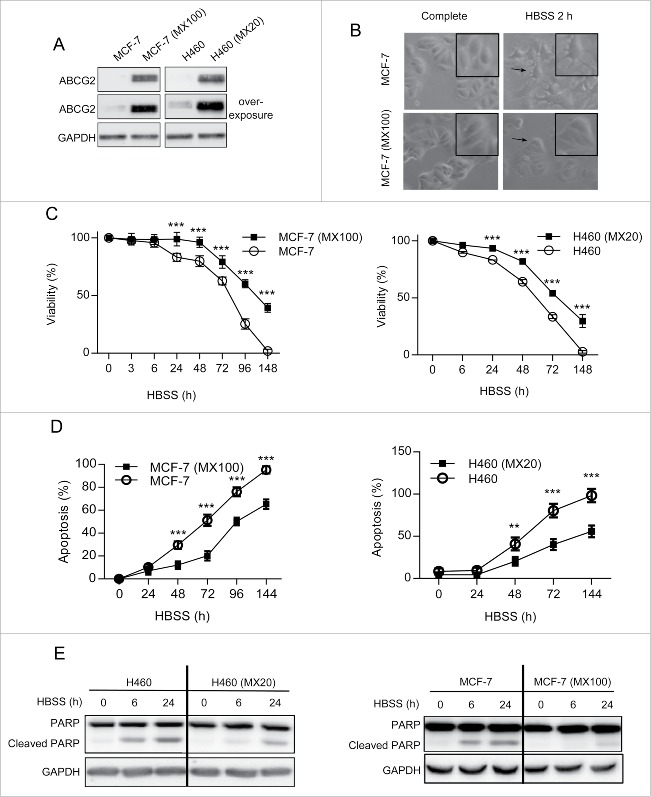
Cancer cell lines selected for resistance to ABCG2 drug substrates, such as mitoxantrone (MX), usually become resistant due to the aberrant overexpression of ABCG2, thereby providing an enhanced mechanism by which tumor cells can eliminate the drug before it initiates a cell death pathway.17 Two such cell lines, MCF-7 (MX100; MCF-7 cells selected with 100 nM MX), and H460 (MX20; H460 cells selected with 20 nM MX) (Fig. 1A) were utilized for these studies. During the course of our investigations on the regulation of ABCG2 expression by methylxanthines in these cells18 we observed that both cell lines were more resistant to this class of compounds than their drug-sensitive parent lines. This result was unexpected, since methylxanthines are not ABCG2 substrates. We therefore considered the possibility that ABCG2 may protect cells against other nonsubstrate stressors. Rather than using another drug, we chose to induce stress via nutrient deprivation, reasoning that this approach would not introduce a new substrate into the analysis. As shown in Figure 1B, when deprived of amino acids via growth in Hank's balanced salt solution (HBSS), parental MCF-7 cells exhibited clear morphological changes associated with apoptosis, including cellular shrinkage, cell convolution, formation of extensions and detachment from the culture surface. In contrast, ABCG2-overexpressing MCF-7 (MX100) cells appeared morphologically unchanged under the same conditions. Similar results were obtained with H460 and H460 (MX20) cells (data not shown). To measure viability, cells were cultured in full medium without MX for at least 7 d prior to incubation in HBSS, in order to eliminate any cytotoxic effect of this chemotherapeutic agent. As shown in Figure 1C, both drug-resistant cell lines (H460 [MX20] and MCF-7 [MX100]) exhibited a higher survival rate than their parental counterparts (H460 and MCF-7), with 20% to 30% survival at 7 d, when no viable parental cells were detected. The degree of apoptosis observed in these cells was consistent with the viability assays; apoptosis, as measured by ANXA5/annexin V and 7AAD, was detected at earlier time points and at higher levels in parental cells compared to their drug-resistant sublines (Fig. 1D). Last, we examined the levels of cleaved PARP, a classic marker for apoptotic enzyme activity (Fig. 1E). Amino acid starvation induced a greater accumulation of the PARP cleavage product in the parental cells indicating a higher apoptosis activity. Taken together, these results suggest that ABCG2 protects cells under certain stress conditions, including nutrient deprivation, and suggested that autophagy, a well-conserved stress response, may play a role in ABCG2-mediated cell survival.
Figure 1.
ABCG2-expressing drug-resistant cells are also more “resistant” to nutrient deprivation. (A) Levels of ABCG2 protein in both cell pairs (MCF-7 and MCF-7 [MX100]; H460 and H460 [MX20]) were examined by western blot. GAPDH was used as loading control. (B) MCF-7 and MCF-7 (MX100) cells exhibit different morphologies following incubation in HBSS. White field images were captured at 10X amplification. Digital amplification of selected areas is shown in the upper right corner. (C) Drug-sensitive and -resistant cell lines were incubated in HBSS for times indicated and assayed for viability. (D) Apoptotic cell death in both parental and resistant cells following incubation in HBSS was measured by the Guava Nexin assay. (E) Western blot analysis of PARP cleavage confirms higher apoptotic activity in parental cells incubated in HBSS as compared to ABCG2-expressing drug-resistant cells. Graphs indicate mean ± SD for data from 3 independent experiments. The multiple Student t test was used to calculate significance and the same was carried out for all figures. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (C and D).
ABCG2 expression increases autophagic activity
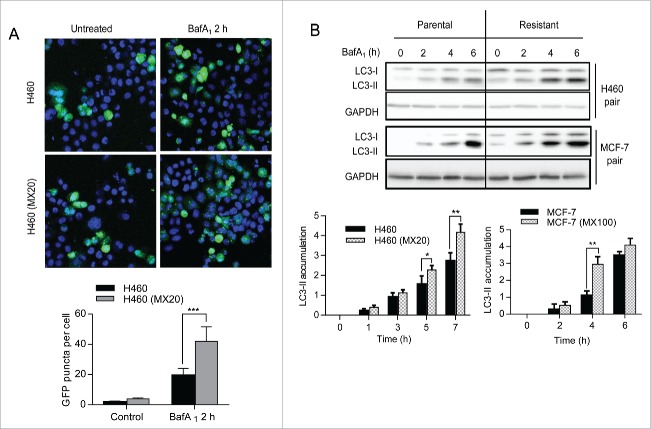
Autophagy is a highly conserved process by which unnecessary or defective macromolecules are shuttled to the lysosome for degradation to basic components that are then recycled to support survival during various types of stress, including nutrient deprivation. To determine whether autophagy played a role in cell survival mediated by ABCG2, we analyzed autophagy activity in the ABCG2 overexpressing drug-resistant cells and their parental cells, using 2 well-established markers: 1) LC3-II, the form of LC3 that is conjugated with phosphotidylethanolamine and incorporated into the membrane of the phagophore (the autophagosome precursor) following autophagy induction, and 2) SQSTM1/p62, a ubiquitin-binding receptor protein that is scavenged and degraded by autophagy.19 The drug-resistant ABCG2-expressing cells exhibited higher basal autophagy activity than their parent cell lines (Fig. 2). Using the GFP-LC3 puncta assay, we then measured the conversion of GFP-tagged LC3 from the cytosolic form (LC3-I) to the lipidated form (LC3-II), which can be visualized as puncta by fluorescence microscopy (Fig. 2A). In both H460 and H460 (MX20) cells, treatment with the lysosomal inhibitor bafilomycin A1 (BafA1), which blocks the turnover of LC3-II, induced a significant cellular accumulation of GFP-LC3 puncta. However, H460 MX20 cells accumulated ∼ 50% more fluorescent puncta than H460 cells, again indicating that ABCG2 expression leads to higher basal autophagy activity. To confirm this apparent increase in autophagy, the accumulation of LC3-II was also measured by western blot. First, note that levels of LC3-II at 0hr were higher in H460 MX20 as compared to H460 cells, indicating higher steady state levels of autophagosome formation in the presence of ABCG2. Importantly, when cells were treated with BafA1 to inhibit lysosomal degradation of autophagosome contents, the accumulation of LC3-II was more rapid and robust in the ABCG2-expressing cells compared to the parental cells (Fig. 2B).
Figure 2.
ABCG2-expressing drug-resistant cells have higher basal levels of autophagy. (A) H460 and H460 (MX20) cells were transiently transfected with a GFP-LC3 plasmid prior to 2 h of BafA1 (20 nM) treatment. Confocal fluorescence microscopic images of green-fluorescent GFP-LC3 were captured and LC3 puncta were quantified and graphed (shown below). Results (mean ± SD) are the average of counts from 25 randomly selected cells for each cell line. (B) Levels of LC3-II in parental and resistant cells were analyzed by western blot after treatment with BafA1. Quantification of LC3-II levels normalized to GAPDH is shown graphically (below).
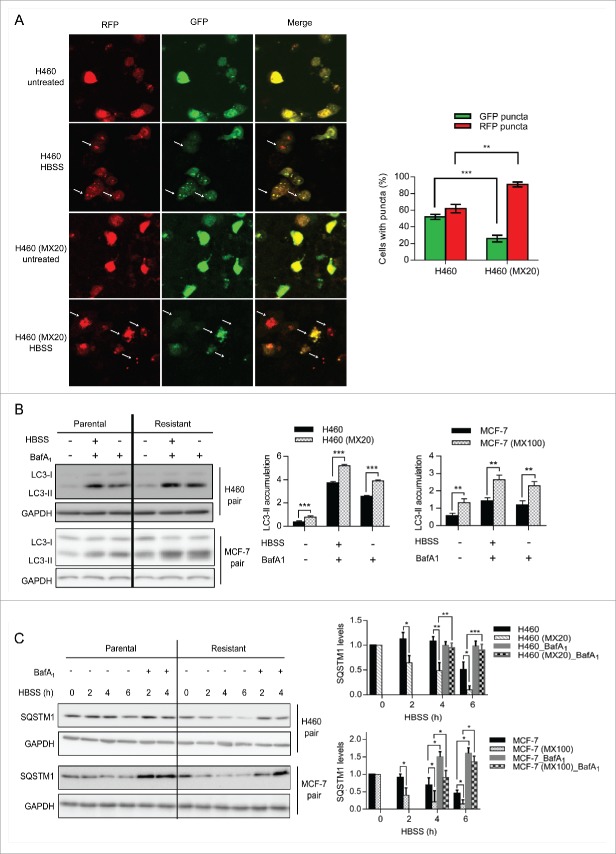
We then analyzed the autophagy activity induced by amino acid starvation in both parental and resistant cells. We used the tandem-fluorescence-tagged LC3 assay (utilizing GFP and RFP) to measure the autophagy flux during incubation in HBSS.20 This assay relies on the instability of GFP in acidic environments; GFP green fluorescence is quenched upon the entry of GFP-LC3 into the autolysosomes and lysosomes, leaving the RFP red fluorescence as a direct measure of the turnover of LC3 in these compartments. Therefore, inhibition of the lysosomal activity is not necessary in this assay. H460 cell lines were transiently transfected with the GFP-RFP-LC3 construct 24 h prior to HBSS incubation. Following treatment, cells were fixed and observed by confocal microscopy. The numbers of cells containing green or red fluorescent dots were counted and averaged from 25 images for both cell lines. As shown in Figure 3A, 2 h of incubation in HBSS resulted in a higher percentage of red dots only containing cells in H460 (MX20) cells compared to H460, suggesting that the rate of fusion of the autophagosome and lysosome, and hence autophagy flux, is higher in the ABCG2 expressing drug-resistant cells. To verify this result, LC3-II was also analyzed by western blot. When cells were incubated in HBSS, higher levels of LC3-II were observed in H460 (MX20) cells compared to H460 cells (Fig. 3B), consistent with the hypothesis that ABCG2-expressing cells have higher levels of autophagy flux.21 Similar results were shown with MCF-7 and MCF-7 (MX100) cells (Fig. 3B). Finally, the rate of degradation of SQSTM1 following incubation in HBSS was significantly increased in drug-selected cell lines (3C, shaded bar), as compared to their parental counterparts (3C, black bar). This degradation was blocked by treatment with the lysosomal inhibitor BafA1, verifying that the loss of SQSTM1 was due to lysosomal degradation.
Figure 3.
ABCG2-expressing drug-resistant cells have higher levels of autophagy following nutrient deprivation. (A) Autophagy flux was measured using a tandem-fluorescence-tagged LC3 assay. Parental and resistant H460 cell lines were transfected with a GFP-RFP-LC3 construct and treated with HBSS for 2 h. Cells were fixed and confocal images were taken. Quantification of the green and red dots is shown on the right. (B) Both MCF-7 and H460 cell pairs were incubated in HBSS alone or HBSS plus Baf A1 for 2 h. LC3 II levels were analyzed using western blot and quantified (right). (C) Levels of SQSTM1 in parental cells and their resistant sublines were determined following treatment with HBSS with or without BafA1 (20 nM). Quantifications are shown on the right.
ABCG2 is necessary and sufficient for the enhancement of stress-induced autophagy flux
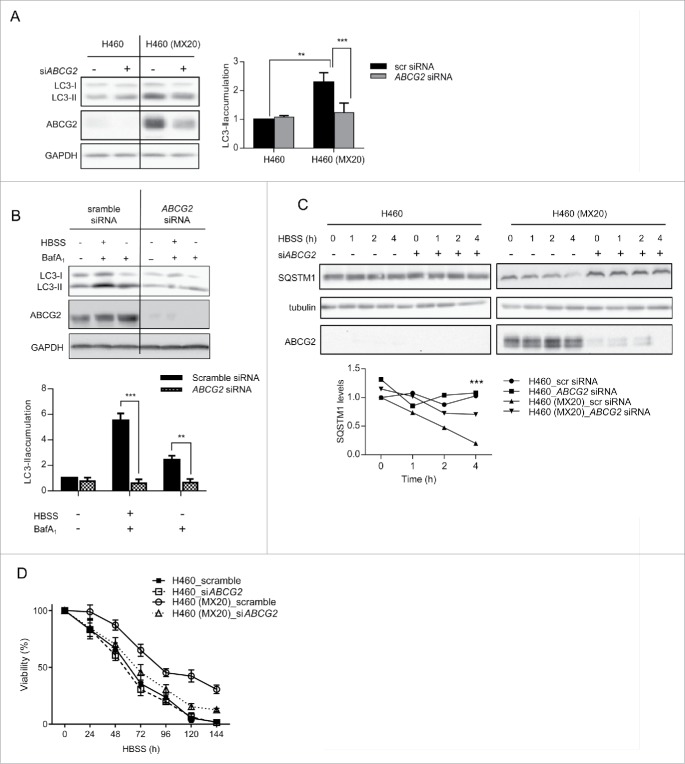
To verify that the increase in autophagy observed in the drug-resistant cells was ABCG2-dependent, siRNA knockdown was used to decrease the expression of ABCG2 in H460 (MX20) cells. As shown in Figure 4A to C the reduction of ABCG2 protein expression significantly reduced the basal and induced levels of autophagy to ones similar to what was observed in the parental H460 cells, indicating that ABCG2 expression was required for enhancement of autophagy. Importantly, the ABCG2-induced reduction in autophagy flux was accompanied by a decreased viability of nutrient-deprived drug-resistant cells, supporting the hypothesis that the enhanced survival of drug-selected cells under stress conditions is ABCG2-dependent (Fig. 4D).
Figure 4.
ABCG2 knockdown leads to reduction of autophagy in drug-resistant cells. (A) ABCG2 knockdown mediated by siRNA was carried out in both H460 and H460 (MX20) cell lines. 48 h after transfection, cells were harvested for analysis of LC3 expression. Quantification of levels of LC3-II is shown below. (B) H460 and H460 (MX20) cells were incubated in HBSS and/or BafA1 for 3 h following knockdown of ABCG2. LC3 expression was analyzed and quantified (C) H460 and H460 (MX20) cells were incubated in HBSS for different time periods following knockdown of ABCG2. SQSTM1, ABCG2 and tubulin were analyzed by western blot. Quantification of levels of SQSTM1 is shown below. All images shown represent results from 3 independent experiments. (D) Cell viability was measured for both H460 and H460(MX20) cells treated with HBSS following ABCG2 knockdown.
We also considered the possibility that drug-selected cell lines may harbor unidentified genetic alterations in addition to ABCG2 overexpression that could contribute to the stress resistance observed. To eliminate this potential complexity, we took advantage of cells that had been stably transfected with an ABCG2 expression vector (HEK293 [ABCG2]). When incubated in HBSS, HEK293 (ABCG2) cells exhibited significant higher autophagy activity compared to mock-transfected control cells (HEK293 [pcDNA] cells), as indicated by the faster degradation of SQSTM1 and more rapid accumulation of LC3-II (Fig. 5A). Similar results were obtained following transient transfection of ABCG2 into the breast cancer cell line MCF-7 (Fig. 5B). Consistent with this observation, HEK293 cells exhibited a significant increase (+15.8%) in early apoptotic cell death within 6 h of incubation in HBSS, whereas HEK293 (ABCG2) cells were only moderately affected (+4.5%). Cytotoxicity assays confirmed this observation, since viability of HEK293 cells decreased to less than 10% by 96 h, while ∼80% of HEK294 (ABCG2) cells remained viable (Fig. 5C and D). Taken together, these data indicate that autophagy induced by stress can be specifically enhanced by ABCG2 expression, thereby delaying cell death and improving cell survival.
Figure 5.
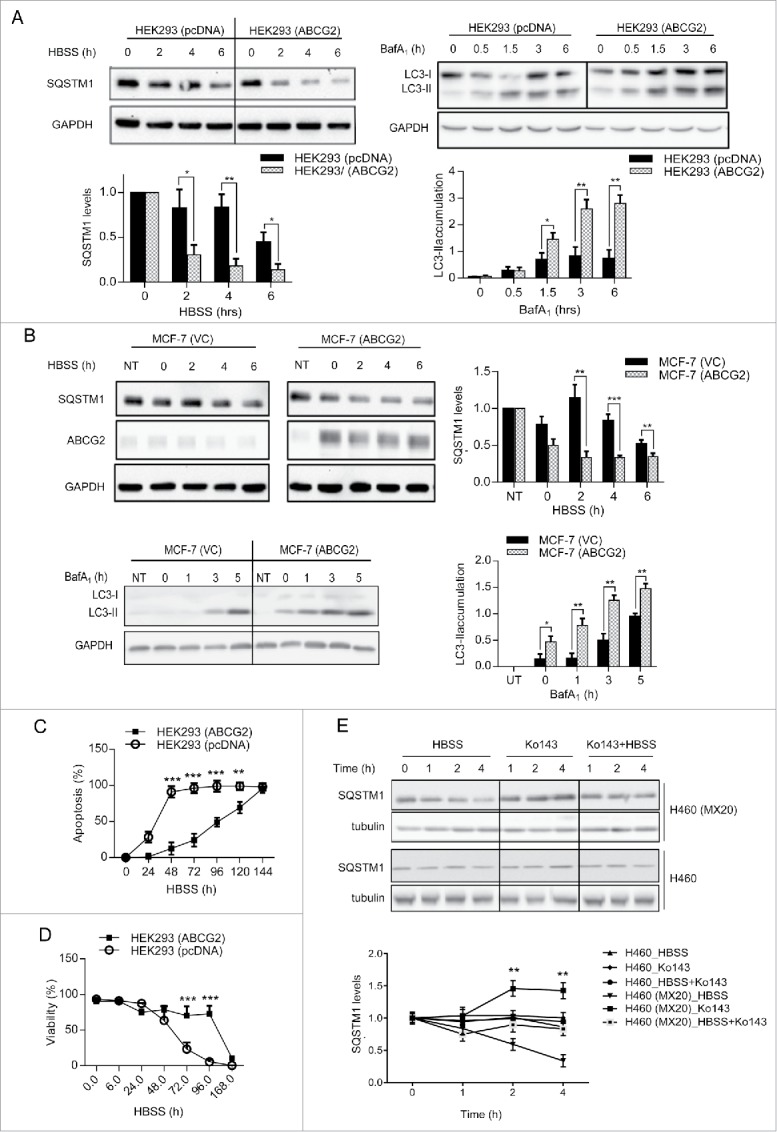
Overexpression of functional ABCG2 leads to higher autophagy activity and better survival under nutrient deprivation. (A) HEK293 cells stably expressing ABCG2 (HEK293 [ABCG2]) were compared with cells stably transfected with empty vector pcDNA (HEK293 [pcDNA]) following incubation in HBSS (for SQSTM1) or BafA1 (for LC3-II). Quantification of SQSTM1 and LC3-II is shown below. (B) MCF-7 cells transiently transfected with ABCG2 (MCF-7 [ABCG2]) were compared to mock-transfected cells (VC: vector control) NT, Nontransfected. Levels of SQSTM1 and LC3-II were analyzed by western blot (quantification on the right). (C) HEK293 (ABCG2) and HEK293 (pcDNA) cells were incubated with HBSS for the indicated times and stained for ANXA5 and 7-AAD to analyze early apoptosis using the Guava EasyCyte flow cytometry system. (D) Cell viability following incubation in HBSS was assayed by trypan blue exclusion. (E) Cells were incubated with HBSS and/or the ABCG2 inhibitor Ko143 (2 uM) for the indicated time. SQSTM1 levels were analyzed by western blot, with tubulin used as loading control. Quantification of SQSTM1 is shown below. Graphs represent mean ± SD for data from 3 independent experiments. Significance was calculated using the multiple Student t tests. ***, P < 0.001.
ABCG2 is known to efflux a variety of hydrophobic substrates, including many chemotherapeutic agents as well as endogenous substances such as folic acid and its polyglutamates,22-24 vitamins (riboflavin and vitamin K3),25,26 heme and its precursors,27,28 and uric acid.29 To determine whether ABCG2 transport activity was required for autophagy regulation, Ko143, a potent and specific inhibitor of ABCG2 activity, was used to block ABCG2-mediated transport during amino acids deprivation. As expected, Ko143 resulted in a rapid inhibition of ABCG2 transport function without altering the expression or localization of ABCG2 (data not shown). As shown in Figure 5E, Ko143 decreased the rate of HBSS-induced SQSTM1 degradation in H460 (MX20) cells to levels similar to what is observed in H460 cells, while the rate of SQSTM1 degradation in parental cells was not significantly changed. Interestingly, Ko143 by itself slightly increased the steady-state level of SQSTM1 in the drug-resistant cells, indicating that inhibition of ABCG2 activity may contribute to a decrease of the basal autophagic activity. Taken together, these results confirm a requirement for ABCG2 transport activity in autophagy regulation.
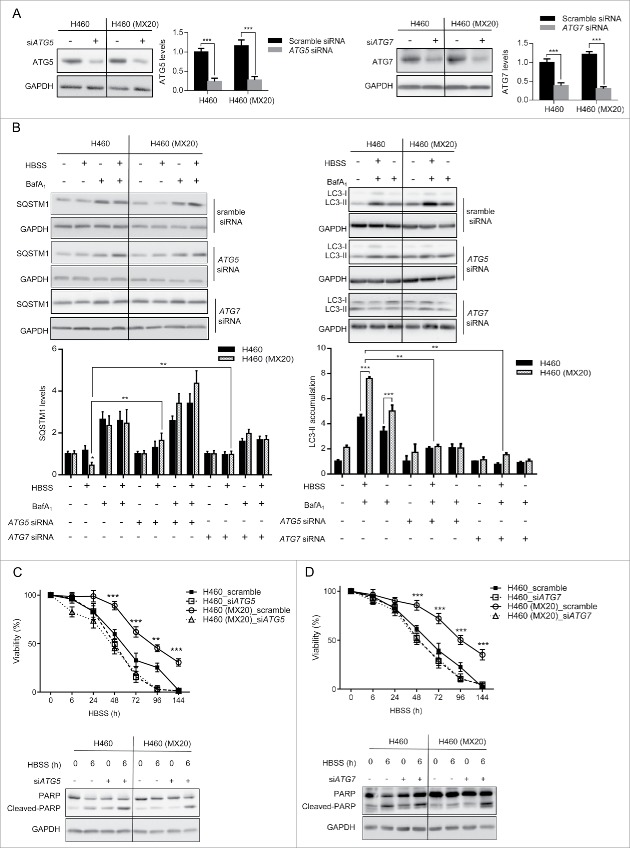
Inhibition of autophagy diminishes the resistance of ABCG2-expressing cells to amino acid starvation. To prove that the ABCG2-mediated stress resistance is a consequence of the increased rate of autophagy, autophagy was inhibited by siRNA-targeted downregulation of ATG5 (autophagy-related 5; part of a ubiquitin ligase essential for phagophore elongation30) or ATG7 (an E1-like enzyme essential for the E2-substrate reaction of LC3 lipidation31). siRNAs were transfected into both H460 and H460 (MX20) cells prior to HBSS treatment. Both cell lines express similar levels of ATG5 and ATG7 protein (Fig. 6A). Seventy-two h post-transfection, when ATG5 or ATG7 was successfully downregulated, cells were incubated in HBSS and the autophagy activity was determined. As previously shown, loss of SQSTM1 expression was more pronounced in H460 (MX20) cells than in H460 cells exposed to HBSS, indicating a higher level of autophagy in the ABCG2-expressing cells. As expected, the knockdown of ATGs inhibited the degradation of SQSTM1 in both cell lines (Fig. 6B), and the accumulation of LC3-II was diminished. To evaluate the effect of decreased autophagy on cell death, viability studies and PARP cleavage assays were carried out. In the presence of HBSS, the percentage of viable cells was much lower in parental H460 cells than in their drug-selected counterparts, indicating their greater sensitivity to amino acid deprivation. Importantly, knockdown of either ATG5 or ATG7 greatly diminished this difference; following 24 h exposure to HBSS, H460 cells and H460 (MX20) cells treated with either ATG5 or ATG7 siRNA, exhibited a similar cytotoxic profile (Fig. 6C) and a similar degree of apoptosis (Fig. 6D). Similar results were also obtained with drug-selected MCF-7 (MX100) cells and stably transfected HEK293 cells (data not shown). Taken together, these results show that knockdown of autophagy sensitized cells to stress and diminished the survival advantage conferred by the expression of ABCG2, validating the hypothesis that ABCG2 protects cancer cells against non-substrate stresses by upregulating autophagy.
Figure 6.
Inhibition of autophagy eliminates the cytoprotective effect of ABCG2 in drug-resistant cells. (A) Both H460 and H460 (MX20) cell lines were transfected with siRNA targeting ATG5 or ATG7. Protein levels of ATG5 or ATG7 were analyzed after 72 h of transfection. Quantifications are shown on the right. (B). Post ATG5 or ATG7 knockdown, cells were treated with HBSS and/or BafA1 for 4 h and SQSTM1 levels were analyzed by western blot; for LC3-II, cells were treated with HBSS with or without BafA1 for 2 h and analyzed by western blot. Quantification of these proteins is shown below. (C, D) Following knockdown of ATG5 or ATG7, cells were treated with HBSS for 6 d and cell viability was analyzed by trypan blue exclusion. Note that H460 (MX20) cell viability was reduced to a level similar to what was observed in H460 cells in response to nutrient deprivation. PARP protein was analyzed by western blot following 8-h HBSS treatment.
ABCG2 protects cells from other stress inducers
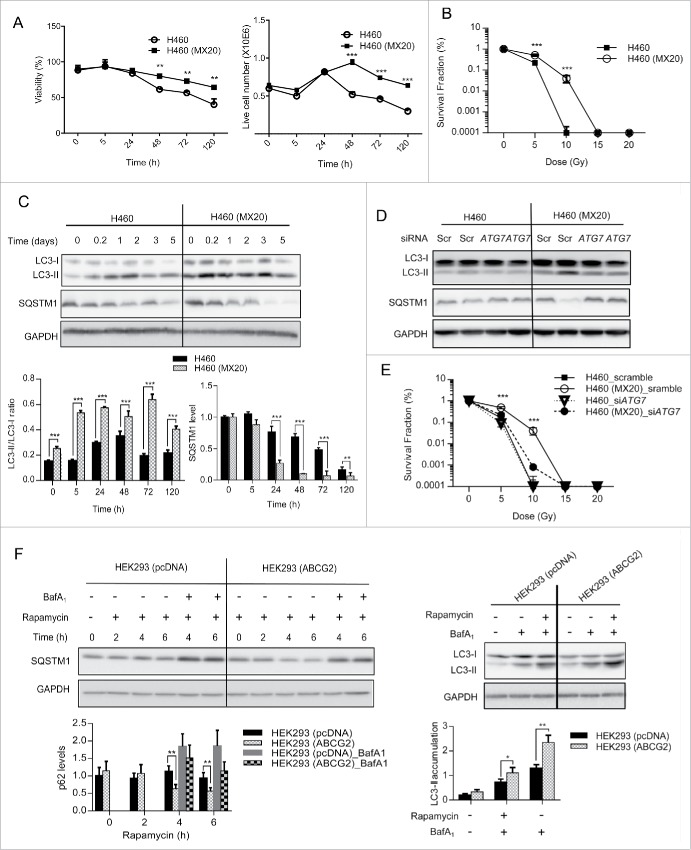
Ionizing radiation (IR) is critical to the treatment of some cancers, including lung and breast tumors, yet radiation resistance is common and can lead to treatment failure. Since IR has been shown to induce autophagy,32,33 we next tested whether the presence of ABCG2 could accelerate IR-induced autophagy, thereby delaying cell death. H460 and H460 (MX20) cells were treated with a single dose of 10 Gy γ-radiation in complete culture medium. Media was changed after treatment to prevent a nonspecific effect of irradiation on media components. Cells were cultured for up to 5 d and measured for viability. As shown in Figure 7, the viability of both parental and resistant H460 cells was affected by IR, but the ABCG2-expressing H460 (MX20) cells showed a marked resistance to cell death over time (Fig. 7A), and continued to proliferate for at least 24 h beyond what was observed for the H460 cells (48 h vs 24 h; Fig. 7A). Clonogenic assays confirmed that the percentage of long-term surviving cells was higher in the ABCG2-expressing cells (Fig. 7B) at doses of 5 Gy and 10 Gy, demonstrating the survival advantage that H460 (MX20) had over H460 cells.
Figure 7.
ABCG2 protects cells from other autophagy inducers: radiation and rapamycin. (A) H460 and H460 (MX20) cells were treated with IR (10 Gy) then cultured for indicated times. Viability was measured by trypan blue exclusion. Quantifications of the viability percentage (left panel) and total live cell numbers (right panel) are shown. (B) Clonogenic assays were carried out with H460 and H460 (MX20) cells treated with increasing doses of ionizing radiation. Survival fractions were calculated for both cell lines. (C) Cell lysates were also collected to analyze levels of LC3-II and SQSTM1. Quantification of the SQSTM1 and LC3-II/LC3-I ratios are shown graphically. (D) Cells were transfected with siRNA targeting ATG7, followed by IR (10 Gy) treatment. Levels of LC3-II and SQSTM1 were analyzed at d 3 post IR treatment. (E) Clonogenic assays were carried out for cells transfected with either scrambled siRNA or siRNA targeted to ATG7. (F). HEK293 (pCDNA) and HEK293 (ABCG2) cells were treated with 200 nM rapamycin. Levels of SQSTM1 and LC3-II were analyzed at the times indicated by western blot. Quantification of the western blots is shown below. GAPDH was used as a loading control.
To determine whether ABCG2-regulated autophagy played a role in radiation resistance, the levels of LC3-II and SQSTM1 were examined in both cell lines following γ-radiation. As shown in Figure 7C, while γ-radiation induced autophagy in both parental and ABCG2-overexpresssing cells; the autophagy response was more profound in the ABCG2-overexpressors, supporting the hypothesis that ABCG2 enhances radiation-induced autophagy, thereby delaying apoptosis and improving cell survival. Furthermore, ATG7 knockdown blocked the autophagy response to radiation treatment in both cell lines, and dramatically decreased the proliferation rate of H460 (MX20) cells in clonogenic assays (Fig. 7E). Given that the IR dose is fractionated in most clinical protocols, this delay in cell death may be sufficient to allow tumor cells to survive radiation-induced stress. This differential response was also observed when autophagy was induced with rapamycin, a drug that induces autophagy by inhibiting the MTORC1 complex, a nutrient-sensing complex that maintains normal homeostasis under nonstress conditions. As shown in Figure 7F, while rapamycin induced autophagy in both HEK293 (pcDNA) and HEK293 (ABCG2) cells, the ABCG2-expressing cells exhibited a much higher rate of autophagy, as indicated by the more rapid decrease in SQSTM1 levels and greater conversion of LC3-I to LC3-II when compared to their mock-transfected counterparts. Taken together, these studies suggest that the ABCG2-mediated elevation of autophagy can be generalized to multiple autophagy inducers, ranging from starvation to DNA damage and drug treatment.
Discussion
One hallmark of cancer is the ability of tumor cells to survive in stressful environments, whether these environs exist within the tumor milieu or are created by therapeutic intervention (i.e. drugs, radiation, etc). A well-studied mechanism of cancer cell survival in the presence of certain chemotherapeutic agents is the enhanced expression of multidrug resistance transporters. Interestingly, while these transporters have long been known to confer resistance to their drug substrates, sporadic evidence has accumulated over the years to suggest that some of these transporters may also contribute to cell survival in the presence of other, nonsubstrate stressors. Most early studies focused on ABCB1, whose enhanced expression conferred a survival advantage in the presence of stressors that were not drug substrates, including Fas ligand, tumor necrosis factor, and ultraviolet (UV) irradiation.34,35 More recently, it has been suggested that ABCG2 may also play a more general role in cell survival. An early indicator of this phenotype is the observation that ABCG2 expression protects placental trophoblasts and placenta choriocarcinoma cells against cytokines (TNF, IFNG) and exogenous ceramides (C6 and C8). In this case, ABCG2 appears to exert its cytoprotective effect upstream of a common terminal pathway of apoptotic death, since cell death induced by 2 intrinsic apoptotic activators, deguelin (iAA1) and 1-(3, 4-dichlorobezyl)-1H-indole-2, 3-dione (iAA2,) is not affected by ABCG2 expression.9 Another study demonstrates that ABCG2-expressing human embryonic stem cells tolerate the physical stress of cell sorting as well as UV irradiation much better than the ABCG2-negative population.11 These in vitro studies have correlates in the clinical setting, where the expression and activity of ABCG2 has been linked to radiotherapy resistance.12 Interestingly, several studies suggest that ABCG2 may also be an indicator of poor prognosis in some tumors, including lung,36 breast,37 and esophageal cancers38 even in the absence of drug treatment. Together, these observations suggest a role for ABCG2 in general tumor cell survival independent of its well-characterized drug efflux function. How this is accomplished has not been previously addressed.
We now report that ABCG2 regulates the rate of autophagy, a function that may contribute to its role in resistance to stress inducers. Under normal growth conditions, ABCG2-overexpressing cells exhibited a higher basal rate of autophagy than their low-expressing counterparts. Under conditions designed to induce autophagy (nutrient starvation, ionizing radiation, inhibition of the MTOR signaling pathway) an even higher rate of autophagy was observed in ABCG2-expressing cells. This effect was seen in multiple ABCG2-containing tumor cell lines regardless of whether ABCG2 overexpression was the result of drug selection or accomplished through transfection, indicating the generality of ABCG2-regulated autophagy in different genotypic/phenotypic backgrounds. Moreover, although the autophagy inducers used in this study were not ABCG2 substrates, the transport activity of ABCG2 was required for the phenotype, suggesting that ABCG2 may transport certain cellular substance(s) involved in autophagy regulation. One potential candidate is glutathione, which has previously been proposed to be a substrate for ABCG2.39 However, a recent study shows that ABCG2 is not able to transport glutathione,40 and our laboratory has also been unable to detect increased glutathione transport in ABCG2-expressing vs. parental cells (data not shown). Other ABCG2 substrates that are potential autophagy mediators are currently under investigation.
The role of autophagy in tumorigenesis is complex and context-dependent. Mice heterozygous for the autophagy mediator beclin 1 (BECN1) exhibit a higher tumor incidence than their wild-type counterparts, suggesting a key role for autophagy in the prevention of tumorigenesis. This hypothesis is supported by the observation that human tumors, including ovarian, breast and prostate cancers, express low levels of BECN1.41,42 In contrast, a recent study using palb2 knockout mice suggests that autophagy promotes mammary tumor growth by suppressing TP53/TRP53/p53 (note that the mouse nomenclature is TRP53, but we use TP53 hereafter to refer to both the human and mouse genes/proteins for simplicity) activation induced by DNA damage.43 Although these 2 roles appear contradictory on the surface, evidence is emerging to suggest that the impact of autophagy in a given tumor will be context-specific, likely influenced by the genotype or phenotype of the tumor as well as the stage of tumorigenesis. That said, in most established tumor models studied to date autophagy is cytoprotective and confers resistance to cancer treatments, including chemotherapy and radiation.44 This finding has led to numerous phase I/II clinical trials to test autophagy inhibitors as chemosensitizers for other antitumor regimens.45 Our studies suggest that this combination could be particularly important for ABCG2-expressing tumors due to their high levels of autophagy. Additionally, it is interesting to note that the majority of these trials employ hydroxychloroquine (HCQ) as the autophagy inhibitor, due to its well-established safety profile. However, HCQ is a known substrate of ABCG2 46 and failed to inhibit autophagy in our ABCG2-expressing cells (data not shown). If these in vitro observations are borne out in the in vivo setting, it may be useful to include ABCG2 as a biomarker for tumor response to HCQ combination therapies.
Considerable progress has been made in identifying components of the autophagy machinery, and in understanding regulatory mechanisms that control the autophagic process. Yet there are several unanswered questions, and many factors that control autophagy remain elusive. The identification of ABCG2 as a regulator of autophagy flux suggests that this transporter may confer a “transient resistance” to tumor cells under stress conditions. Recently, both ABCC1 and ABCB1 have also been suggested to play a role in autophagy,47,48 suggesting that there may be a general role of ABC transprorters in cell survival that has not been recognized and exploited. This may be particularly important for ABCG2, since this transporter, along with ABCB1, is highly expressed in most cancer stem cells (CSCs), where it has been used for both CSC isolation and as a CSC biomarker.49 Evidence is also accumulating to suggest that ABCG2, unlike ABCB1, may be required for the maintenance of “stemness.”8 Since CSCs have been shown to contribute to the pathology of many cancers with respect to both cell survival and metastases,50 it is intriguing to speculate that ABCG2, in addition to protecting CSCs against chemotherapeutic substrates, may also play a role in autophagy regulation in these progenitor cells. While this remains to be tested, it suggests that ABCG2 may protect CSCs against a variety of microenvironmental stressors, adding to the inherent resistance of these cells to both unfavorable milieus and standard anticancer regimens.
Our finding that ABCG2 expression enhances stress-induced autophagy and cell survival in multiple tumor cell types indicates a novel role of this transporter beyond the conventional drug-efflux function. An understanding of the mechanism by which ABCG2 regulates autophagy will allow us to better define the tumor milieu in which increased autophagy flux leads to increased tumor survival. This in turn may allow us to predict those tumors in which autophagy-targeted therapy should be exploited, and provide a new target for the elimination of CSC populations.
Materials and methods
Cell culture and chemicals
The human breast adenocarcinoma cell line MCF-7 and its mitoxantrone (MX)-resistant subline MCF-7 (MX100) as well as the human large cell lung carcinoma cell line NCI-H460 and its MX-resistant subline NCI-H460 (MX20) were kindly provided by Dr. Susan Bates (National Institutes of Health, Bethesda, MD). Both MCF-7 cell lines were grown in improved minimum essential medium (IMEM; Invitrogen, 10373-017) containing 2 g/l glucose, 2 mM L-glutamine, 1 mM sodium pyruvate and 10% fetal bovine serum at 37°C in 5% (v/v) CO2. MCF-7 (MX100) cells were maintained in the presence of 100 nM MX (Sigma, M6545).51 Both H460 cell lines were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum and 2 mM L-glutamine at 37˚C in 5% (v/v) CO2. H460 (MX20) cells were maintained in the presence of 20 nM MX.51 HEK293 cells stably transfected with either pcDNA or pcDNA-ABCG2 were maintained in Dulbecco's modified Eagle's medium (DMEM, Cellgro, 15-017-CV) containing 10% fetal bovine serum and 2 mM L-glutamine, and supplemented with 2 mg/ml geneticin (Invitrogen, 10131-035). Hank's Balanced Salt Solution was purchased from Thermo Fisher Scienctific (24020117). Bafilomycin A1 (B1793) and rapamycin (R8781) were purchased from Sigma-Aldrich. The ABCG2 specific inhibitor, Ko143, was purchased from Tocris Bioscience (3241).
Transient transfection
MCF-7 cells were seeded at 2.5×105 per well in 6-well plates and cultured overnight before transfection. Two µg of plasmid pCMV-V6 (empty vector control) or pCMV-ABCG2 (Origene, SC320948) were mixed with Lipofectamine 2000 (Life Technologies, 11668027) at a 1:3 ratio according to the manufacturer's instructions and added to the culture media. Transfected cells were incubated at 37°C for 24 h before treatment and analysis.
Western blot analysis
Western blot assays for the autophagy markers LC3B (this is the only isoform of LC3 tested, referred as LC3 in this study) and SQSTM1 were conducted according to the protocol described previously,18 with specific modifications for LC3. Immunoblotting was accomplished using a rabbit monoclonal antibody against LC3 (1:1,000; Cell Signaling Technology, 2775) and immunoreactive proteins were visualized using an enhanced chemiluminescent system (Super Signal West Femto Chemiluminescent Substrate; Thermo Scientific, 34095) according to the manufacturer's recommendations. SQSTM1was detected with guinea pig monoclonal anti- SQSTM1 antibody (Progen, GP62-C). Mouse monoclonal antibody against ABCG2 was purchased from Kamiya Biomedical (MC-177) and rabbit monoclonal antibodies against GAPDH (2118), PARP (9542), ATG7 (8558) and ATG5 (2630) were purchased from Cell Signaling Technology.
Apoptosis assay
The Guava EasyCyte flow cytometry analysis system (Guava Technologies, Millipore) was utilized to determine the percentage of apoptotic cells. Assays were conducted according to the manufacturer's instructions. Cells were pretreated with HBSS for different time periods prior to the assay. Pretreated cells were trypsinized and collected in ice-cold phosphate-buffered saline (Cellgro, 231-031-CV) to a final concentration of 2*105/ml. Prior to analysis, 5 µL of ANXA5-phycoerythrin, a marker for early apoptosis, and 5 µL of 7-amino-actinomycin (7-AAD), a cell-impermeant dye indicating late apoptosis or dead cells (Guava Nexin reagent, Millipore, 4500-0455), were added to cell suspensions in ice-cold phosphate-buffered saline. After 20 min incubation and thorough mixing, 200 ul of each sample was added to a 96-well plate and analyzed.
Immunocytochemistry
Cells were transfected with either GFP-LC3 (for LC3 puncta assay) or GFP-mRFP-LC3 (for tandem fluorescence LC3 assay) constructs 24 h prior to treatment. Cells were then washed and fixed in 4% paraformaldehye solution for 10 min. Hoechst 33258 (50 ng/ml) were used to stain the nucleus. Coverslips with cells were then mounted, sealed on glass slides with mounting medium (Life Technologies, S36937) and examined using a Nikon A1+ confocal laser microscope system (Melville, NY).
Ionizing radiation and clonogenic assays
Cells were seeded in 60-mm dishes at 4×105 per dish the day before treatment. H460 and H460 (MX20) cells were treated with gamma-irradiation in a Nordion JS Gamma Irradiator (447 March Rd, Ottawa, ON, Canada, K2K 1X8) at a single dosage of 10 Gy. Immediately after the treatment, cells were cultured for 1 to 5 d and analyzed for survival using the apoptosis assay. For clonogenic assays, cells were plated at predetermined concentrations in 6-well dishes (e.g., 1000 cells/well) and treated with gamma-irradiation at dosages of 0, 5, 10, 15, and 20 Gy. Cells were then cultured for 10 d before fixation and staining with crystal violet (0.5%). Colonies with more than 50 cells were counted manually using a stereomicroscope or automatically using ImageJ software. Plating efficiency (PE) and survival fractions were calculated as follow: PE = no. of colonies formed/no. of cells seeded × 100%; survival fractions = no. of colonies formed after treatment/no. of cells seeded × PE.
siRNA knockdown
The customized ON-Target plus siRNA (synthesized by Thermo Scientific according to a previously identified sequence targeting exon 7 in ABCG2 mRNA 52) was used to downregulate ABCG2 in H460 or H460 (MX20) cells. Cells were seeded at 3×105 per well in 6-well plates in RPMI medium without antibiotics the day before transfection. Either ABCG2 siRNA (100 nM) or scrambled siRNA (100 nM) (siGENOME Nontargeting siRNA; Thermo Scientific, D-001210-05-20) was transfected into cells using 10 µl Lipofectamine 2000. Twenty-four h after transfection, cells were washed with media and further cultured for 24 h before treatment. A siGENOME SMARTpool siRNA mixture (Dharmacon/GE, M-004374-04-0005) was used to downregulate ATG5 (M-020112-01-0005 for ATG7), using a procedure similar to that used to knock down ABCG2.
Statistical analyses
One-way ANOVA was used for the statistical analyses in the apoptosis and cell viability studies. The Student t test was used for the statistical analyses in GFP-LC3 puncta quantifications and the clonogenic assay. Multiple Student t tests were used for the statistical analyses for the western blot quantification. Data are presented as the means ± s.d., and statistical significance is denoted as the P value.
Abbreviations
- ABC
ATP-binding cassette
- ABCB1
ATP-binding cassette subfamily B member 1
- ABCG2
ATP-binding cassette subfamily G member 2 (junior blood group)
- ATG5
autophagy-related 5
- ATG7
autophagy-related 7
- BafA1
bafilomycin A1
- CSC
cancer stem cell
- GFP
green fluorescent protein
- HBSS
Hank's balanced salt solution
- HCQ
hydroxychloroquine
- IR
ionizing radiation
- LC3B
microtubule-associated protein 1 light chain 3 β
- MX
mitoxantrone
- PARP
poly(ADP-ribose) polymerase
- RFP
red fluorescent protein
- SQSTM1
sequestosome 1
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Joseph Bertino (CINJ) for valuable discussions and Dr. Cen Zhang (CINJ) for help with the gamma irradiation experiments.
Funding
This work was supported in part by NCI CCSG - P30 CA072720.
References
- [1].Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 2001; 11:1156-66; PMID:11435397; http://dx.doi.org/ 10.1101/gr.GR-1649R [DOI] [PubMed] [Google Scholar]
- [2].Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene 2003; 22:7496-511; PMID:14576854; http://dx.doi.org/ 10.1038/sj.onc.1206950 [DOI] [PubMed] [Google Scholar]
- [3].Diestra JE, Scheffer GL, Catala I, Maliepaard M, Schellens JH, Scheper RJ, Germa-Lluch JR, Izquierdo MA. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol 2002; 198:213-9; PMID:12237881; http://dx.doi.org/ 10.1002/path.1203 [DOI] [PubMed] [Google Scholar]
- [4].Turner JG, Gump JL, Zhang C, Cook JM, Marchion D, Hazlehurst L, Munster P, Schell MJ, Dalton WS, Sullivan DM. ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood 2006; 108:3881-9; PMID:16917002; http://dx.doi.org/ 10.1182/blood-2005-10-009084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Britton KM, Eyre R, Harvey IJ, Stemke-Hale K, Browell D, Lennard TWJ, Meeson AP. Breast cancer, side population cells and ABCG2 expression. Cancer Lett 2012; 323:97-105; PMID:22521545; http://dx.doi.org/ 10.1016/j.canlet.2012.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem cells (Dayton, Ohio) 2006; 24:506-13; PMID:16239320; http://dx.doi.org/ 10.1634/stemcells.2005-0282 [DOI] [PubMed] [Google Scholar]
- [7].Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005; 5:275-84; PMID:15803154; http://dx.doi.org/ 10.1038/nrc1590 [DOI] [PubMed] [Google Scholar]
- [8].Bhattacharya S, Das A, Mallya K, Ahmad I. Maintenance of retinal stem cells by Abcg2 is regulated by notch signaling. J Cell Sci 2007; 120:2652-62; PMID:17635990; http://dx.doi.org/ 10.1242/jcs.008417 [DOI] [PubMed] [Google Scholar]
- [9].Evseenko DA, Murthi P, Paxton JW, Reid G, Emerald BS, `Mohankumar KM, Lobie PE, Brennecke SP, Kalionis B, Keelan JA. The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. Faseb J 2007; 21:3592-605; PMID:17595345; http://dx.doi.org/ 10.1096/fj.07-8688com [DOI] [PubMed] [Google Scholar]
- [10].Evseenko DA, Paxton JW, Keelan JA. The xenobiotic transporter ABCG2 plays a novel role in differentiation of trophoblast-like BeWo cells. Placenta 2007; 28(Suppl A):S116-20; PMID:17275084; http://dx.doi.org/ 10.1016/j.placenta.2006.12.003 [DOI] [PubMed] [Google Scholar]
- [11].Erdei Z, Sarkadi B, Brozik A, Szebenyi K, Varady G, Mako V, Pentek A, Orban TI, Apati A. Dynamic ABCG2 expression in human embryonic stem cells provides the basis for stress response. Eur Biophys J 2013; 42:169-79; PMID:22851001; http://dx.doi.org/ 10.1007/s00249-012-0838-0 [DOI] [PubMed] [Google Scholar]
- [12].Shien K, Toyooka S, Ichimura K, Soh J, Furukawa M, Maki Y, Muraoka T, Tanaka N, Ueno T, Asano H, et al.. Prognostic impact of cancer stem cell-related markers in non-small cell lung cancer patients treated with induction chemoradiotherapy. Lung cancer 2012; 77:162-7; PMID:22387005; http://dx.doi.org/ 10.1016/j.lungcan.2012.02.006 [DOI] [PubMed] [Google Scholar]
- [13].Ingram WJ, Crowther LM, Little EB, Freeman R, Harliwong I, Veleva D, Hassall TE, Remke M, Taylor MD, Hallahan AR. ABC transporter activity linked to radiation resistance and molecular subtype in pediatric medulloblastoma. Exp Hematol Oncol 2013; 2:26; PMID:24219920; http://dx.doi.org/ 10.1186/2162-3619-2-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 2009; 43:67-93; PMID:19653858; http://dx.doi.org/ 10.1146/annurev-genet-102808-114910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008; 451:1069-75; PMID:18305538; http://dx.doi.org/ 10.1038/nature06639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 2012; 12:401-10; PMID:22534666; http://dx.doi.org/ 10.1038/nrc3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res 2001; 7:145-52; PMID:11205902 [PubMed] [Google Scholar]
- [18].Ding R, Shi J, Pabon K, Scotto KW. Xanthines down-regulate the drug transporter ABCG2 and reverse multidrug resistance. Mol Pharmacol 2012; 81:328-37; PMID:22113078; http://dx.doi.org/ 10.1124/mol.111.075556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rusten TE, Stenmark H. p62, an autophagy hero or culprit? Nat Cell Biol 2010; 12:207-9; PMID:20190829; http://dx.doi.org/ 10.1038/ncb0310-207 [DOI] [PubMed] [Google Scholar]
- [20].Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al.. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012; 8:445-544; PMID:22966490; http://dx.doi.org/ 10.4161/auto.19496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Menzies FM, Moreau K, Puri C, Renna M, Rubinsztein DC. Measurement of autophagic activity in mammalian cells. Curr Protoc Cell Biol 2012; Chapter 15:Unit 15 6; PMID:22422474 [DOI] [PubMed] [Google Scholar]
- [22].Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, Ross DD, Bates SE, Kruh GD. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res 2003; 63:4048-54; PMID:12874005 [PubMed] [Google Scholar]
- [23].Ifergan I, Shafran A, Jansen G, Hooijberg JH, Scheffer GL, Assaraf YG. Folate deprivation results in the loss of breast cancer resistance protein (BCRP/ABCG2) expression. A role for BCRP in cellular folate homeostasis. J Biol Chem 2004; 279:25527-34; PMID:15047700; http://dx.doi.org/ 10.1074/jbc.M401725200 [DOI] [PubMed] [Google Scholar]
- [24].Breedveld P, Pluim D, Cipriani G, Dahlhaus F, van Eijndhoven MA, de Wolf CJ, Kuil A, Beijnen JH, Scheffer GL, Jansen G, et al.. The effect of low pH on breast cancer resistance protein (ABCG2)-mediated transport of methotrexate, 7-hydroxymethotrexate, methotrexate diglutamate, folic acid, mitoxantrone, topotecan, and resveratrol in in vitro drug transport models. Mol Pharmacol 2007; 71:240-9; PMID:17032904; http://dx.doi.org/ 10.1124/mol.106.028167 [DOI] [PubMed] [Google Scholar]
- [25].van Herwaarden AE, Wagenaar E, Merino G, Jonker JW, Rosing H, Beijnen JH, Schinkel AH. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol Cel Biol 2007; 27:1247-53; PMID:17145775; http://dx.doi.org/ 10.1128/MCB.01621-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shukla S, Wu CP, Nandigama K, Ambudkar SV. The naphthoquinones, vitamin K3 and its structural analogue plumbagin, are substrates of the multidrug resistance linked ATP binding cassette drug transporter ABCG2. Mol Cancer Ther 2007; 6:3279-86; PMID:18065489; http://dx.doi.org/ 10.1158/1535-7163.MCT-07-0564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, et al.. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A 2002; 99:15649-54; PMID:12429862; http://dx.doi.org/ 10.1073/pnas.202607599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem 2004; 279:24218-25; PMID:15044468; http://dx.doi.org/ 10.1074/jbc.M313599200 [DOI] [PubMed] [Google Scholar]
- [29].Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A 2009; 106:10338-42; PMID:19506252; http://dx.doi.org/ 10.1073/pnas.0901249106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, Inagaki F, Ohsumi Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem 2007; 282:37298-302; PMID:17986448; http://dx.doi.org/ 10.1074/jbc.C700195200 [DOI] [PubMed] [Google Scholar]
- [31].Tanida I, Yamasaki M, Komatsu M, Ueno T. The FAP motif within human ATG7, an autophagy-related E1-like enzyme, is essential for the E2-substrate reaction of LC3 lipidation. Autophagy 2012; 8:88-97; PMID:22170151; http://dx.doi.org/ 10.4161/auto.8.1.18339 [DOI] [PubMed] [Google Scholar]
- [32].Ito H, Daido S, Kanzawa T, Kondo S, Kondo Y. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. International journal of oncology 2005; 26:1401-10; PMID:15809734 [PubMed] [Google Scholar]
- [33].Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res 2008; 68:1485-94; PMID:18316613; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0562 [DOI] [PubMed] [Google Scholar]
- [34].Johnstone RW, Cretney E, Smyth MJ. P-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell death. Blood 1999; 93:1075-85; PMID:9920858 [PubMed] [Google Scholar]
- [35].Smyth MJ, Krasovskis E, Sutton VR, Johnstone RW. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc Natl Acad Sci U S A 1998; 95:7024-9; PMID:9618532; http://dx.doi.org/ 10.1073/pnas.95.12.7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li F, Zeng H, Ying K. The combination of stem cell markers CD133 and ABCG2 predicts relapse in stage I non-small cell lung carcinomas. Med Oncol 2011; 28:1458-62; PMID:20717756; http://dx.doi.org/ 10.1007/s12032-010-9646-5 [DOI] [PubMed] [Google Scholar]
- [37].Omran OM. The prognostic value of breast cancer resistance protein (BCRB/ABCG2) expression in breast carcinomas. J Environ Pathol Toxicol Oncol 2012; 31:367-76; PMID:23394449; http://dx.doi.org/ 10.1615/JEnvironPatholToxicolOncol.2013006767 [DOI] [PubMed] [Google Scholar]
- [38].Tsunoda S, Okumura T, Ito T, Kondo K, Ortiz C, Tanaka E, Watanabe G, Itami A, Sakai Y, Shimada Y. ABCG2 expression is an independent unfavorable prognostic factor in esophageal squamous cell carcinoma. Oncology 2006; 71:251-8; PMID:17671398; http://dx.doi.org/ 10.1159/000106787 [DOI] [PubMed] [Google Scholar]
- [39].Brechbuhl HM, Gould N, Kachadourian R, Riekhof WR, Voelker DR, Day BJ. Glutathione transport is a unique function of the ATP-binding cassette protein ABCG2. J Biol Chem 2010; 285:16582-7; PMID:20332504; http://dx.doi.org/ 10.1074/jbc.M109.090506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gauthier C, Ozvegy-Laczka C, Szakacs G, Sarkadi B, Di Pietro A. ABCG2 is not able to catalyze glutathione efflux and does not contribute to GSH-dependent collateral sensitivity. Front Pharmacol 2013; 4:138; PMID:24312054; http://dx.doi.org/ 10.3389/fphar.2013.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shimizu S, Yoshida T, Tsujioka M, Arakawa S. Autophagic cell death and cancer. Int J Mol Sci 2014; 15:3145-53; PMID:24566140; http://dx.doi.org/ 10.3390/ijms15023145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al.. Autophagy suppresses tumorigenesis through elimination of p62. Cell 2009; 137:1062-75; PMID:19524509; http://dx.doi.org/ 10.1016/j.cell.2009.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Huo Y, Cai H, Teplova I, Bowman-Colin C, Chen G, Price S, Barnard N, Ganesan S, Karantza V, White E, et al.. Autophagy opposes p53-mediated tumor barrier to facilitate tumorigenesis in a model of PALB2-associated hereditary breast cancer. Cancer Discov 2013; 3:894-907; PMID:23650262; http://dx.doi.org/ 10.1158/2159-8290.CD-13-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gewirtz DA. The four faces of autophagy: implications for cancer therapy. Cancer Res 2014; 74:647-51; PMID:24459182; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-2966 [DOI] [PubMed] [Google Scholar]
- [45].Carew JS, Kelly KR, Nawrocki ST. Autophagy as a target for cancer therapy: new developments. Cancer Manag Res 2012; 4:357-65; PMID:23091399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Marki-Zay J, Tauberne Jakab K, Szeremy P, Krajcsi P. MDR-ABC transporters: biomarkers in rheumatoid arthritis. Clin Exp Rheumatol 2013; 31:779-87; PMID:23711386 [PubMed] [Google Scholar]
- [47].Desideri E, Filomeni G, Ciriolo MR. Glutathione participates in the modulation of starvation-induced autophagy in carcinoma cells. Autophagy 2012; 8:1769-81; PMID:22964495; http://dx.doi.org/ 10.4161/auto.22037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mazzanti R, Platini F, Bottini C, Fantappie O, Solazzo M, Tessitore L. Down-regulation of the HGF/MET autocrine loop induced by celecoxib and mediated by P-gp in MDR-positive human hepatocellular carcinoma cell line. Biochem Pharmacol 2009; 78:21-32; PMID:19447220; http://dx.doi.org/ 10.1016/j.bcp.2009.03.013 [DOI] [PubMed] [Google Scholar]
- [49].Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer 2010; 10:147-56; PMID:20075923; http://dx.doi.org/ 10.1038/nrc2789 [DOI] [PubMed] [Google Scholar]
- [50].Li F, Tiede B, Massague J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res 2007; 17:3-14; PMID:17179981; http://dx.doi.org/ 10.1038/sj.cr.7310118 [DOI] [PubMed] [Google Scholar]
- [51].Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, Litman T, Dean M, Bates SE. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res 2001; 61:6635-9; PMID:11559526. [PubMed] [Google Scholar]
- [52].Ee PL, He X, Ross DD, Beck WT. Modulation of breast cancer resistance protein (BCRP/ABCG2) gene expression using RNA interference. Mol Cancer Ther 2004; 3:1577-83; PMID:15634651. [PubMed] [Google Scholar]