The periplasmic sensing domain of V. fischeri chemoreceptor protein A was purified and crystallized. Analysis of an X-ray diffraction data set collected to 1.8 Å resolution is presented.
Keywords: bacterial chemotaxis, receptor, sensing domain, methyl-accepting protein
Abstract
Flagella-mediated motility and chemotaxis towards nutrients are important characteristics of Vibrio fischeri that play a crucial role in the development of its symbiotic relationship with its Hawaiian squid host Euprymna scolopes. The V. fischeri chemoreceptor A (VfcA) mediates chemotaxis toward amino acids. The periplasmic sensory domain of VfcA has been crystallized by the hanging-drop vapour-diffusion method using polyethylene glycol 3350 as a precipitating agent. The crystals belonged to space group P1, with unit-cell parameters a = 39.9, b = 57.0, c = 117.0 Å, α = 88.9, β = 80.5, γ = 89.7°. A complete X-ray diffraction data set has been collected to 1.8 Å resolution using cryocooling conditions and synchrotron radiation.
1. Introduction
The marine bacterium Vibrio fischeri forms mutualistic and pathogenic interactions with animal hosts (Nealson & Hastings, 1991 ▸). V. fischeri exists in two forms: (i) a free-living organism in the gut of many marine mammals and (ii) a light-organ symbiont in certain species of squid and fish (Nealson & Hastings, 1991 ▸).
Bacterial flagella-mediated motility and chemotaxis are crucial for the establishment of symbiotic relationships between bacteria and other organisms (Manson et al., 1998 ▸; Norsworthy & Visick, 2013 ▸; Sourjik & Wingreen, 2012 ▸). Chemotaxis appears to play a role in guiding V. fischeri towards the colonization-permissive sites in its symbiotic Hawaiian squid host Euprymna scolopes (Norsworthy & Visick, 2013 ▸). Membrane-embedded methyl-accepting chemotaxis proteins (MCPs) serve as receptors that recognize chemoattractants or repellents. Upon ligand binding, MCPs transduce the signal through the CheAY two-component system to guide bacterial motility toward chemoattractants and away from repellents (Norsworthy & Visick, 2013 ▸). Of the 43 putative MCPs encoded in the genome of V. fischeri, the involvement in chemotaxis has been so far studied for three: VfcA (V. fischeri chemoreceptor A), VfcB and VfcB2 (Nikolakakis et al., 2016 ▸; Brennan et al., 2013 ▸). VfcA mediates chemotaxis towards four amino acids (serine, alanine, cysteine and threonine), while both VfcB and VfcB2 are responsible for mediating chemoattraction to short-chain and medium-chain fatty acids (Norsworthy & Visick, 2013 ▸; Brennan et al., 2013 ▸). Previous mutagenesis studies have suggested that vfcA mutant V. fischeri is defective in amino-acid chemotaxis (Brennan et al., 2013 ▸).
The mechanism by which VfcA recognizes its signal molecules and the structural basis behind its ligand specificity remain unknown. A BLAST search against the known structures available in the Protein Data Bank (PDB) revealed that the periplasmic sensing domain of VfcA (VfcAperi) has less than 26% amino-acid sequence homology to any structurally characterized receptor [25 and 20% sequence similarity to the V. cholerae chemoreceptor MCP37 (PDB entry 3c8c; New York SGX Research Center for Structural Genomics, unpublished work) and to histidine kinase from Methanosarcina mazei (PDB entries 3li8, 3li9, 3lia and 3lib; Zhang & Hendrickson, 2010 ▸), respectively]. According to a Pfam analysis (Finn et al., 2016 ▸) of its primary sequence, VfcA contains a Cache_1 (calcium channels and chemotaxis receptors) motif located between residues 107 and 185 (Anantharaman & Aravind, 2001 ▸) and thus belongs to the family of tandem Per–Arnt–Sim (tandem PAS) sensing domains. We have recently reported the crystal structure of the periplasmic domain of Campylobacter jejuni Tlp3 (22% amino-acid sequence identity to VfcAperi), which is a different member of the family of MCP receptors for amino acids that contains a tandem PAS sensing domain (Liu et al., 2015 ▸). To investigate whether members of this family share a common mechanism of amino-acid recognition, we have initiated structural studies on VfcAperi. Here, we report the expression, purification and co-crystallization of VfcAperi with one of its putative signal molecules.
2. Materials and methods
2.1. Cloning, protein expression and purification
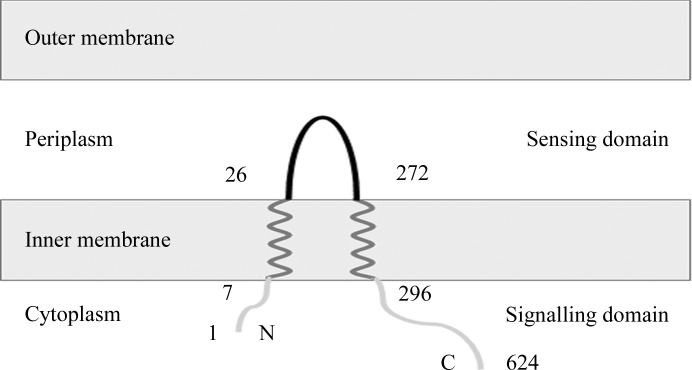
The two transmembrane helices of VfcA from V. fischeri strain ES114 (UniProtKB Q5E6S4) were predicted to comprise residues 7–25 and 273–296 using the TMHMM server v.2.0 (http://www.cbs.dtu.dk/services/TMHMM-2.0/; Krogh et al., 2001 ▸; Fig. 1 ▸). The Escherichia coli codon-optimized DNA fragment encoding the periplasmic sensory domain of VfcA (VfcAperi; amino-acid residues 26–272) was synthesized and ligated into the expression vector pET151/D-TOPO (Invitrogen) by GenScript.
Figure 1.
The predicted membrane topology of VfcA and the boundaries (amino-acid residue numbers) of the periplasmic sensory domain VfcAperi characterized in this study.
The recombinant protein used for crystallization comprised residues 26–272 of VfcA plus six additional residues (GIDPFT) originating from the Tobacco etch virus (TEV) protease cleavage site and vector. The recombinant VfcAperi was expressed in E. coli BL21 (DE3) cells (Novagen) grown at 37°C in Luria–Bertani broth containing 50 mg l−1 ampicillin (Aspen Pharmacare) to an OD600 mm of 0.6 and then induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (Thermo Scientific) for 3.5 h. The cells were harvested, resuspended in a buffer consisting of 20 mM Tris–HCl pH 8.0, 100 mM NaCl, 1 mM phenylmethylsulfonyl fluoride and lysed using an EmulsiFlex-C5 high-pressure homogenizer (Avestin). The cell lysate was clarified by centrifugation at 12 000g for 30 min at 4°C. NaCl and imidazole were then added to the supernatant to final concentrations of 500 and 15 mM, respectively, before loading it onto a 5 ml HiTrap Chelating HP column (GE Healthcare) pre-washed with buffer A (20 mM Tris–HCl pH 8.0, 500 mM NaCl, 20 mM imidazole). The column was washed with 20 column volumes of buffer A and the protein was eluted with buffer A supplemented with 500 mM imidazole. The His6 tag was cleaved with His6-TEV protease (Invitrogen) whilst dialyzing the sample overnight at 4°C against buffer B [50 mM Tris–HCl pH 8.0, 2 mM dithiothreitol, 200 mM NaCl, 1%(v/v) glycerol]. NaCl and imidazole were then added to the sample to final concentrations of 500 and 20 mM, respectively, and the TEV protease and the uncleaved protein were removed by passing the sample through a HiTrap Chelating HP column. The flowthrough fractions were collected and concentrated to 2 ml in a Vivaspin 10 kDa cutoff concentrator and further purified by passing them through a Superdex 200 HiLoad 26/60 gel-filtration column (GE Healthcare) equilibrated with buffer C (10 mM Tris–HCl pH 8.0, 200 mM NaCl). The protein was concentrated to 10 mg ml−1 (the concentration was determined using the Bradford assay; Bradford, 1976 ▸). The protein purity was estimated to be greater than 98% by quantifying the protein in the VfcA band and the impurities on an SDS–PAGE gel with ImageJ (https://imagej.nih.gov/ij/; Fig. 2 ▸). The oligomeric state of VfcAperi was calculated using a calibration plot of log(molecular weight) versus retention volume [V retention (ml) = 549.3–73.9 × log(molecular weight)] available at the EMBL Protein Expression and Purification Core Facility website (http://www.embl.de/pepcore/pepcore_services/protein_purification/chromatography/hiload26-60_superdex200/index.html).
Figure 2.
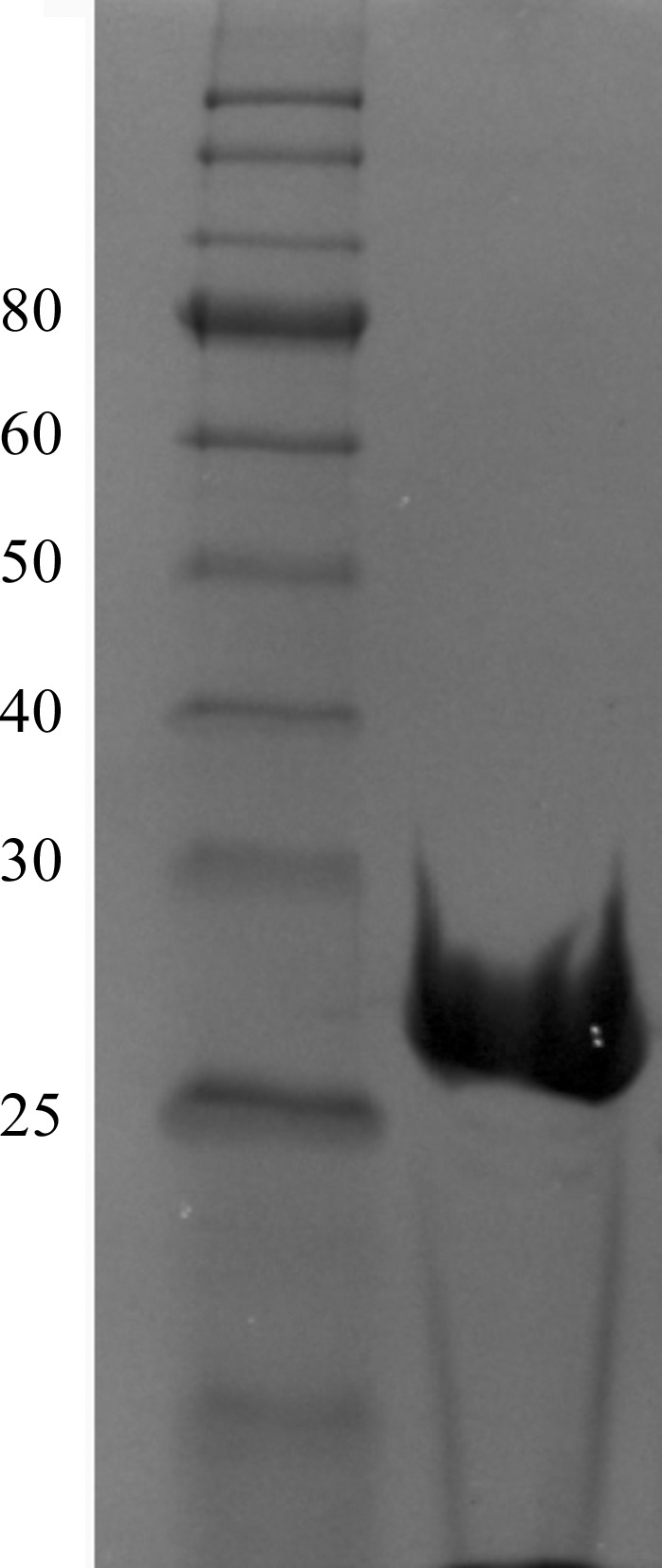
Coomassie Blue-stained 12% SDS–PAGE gel of recombinant VfcAperi. The left lane contains molecular-weight marker (labelled in kDa).
2.2. Crystallization
Prior to crystallization, VfcA (10 mg ml−1) was mixed with alanine (final concentration 5 mM) and then centrifuged for 20 min at 13 000g to clarify the solution. The search for crystallization conditions was performed using commercially available screens. The initial crystallization droplets consisted of 100 nl protein solution mixed with 100 nl reservoir solution and equilibrated against 50 µl reservoir solution in a 96-well Art Robbins CrystalMation Intelli-Plate (Hampton Research). Crystals appeared after 1 d from condition No. 79 of Index Screen HT (Fig. 3 ▸), which consisted of 200 mM ammonium acetate, 100 mM bis-tris pH 6.5, 25%(w/v) polyethylene glycol (PEG) 3350. This condition was further optimized to improve the crystal quality, yielding an optimal crystallization reservoir solution consisting of 200 mM ammonium acetate, 100 mM HEPES pH 7.0, 25%(w/v) PEG 3350. Crystallization conditions are detailed in Table 1 ▸.
Figure 3.
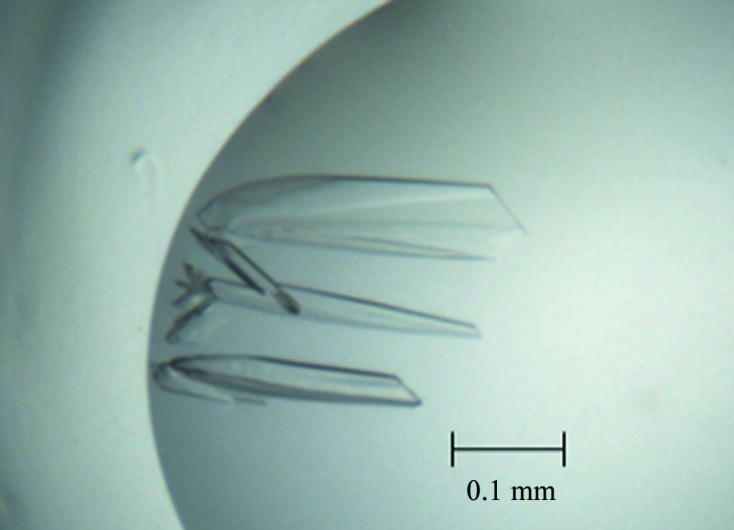
Crystals of a putative complex of VfcAperi with alanine.
Table 1. Crystallization.
| Method | Hanging-drop vapour diffusion |
| Plate type | 96-well Art Robbins CrystalMation Intelli-Plate (Hampton Research) |
| Temperature (K) | 293 |
| Protein concentration (mg ml−1) | 10 |
| Buffer composition of protein solution | 100 mM ammonium acetate, 12.5%(w/v) PEG 3350, 50 mM HEPES pH 7.0 |
| Composition of reservoir solution | 200 mM ammonium acetate, 25%(w/v) PEG 3350, 100 mM HEPES pH 7.0 |
| Volume and ratio of drop | 1 µl, 1:1 |
| Volume of reservoir (µl) | 500 |
2.3. Data collection and processing
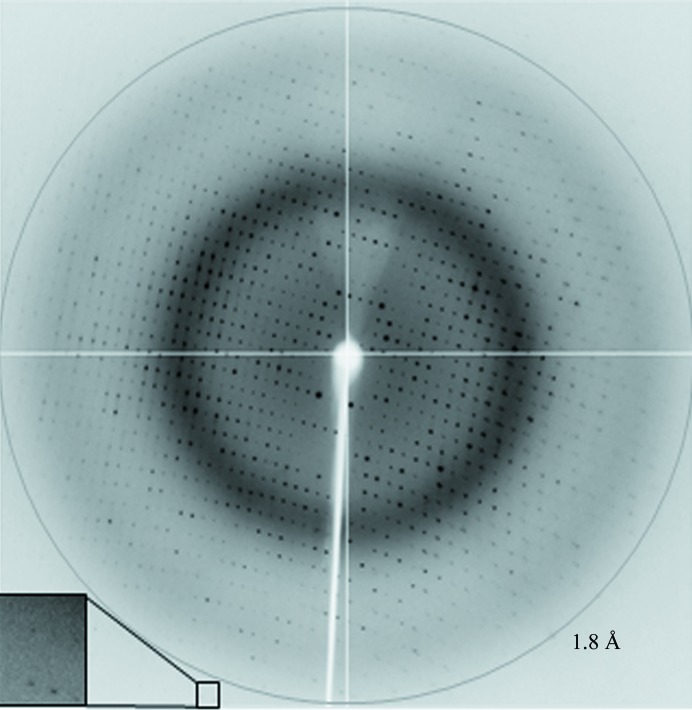
The crystals were briefly soaked in a cryostabilizing solution consisting of 220 mM ammonium acetate, 120 mM HEPES pH 7.0, 25%(w/v) PEG 3350, 10 mM alanine, 10%(v/v) glycerol and flash-cooled in liquid nitrogen prior to data collection. X-ray diffraction data were collected to 1.8 Å resolution on the MX1 beamline of the Australian Synchrotron (AS; Fig. 4 ▸). A total of 360 images were collected using a 0.5° oscillation width. The data were processed and scaled using iMosflm (Battye et al., 2011 ▸) and AIMLESS (Evans & Murshudov, 2013 ▸) from the CCP4 suite (Winn et al., 2011 ▸). Calculation of the self-rotation function was performed using POLARRFN (Winn et al., 2011 ▸). The statistics of data collection are summarized in Table 2 ▸.
Figure 4.
A representative 0.5° oscillation image of the data collected using an ADSC Quantum 210r CCD detector at station MX1, Australian Synchrotron, Victoria, Australia. The magnified rectangle shows diffraction spots beyond the 1.8 Å resolution ring.
Table 2. Data collection and processing.
Values in parentheses are for the outer shell.
| Diffraction source | MX1 beamline, AS |
| Wavelength (Å) | 1.0 |
| Temperature (K) | 100 |
| Detector | ADSC Quantum 210r |
| Rotation range per image (°) | 0.5 |
| Total rotation range (°) | 180 |
| Exposure time per image (s) | 1.0 |
| Space group | P1 |
| a, b, c (Å) | 39.9, 57.0, 117.0 |
| α, β, γ (°) | 88.9, 80.5, 90.0 |
| Mosaicity (°) | 0.4 |
| Resolution range (Å) | 41.0–1.8 (1.9–1.8) |
| Total No. of reflections | 169704 (7009) |
| No. of unique reflections | 86241 (3821) |
| Completeness (%) | 96 (81) |
| Multiplicity | 2.0 (1.8) |
| 〈I/σ(I)〉 | 12.5 (3.9) |
| R r.i.m. | 0.075 (0.223) |
| Overall B factor from Wilson plot (Å2) | 15.6 |
3. Results and discussion
Recombinant VfcAperi was overexpressed in E. coli BL21 (DE3) cells harbouring the pET151/D-TOPO plasmid upon induction with T7 polymerase. The protein was purified to >98% electrophoretic homogeneity based on Coomassie Blue staining of SDS–PAGE gels. The recombinant protein consisted of amino-acid residues 27–272 of VfcAperi with six additional amino acids (GIDPFT) at the N-terminus originating from the TEV cleavage site and expression vector. The protein migrated on SDS–PAGE with an apparent molecular weight of 27 kDa, which is close to the value calculated from the amino-acid sequence (27.7 kDa). When subjected to gel filtration, the protein eluted as a single peak with an apparent molecular weight of approximately 27 kDa, indicating that VfcAperi is monomeric in solution. This result is consistent with previous reports on other tandem PAS sensing domains, which also behave as monomers in solution (Rico-Jiménez et al., 2013 ▸; Machuca, Liu & Roujeinikova, 2015 ▸; Machuca, Liu, Beckham et al., 2015 ▸; Liu et al., 2015 ▸).
Crystals of VfcAperi were obtained in the presence of its putative ligand (alanine) using a sparse-matrix crystallization approach. Images collected from a cryocooled crystal at the Australian Synchrotron showed diffraction to 1.8 Å resolution (Fig. 4 ▸). Analysis of the X-ray diffraction data using the autoindexing routine in iMosflm is consistent with a P triclinic crystal system (space group P1), with unit-cell parameters a = 39.9, b = 57.0, c = 117.0 Å, α = 88.9, β = 80.5, γ = 89.7°. The average I/σ(I) value is 12.5 for all reflections (resolution range 41.0–1.8 Å) and 3.9 in the highest resolution shell (1.9–1.8 Å). A total of 169 704 measurements were made of 86 241 independent reflections. Data processing gave an R p.i.m. of 0.075 for intensities (0.223 in the 1.9–1.8 Å resolution shell) and the data were 96% complete (81% completeness in the highest resolution shell).
Calculations of the Matthews coefficient (Matthews, 1977 ▸) for two, three and four molecules in the asymmetric unit gave values of 4.7, 3.2 and 2.4 Å3 Da−1, corresponding to solvent contents of approximately 74, 61 and 48%, respectively. These values fall within the range observed for protein crystals by Matthews (1977 ▸). Analysis of the intensity distribution using the phenix.xtriage program (Adams et al., 2010 ▸) resulted in a multivariate Z-score of 3.95 from the L-test, which was higher than the value expected for untwinned data (3.5) and suggested the presence of pseudo-merohedral twinning with operators (h, −k, h − l; −h, k, −l; –h, −k, −h + l). A self-rotation function calculated by POLARRFN using diffraction data between 10 and 5 Å resolution and an integration radius of 25 Å showed no dominant features that could be confidently assigned to noncrystallographic axes. Thus, we are currently unable to determine the protein content of the asymmetric unit. Phasing by molecular replacement has not been possible owing to the low sequence similarity to known structures deposited in the PDB. A search for heavy-atom derivatives and the solution of the structure using multiple isomorphous replacement and/or multiwavelength anomalous dispersion methods is under way.
Acknowledgments
Part of this research was undertaken on the MX1 beamline of the AS, Victoria, Australia. We thank the AS staff for their assistance with data collection. We are also grateful to Dr Danuta Maksel and Dr Robyn Gray at the Monash University Protein Crystallography Unit for assistance with the robotic crystallization trials.
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Anantharaman, V. & Aravind, L. (2001). Trends Biochem. Sci. 26, 579–582. [DOI] [PubMed]
- Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. (2011). Acta Cryst. D67, 271–281. [DOI] [PMC free article] [PubMed]
- Bradford, M. M. (1976). Anal. Biochem. 72, 248–254. [DOI] [PubMed]
- Brennan, C. A., DeLoney-Marino, C. R. & Mandel, M. J. (2013). Appl. Environ. Microbiol. 79, 1889–1896. [DOI] [PMC free article] [PubMed]
- Evans, P. R. & Murshudov, G. N. (2013). Acta Cryst. D69, 1204–1214. [DOI] [PMC free article] [PubMed]
- Finn, R. D., Coggill, P., Eberhardt, R. Y., Eddy, S. R., Mistry, J., Mitchell, A. L., Potter, S. C., Punta, M., Qureshi, M., Sangrador-Vegas, A., Salazar, G. A., Tate, J. & Bateman, A. (2016). Nucleic Acids Res. 44, D279–D285. [DOI] [PMC free article] [PubMed]
- Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E. L. (2001). J. Mol. Biol. 305, 567–580. [DOI] [PubMed]
- Liu, Y. C., Machuca, M. A., Beckham, S. A., Gunzburg, M. J. & Roujeinikova, A. (2015). Acta Cryst. D71, 2127–2136. [DOI] [PubMed]
- Machuca, M. A., Liu, Y. C., Beckham, S. A. & Roujeinikova, A. (2015). Acta Cryst. F71, 211–216. [DOI] [PMC free article] [PubMed]
- Machuca, M. A., Liu, Y. C. & Roujeinikova, A. (2015). Acta Cryst. F71, 110–113. [DOI] [PMC free article] [PubMed]
- Manson, M. D., Armitage, J. P., Hoch, J. A. & Macnab, R. M. (1998). J. Bacteriol. 180, 1009–1022. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1977). The Proteins, 3rd ed., edited by H. Neurath & R. L. Hill, Vol. 3, pp. 468–477. New York: Academic Press.
- Nealson, K. & Hastings, J. (1991). The Prokaryotes, edited by A. Balows, H. G. Trüper, M. Dworkin, W. Harder & K.-H. Schleifer, pp. 625–639. Berlin, Heidelberg, New York: Springer.
- Nikolakakis, K., Monfils, K., Moriano-Gutierrez, S., Brennan, C. & Ruby, E. (2016). Appl. Environ. Microbiol. 82, 696–704. [DOI] [PMC free article] [PubMed]
- Norsworthy, A. N. & Visick, K. L. (2013). Front. Microbiol. 4, 356. [DOI] [PMC free article] [PubMed]
- Rico-Jiménez, M., Muñoz-Martínez, F., García-Fontana, C., Fernandez, M., Morel, B., Ortega, Á., Ramos, J. L. & Krell, T. (2013). Mol. Microbiol. 88, 1230–1243. [DOI] [PubMed]
- Sourjik, V. & Wingreen, N. S. (2012). Curr. Opin. Cell Biol. 24, 262–268. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Zhang, Z. & Hendrickson, W. A. (2010). J. Mol. Biol. 400, 335–353. [DOI] [PMC free article] [PubMed]