Bile salt hydrolase is an enzyme that is produced by various commensal bacteria in the intestine and is also a key mechanistic microbiome target for enhanced animal and human health.
Keywords: bile salt hydrolase, Lactobacillus, lipid metabolism, gut microbiome, crystal structure
Abstract
Bile salt hydrolase (BSH) is a gut-bacterial enzyme that negatively influences host fat digestion and energy harvesting. The BSH enzyme activity functions as a gateway reaction in the small intestine by the deconjugation of glycine-conjugated or taurine-conjugated bile acids. Extensive gut-microbiota studies have suggested that BSH is a key mechanistic microbiome target for the development of novel non-antibiotic food additives to improve animal feed production and for the design of new measures to control obesity in humans. However, research on BSH is still in its infancy, particularly in terms of the structural basis of BSH function, which has hampered the development of BSH-based strategies for improving human and animal health. As an initial step towards the structure–function analysis of BSH, C-terminally His-tagged BSH from Lactobacillus salivarius NRRL B-30514 was crystallized in this study. The 1.90 Å resolution crystal structure of L. salivarius BSH was determined by molecular replacement using the structure of Clostridium perfringens BSH as a starting model. It revealed this BSH to be a member of the N-terminal nucleophile hydrolase superfamily. Crystals of apo BSH belonged to space group P21212, with unit-cell parameters a = 90.79, b = 87.35, c = 86.76 Å (PDB entry 5hke). Two BSH molecules packed perfectly as a dimer in one asymmetric unit. Comparative structural analysis of L. salivarius BSH also identified potential residues that contribute to catalysis and substrate specificity.
1. Introduction
Microbiota residing in the intestine affect host physiology and growth performance via food digestion, nutrient utilization and host immunity modulation. Recent studies have indicated that gut microbiota are implicated in host energy regulation and the development of obesity in humans; thus, manipulating specific gut microbial functions may be one means to control obesity and its associated chronic diseases (DiBaise et al., 2008 ▸; Tilg et al., 2009 ▸). The intestinal bile salt hydrolase (BSH), an enzyme produced by diverse gut microflora, catalyzes the essential gateway reaction in the metabolism of bile acids in the small intestine and plays an important role in host metabolism and energy harvesting (Begley et al., 2006 ▸; Jones et al., 2008 ▸; Joyce, Shanahan et al., 2014 ▸; Martoni et al., 2015 ▸). Using a controlled system, Joyce, MacSharry et al. (2014 ▸) recently obtained direct evidence demonstrating that BSH activity alone can significantly influence host lipid metabolism and weight gain. Consistent with the findings from this research in humans and mice, extensive research using food animals has shown that the growth-promoting effect of antibiotic growth promoters (AGPs) is highly correlated with decreased BSH activity as well as a significantly reduced population of Lactobacillus species, which are the major BSH producers in the intestine (Lin, 2014 ▸). Thus, BSH inhibitors have been proposed as promising feed additives to replace AGPs in order to enhance food safety and the productivity of food animals (Lin, 2014 ▸; Wang et al., 2012 ▸). Together, these recent findings have strongly suggested that BSH is a key mechanistic microbiome target for the development of novel alternatives to AGPs to enhance animal production and of new measures to control obesity in humans.
The BSH enzyme catalyzes the deconjugation of glycine-conjugated or taurine-conjugated bile acids, which is an essential gateway reaction in the metabolism of bile acids in the small intestine (Begley et al., 2006 ▸). The bile acids have dual digestive and signalling roles in the host; therefore, it has increasingly been recognized that intestinal BSH activity has a significant impact on host physiology by disturbing conjugated bile acid-mediated fat metabolism and endocrine functions (Begley et al., 2006 ▸; Jones et al., 2008 ▸; Joyce, Shanahan et al., 2014 ▸; Martoni et al., 2015 ▸). A number of BSH enzymes have been identified from different commensal bacteria, and Lactobacillus populations are the major BSH producers in the intestine. Despite recent significant progress in the characterization of diverse BSH enzymes, research on BSH is still in its infancy, particularly in terms of the structural basis of BSH function (Begley et al., 2006 ▸; Patel et al., 2010 ▸). To date, crystal structures of BSH enzymes from only two specific species, Bifidobacterium longum and Clostridium perfrigens, have been reported (Kumar et al., 2006 ▸; Rossocha et al., 2005 ▸). Given the ecological diversity of BSH in the gut microbiome, structural analyses of BSH enzymes from various species are warranted, and would lead to the discovery of the critical residues in catalysis and provide key information on the substrate selectivity of BSH enzymes (Begley et al., 2006 ▸). Clearly, structural studies on BSH will also directly facilitate future translational research, such as the use of molecular docking to develop BSH inhibitor-based alternatives to AGPs for growth promotion in food animals (Lin, 2014 ▸).
Recently, we have identified and characterized a BSH enzyme from L. salivarius NRRL B-30514 (Wang et al., 2012 ▸). L. salivarius BSH (lsBSH) was able to efficiently hydrolyze both glycoconjugated and tauroconjugated bile salts. Thus, unlike many BSH enzymes from other bacteria, which have a narrow substrate spectrum, this BSH displayed potent hydrolytic activity towards a broad range of substrates (Wang et al., 2012 ▸). The broad substrate specificity of lsBSH makes it an ideal candidate for structure–function analysis and for the identification of desired BSH inhibitors using computational techniques. Here, we report the crystallization, X-ray diffraction analysis and structure of lsBSH.
2. Materials and methods
2.1. Macromolecule production
Recombinant lsBSH was produced in Escherichia coli using the pET-21b vector (Novagen). The cloning and purification were described in a recent publication (Wang et al., 2012 ▸). The key information for lsBSH production is briefly summarized in Table 1 ▸. Recombinant lsBSH protein, containing a 6×His tag at the C-terminus, was overproduced in E. coli BL21 (DE3) cells and subsequently purified using a modified procedure. Briefly, the E. coli cells were grown in LB medium containing 100 µg ml−1 ampicillin at 37°C until the OD600 reached 0.6–0.8. Expression of lsBSH was induced by the addition of isopropyl β-d-1-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM. The growth temperature was decreased to 15°C after induction and the culture was further grown for approximately 16 h. Subsequently, the cells were harvested by centrifugation at 5000g at 4°C for 20 min and the pellets were resuspended in lysis buffer consisting of 50 mM Tris–HCl pH 7.0, 500 mM NaCl, 5%(v/v) glycerol, 50 mM imidazole. The resuspended cells were then lysed using a Microfluidics high-pressure homogenizer and centrifuged at 18 000 rev min−1 for 1 h at 277 K. The supernatant was subjected to the following stepwise purification. Firstly, the supernatant was loaded onto an Ni–NTA column and washed with a buffer consisting of 50 mM Tris–HCl pH 7.0, 50 mM NaCl, 5%(v/v) glycerol, 50 mM imidazole. The His-tagged lsBSH was eluted with a buffer consisting of 50 mM Tris–HCl pH 7.0, 50 mM NaCl, 5%(v/v) glycerol, 150 mM imidazole. The purified lsBSH fractions from the Ni–NTA column were then subjected to Mono Q chromatography and eluted with a gradient of sodium chloride [the buffer consisted of 50 mM Tris–HCl pH 7.0, 1 M NaCl, 5%(v/v) glycerol, 2 mM DTT with a 1–60% gradient of sodium chloride in 20 column volumes (CV)]. Subsequently, the pooled lsBSH fractions were further purified using hydrophobic interaction chromatography on a phenyl column; the column was washed with buffer consisting of 50 mM Tris pH 7.0, 0.5 M NaCl, 5% glycerol, 2 mM DTT, and lsBSH was eluted using a 10 CV gradient to a buffer consisting of 50 mM Tris–HCl pH 7.0, 5%(v/v) glycerol. Finally, the lsBSH fractions were pooled and concentrated to about 3 mg ml−1 for purification by Superdex 200 chromatography. lsBSH protein with high purity was eluted with buffer consisting of 10 mM sodium acetate pH 5.5, 400 mM NaCl, 1 mM DTT, 1 mM EDTA, 10%(v/v) glycerol.
Table 1. Macromolecule-production information for lsBSH.
| Source organism | L. salivarius NRRL B-30514 |
| DNA source | Genomic DNA |
| Forward primer† | 5′-CGCGGATCCATGTGTACAGCAATTACTTT-3′ |
| Reverse primer‡ | 5′-CCGCTCGAGATTCAACTTATTTATTATTTGT-3′ |
| Cloning vector | pET-21b |
| Expression vector | pET-21b |
| Expression host | E. coli BL21 (DE3) |
| Complete amino-acid sequence of the construct produced§ | MCTAITLNGNSNYFGRNLDLDFSYGEEVIITPAEYEFKFRKEKAIKNHKSLIGVGIVANDYPLYFDAINEDGLGMAGLNFPGNAYYSDALENDKDNITPFEFIPWILGQCSDVNEARNLVEKINLINLSFSEQLPLAGLHWLIADREKSIVVEVTKSGVHIYDNPIGILTNNPEFNYQMYNLNKYRNLSISTPQNTFSDSVDLKVDGTGFGGIGLPGDVSPESRFVRATFSKLNSSKGMTVEEDITQFFHILGTVEQIKGVNKTESGKEEYTVYSNCYDLDNKTLYYTTYENRQIVAVTLNKDKDGNRLVTYPFERKQIINKLNLERHHHHHH |
The BamHI site is underlined.
The XhoI site is underlined.
The His tag is underlined.
After purification, the purity of the lsBSH was judged using 12% SDS–PAGE as described previously (Wang et al., 2012 ▸). The purified lsBSH was extensively dialysed against buffer consisting of 10 mM sodium acetate pH 5.5, 400 mM NaCl, 1 mM DTT, 1 mM EDTA, 10%(v/v) glycerol and was then concentrated to 16.0 mg ml−1 for crystallization as described below.
2.2. Crystallization
Crystal screening was performed at 293 K by the sitting-drop vapour-diffusion method. 200 nl purified lsBSH at a final concentration of 16.0 mg ml−1 in buffer consisting of 10 mM sodium acetate pH 5.5, 400 mM NaCl, 1 mM DTT, 1 mM EDTA, 10% glycerol was mixed with 200 nl reservoir solution and equilibrated against 15 µl reservoir solution using a Mosquito LCP (TTP Labtech). Commercial crystallization kits from Hampton Research and Qiagen were used for crystal screening. Initial crystals of lsBSH were obtained in a condition consisting of 0.2 M KH2PO4 pH 4.8, 20%(w/v) polyethylene glycol 3350 and further optimization was carried out by micro-seeding under the same condition. Crystallization information is summarized in Table 2 ▸.
Table 2. Crystallization conditions for lsBSH.
| Method | Sitting-drop vapour diffusion |
| Plate type | Swissci SD-3 |
| Temperature (K) | 293 |
| Protein concentration (mg ml−1) | 16.0 |
| Buffer composition of protein solution | 10 mM sodium acetate pH 5.5, 400 mM NaCl, 1 mM DTT, 1 mM EDTA, 10% glycerol |
| Composition of reservoir solution | 20% polyethylene glycol 3350, 0.2 M potassium dihydrogen phosphate pH 4.8 |
| Volume and ratio of drop | 200 nl, 1:1 |
| Volume of reservoir (µl) | 15 |
2.3. Data collection and processing
All crystals were flash-cooled with the addition of 25% glycerol as a cryoprotectant and diffraction data were collected at Biortus, Jiangyin, People’s Republic of China with a home-source diffraction system consisting of a Rigaku F-RE++ generator and a Saturn 944 CCD detector. The data-collection statistics are shown in Table 3 ▸.
Table 3. Data-collection and processing statistics for lsBSH.
Values in parentheses are for the outer shell.
| Diffraction source | Rigaku F-RE++ |
| Wavelength (Å) | 1.5418 |
| Temperature (K) | 100 |
| Detector | Saturn 944 CCD |
| Crystal-to-detector distance (mm) | 50 |
| Rotation range per image (°) | 0.75 |
| Total rotation range (°) | 150 |
| Exposure time per image (s) | 30 |
| Space group | P21212 |
| a, b, c (Å) | 90.79, 87.36, 86.77 |
| α, β, γ (°) | 90, 90, 90 |
| Mosaicity (°) | 0.695 |
| Resolution range (Å) | 50.0–1.90 (1.93–1.90) |
| Total No. of reflections | 312064 |
| No. of unique reflections | 54757 |
| Completeness (%) | 99.3 (90.0) |
| Multiplicity | 5.7 (3.6) |
| 〈I/σ(I)〉 | 28.9 (4.91) |
| R r.i.m. | 0.060 (0.278) |
| Overall B factor from Wilson plot (Å2) | 13.0 |
2.4. Structure solution and refinement
The structure of lsBSH was determined by the molecular-replacement method using Phaser (McCoy et al., 2007 ▸) with the C. perfringens BSH (cpBSH) structure as a search model (Rossocha et al., 2005 ▸; PDB entry 2bjf; 37% sequence identity). Structure refinement was performed with Coot (Emsley et al., 2010 ▸) and REFMAC5 (Murshudov et al., 2011 ▸) and is summarized in Table 4 ▸.
Table 4. Structure solution and refinement of lsBSH.
Values in parentheses are for the outer shell.
| PDB code | 5hke |
| Resolution range (Å) | 45.50–1.90 (1.97–1.90) |
| Completeness (%) | 99.20 (91.47) |
| σ Cutoff | 2.0 |
| No. of reflections, working set | 52091 (2619) |
| No. of reflections, test set | 2619 (213) |
| Final R cryst | 0.152 (0.194) |
| Final R free | 0.185 (0.218) |
| No. of non-H atoms | |
| Protein | 5132 |
| Ligand | 20 |
| Water | 400 |
| Total | 5552 |
| R.m.s. deviations | |
| Bonds (Å) | 0.020 |
| Angles (°) | 1.877 |
| Average B factors (Å2) | |
| Overall | 19.8 |
| Protein | 19.7 |
| Water | 24.9 |
| Ramachandran plot | |
| Most favoured (%) | 97.6 |
| Allowed (%) | 2.2 |
| Disallowed (%) | 0.2 |
3. Results and discussion
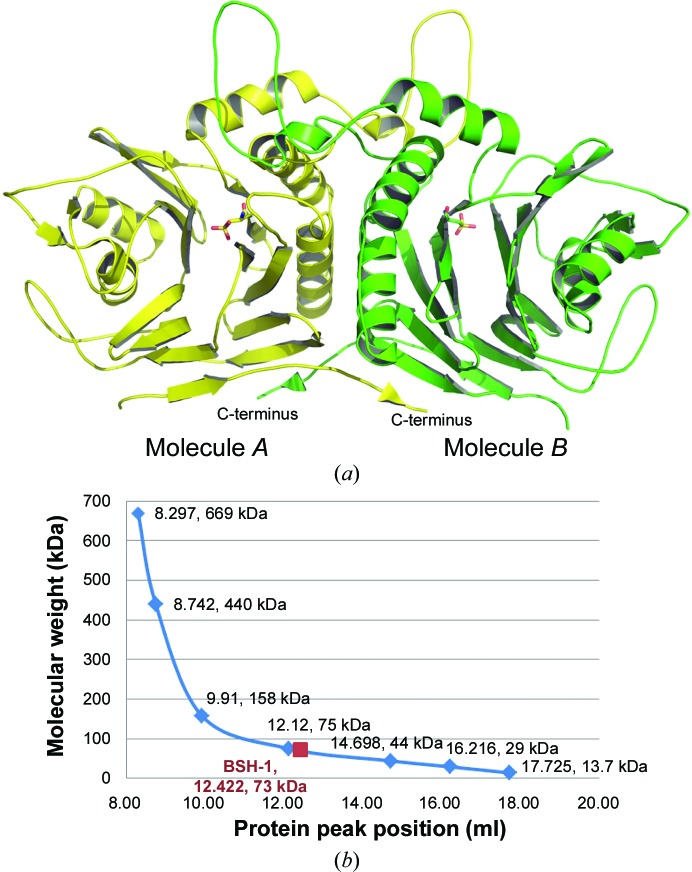
We have determined the 1.90 Å resolution crystal structure of lsBSH in space group P21212 (PDB entry 5hke). It showed two lsBSH molecules packed perfectly as a dimer in the asymmetric unit (Fig. 1 ▸ a). The presence of the dimer in solution was confirmed by gel filtration with Superdex 200 (Fig. 1 ▸ b). Analysis of the protein interfaces with PISA showed that lsBSH can be stable as a tetramer and as a dimer in solution (Krissinel & Henrick, 2007 ▸).
Figure 1.
(a) Two lsBSH molecules packed in one asymmetric unit. The N-terminal Cys2 was oxidized to a cysteinesulfonic acid. (b) The dimeric nature of apo lsBSH was confirmed by gel filtration on Superdex 200. The solid maroon square shows the position of lsBSH (labelled ‘BSH-1’ in the figure) with an estimated molecular weight of 73 kDa.
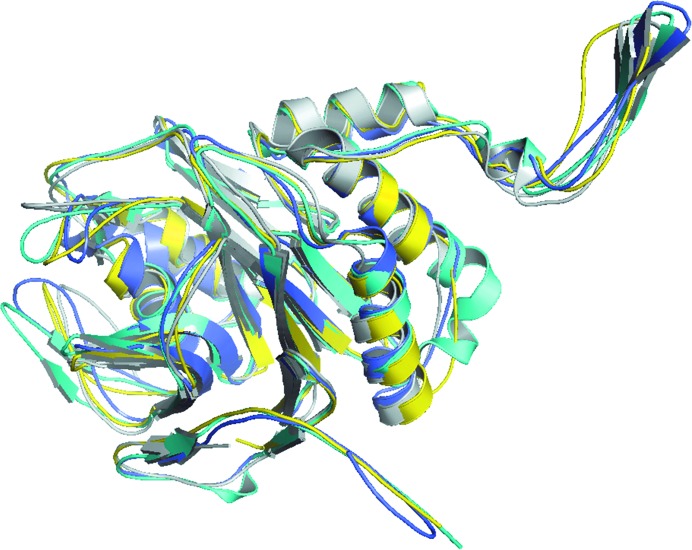
Except for residues 1 and 301–305, which are missing from the structure of lsBSH, all amino acids are well defined, including Cys2, which was oxidized to a cysteinesulfonic acid. The overall structure consisted of a four-layered αββα core and showed an N-terminal nucleophile (Ntn) hydrolase-like fold, similar to the previously reported structures of C. perfrigens BSH (cpBSH), B. longum BSH (blBSH) and Lysinibacillus sphaericus penicillin V acylase (bsPVA) (Kumar et al., 2006 ▸; Rossocha et al., 2005 ▸; Suresh et al., 1999 ▸; Fig. 2 ▸).
Figure 2.
Structural superimposition of lsBSH (yellow; PDB entry 5hke) with cpBSH (cyan; PDB entry 2bjf), blBSH (grey; PDB entry 2hf0) and L. sphaericus penicillin V acylase (slate; PDB entry 3pva).
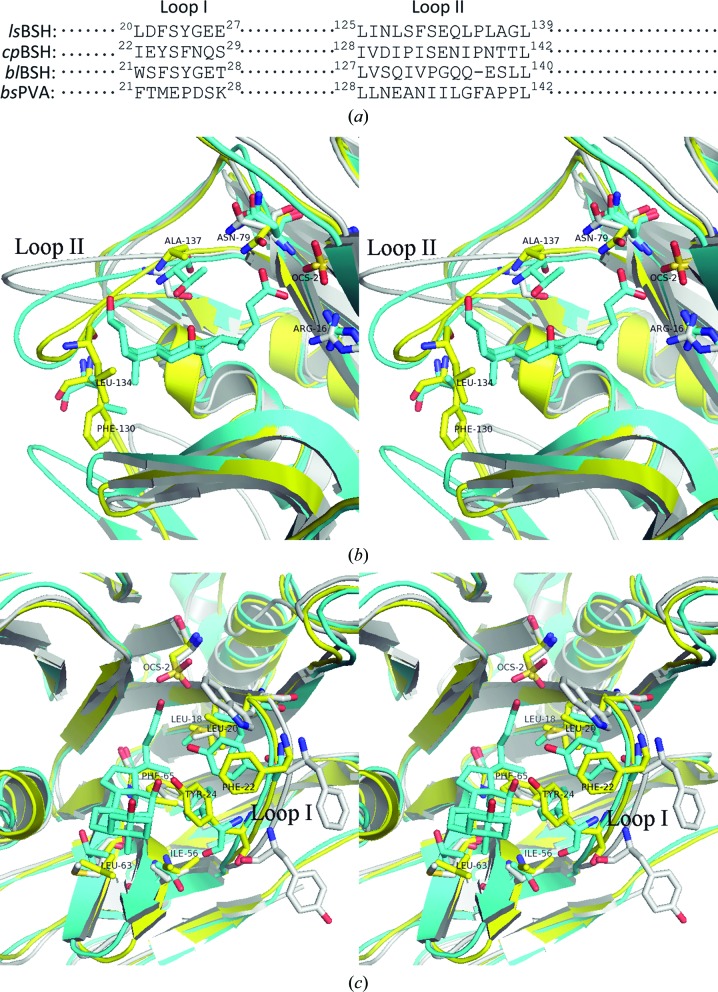
A superimposition of the structure of lsBSH with those of cpBSH and blBSH shows that they share the conserved catalytic active centre containing the cysteine nucleophile (Cys2) and its coordinated neighbouring amino acids (Kumar et al., 2006 ▸; Rossocha et al., 2005 ▸). However, the amino acids surrounding the binding pocket are inconsistent (Fig. 3 ▸). Differences were mainly observed in the amino acids within two loops: loop I consisting of amino acids 20–27 and loop II consisting of amino acids 125–139 (residue numbers from lsBSH; Fig. 3 ▸ a). Loop II of lsBSH is closer to the taurodeoxycholate than that in the cpBSH complex structure (PDB entry 2bjf). In loop II the hydrophobic residue Leu134 in lsBSH intrudes into the pocket and condenses the entrance to the substrate-binding pocket (Fig. 3 ▸ b). Phe130 may also contribute to this restrained spatial configuration (Fig. 3 ▸ b). In loop I, Tyr24 in lsBSH (corresponding to Phe26 in cpBSH), along with Phe65 (corresponding to Ala68 in cpBSH), also intrudes into the binding pocket (Fig. 3 ▸ c). These observations suggest that these amino acids may force the substrate to bind in a different orientation, such as that rotated by 90°, and to sit deeply in the binding pocket, which will lead to different enzyme–substrate interactions and is obviously different from what was observed in cpBSH (Rossocha et al., 2005 ▸). In blBSH, which exhibits a preference for glycoconjugated bile salts over tauroconjugated bile salts (Kumar et al., 2006 ▸), Tyr24 is present in loop I as observed in lsBSH; however, the large hydrophobic amino acid Trp21 (corresponding to Leu20 in lsBSH and Ile22 in cpBSH) seems to make this tyrosine point outwards from the binding pocket (Fig. 3 ▸ c). In addition to the differences observed in these two loops as described above, comparison of lsBSH with cpBSH and blBSH also identified differences in other surrounding amino acids in lsBSH, including Leu63 (corresponding to Thr66 in cpBSH and Met65 in blBSH) and Ile56 (corresponding to Thr59 in cpBSH and Val58 in blBSH) located at the bottom of the binding pocket, and Phe22 (corresponding to Tyr24 in cpBSH and Phe23 in blBSH) and Leu18 (corresponding to Met20 in cpBSH and Leu19 in blBSH) located in loop I (Fig. 3 ▸ c). These differences may also contribute to the different enzyme–substrate interactions, consequently determining the different substrate specificities. Together, unlike the binding pocket in cpBSH that shows an open entrance with a shallow bottom, a number of unique residues in lsBSH make lsBSH display a narrow entrance to the binding pocket and an increased inner capacity of the binding pocket, which may enable the substrate to sit deeply in the pocket with a different conformation and lead to the different enzyme–substrate interaction (broad spectrum of specificity); these residues are summarized in Table 5 ▸.
Figure 3.
Comparison of the substrate-binding pocket of lsBSH (yellow) with those of cpBSH (cyan) and blBSH (grey). A reference taurodeoxycholate molecule (cyan) from the cpBSH complex structure (PDB entry 2bjf) is shown. (a) Sequence alignment of loop I and loop II that surround the binding pocket. (b) Cross-eyed stereoview showing the significant difference in loop II. Only the residue numbers for lsBSH are shown. (c) Cross-eyed stereoview showing the difference in loop I. Only the residue numbers for lsBSH are shown.
Table 5. Major amino-acid residues of lsBSH that are potentially involved in catalysis and substrate specificity based on comparative structural analysis.
| Residue | Specific location | Speculation |
|---|---|---|
| Tyr24 | Loop I | Along with Phe65, may force the substrate to sit deeply in the binding pocket |
| Leu18 | Loop I | Given their differences when compared with the structures of cpBSH and blBSH, they may contribute to different enzyme–substrate interactions |
| Phe22 | Loop I | |
| Leu134 | Loop II | Both residues contribute to restraint of the spatial configuration by condensing the substrate-binding pocket entrance |
| Phe130 | Loop II | |
| Ile56 | Bottom of the binding pocket | Compared with the structures of cpBSH and blBSH, these two residues may determine the differing substrate specificities |
| Leu63 | Bottom of the binding pocket | |
| Phe65 | Bottom of the binding pocker | Along with Tyr24, may force the substrate to sit deeply in the binding pocket |
Previous comparative genomics and structural studies have identified several conserved, catalytically important residues in the active site of BSH (Cys2, Arg16, Asp19, Asn79, Asn171 and Arg224); however, this conclusion was primarily based on comparison of the structure of BSH with that of penicillin V acylase (Begley et al., 2006 ▸; Kumar et al., 2006 ▸; Wang et al., 2012 ▸). To date, Cys2 is the only residue that has been subjected to site-directed mutagenesis and validated for its essential role in the activity of BSH (Kumar et al., 2006 ▸). Therefore, future in-depth structural analysis of lsBSH (e.g. in complex with a specific substrate) in conjunction with comprehensive amino-acid substitution mutagenesis would help us to discover the critical residues in catalysis and to understand why lsBSH displays a potent catalytic activity towards a broad spectrum of substrates including both glycoconjugated and tauroconjugated bile salts.
Supplementary Material
PDB reference: bile salt hydrolase, 5hke
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31172344), the Special Program on Science and Technology Innovation Capacity Building of BAAFS (KJCX20150703) and AgResearch at The University of Tennessee. We also thank the staff of Biortus, Jiangyin, People’s Republic of China for assisting us with the crystallization and the collection of high-resolution X-ray data.
References
- Begley, M., Hill, C. & Gahan, C. G. M. (2006). Appl. Environ. Microbiol. 72, 1729–1738. [DOI] [PMC free article] [PubMed]
- DiBaise, J. K., Zhang, H., Crowell, M. D., Krajmalnik-Brown, R., Decker, G. A. & Rittmann, B. E. (2008). Mayo Clin. Proc. 83, 460–469. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Jones, B. V., Begley, M., Hill, C., Gahan, C. G. M. & Marchesi, J. R. (2008). Proc. Natl Acad. Sci. USA, 105, 13580–13585. [DOI] [PMC free article] [PubMed]
- Joyce, S. A., MacSharry, J., Casey, P. G., Kinsella, M., Murphy, E. F., Shanahan, F., Hill, C. & Gahan, C. G. M. (2014). Proc. Natl Acad. Sci. USA, 111, 7421–7426. [DOI] [PMC free article] [PubMed]
- Joyce, S. A., Shanahan, F., Hill, C. & Gahan, C. G. M. (2014). Gut Microbes, 5, 669–674. [DOI] [PMC free article] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Kumar, R. S., Brannigan, J. A., Prabhune, A. A., Pundle, A. V., Dodson, G. G., Dodson, E. J. & Suresh, C. G. (2006). J. Biol. Chem. 281, 32516–32525. [DOI] [PubMed]
- Lin, J. (2014). Front. Microbiol. 5, 33. [DOI] [PMC free article] [PubMed]
- Martoni, C. J., Labbé, A., Ganopolsky, J. G., Prakash, S. & Jones, M. L. (2015). Gut Microbes, 6, 57–65. [DOI] [PMC free article] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Patel, A. K., Singhania, R. R., Pandey, A. & Chincholkar, S. B. (2010). Appl. Biochem. Biotechnol. 162, 166–180. [DOI] [PubMed]
- Rossocha, M., Schultz-Heienbrok, R., von Moeller, H., Coleman, J. P. & Saenger, W. (2005). Biochemistry, 44, 5739–5748. [DOI] [PubMed]
- Suresh, C. G., Brannigan, J. A., Pundle, A. V., SivaRaman, H., Rao, K. N., McVey, C. E., Verma, C. S., Dauter, Z., Dodson, E. J. & Dodson, G. G. (1999). Nature Struct. Biol. 6, 414–416. [DOI] [PubMed]
- Tilg, H., Moschen, A. R. & Kaser, A. (2009). Gastroenterology, 136, 1476–1483. [DOI] [PubMed]
- Wang, Z., Zeng, X., Mo, Y., Smith, K., Guo, Y. & Lin, J. (2012). Appl. Environ. Microbiol. 78, 8795–8802. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: bile salt hydrolase, 5hke