Abstract
Background
Investigations of age effects on youth anxiety outcomes in randomized trials (RCTs) of cognitive behavior therapy (CBT) have failed to yield a clear result due to inadequate statistical power and methodologic weaknesses. We conducted an individual patient data metaanalysis to address this gap.
Question
Does age moderate CBT effect size, measured by a clinically and statistically significant interaction between age and CBT exposure?
Methods
All English language RCTs of CBT for anxiety in 6–19 year olds were identified using systematic review methods. Investigators of eligible trials were invited to submit their individual patient data. The anxiety disorder interview schedule (ADIS) primary diagnosis severity score was the primary outcome. Age effects were investigated using multilevel modeling to account for study level data clustering and random effects.
Results
Data from 17 of 23 eligible trials were obtained (74%); 16 studies and 1,171 (78%) cases were available for the analysis. No interaction between age and CBT exposure was found in a model containing age, sex, ADIS baseline severity score, and comorbid depression diagnosis (power ≥ 80%). Sensitivity analyses, including modeling age as both a categorical and continuous variable, revealed this result was robust.
Conclusions
Adolescents who receive CBT in efficacy research studies show benefits comparable to younger children. However, CBT protocol modifications routinely carried out by expert trial therapists may explain these findings. Adolescent CBT protocols are needed to facilitate the transportability of efficacy research effects to usual care settings where therapists may have less opportunity for CBT training and expertise development.
Keywords: anxiety disorders, cognitive behavior therapy, child/adolescent, treatment, empirical supported treatment
INTRODUCTION
Anxiety disorders affect 6–7% of those under 18 years of age[1, 2] and are associated with debilitating consequences. These include poor school performance, disrupted relationships with peers and adults, and diminished participation in the normal activities of childhood and adolescence.[3] Relatively few children and youth are affected by only one type of anxiety disorder,[4] and there is frequent comorbidity with other disorders, particularly depression and substance abuse.[5] Anxiety that begins before age 18 often persists into adulthood,[6–8] especially in the presence of comorbid conditions such as depression,[9] resulting in a lifetime of diminished life quality characterized by failed adult relationships, poor labor market participation, reduced income, and increased need for social welfare assistance.
Cognitive behavioral therapy (CBT) is the psychotherapeutic treatment of choice for children and youth with anxiety and mood disorders. Eight research syntheses,[10–17] including three metaanalyses, report that compared to waitlist or attention controls in randomized controlled trials (RCTs), CBT is an efficacious treatment when administered by highly trained experts to carefully screened samples of children and youth under ideal conditions. These authors also report that the efficacy of individual and group CBT treatment approaches (either with or without parent involvement) is not significantly different. Despite this strong research base, a number of authors have questioned whether the levels of benefit observed (e.g. anxiety diagnosis remission rates) apply equally to children and adolescents.[12] First, there is reason to believe that the CBT protocols evaluated in these trials may be developmentally inappropriate for adolescents because many were created for children under age 12 and then adapted for use in adolescents. Consequently, effect size estimates based on all children aged 6–19 may mask age-related clinical heterogeneity and thus overestimate the benefits of CBT in adolescents. Second, adolescents may have increased severity of disorder and be less responsive to treatment. For example, teens may be struggling with the effects of prolonged untreated anxiety including entrenched anxiety-related avoidance, chronic depression, substance abuse, and more severe anxiety.[3] Finally, high need for autonomy by teens, compared to younger children, may result in poor engagement and compliance leading to reduced treatment benefit in adolescents.
Three of the available research syntheses consider the issue of developmental appropriateness, but conclusions regarding whether age moderates CBT treatment effect cannot be drawn. Compton et al.[11] reports that one of 21 studies reviewed investigated the effect of age and reported a significant positive effect. James et al.[15] calls for further studies that include adolescents and investigate age effects. Hudson[12] concludes some evidence exists that age moderates CBT effect size, but calls for further studies. Finally, it is worthy of note that despite lack of clarity regarding age effects, the recent CAMS trial (Child/adolescent anxiety multimodal study) used a separate CBT protocol for adolescents.[18, 19]
When we reviewed the individual CBT RCTs included in the available reviews, we found six studies that reported findings regarding the influence of age on CBT treatment outcomes. Most reports suffered from significant methodologic weaknesses, namely the conduct of unplanned subgroup analyses with insufficient power to rigorously test for an interaction, the appropriate statistical method for assessing treatment effect moderators.[20] In fact, only three of the six studies tested for an interaction between age and CBT treatment effect with one of the three reporting a statistically significant positive finding.
It is widely recognized that individual trials are rarely designed with adequate levels of power to conduct subgroup analyses and test for interaction effects.[21] However, the availability of a substantial number of rigorous trials of a specific intervention, such as CBT for child and adolescent anxiety, offers great potential to advance knowledge through the conduct of an individual patient data metaanalysis (IPDMA). This gold standard meta-analysis technique calls for patient level data to be obtained for all relevant RCTs and combined in a common dataset.[22–24] The resulting larger sample size provides increased power and enables a more meaningful investigation of treatment effect moderators because findings based on individual trials might change in IPDMA analyses using larger sample sizes.
Accordingly, we conducted an IPDMA to address the question: Does age moderate CBT effect size, as measured by a clinically and statistically significant interaction between age and the effect of CBT exposure? In particular, is CBT effect size attenuated in adolescents compared to younger children in the currently available RCTs? Our goal was to clarify the extent to which the benefit of CBT extends equally to children and adolescents. An answer to this question is relevant to good clinical practice. It is also relevant to the development of knowledge translation strategies that aim to ensure the transportability of CBT treatment effects observed in efficacy studies to anxious adolescents who seek help in usual care settings.[25]
METHODS
TRIAL SEARCH STRATEGY
Cochrane[26] and PRISMA[27] (preferred reporting items for systematic reviews and metaanalyses) methods were used to identify eligible CBT RCTs as follows: (i) electronic data-bases (OVID-Medline, OVID-Embase, OVID-Cochrane Central, OVID-PsycINFO, and EBSCO CINAHL) were searched for the period 1990 to 2011 to identify existing systematic reviews and metaanalyses of the efficacy of CBT in child and adolescent anxiety (key words and a replicable strategy created by an experienced research librarian available from the author); (ii) reference lists of the eight published systematic reviews/metaanalyses identified were hand searched to identify RCTs; these existing reviews included potentially eligible primary RCTs published from 1966 to 2005; (iii) additional RCTs published from 2005 onwards were then identified by searching the same electronic databases (key words and search strategy available from the author); (iv) reference lists of all eligible RCTs identified were hand searched; and (v) all collaborating authors reviewed the list and noted omissions.
STUDY ELIGIBILITY
Published primary studies that met the following criteria were eligible: (i) RCT comparing CBT to waitlist or attention controls; (ii) common CBT protocol used for all study participants; (iii) English language; (iv) participants aged 6–19; (v) baseline diagnosis: anxiety disorder (excluding OCD and PTSD) or clinically elevated levels of anxiety as defined by the author; (vi) outcome assessments include one or more of: (a) presence of anxiety diagnosis; (b) severity of anxiety diagnosis; (c) self-report or parent-report measures of anxiety symptoms; (vii) face-to-face CBT implemented by any service provider (excluding self-help and parent implemented CBT).
STUDY SELECTION AND DATA EXTRACTION
K.B. and S.D. screened potentially eligible studies (titles and abstracts) and extracted study level data from all eligible studies. Disagreements were resolved through discussion.
COMMON DATASET
Senior investigators of all eligible RCTs were invited to contribute their individual patient data using a common template. Study level variables (e.g. number of CBT sessions, service provider, setting, parental involvement in CBT sessions, etc.) were extracted from published study reports by two reviewers (K.B. and S.D.). Disagreements were resolved through discussion.
INDIVIDUAL PATIENT ELIGIBILITY
Participants in obtained eligible studies were included in the IPDMA if they had complete data for the variables required for the primary analysis (see below).
VARIABLES AND MEASURES—PRIMARY ANALYSIS
Anxiety Disorder Severity Score
The primary outcome measure was the anxiety disorder interview schedule[28] (ADIS) clinical severity rating score at posttreatment for the primary, targeted diagnosis (identified at baseline), hereafter denoted as ADIS-CSR-PT. The baseline ADIS clinical severity rating score was used as a covariate, hereafter denoted as ADIS-CSR-B. The ADIS is a semistructured interview that assesses anxiety, mood, and externalizing disorders and generates impairment/severity ratings measured on a scale of 0–8 for each diagnosis. Child and parent versions are available. Inter-rater reliability is good[28] and evidence of responsiveness to treatment effect is available.[29, 30]
Age
Four categories (6–7; 8–11; 12–15; and 16–19 years of age) were selected a priori, reflecting the theoretical basis of our hypothesis that developmental stage moderates CBT treatment effect. The reference category was 8–11 years because CBT protocols were developed for this age group. We hypothesized that those who are younger or older then 8–11 years would respond differently to CBT (i.e. cognitive limitations in the younger children and the special challenges associated with treating adolescents as outlined in the introduction). In addition to this theoretical foundation, a categorical representation provides maximal power to detect an interaction between age and treatment effect.
Sex
Sex was included as a covariate, since female sex predicts worse treatment outcomes.[3, 5]
Depression
Baseline ADIS depression diagnosis was included as a covariate, since comorbid depression predicts worse anxiety treatment outcomes.[9, 31]
CBT Exposure
Study participants who received individual or group CBT, with or without parent involvement, of any duration, and by any type of service provider were classified as exposed to CBT.
Control Group Conditions
Study participants assigned to a waitlist or attention control group were classified as controls.
SENSITIVITY ANALYSIS VARIABLES AND MEASURES
The robustness of our results was tested using alternative measures of (i) anxiety disorder/symptoms and (ii) age as follows:
Anxiety Disorder Status
Presence of an ADIS anxiety diagnosis (yes/no) at posttreatment was used.
Anxiety Symptoms
The child behavior checklist (CBCL) internalizing t-scores (parent report) and the revised child manifest anxiety scale (RCMAS) total anxiety t-scores (self-report) at posttreatment were used as alternative outcome measures in multi-indicator sensitivity analyses, because these were the most frequently applied symptom scales in the trials. Baseline CBCL or RCMAS were included as covariates when these variables were used as outcomes. Both the CBCL and RCMAS are psychometrically valid and reliable measures of child and adolescent anxiety.[32, 33]
Age
Sensitivity analyses were conducted using age as a continuous variable (including linear, quadratic, and cubic terms).
STATISTICAL METHODS
Data from each trial were received and checked for completeness and missing values. Final analyses were based on all patients randomized using the intention to treat principle. Any queries raised were resolved by contacting the trial investigator. Analyses were performed on the data as provided with no imputation for missing data. In stage one of the metaanalysis of individual patient data, random-effects linear regression models stratified by study were fitted, and included the interaction of the effect of CBT with age group on ADIS-CSR-PT at the study level. Individual study results were presented using forest plots.[34] In stage two analyses, linear random-effects models for the aggregate effects of CBT exposure on ADIS-CSR-PT and age group subgroup analyses were investigated with random-effects using multilevel modeling (to allow for treatment heterogeneity between studies).[35] Multilevel regression models were constructed using restricted maximum likelihood where individuals (level 1) were nested within studies (level 2). Between study differences were assessed through two models: (1) random intercept model at the study level; (2) random-effects model where CBT treatment effect was allowed to vary between studies. Our study question was addressed by conducting a series of prespecified models that evaluated CBT treatment effect and the age group by CBT interaction term on ADIS-CSR-PT, adjusting for ADIS-CSR-B, age group, baseline depression diagnosis, and sex. The estimated residual variance was calculated for each model. Formal comparisons of fit between simpler and more complex models were conducted using the Bayesian information criterion (BIC) statistic. The BIC accounts for model complexity (number of parameters included), and enables the derivation of likelihood ratio tests for pairs of models that are hierarchically structured.[36] All analyses were conducted using SAS v. 9.2.[37] Significance was considered at P < .05; multiple comparisons were adjusted using the Bonferroni method.
Power calculations postulated differences in treatment effect between the 8–11-year-old reference group, and each of the 6–7, 12–15, and 16–19-year-old groups. Mean differences of =2 on the ADIS-CSR-PT were judged to be a minimum clinically important difference. We assumed the estimated group means were normally distributed, and used a derived value of 0.34 for the residual (between person) standard deviation on the ADIS-CSR-PT. Study power was then calculated in the usual way from the standard normal distribution theory, adopting a null hypothesis of equal treatment effects in each pair of age groups being compared.[21]
RESULTS
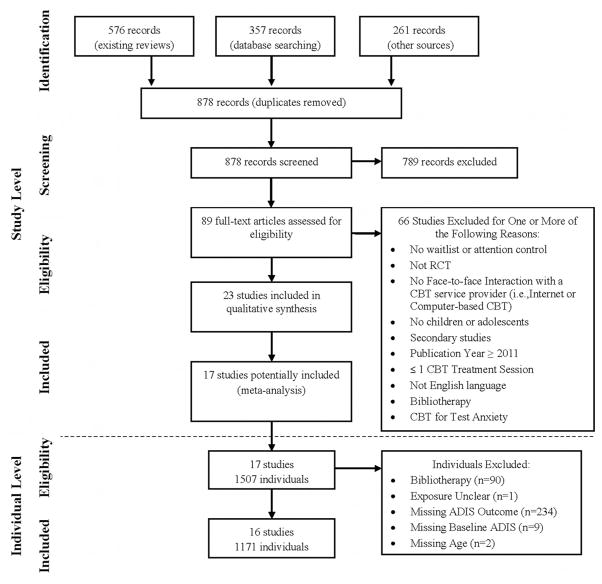
Figure 1 shows the PRISMA flow diagram. Our search strategy identified 878 records after duplicates were removed; 89 records remained after level 1 and 2 screening of titles and abstracts. Following full-text screening, 23 RCT’s met our inclusion criteria. We obtained datasets for 17 of them (74%) (see Appendix for reference list). Among the 1,507 cases included in the 17 studies, 91 were excluded because they received self-help CBT or their exposure status could not be confirmed; 245 cases were excluded due to missing data (Fig. 1 contains specific reasons). The remaining 1,171 cases included 16 of the trials and 77.7% of all cases potentially available in the 17 studies obtained. The six eligible but not obtained datasets included 265 participants (147 CBT; 118 control subjects) or 14.9% of all individuals who could potentially be included in our analyses. Reasons for non-inclusion were: the data was no longer available (4); non-response of author (2).
Figure 1.
PRISMA flow diagram of eligible studies (and individuals).
Table 1 presents descriptive information for the 17 obtained studies. The percentage of participants aged ≥ 12 years varied between studies from 4.1 to 100%. Similarly, the percentage with comorbid depression varied from 0 to 22.2% and the number of CBT sessions provided varied from 9 to 18 between studies. CBT providers were: psychologists and graduate students in 88% of studies, psychiatric social workers, and psychiatry residents in 6% of studies, and health care psychologists, psychotherapists, and behavioral therapists in the remaining 6%. Regarding setting, 76.5% of studies provided treatment in a clinic setting while 23.5% utilized school settings. The anxiety disorders included are listed for each study. All studies used the ADIS to assess anxiety status. Ten (59%) also assessed anxiety symptoms using the CBCL parent report; while 8 (47%) also used the RCMAS (child report). Ethnicity could not be included in our analyses because of substantive measurement differences between trials, and 29% did not report it at all. Similarly, only 53% of studies reported on family structure or income, precluding the inclusion of indicators of socioeconomic status (SES) in our analytic models. All but one study included some type of parental involvement in the CBT intervention tested, so this variable was not included as a covariate. The most commonly reported exclusion criteria were as follows; 11/17 studies excluded participants with psychosis; 10/17 studies excluded participants taking psychotropic medication; 3/17 studies excluded participants who experienced a change to their dose of psychotropic medication; and 8/17 studies excluded participants with physical or intellectual disabilities. The study level characteristics of the six unobtained studies were summarized similarly (see Table 3 in the Appendix). Their exclusion appears unlikely to have introduced bias into the IPDMA results.
TABLE 1.
Individual study characteristics
| Study* | Groups** (n) | Age years | ≥12 years (%) | Female (%) | Anxiety disorders included*** | Comorbid depression (%) | CBT sessions (child) | Provider qualifications**** | Setting
|
|
|---|---|---|---|---|---|---|---|---|---|---|
| School | Clinic | |||||||||
| 1. | Group CBT (6) Waitlist (6) |
13–18 | 100.0 | 58.3 | SP with 67% having other comorbid anxiety disorder | 0.0 | 12 | A | √ | |
| 2. | Child CBT (28) CBT + FAM1 (25) Waitlist (26) |
6–14 | 21.5 | 43.0 | SAD OAD SP |
Data not available | 12 | B | √ | |
| 3. | Child CBT (53) Family CBT (57) Waitlist (25) |
8–17 | 54.8 | 59.3 | All | 20.0 | 13 | C | √ | |
| 4. | Group CBT (61) Control (67) |
7–13 | 12.5 | 72.7 | All | 0.0 | 10 | B | √ | |
| 5. | Individual CBT (12) Group CBT (10) Waitlist (15) |
8–14 | 32.4 | 48.6 | GAD SP SAD |
5.4 | 18 | B | √ | |
| 6. | Group CBT (4) Group attention (5) |
15–18 | 100.0 | 88.9 | All | 22.2 | 10 | B | √ | |
| 7. | Group CBT (60) Group attention (52) |
6–16 | 32.1 | 42.9 | All | 2.7 | 10 | B | √ | |
| 8. | Individual CBT (55) Family CBT (56) Family attention (50) |
7–14 | 24.2 | 44.1 | GAD SAD SP |
9.9 | 16 | B | √ | |
| 9. | Individual CBT (29) Waitlist (24) |
9–14 | 41.5 | 41.5 | OAD SAD AD |
13.2 | 16 | B | √ | |
| 10. | Individual CBT (71) Waitlist (54) |
8–14 | 36.8 | 42.4 | OAD SAD AD |
5.6 | 16 | B | √ | |
| 11. | SASS2 (21) Waitlist (21) |
13–17 | 100.0 | 73.8 | SP with ≥43% having other comorbid anxiety disorder | 0.0 | 12 | B | √ | |
| 12. | SASS2 (19) Group attention (17) |
14–16 | 100.0 | 83.3 | SP with 33% having other comorbid anxiety disorder | 13.9 | 12 | B | √ | |
| 13. | Child CBT (29) Child and parents CBT (30) Waitlist (20) |
7–17 | 38.0 | 50.6 | SAD SP GAD PD ± A |
21.5 | 12 | B | √ | |
| 14. | Group CBT (90) Bibliotherapy (90) Waitlist (87) |
6–15 | 4.1 | 39.7 | All | 7.9 | 9 | B | √ | |
| 15. | Group CBT (37) Waitlist (19) |
5–18 | 28.6 | 41.1 | SP OAD GAD |
7.1 | 12 | B | √ | |
| 16. | EBCM3 (40) EBCSC4 (41) ES5 (23) |
6–16 | 20.2 | 48.1 | Phobias (simple, social, and Agoraphobia) with ≥49% having other comorbid anxiety disorder | 5.8 | 10 | B | √ | |
| 17. | Clinic CBT (22) Clinic and internet CBT (27) Waitlist (23) |
7–14 | 19.4 | 41.7 | All | 1.4 | 10 | B | √ | |
see Appendix;
1, family anxiety management training; 2, skills for academic and social success; 3, exposure-based contingency management; 4, exposure-based cognitive self-control; 5, education support control;
AD, avoidant disorder; GAD, generalized anxiety disorder; OAD, over-anxious disorder; PD ± A, panic disorder with or without Agoraphobia; SAD, separation anxiety disorder; SP, social phobia;
A, psychiatric social worker and psychiatry residents; B, psychologist(s) and graduate student(s); C, health care psychologists, psychotherapists, and behavioral therapists.
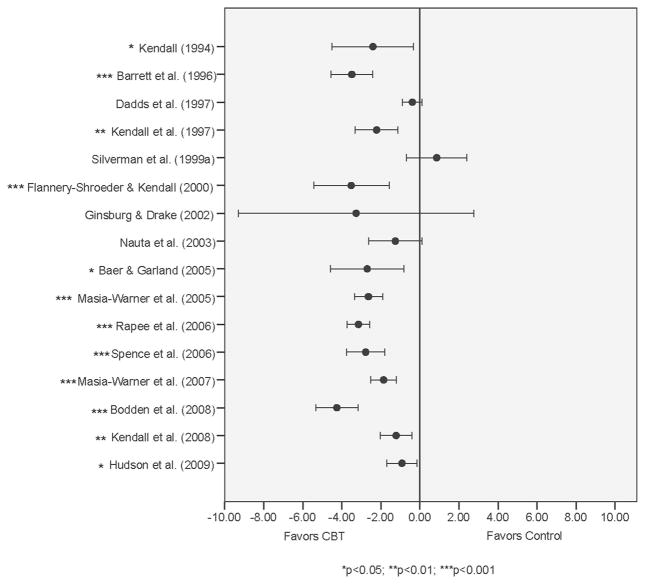
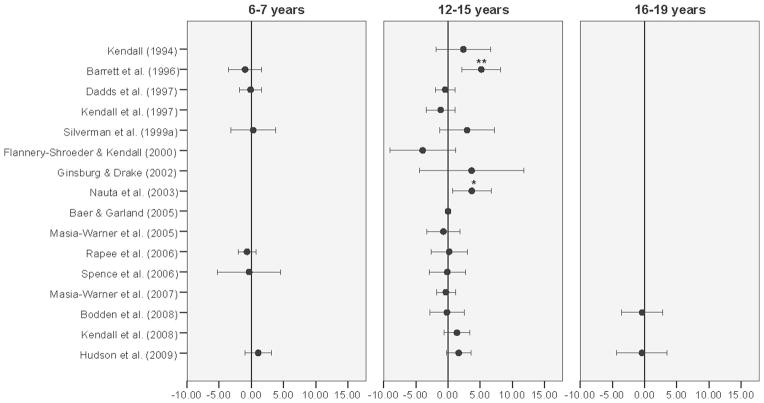
Figure 2 presents a forest plot of study level results for our primary outcome measure—between group mean difference in ADIS-CSR-PT and the 95% confidence interval (adjusted for ADIS-CSR-B). Twelve (75%) of the 16 study level results were statistically significant. Figure 3 presents a forest plot of study level results for age group by CBT treatment interaction (adjusted for ADIS-CSR-B). Only two studies revealed statistically significant interactions corresponding to differences between the 12–15 year and the 8–11-year-old age groups. Only one study included participants in all four age groups in both the CBT and control groups, but no statistically significant age by CBT treatment interaction was found between any of the three other age groups and the 8–11 year reference age group. The variability in interaction estimates within studies was evident and called for pooling study results in phase 2 of the analysis.
Figure 2.
Study level mean differences in ADIS-CSR-PT adjusted by ADIS-CSR-B.
Figure 3.
Treatment effects by age group (adjusted for ADIS-CSR-B).
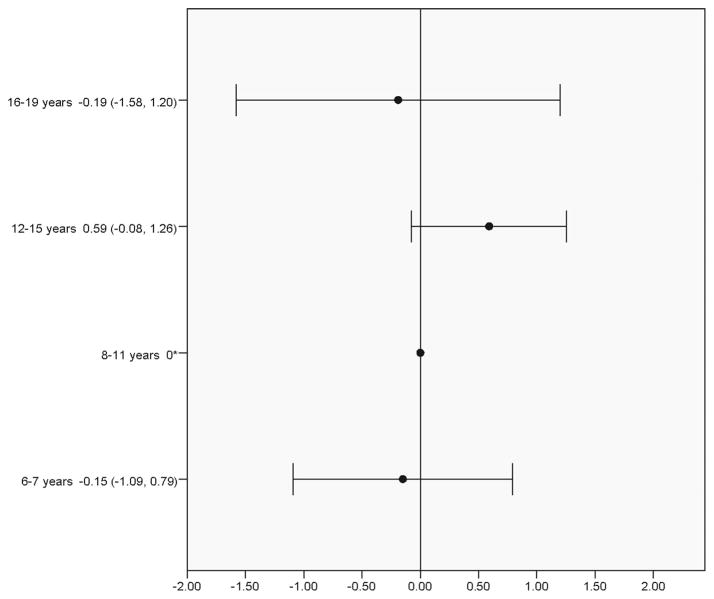
Table 2 shows results for the seven models evaluated in the phase 2 pooled analysis. Results for each model show, as appropriate, the variance between studies for the intercept (between-study differences in the mean ADIS-CSR-PT), and treatment slope (between study variability of treatment effect). The main effects of treatment (included in models 1–7) and ADIS-CSR-PT (included in models 3–7) are statistically significant in all models tested. There is also significant variation between studies in treatment effect (slope), representing 13% of the total variance explained. The age group by CBT treatment interaction tested in model 5 was not statistically significant and had little to no effect on the BIC and other model parameters compared to model 4. Figure 4 in the Appendix presents a forest plot of the age group findings relative to the 8–11 year age group.
TABLE 2.
Random effects model for ADIS-CSR-PT
| Variable | Model
|
||||||
|---|---|---|---|---|---|---|---|
| 1 Treatment (random intercept) |
2 Treatment (random treatment slope) |
3 Treatment, and ADIS-CSR-B |
4 Treatment, ADIS-CSR-B, and Age |
5 Treatment, ADIS-CSR-B, Age and interaction |
6 Treatment, ADIS-CSR-B, age, sex, depression, and interaction |
7 Treatment, ADIS-CSR-B, age, sex, and and depression |
|
| Intercept, beta (SE) | 4.53 (0.25)*** | 4.62 (0.29)*** | 4.62 (0.25)*** | 4.52 (0.25)*** | 4.61 (0.27)*** | 4.46 (0.28)*** | 4.38 (0.26)*** |
| CBT treatment, beta (SE) | −1.98 (0.14)*** | −2.11 (0.35)*** | −2.17 (0.34)*** | −2.16 (0.34)*** | −2.32 (0.37)*** | −2.18 (0.37)*** | −2.05 (0.33)*** |
| ADIS-CSR-B, beta (SE) | – | – | 0.47 (0.06)*** | 0.47 (0.06)*** | 0.47 (0.06)*** | 0.49 (0.06)*** | 0.48 (0.06)*** |
| Age (years; ref: 8–11), beta (SE) | – | – | – | 0 (−) | 0 (−) | 0 (−) | 0 (−) |
| 6–7 | – | – | – | 0.04 (0.24) | 0.11 (0.35) | −0.09 (0.38) | 4.96 × 10−3 (0.26) |
| 12–15 | – | – | – | 0.11 (0.16) | −0.28 (0.27) | −0.15 (0.28) | 0.14 (0.17) |
| 16–19 | – | – | – | 0.71 (0.33)* | 0.84 (0.59) | 0.86 (0.59) | 0.66 (0.34) |
| Depression (ref: absent), beta (SE) | – | – | – | – | – | 0.41 (0.26) | 0.42 (0.26) |
| Sex (ref: female), beta (SE) | – | – | – | – | – | 0.22 (0.14) | 0.22 (0.14) |
| Interactions | – | – | – | – | |||
| Treatment * age group (years; ref: 8–11), beta (SE) | – | – | – | – | 0 (−) | 0 (−) | – |
| 6–7 | – | – | – | – | −0.15 (0.48) | 0.16 (0.52) | – |
| 12–15 | – | – | – | – | 0.59 (0.34) | 0.45 (0.35) | – |
| 16–19 | – | – | – | – | 0.19 (0.71) | −0.28 (0.71) | – |
| Random effects | |||||||
| Variance between studies (SE) | 0.77 (0.31)** | 0.38 (0.35) | 0 (−) | 0 (−) | 0 (−) | 0 (−) | 0 (−) |
| Variance of treatment slopes (SE) | – | 0.75 (0.34)* | 0.72 (0.23)** | 0.69 (0.23)** | 0.72 (0.24)** | 0.63 (0.22)** | 0.62 (0.22)** |
| Residual variance (SE) | 5.35 (0.22)*** | 5.02 (0.21)*** | 4.78 (0.20)*** | 4.78 (0.20)*** | 4.77 (0.20)*** | 4.81 (0.21)*** | 4.81 (0.21)*** |
| Model parameters | |||||||
| BIC | 5,329.1 | 5,282.1 | 5,218.4 | 5,217.1 | 5,212.1 | 4,888.4 | 4,892.0 |
P-value <.05,
P<.01,
P<.001.
Note: Baseline severity centered.
Similar findings emerged when baseline depression diagnosis and sex were added (model 6). These covariates remain statistically insignificant when the treatment by age interaction is removed (model 7). The coeffi-cients for CBT treatment and ADIS-CSR-B are stable across all models, indicating no substantial confounding of the main effects by other variables. As can be seen, the BIC changes very little as additional variables are introduced into the model. Hence, these additional variables, while themselves potentially statistically significant, do not substantially increase the amount of explained variation in the outcome data.
SENSITIVITY ANALYSES
The Table 2 analyses were repeated using: (i) three alternative treatment outcome measures [posttreatment presence of the primary targeted ADIS anxiety diagnosis (binary outcome: yes/no), CBCL and RCMAS symptom scale t-scores]; and (ii) age as a continuous variable. All results confirmed the findings of the main analysis were robust; that is, no statistically significant age by CBT treatment effect interaction (results available from author). Finally, we investigated the possibility of a three-way interaction between sex, age, and CBT treatment, but it was not significant (details not shown).
POWER
Power calculations carried out before examination of the data revealed the following. With the sample size of 1,171 achieved and considering age group comparisons with the 8–11 year group, we estimated the power to detect a clinically important difference of 2 scale points on the ADIS-CSR to be: 99% (6–7 years), >99% (12–15 years), and 80% (16–19 years). For a 3-scale point change: 99% (6–7 years), 99% (12–15 years), and 99% (16–19 years). Finally for completeness, for a one-scale point change, power was estimated at 55% (6–7 years), 84% (12–15 years), and 29% (16–19 years).
DISCUSSION
Individual trials of CBT for child and adolescent anxiety have not clarified whether adolescents enjoy the same level of benefit from CBT as do younger children primarily due to inadequate power. The IPDMA reported here addresses this knowledge gap. We obtained data from 16 (74%) of all eligible RCTs that compared CBT to a waitlist or attention control group in youth aged 6–19 years with an anxiety diagnosis. Tests for an interaction between age and the effect of CBT exposure (n = 1,171 cases) were not statistically significant showing that age does not moderate CBT treatment outcomes in carefully controlled efficacy studies.
The lack of evidence for an age effect is encouraging in that outcomes for children and youth treated by therapists in efficacy RCTs appear unlikely to be affected by possible developmental mismatches associated with the content of CBT protocols. However, the absence of an interaction effect may be because therapists who participate in efficacy studies have the skills needed to adapt available treatment manuals to the needs of adolescents, and accordingly ensure the efficacy of the CBT experience. In fact, 10 of 17 investigators who contributed data to our IPDMA confirmed that their study therapists routinely tailored CBT protocols to meet adolescent needs. Consequently, despite the absence of an age effect in our IPDMA of efficacy RCTs, critical questions remain regarding the influence of age on CBT outcomes in usual care. It is possible that clinicians working in these settings may be less skilled in adapting CBT protocols to adolescents, or be unaware of the need to do so. For example, the scant research available suggests that most children and youth with mental health problems are not seen by specialist providers.[38] Moreover, it is known that the quality of usual child and adolescent mental health care is uneven and that the benefits observed in efficacy research may not be realized in routine, community care settings due to a range of barriers to transportability.[25, 39, 40] In fact, some evidence is beginning to emerge that treatment outcomes may be better in university compared to community settings, but few studies are currently available.[41]
STRENGTHS AND LIMITATIONS
The strengths of our study include the following. First, the sample size required to test interaction effects in individual trials is approximately four times greater than that needed to assess main effects,[21] and it is often difficult in grant requests to provide a compelling scientific rationale to justify the associated increased costs. IPDMA methods provide a much-needed alternative that enables the achievement of greater power by combining individual patient data from individual RCTs.
Second, IPDMA is the gold standard approach to metaanalysis and we were able to achieve the high level of collaboration needed for success. The high response rate from eligible investigators, with 17 of 23 (74%) requested datasets obtained, reflects the high enthusiasm in the field for projects like this. In fact, four more datasets would have been contributed, had they not been lost. Third, the high response rate also limits the potential for bias in our findings. When we examined the published results of the unobtained datasets, we found no compelling evidence that they might have changed our results. Similarly, among the eligible studies obtained we were able to include 78% of the individual cases in our primary analysis. The availability of the ADIS in all studies was a fourth strength, allowing us to avoid the methodologic complications associated with creating a common outcome measure derived from different measures of the same construct. Sensitivity analyses conducted using other measures of anxiety outcomes (RC-MAS, CBCL, and presence/absence of anxiety disorder) revealed that our results were robust to the choice of anxiety outcome. Fifth, when we repeated our analyses using age as a continuous variable, the results did not change. Sixth, we included key covariates in the models tested including baseline anxiety severity and the presence of depression. Finally, we used random effects models that provide a conservative approach to the analysis.
At the same time, our study is not without limitations. It was unfortunate that we could not include key socio-demographic variables (e.g. SES and ethnicity) in the models we tested due to lack of data in original studies. Investigators are encouraged to increase the attention paid to including them in future studies. Second, we did not look at age effects for specific diagnoses because the potential to do so was limited in the available studies, and the careful analysis required was beyond the scope of the present report. Although there is some variation in the prevalence of specific disorders at different ages, it is possible that the high comorbidity among anxiety disorders in children and the frequent inclusion of multiple disorders in the same treatment protocols could obscure age-related diagnostic effects. Third, we could be criticized for not conducting multiple exploratory subgroup analyses to consider the full range of possible CBT moderators. In contrast, supported with peer-reviewed funding,1 our objective was to address a single, theory driven research question established a priori, and to obtain a methodologically rigorous result of direct relevance to the quality of clinical care and knowledge translation priorities (i.e. barriers to effective, evidence-based practice). Numerous authors have pointed out the pitfalls of subgroup analysis, including the risk of false negatives (due to inadequate power) and false positives (due to multiple significance testing).[21, 42] To have considered multiple possible moderators is at best exploratory and at worst simply data dredging. Fourth, we could not include the CAMS trial because two different CBT protocols were used—one for children and one for adolescents—and therefore CAMS does not provide a test of age effects. Fifth, our analysis focused on end-of-treatment outcomes only. Age effects at posttreatment follow-up were not investigated. Sixth, we did not consider the effect of family/parent involvement because all trials except one included some type of family/parent component in their CBT protocol. It is worthy of note that three of the 17 included studies identified the use of family CBT (study 2, 3, and 8). However, since the age ranges in these studies (i.e. 6–14, 8–17, and 7–14) are not different from the age ranges in the remaining studies, it is unlikely that their inclusion had an impact on our results. A final point concerns the influence of parental anxiety and accommodation on our results. Both of these factors may vary by age (e.g. interfering with exposure in younger children; limiting age-appropriate autonomy in adolescents) and are therefore worthy of therapeutic attention at all ages. It is unclear whether or not their influence would interact with age in predicting outcome.
CONCLUSIONS
Our IPDMA results are encouraging and show that when adolescents receive CBT in efficacy research studies they benefit at a level similar to younger children. However, CBT protocol modifications routinely carried out by therapists who participate in RCTs may explain these findings. The development and evaluation of protocols tailored to the unique needs of adolescents could facilitate the transportability of effective CBT to adolescents seen in usual care settings, where therapists may have less opportunity for CBT training and development of the needed expertise. First, protocols need to consider adolescent increased cognitive sophistication, skepticism of adults’ ideas, desire for autonomy, and focus on interpersonal (especially peer) issues relative to younger children.[43] Second, they need to recognize that complex presentations are common in usual care settings (in contrast to efficacy studies), are characterized by increased anxiety severity and comorbidity, and necessitate the provision of CBT in the context of a larger treatment plan that may include other interventions.[40] Finally, therapist training and supervision is generally less in routine compared to academic settings, and therapists in usual care may face organizational and financial constraints not found in academic centers.[44, 45] All of these factors have the potential to significantly affect the transportability of currently available CBT protocols and hence, the potential for adolescents with anxiety to benefit.
Anxiety disorders are a significant mental health problem among children and youth. They are of particular concern for adolescents who may be struggling with the effects of prolonged untreated disorder and may result in the cooccurrence of depression and substance abuse. A better understanding of the factors that are most likely to influence the transportability of CBT to usual care settings holds great promise for better anxiety outcomes and improved quality of life in adolescence and adulthood. We hope the IPDMA reported here stimulates the field to move forward in this direction, and to engage in knowledge translation research that aims to ensure the benefits of CBT are reaped broadly by children, youth, and families.
Acknowledgments
The Canadian Institutes of Health Research provided funding. Dr. Walter and Ms. Diaz-Granados provided statistical expertise. Maureen Rice, our research librarian, designed, and conducted the electronic database searches. We thank the following individuals for their contributions: Alexa Bagnell, Dalhousie University; Jayne Barker, Mental Health Commission of Canada; Deborah Beidel, University of Central Florida; Jane Garland, University of British Columbia; and Chandra Magill, McGill University.
APPENDIX
Table 3.
Individual study characteristics—unobtained studies*
| Study | Groups (n) | Age (years) | ≥12 years (%) | Female (%) | Anxiety disorders included** |
Comorbid depression |
Provider qualifications |
Tx setting
|
|
|---|---|---|---|---|---|---|---|---|---|
| School | Clinic | ||||||||
| Barrett | Group CBT (23) Group CBT + FAM (17) Waitlist (20) |
7–14 | N/A | 47% | SAD OAD SP |
2% depression (4% avoidant) | Registered clinical psychologists | √ | |
| Hayward et al. | Group CBT (12) No Tx (23) |
Adolescents (age range not reported) | N/A | 100% | SP | 0% current depression (although unknown # of participants had past history of depression) | Clinical psychologist or child psychiatrist (research assistant co-led) | √ | |
| King et al. | CBT (17) Waitlist (17) |
5–15 | N/A | 47% | SAD OAD AdjD SP SiP Anx NOS OCD Dysthymia Avoidant PD (see note 1) |
Not reported | Registered psychologists | √ | |
| Last et al. | CBT (32) Attention control (24) |
6–17 | N/A | ~67% | SiP SP SAD Avoidant PD OAD PD |
0% | Not reported | √ | |
| Muris et al. | Group CBT (10) ED (10) No Tx (10) |
9–12 | N/A | 67% | SAD GAD SP (see note 2) |
Not reported | Inexperienced clinical psychology student (supervised by experienced child psychologist) | √ | |
| Spence et al. | CBT (19) CBT + parent sessions (17) Waitlist (14) |
7–14 | N/A | 38% | SP | 8% dysthymia | Psychologists | √ | |
Note 1: School attendance problems were main reason for inclusion in the study; 74% had a 1· anxiety diagnosis.
Note 2: Anxiety disorder status not assessed in control participants.
Since individual patient data were not obtained for these studies, all data in this table are study level data as reported in the published papers.
AdjD, adjustment disorder; Anx NOS, anxiety not otherwise specified; Avoidant PD, avoidant personality disorder; GAD, generalized anxiety disorder; OAD, overanxious disorder; OCD, obsessive compulsive disorder; PD, personality disorder; SAD, separation anxiety disorder; SiP, simple phobia; SP, social phobia.
Figure 4.
Age x treatment group interaction for four developmental age groups (model 5).
ELIGIBLE PRIMARY STUDIES—DATA OBTAINED
- 1.Baer S, Garland EJ. Pilot study of community-based cognitive behavioral group therapy for adolescents with social phobia. J Am Acad Child Adolesc Psychiatry. 2005;44:258–264. doi: 10.1097/00004583-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Barrett PM, Dadds MR, Rapee RM. Family treatment of childhood anxiety: a controlled trial. J Consult Clin Psychol. 1996;64:333–342. doi: 10.1037//0022-006x.64.2.333. [DOI] [PubMed] [Google Scholar]
- 3.Bodden DHM, Bogels SM, Nauta MH, et al. Child ¨ versus family cognitive-behavioral therapy in clinically anxious youth: an efficacy and partial effectiveness study. J Am Acad Child Adolesc Psychiatry. 2008;47:1384–1394. doi: 10.1097/CHI.0b013e318189148e. [DOI] [PubMed] [Google Scholar]
- 4.Dadds MR, Spence SH, Holland DE, et al. Prevention and early intervention for anxiety disorders: a controlled trial. J Consult Clin Psychol. 1997;65:627–635. doi: 10.1037//0022-006x.65.4.627. [DOI] [PubMed] [Google Scholar]
- 5.Flannery-Schroeder EC, Kendall PC. Group and individual cognitive-behavioral treatments for youth with anxiety disorders: a randomized clinical trial. Cognitive Ther Res. 2000;24:251–278. [Google Scholar]
- 6.Ginsburg GS, Drake KL. School-based treatment for anxious African-American adolescents: a controlled pilot study. J Am Acad Child Adolesc Psychiatry. 2002;41:768–775. doi: 10.1097/00004583-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hudson JL, Rapee RM, Deveney C, et al. Cognitive-behavioral treatment versus an active control for children and adolescents with anxiety disorders: a randomized trial. J Am Acad Child Adolesc Psychiatry. 2009;48:533–544. doi: 10.1097/CHI.0b013e31819c2401. [DOI] [PubMed] [Google Scholar]
- 8.Kendall PC, Hudson JL, Gosch E, et al. Cognitive-behavioral therapy for anxiety disordered youth: a randomized clinical trial evaluating child and family modalities. J Consult Clin Psychol. 2008;76:282–297. doi: 10.1037/0022-006X.76.2.282. [DOI] [PubMed] [Google Scholar]
- 9.Kendall PC. Treating anxiety disorders in children: results of a randomized clinical trial. J Consult Clin Psychol. 1994;62:100–110. doi: 10.1037//0022-006x.62.1.100. [DOI] [PubMed] [Google Scholar]
- 10.Kendall PC, Flannery-Schroeder E, Panichelli-Mindel SM, et al. Therapy for youths with anxiety disorders: a second randomized clinical trial. J Consult Clin Psychol. 1997;65:366–380. doi: 10.1037//0022-006x.65.3.366. [DOI] [PubMed] [Google Scholar]
- 11.Masia-Warner C, Klein RG, Dent HC, et al. School-based intervention for adolescents with social anxiety disorder: results of a controlled study. J Abnorm Child Psychol. 2005;33:707–722. doi: 10.1007/s10802-005-7649-z. [DOI] [PubMed] [Google Scholar]
- 12.Masia Warner C, Fisher PH, Shrout PE, et al. Treating adolescents with social anxiety disorder in school: an attention control trial. J Child Psychol Psychiatry. 2007;48:676–686. doi: 10.1111/j.1469-7610.2007.01737.x. [DOI] [PubMed] [Google Scholar]
- 13.Nauta MH, Scholing A, Emmelkamp PMG, Minderaa RB. Cognitive-behavioral therapy for children with anxiety disorders in a clinical setting: no additional effect of a cognitive parent training. J Am Acad Child Adolesc Psychiatry. 2003;42:1270–1278. doi: 10.1097/01.chi.0000085752.71002.93. [DOI] [PubMed] [Google Scholar]
- 14.Rapee RM, Abbott MJ, Lyneham HJ. Bibliotherapy for children with anxiety disorders using written materials for parents: a randomized controlled trial. J Consult Clin Psychol. 2006;74:436–444. doi: 10.1037/0022-006X.74.3.436. [DOI] [PubMed] [Google Scholar]
- 15.Silverman WK, Kurtines WM, Ginsburg GS, et al. Treating anxiety disorders in children with group cognitive–behaviorial therapy: a randomized clinical trial. J Consult Clin Psychol. 1999a;67:995–1003. doi: 10.1037//0022-006x.67.6.995. [DOI] [PubMed] [Google Scholar]
- 16.Silverman WK, Kurtines WM, Ginsburg GS, et al. Contingency management, self-control, and education support in the treatment of childhood phobic disorders: a randomized clinical trial. J Consult Clin Psychol. 1999b;67:675–687. doi: 10.1037//0022-006x.67.5.675. [DOI] [PubMed] [Google Scholar]
- 17.Spence SH, Holmes JM, March S, Lipp OV. The feasibility and outcome of clinic plus internet delivery of cognitive-behavior therapy for childhood anxiety. J Consult Clin Psychol. 2006;74:614–621. doi: 10.1037/0022-006X.74.3.614. [DOI] [PubMed] [Google Scholar]
Eligible primary studies—Data not obtained
- 1.Barrett PM. Evaluation of cognitive-behavioral group treatments for childhood anxiety disorders. J Clin Child Psychol. 1998;27:459–468. doi: 10.1207/s15374424jccp2704_10. [DOI] [PubMed] [Google Scholar]
- 2.Hayward C, Varady S, Albano AM, et al. Cognitive-behavioral group therapy for social phobia in female adolescents: results of a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39:721–726. doi: 10.1097/00004583-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 3.King NJ, Tonge BJ, Heyne D, et al. Cognitive-behavioral treatment of school-refusing children: a controlled evaluation. J Am Acad Child Adolesc Psychiatry. 1998;37:395–403. doi: 10.1097/00004583-199804000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Last CG, Hansen C, Franco N. Cognitive-behavioral treatment of school phobia. J Am Acad Child Adolesc Psychiatry. 1998;37:404–411. doi: 10.1097/00004583-199804000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Muris P, Meesters C, van Melick M. Treatment of childhood anxiety disorders: a preliminary comparison between cognitive-behavioral group therapy and a psychological placebo intervention. J Behav Ther Exp Psychiatry. 2002;33:143–158. doi: 10.1016/s0005-7916(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 6.Spence SH, Donovan C, Brechman-Toussaint M. The treatment of childhood social phobia: the effectiveness of a social skills training-based, cognitive-behavioural intervention, with and without parental involvement. J Child Psychol Psychiat. 2000;41:713–726. [PubMed] [Google Scholar]
Footnotes
Bennett K. Prevention and intervention in adolescent anxiety: a research synthesis. Canadian Institutes of Health Research, Research Synthesis Grant ($100,000). 2008.
Institution at which the work was performed: McMaster University.
Conflict of interest: Dr. Manassis reports book royalties from Rout-ledge Publishing. Dr. Kendall reports royalties from sales of Coping CAT and related treatment materials. Remaining authors have no relevant conflicts of interest.
References
- 1.Waddell C, Offord DR, Shepherd CA, et al. Child psychiatric epidemiology and Canadian public policy-making: the state of the science and the art of the possible. Can J Psychiatry. 2002;47:825–832. doi: 10.1177/070674370204700903. [DOI] [PubMed] [Google Scholar]
- 2.Costello EJ, Foley DL, Angold A. 10-year research update review: the epidemiology of child and adolescent psychiatric disorders: II. Developmental epidemiology. J Am Acad Child Adolesc Psychiatry. 2006;45:8–25. doi: 10.1097/01.chi.0000184929.41423.c0. [DOI] [PubMed] [Google Scholar]
- 3.Connolly SD, Bernstein GA the Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:267–283. doi: 10.1097/01.chi.0000246070.23695.06. [DOI] [PubMed] [Google Scholar]
- 4.The Research Units on Paediatric Psychopharmacology Anxiety Group. Fluvoxamine treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344:1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- 5.Manassis K, Avery D, Butalia S, Mendlowitz S. Cognitive-behavioral therapy with childhood anxiety disorders: functioning in adolescence. Depress Anxiety. 2004;19:209–216. doi: 10.1002/da.10133. [DOI] [PubMed] [Google Scholar]
- 6.Kim-Cohen J, Caspi A, Moffitt TE, et al. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national co-morbidity survey replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 8.Woodward LJ, Fergusson DM. Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40:1086–1093. doi: 10.1097/00004583-200109000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Last CG, Hansen C, Franco N. Anxious children in adulthood: a prospective study of adjustment. J Am Acad Child Adolesc Psychiatry. 1997;36:645–652. doi: 10.1097/00004583-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Cartwright-Hatton S, Roberts C, Chitsabesan P, et al. Systematic review of the efficacy of cognitive behaviour therapies for childhood and adolescent anxiety disorders. Br J Clin Psychol. 2004;43:421–436. doi: 10.1348/0144665042388928. [DOI] [PubMed] [Google Scholar]
- 11.Compton SN, March JS, Brent D, et al. Cognitive-behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry. 2004;43:930–959. doi: 10.1097/01.chi.0000127589.57468.bf. [DOI] [PubMed] [Google Scholar]
- 12.Hudson JL. Efficacy of cognitive-behavioural therapy for children and adolescents with anxiety disorders. Behav Change. 2005;22:55–70. [Google Scholar]
- 13.In-Albon T, Schneider S. Psychotherapy of childhood anxiety disorders: a meta-analysis. Psychother Psychosom. 2007;76:15–24. doi: 10.1159/000096361. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa S, Okajima I, Matsuoka H, Sakano Y. Cognitive behavioural therapy for anxiety disorders in children and adolescents: a meta-analysis. Child Adolesc Ment Health. 2007;12:164–172. doi: 10.1111/j.1475-3588.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 15.James A, Soler A, Weatherall R. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev. 2005;4:1–35. doi: 10.1002/14651858.CD004690.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Waddell C, Godderis R, Hua J, et al. Preventing and Treating Anxiety Disorders in Children and Youth. Vancouver, BC: University of British Columbia; 2004. [Google Scholar]
- 17.Silverman WK, Pina AA, Viswesvaran C. Evidence-based psychosocial treatments for phobic and anxiety disorders in children and adolescents. J Clin Child Adolesc Psychol. 2008;37:105–130. doi: 10.1080/15374410701817907. [DOI] [PubMed] [Google Scholar]
- 18.Compton S, Walkup J, Albano AM, et al. Child/adolescent anxiety multimodal study (CAMS): rationale, design, and methods. Child Adolesc Psychiatry Ment Health. 2010;4:1. doi: 10.1186/1753-2000-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kendall PC, Choudhury M, Hudson JL, Webb A. The C.A.T. Project Workbook. Ardmore, PA: Workbook Publishing; 2002. [Google Scholar]
- 20.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. 2010;340:850–854. doi: 10.1136/bmj.c117. [DOI] [PubMed] [Google Scholar]
- 21.Brookes ST, Whitely E, Egger M, et al. Subgroup analyses in randomized trials: risks of subgroup-specific analyses. J Clin Epidemiol. 2004;57:229–236. doi: 10.1016/j.jclinepi.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Sutton AJ, Higgins JPT. Recent developments in meta-analysis. Stat Med. 2008;27(5):625–650. doi: 10.1002/sim.2934. [DOI] [PubMed] [Google Scholar]
- 23.Sud S, Douketis J. The devil is in the details . . or not? A primer on individual patient data meta-analysis. Evid Based Med. 2009;14:100–101. doi: 10.1136/ebm.14.4.100. [DOI] [PubMed] [Google Scholar]
- 24.Simmonds MC, Higgins JP, Stewart LA, et al. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials. 2005;2:209–217. doi: 10.1191/1740774505cn087oa. [DOI] [PubMed] [Google Scholar]
- 25.Schoenwald SK, Hoagwood K. Effectiveness, transportability, and dissemination of interventions: what matters when? Psychiatr Serv. 2001;52:1190–1197. doi: 10.1176/appi.ps.52.9.1190. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Green S. [Accessed 02/06, 2011];Cochrane handbook for systematic reviews of interventions version 5.1.0. Available at: www.cochrane-handbook.org. Updated 2011.
- 27.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Silverman WK, Nelles WB. The anxiety disorders interview schedule for children. J Am Acad Child Adolesc Psychiatry. 1988;27:772–778. doi: 10.1097/00004583-198811000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Kendall PC, Flannery-Schroeder E, Panichelli-Mindel SM, et al. Therapy for youths with anxiety disorders: a second randomized clinical trial. J Consult Clin Psychol. 1997;65:366–380. doi: 10.1037//0022-006x.65.3.366. [DOI] [PubMed] [Google Scholar]
- 30.Silverman WK, Kurtines WM, Ginsburg GS, et al. Treating anxiety disorders in children with group cognitive-behaviorial therapy: a randomized clinical trial. J Consult Clin Psychol. 1999;67:995–1003. doi: 10.1037//0022-006x.67.6.995. [DOI] [PubMed] [Google Scholar]
- 31.Ollendick TH, Jarrett MA, Grills-Taquechel AE, et al. Comorbidity as a predictor and moderator of treatment outcome in youth with anxiety, affective, attention deficit/hyperactivity disorder, and oppositional/conduct disorders. Clin Psychol Rev. 2008;28:1447–1471. doi: 10.1016/j.cpr.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Seligman LD, Ollendick TH, Langley AK, Baldacci HB. The utility of measures of child and adolescent anxiety: a meta-analytic review of the revised children’s manifest anxiety scale, the state-trait anxiety inventory for children, and the child behavior checklist. J Clin Child Adolesc Psychol. 2004;33:557–565. doi: 10.1207/s15374424jccp3303_13. [DOI] [PubMed] [Google Scholar]
- 33.Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 34.Schriger DL, Altman DG, Vetter JA, et al. Forest plots in reports of systematic reviews: a cross-sectional study reviewing current practice. Int J Epidemiol. 2010;39:421–429. doi: 10.1093/ije/dyp370. [DOI] [PubMed] [Google Scholar]
- 35.Boyle MH, Douglas Willms J. Multilevel modelling of hierarchical data in developmental studies. J Child Psychol Psychiat. 2001;42:141–162. [PubMed] [Google Scholar]
- 36.Rice K. Bayesian methods for model comparison. In: Armitage P, Colton T, editors. Encyclopedia of Biostatistics. 2. UK: Wiley; 2005. pp. 334–338. [Google Scholar]
- 37.SAS Institute Inc. SAS version 9.2. 2008. [Google Scholar]
- 38.Ford T. Practitioner review: how can epidemiology help us plan and deliver effective child and adolescent mental health services. J Child Psychol Psychiatry. 2008;49:900–914. doi: 10.1111/j.1469-7610.2008.01927.x. [DOI] [PubMed] [Google Scholar]
- 39.Sexton T, Kelley S. Finding the common core: evidence-based practices, clinically relevant evidence, and core mechanisms of change. Adm Policy Ment Health. 2010;37:81–88. doi: 10.1007/s10488-010-0277-0. [DOI] [PubMed] [Google Scholar]
- 40.Kazdin AE. Evidence-based treatment and practice: new opportunities to bridge clinical research and practice, enhance the knowledge base, and improve patient care. Am Psychol. 2008;63:146–159. doi: 10.1037/0003-066X.63.3.146. [DOI] [PubMed] [Google Scholar]
- 41.Neil AL, Christensen H. Efficacy and effectiveness of school-based prevention and early intervention programs for anxiety. Clin Psychol Rev. 2009;29:208–215. doi: 10.1016/j.cpr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Groenwold RHH, Donders ART, van der Heijden GJMG, et al. Confounding of subgroup analyses in randomized data. Arch Intern Med. 2009;169:1532–1534. doi: 10.1001/archinternmed.2009.250. [DOI] [PubMed] [Google Scholar]
- 43.Scapillato D, Manassis K, Jellinek MS. Cognitive-behavioral/interpersonal group treatment for anxious adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41:739–741. doi: 10.1097/00004583-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 44.La Greca AM, Silverman WK, Lochman JE. Moving beyond efficacy and effectiveness in child and adolescent intervention research. J Consult Clin Psychol. 2009;77:373–382. doi: 10.1037/a0015954. [DOI] [PubMed] [Google Scholar]
- 45.Manassis K. Cognitive Behavioral Therapy with Children: A Guide for the Community Practitioner. New York, NY: Routledge; 2009. [Google Scholar]