The crystal structure of ketopantoate reductase complexed with NADP+ was determined at 2.3 Å resolution.
Keywords: coenzyme A, feedback inhibition, competitive inhibition, hyperthermophilic archaea
Abstract
Coenzyme A (CoA) plays pivotal roles in a variety of metabolic pathways in all organisms. The biosynthetic pathway of CoA is strictly regulated by feedback inhibition. In the hyperthermophilic archaeon Thermococcus kodakarensis, ketopantoate reductase (KPR), which catalyzes the NAD(P)H-dependent reduction of 2-oxopantoate, is a target of feedback inhibition by CoA. The crystal structure of KPR from T. kodakarensis (Tk-KPR) complexed with CoA and 2-oxopantoate has previously been reported. The structure provided an explanation for the competitive inhibition mechanism. Here, further biochemical analyses of Tk-KPR and the crystal structure of Tk-KPR in complex with NADP+ are reported. A mutational analysis implies that the residues in the binding pocket cooperatively contribute to the recognition of CoA. The structure reveals the same dimer architecture as the Tk-KPR–CoA–2-oxopantoate complex. Moreover, the positions of the residues involved in the dimer interaction are not changed by the binding of CoA and 2-oxopantoate, suggesting individual conformational changes of Tk-KPR monomers.
1. Introduction
Coenzyme A (CoA) plays pivotal roles in a variety of metabolic pathways in all three domains of life (Genschel, 2004 ▸; Leonardi et al., 2005 ▸; Spry et al., 2008 ▸). In bacterial and eukaryotic biosynthetic pathways of CoA, ketopantoate reductase (KPR) catalyzes the reduction of 2-oxopantoate to d-pantoate by utilizing NAD(P)H (Shimizu et al., 1988 ▸; Ottenhof et al., 2004 ▸; Webb et al., 2004 ▸). After the reaction catalyzed by KPR, pantothenate synthetase (PS) and pantothenate kinase (PanK) produce d-4′-phosphopantothenate, a precursor of CoA (Webb et al., 2004 ▸; Genschel et al., 1999 ▸; Falk & Guerra, 1993 ▸; Calder et al., 1999 ▸). In contrast, CoA is synthesized in an alternative pathway in archaea. Although archaea produce d-pantoate by utilizing KPR similarly to bacteria (Tomita et al., 2013 ▸), they utilize pantoate kinase (PoK) and phosphopantothenate synthetase (PPS), enzymes that are nonhomologous to PS and PanK, for the synthesis of d-4′-phosphopantothenate (Yokooji et al., 2009 ▸).
The biosynthetic pathway of CoA from 2-oxopantoate is a costly process that consumes one NAD(P)H molecule and five ATP molecules. Therefore, the pathway is regulated by feedback inhibition. The targets of feedback inhibition are also different in bacteria/eukaryotes and archaea. In bacteria and eukaryotes, PanK is the primary target of feedback inhibition by CoA (Vallari et al., 1987 ▸; Rock et al., 2000 ▸, 2002 ▸, 2003 ▸; Zhang et al., 2005 ▸). On the other hand, PoK and PPS are not affected by CoA, and PanK is not present in archaea (Ishibashi et al., 2012 ▸; Tomita et al., 2012 ▸). Notably, archaeal KPR is inhibited by CoA in a competitive manner with NAD(P)H (Tomita et al., 2013 ▸).
Although a detailed reaction mechanism for KPR from Escherichia coli (Ec-KPR) has been proposed from crystallographic and biochemical studies (Zheng & Blanchard, 2000a ▸,b ▸, 2003 ▸; Matak-Vinković et al., 2001 ▸; Lobley et al., 2005 ▸; Ciulli et al., 2007 ▸), the inhibition mechanism of archaeal KPR is insufficiently understood. We previously determined the crystal structure of KPR from the hyperthermophilic archaeon Thermococcus kodakarensis (Tk-KPR) in complex with its feedback inhibitor CoA and the substrate 2-oxopantoate to reveal the feedback-inhibition mechanism (Aikawa et al., 2016 ▸). CoA and 2-oxopantoate are bound to one of the two monomers, while NADP+ is bound to the opposite monomer. The competitive inhibition mechanism was explained by an overlap of the binding sites for CoA and NADP+. Moreover, CoA and 2-oxopantoate induce conformational closure by cooperative binding to an activity pocket composed of the N-terminal and C-terminal domains. CoA is bound by several hydrogen bonds and hydrophobic interactions. In particular, a disulfide bond to Cys84 is observed. Mutation of Cys84 resulted in decreased inhibition efficiency, suggesting the importance of the disulfide bond for the binding of CoA.
In this paper, we performed further biochemical analyses to evaluate the importance of the Tyr60 and Trp129 residues that form a hydrophobic binding pocket for CoA. A mutational study implies that these residues in the binding pocket cooperatively recognize CoA. We also determined the crystal structure of Tk-KPR in complex with NADP+ to further elucidate the inhibition mechanism. The structure reveals the same homodimer architecture as Tk-KPR in complex with CoA and 2-oxopantoate. Moreover, the positions of the residues involved in the dimer interaction are not changed on the binding of CoA and 2-oxopantoate, suggesting individual conformational changes of Tk-KPR monomers.
2. Materials and methods
2.1. Plasmid construction
Site-directed mutagenesis was performed to introduce Y60A or W129A mutations into wild-type (WT) Tk-KPR inserted into pET-21a (Tomita et al., 2013 ▸). PCRs were carried out with primers 5′-CCACAATCGCTGCTCCAGAGGAGCCGCCC-3′ and 5′-CTCTGGAGCAGCGATTGTGGCCTTTGGCTTC-3′ for Y60A and 5′-TGGTTGAAGCTGGAAAAGTTCTCTGGGCAG-3′ and 5′-ACTTTTCCAGCTTCAACCAGCATCGCCCC-3′ for W129A. The PCR products were treated with DpnI and were transformed into E. coli NovaBlue GigaSingles (Novagen, Darmstadt, Germany). The plasmids were purified using a QIAprep Spin Miniprep Kit (Qiagen, Hilden, Germany). The expression plasmid for C84A Tk-KPR inserted into pET-21a had previously been constructed (Aikawa et al., 2016 ▸).
2.2. Protein expression and purification
The Y60A, C84A and W129A mutants of Tk-KPR were expressed and purified as described previously for the WT (Aikawa et al., 2016 ▸). To prepare a Tk-KPR dimer carrying a His6 tag and a Strep-tag on each monomer for a dissociation experiment, His6-tagged and Strep-tagged Tk-KPRs were co-expressed and purified as described previously (Aikawa et al., 2016 ▸).
2.3. Activity-inhibition assay
An activity-inhibition assay was performed as described previously (Aikawa et al., 2016 ▸) with slight modifications. Reaction mixtures containing 0.2 mM NADH, 1.0 µg ml−1 Tk-KPR variant and 50 mM 2-morpholinoethanesulfonic acid pH 6.4 were pre-incubated at 343 K for 2 min in the presence of 0–100 µM CoA. The reactions were initialized by the addition of 0.2 mM 2-oxopantoate, and the rate of decrease in the absorption at 340 nm deriving from NADH was measured using a spectrophotometer. The rate of decrease without enzyme was subtracted from each assay result. The rate of decrease in the absence of CoA was defined as 100% of the residual activity. The measurements of the residual activities were duplicated and the average values were plotted.
2.4. Dimer-dissociation experiment
The buffer for the Tk-KPR dimer carrying a His6 tag and a Strep-tag on each monomer was exchanged to buffer A (50 mM Tris–HCl pH 7.5, 150 mM NaCl) and aliquoted into two tubes. Both tubes were incubated for one week at 277 K. One tube was incubated for a further 10 min at 343 K. The incubated samples were loaded onto 0.5 ml Strep-Tactin Superflow columns (IBA, Göttingen, Germany). The columns were washed with 5 ml buffer B (50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid). The proteins were eluted with 3 ml buffer B supplemented with 2.5 mM desthiobiotin. The fractions were analyzed by SDS–PAGE followed by staining with Coomassie Brilliant Blue.
2.5. Crystallization
The buffer of the purified C84A sample was exchanged to buffer C (10 mM Tris–HCl pH 8.0, 1 mM dithiothreitol). Before crystallization, 10 mg ml−1 Tk-KPR C84A mutant was mixed with 1 mM NADH and 1 mM 2-oxopantoate at room temperature. 1 µl of the sample solution and 1 µl reservoir solution [100 mM sodium acetate pH 5.5, 10%(w/v) polyethylene glycol 3350, 20%(v/v) 2-propanol] were mixed and equilibrated against 500 µl reservoir solution by the hanging-drop vapour-diffusion method at 293 K. Needle-like crystals with dimensions of 0.3 × 0.01 × 0.01 mm were obtained within a week.
2.6. X-ray data collection and structure determination
Crystals were soaked in reservoir solution supplemented with 20%(v/v) ethylene glycol and flash-cooled in a nitrogen-gas stream at 95 K. X-ray diffraction experiments were performed on BL38B1 and BL41XU at SPring-8, Hyogo, Japan and BL1A and NW12 at Photon Factory, Tsukuba, Japan. A data set consisting of 900 frames collected at a wavelength of 1.0000 Å using a Pilatus3 6M detector (Dectris, Baden, Switzerland) with an oscillation angle of 0.2° per frame was processed and scaled with the HKL-2000 program package (Otwinowski & Minor, 1997 ▸). The initial phase was determined by the molecular-replacement method with Phaser (McCoy et al., 2007 ▸) in PHENIX (Adams et al., 2010 ▸) using the N-terminal (residues 1–165) and C-terminal (residues 173–294) domains of the Tk-KPR structure (Aikawa et al., 2016 ▸; PDB entry 5ayv) as separate search models. The best solution had four monomers of Tk-KPR in the asymmetric unit (TFZ = 47.8 and R = 39.3%). Models were built with Coot (Emsley et al., 2010 ▸) and refined with phenix.refine (Adams et al., 2010 ▸). The data-collection and refinement statistics are summarized in Table 1 ▸. Figure preparation and superposition were performed using PyMOL (Schrödinger).
Table 1. Data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| Data collection | |
| Space group | P1 |
| a, b, c (Å) | 37.6, 45.0, 183.1 |
| α, β, γ (°) | 84.9, 87.8, 65.2 |
| Mosaicity (°) | 0.59–0.81 |
| Resolution range (Å) | 50–2.30 (2.38–2.30) |
| Total No. of reflections | 80312 |
| No. of unique reflections | 42722 |
| Completeness (%) | 88.4 (79.7) |
| Multiplicity | 1.9 (1.7) |
| 〈I/σ(I)〉 | 9.4 (2.1) |
| R p.i.m. † (%) | 8.1 (32.6) |
| Refinement | |
| Resolution (Å) | 50–2.30 (2.35–2.30) |
| No. of reflections used for the R free set | 2164 |
| R work/R free (%) | 22.2/26.0 (28.0/31.7) |
| No. of Tk-KPR monomers in asymmetric unit | 4 |
| No. of non-H atoms in asymmetric unit | |
| Protein | 9330 |
| Ligand/ion | 144 |
| Water | 345 |
| B factors (Å2) | |
| Average | 37.2 |
| Wilson | 29.2 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.004 |
| Bond angles (°) | 0.815 |
| Ramachandran plot | |
| Favoured regions (%) | 96.4 |
| Allowed regions (%) | 3.6 |
| Rotamer outliers (%) | 1.7 |
R
p.i.m. = 
 , where Ii(hkl) is the ith intensity measurement of reflection hkl, 〈I(hkl)〉 is the mean intensity for this reflection and N(hkl) is the multiplicity.
, where Ii(hkl) is the ith intensity measurement of reflection hkl, 〈I(hkl)〉 is the mean intensity for this reflection and N(hkl) is the multiplicity.
2.7. Accession code
The atomic coodinates and structure factors for the crystal structure reported here have been deposited in the Protein Data Bank (PDB) with accession code 5hws.
3. Results and discussion
3.1. Mutation study
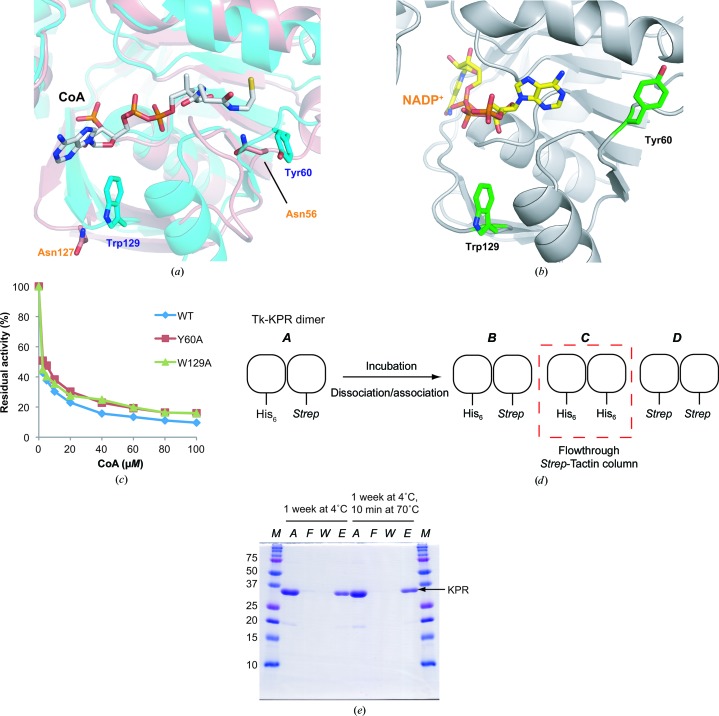
The crystal structure of the Tk-KPR–CoA–2-oxopantoate complex provided an explanation for the competitive inhibition mechanism by CoA (Aikawa et al., 2016 ▸). Tyr60 and Trp129 form the hydrophobic binding pocket for the inhibitor CoA (Fig. 1 ▸ a), but not for the cofactor NADP+ (Fig. 1 ▸ b). These residues are not conserved in Ec-KPR, which is not a target of feedback inhibition (Fig. 1 ▸ a). Therefore, we examined the effects of these residues on the recognition of CoA. We introduced a Y60A or W129A mutation to reduce hydrophobic interactions and performed an activity-inhibition assay (Fig. 1 ▸ c). The results showed that the residual activities of the Y60A and W129A mutants were slightly higher than that of the WT. These observations imply that Tyr60 and Trp129 have slight effects on the recognition of CoA and the inhibition efficiency. Tyr60 and Trp129, as well as other residues in the binding pocket, may cooperatively contribute to the recognition of CoA. The conservation of Tyr60 and Trp129 may be a cue to explore species in which KPR is the target of feedback inhibition by CoA.
Figure 1.
Biochemical analyses of Tk-KPR. (a) Binding mode of CoA. The structures of Tk-KPR–CoA–2-oxopantoate (Aikawa et al., 2016 ▸; PDB entry 5ayv) and Ec-KPR (Ciulli et al., 2007 ▸; PDB entry 2ofp) are superposed and coloured cyan and salmon, respectively. CoA is indicated by a stick model with C atoms coloured grey. Tyr60 and Trp129 of Tk-KPR and Asn56 and Asn127 of Ec-KPR are shown in stick models with C atoms coloured as in the respective chains. (b) Binding mode of NADP+ in the Tk-KPR–CoA–2-oxopantoate structure (Aikawa et al., 2016 ▸; PDB entry 5ayv). Tk-KPR is coloured grey. NADP+ molecules are indicated by stick models with C atoms coloured yellow. Tyr60 and Trp129 are indicated in stick models with C atoms coloured green. (c) Activity-inhibition assay. The residual activities of the WT and the Y60A and W129A mutants were plotted against the concentration of CoA. Blue, WT; red, Y60A; light green, W129A. (d) Schematic representation of the dimer-dissociation experiment. Dissociation and association of a Tk-KPR dimer carrying a His6 tag and a Strep-tag on each monomer (A) generates three specimens B, C and D. After loading onto a Strep-Tactin column, elution of specimen C in the flowthrough fraction was checked by SDS–PAGE. (e) Dimer-dissociation experiment. Samples were loaded onto Strep-Tactin columns after incubation in the conditions indicated above the gel. The fractions were analyzed by SDS–PAGE. Lane M, molecular-weight marker (labelled in kDa); lane A, applied sample; lane F, flowthrough; lane W, wash; lane E, elution.
3.2. Stability of the Tk-KPR dimer
The dimerization of Tk-KPR has been confirmed by size-exclusion chromatography (Tomita et al., 2013 ▸). We further elucidated the stability of the Tk-KPR dimer by a dissociation experiment (Figs. 1 ▸ d and 1 ▸ e). A Tk-KPR dimer carrying a His6 tag and a Strep-tag on each monomer was prepared (specimen A; Fig. 1 ▸ d). The samples were incubated for a long period at low temperature and for a short period at high temperature. If dissociation and association of the dimer occur during incubation, specimens B, C and D would be generated. Therefore, we checked whether specimen C appeared in the flowthrough fractions after loading onto Strep-Tactin columns (Fig. 1 ▸ e). The results showed that such a species did not appear in the flowthrough fractions, indicating that specimen C carrying a His6 tag on both monomers was not generated during the incubation. These observations indicate that dissociation and association of the Tk-KPR dimer does not occur, suggesting that the Tk-KPR dimer is stable.
3.3. Overall structure
The previously determined crystal structure of the Tk-KPR–CoA–2-oxopantoate complex is a heterologous dimer in which CoA and 2-oxopantoate are bound to a monomer in the closed form and NADP+ is bound to a monomer in the open form (Aikawa et al., 2016 ▸). To determine a structure of Tk-KPR with a different crystal packing, Tk-KPR was co-crystallized with its cofactor NADH and the substrate 2-oxopantoate. Since the crystal structure of the Tk-KPR–CoA–2-oxopantoate complex showed a disulfide bond between CoA and Cys84 (Aikawa et al., 2016 ▸), the C84A mutant of Tk-KPR was used for crystallization to prevent the covalent binding of intrinsic CoA derived from the expression host cells. We determined the structure at 2.3 Å resolution (Fig. 2 ▸ a). The crystals belonged to space group P1, which is distinct from that of the previous crystals of the Tk-KPR–CoA–2-oxopantoate complex (Aikawa et al., 2016 ▸). Despite the different space groups, the structure has the same dimer architecture as the structure of the Tk-KPR–CoA–2-oxopantoate complex, suggesting that the dimer structure is not an artifact of crystallization. Calculations using the PISA server (Krissinel & Henrick, 2007 ▸) show that the interface involved in dimerization buries 4190 Å2 of surface area, suggesting that the dimer is stable in solution. Moreover, a dimer-dissociation experiment indicated that the Tk-KPR dimer does not easily dissociate (Figs. 1 ▸ d and 1 ▸ e). Therefore, Tk-KPR would adopt a similar dimer structure in solution to the crystal structure of the Tk-KPR–CoA–2-oxopantoate complex. Four monomers (two dimers) are present in the asymmetric unit. These monomers show almost the same conformations, suggesting that the four monomers are in the same state (Fig. 2 ▸ b, Table 2 ▸). Each monomer is composed of N-terminal (residues 1–173) and C-terminal (residues 174–309) domains (Fig. 2 ▸ a). The N-terminal domain possesses a Rossmann-type αβ fold, and the C-terminal domain consists of seven α-helices. These domains form an activity pocket between them. Electron density for cofactors was observed in the activity pockets. Although NADH and 2-oxopantoate were added to the crystallization samples, the binding cofactor was NADP+ derived from the expression host, as determined previously (Aikawa et al., 2016 ▸). This indicates that the structure is in a reaction-completed state with an open form. The binding mode of NADP+ was similar to that of the NADP+-bound monomer in the Tk-KPR–CoA–2-oxopantoate complex structure (Fig. 2 ▸c).
Figure 2.
Structure of the Tk-KPR–NADP+ complex. (a) Overall structure of the Tk-KPR–NADP+ complex. The two monomers are coloured deep blue and pink. NADP+ molecules are shown as sticks with C atoms coloured as in the respective monomers. An enlarged view of the region in the square is indicated in (c). (b) Superposition of the four monomers in the asymmetric unit. Chains A, B, C and D are coloured green, deep blue, pink and magenta, respectively. (c) Binding site of NADP+. NADP+ is indicated as a stick model with C atoms coloured pink. Residues interacting with NADP+ are shown as grey sticks. Hydrogen bonds are shown as yellow dashed lines. Hydrogen bonds mediated by water molecules are not shown for clarity.
Table 2. R.m.s.d.s of Tk-KPR monomers in the asymmetric unit.
The number of amino-acid residues in each monomer is shown in parentheses.
| Superposed monomers | R.m.s.d. (Å) | No. of Cα atoms used |
|---|---|---|
| Chain A (298) and chain B (298) | 0.228 | 298 |
| Chain A and chain C (303) | 0.386 | 298 |
| Chain A and chain D (297) | 0.298 | 297 |
| Chain B and chain C | 0.358 | 298 |
| Chain B and chain D | 0.307 | 297 |
| Chain C and chain D | 0.446 | 297 |
3.4. Comparison of the dimer interface
The structures of Tk-KPR–CoA–2-oxopantoate and Tk-KPR–NADP+ were superposed to elucidate the difference in their conformations (Fig. 3 ▸ a). The C-terminal domains of the NADP+-bound monomers (yellow and deep blue) were used as probes of superposition. These monomers adopt almost the same open form. Although the opposite monomers (cyan and pink) show different conformations, these overall structures have the same dimer architecture. In both structures the dimer interactions are mediated by several hydrophobic residues from the C-terminal domains (Fig. 3 ▸ b). The positions of these residues are almost the same in the two structures, indicating that the dimer-interaction modes do not change upon the binding of CoA and 2-oxopantoate. Thus, the effect of CoA/2-oxopantoate binding to one monomer does not propagate to the opposite monomer via the C-terminal domains. Moreover, there are no other direct interactions between the monomers. These findings suggest that KPR monomers individually change their conformation on the binding of CoA and 2-oxopantoate.
Figure 3.
Superposition of Tk-KPR in CoA/2-oxopantoate-bound and NADP+-bound forms. (a) Comparison of dimer architecture. The open and closed monomers of the Tk-KPR–CoA–2-oxopantoate structure (Aikawa et al., 2016 ▸; PDB entry 5ayv) are coloured yellow and cyan, respectively. The dimeric structure of Tk-KPR–NADP+ is coloured as in Fig. 2 ▸(a). The region involved in dimer interaction is indicated by a square. (b) Enlarged view of the region in the square in (a). Residues involved in dimerization are shown as sticks and coloured as in (a).
In summary, we performed biochemical studies of Tk-KPR and determined the structure of Tk-KPR complexed with NADP+. The dimer-dissociation experiment suggests a stable dimer interaction for Tk-KPR. Moreover, the crystal structure of Tk-KPR–NADP+ shows the same dimer architecture as that of the Tk-KPR–CoA–2-oxopantoate complex despite belonging to a different space group. These observations suggest that the monomers of Tk-KPR are individually inhibited by CoA. Furthermore, the mutational study suggests that Tyr60 and Trp129 cooperatively contribute to the recognition of CoA.
Supplementary Material
PDB reference: ketopantoate reductase complexed with NADP+, 5hws
Acknowledgments
The authors are grateful to the beamline staff at SPring-8 and Photon Factory for their help during the X-ray diffraction experiment.
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Aikawa, Y., Nishitani, Y., Tomita, H., Atomi, H. & Miki, K. (2016). Proteins, 84, 374–382. [DOI] [PubMed]
- Calder, R. B., Williams, R. S., Ramaswamy, G., Rock, C. O., Campbell, E., Unkles, S. E., Kinghorn, J. R. & Jackowski, S. (1999). J. Biol. Chem. 274, 2014–2020. [DOI] [PubMed]
- Ciulli, A., Chirgadze, D. Y., Smith, A. G., Blundell, T. L. & Abell, C. (2007). J. Biol. Chem. 282, 8487–8497. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Falk, K. L. & Guerra, D. J. (1993). Arch. Biochem. Biophys. 301, 424–430. [DOI] [PubMed]
- Genschel, U. (2004). Mol. Biol. Evol. 21, 1242–1251. [DOI] [PubMed]
- Genschel, U., Powell, C. A., Abell, C. & Smith, A. G. (1999). Biochem. J. 341, 669–678. [DOI] [PMC free article] [PubMed]
- Ishibashi, T., Tomita, H., Yokooji, Y., Morikita, T., Watanabe, B., Hiratake, J., Kishimoto, A., Kita, A., Miki, K., Imanaka, T. & Atomi, H. (2012). Extremophiles, 16, 819–828. [DOI] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Leonardi, R., Zhang, Y.-M., Rock, C. O. & Jackowski, S. (2005). Prog. Lipid Res. 44, 125–153. [DOI] [PubMed]
- Lobley, C. M., Ciulli, A., Whitney, H. M., Williams, G., Smith, A. G., Abell, C. & Blundell, T. L. (2005). Biochemistry, 44, 8930–8939. [DOI] [PubMed]
- Matak-Vinković, D., Vinković, M., Saldanha, S. A., Ashurst, J. L., von Delft, F., Inoue, T., Miguel, R. N., Smith, A. G., Blundell, T. L. & Abell, C. (2001). Biochemistry, 40, 14493–14500. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Ottenhof, H. H., Ashurst, J. L., Whitney, H. M., Saldanha, S. A., Schmitzberger, F., Gweon, H. S., Blundell, T. L., Abell, C. & Smith, A. G. (2004). Plant J. 37, 61–72. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Rock, C. O., Calder, R. B., Karim, M. A. & Jackowski, S. (2000). J. Biol. Chem. 275, 1377–1383. [DOI] [PubMed]
- Rock, C. O., Karim, M. A., Zhang, Y.-M. & Jackowski, S. (2002). Gene, 291, 35–43. [DOI] [PubMed]
- Rock, C. O., Park, H.-W. & Jackowski, S. (2003). J. Bacteriol. 185, 3410–3415. [DOI] [PMC free article] [PubMed]
- Shimizu, S., Kataoka, M., Chung, M.-C. & Yamada, H. (1988). J. Biol. Chem. 263, 12077–12084. [PubMed]
- Spry, C., Kirk, K. & Saliba, K. J. (2008). FEMS Microbiol. Rev. 32, 56–106. [DOI] [PubMed]
- Tomita, H., Imanaka, T. & Atomi, H. (2013). Mol. Microbiol. 90, 307–321. [DOI] [PubMed]
- Tomita, H., Yokooji, Y., Ishibashi, T., Imanaka, T. & Atomi, H. (2012). J. Bacteriol. 194, 5434–5443. [DOI] [PMC free article] [PubMed]
- Vallari, D. S., Jackowski, S. & Rock, C. O. (1987). J. Biol. Chem. 262, 2468–2471. [PubMed]
- Webb, M. E., Smith, A. G. & Abell, C. (2004). Nat. Prod. Rep. 21, 695–721. [DOI] [PubMed]
- Yokooji, Y., Tomita, H., Atomi, H. & Imanaka, T. (2009). J. Biol. Chem. 284, 28137–28145. [DOI] [PMC free article] [PubMed]
- Zhang, Y.-M., Rock, C. O. & Jackowski, S. (2005). J. Biol. Chem. 280, 32594–32601. [DOI] [PubMed]
- Zheng, R. & Blanchard, J. S. (2000a). Biochemistry, 39, 3708–3717. [DOI] [PubMed]
- Zheng, R. & Blanchard, J. S. (2000b). Biochemistry, 39, 16244–16251. [DOI] [PubMed]
- Zheng, R. & Blanchard, J. S. (2003). Biochemistry, 42, 11289–11296. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: ketopantoate reductase complexed with NADP+, 5hws