Abstract
Background
Subsets of human blood monocytes can be defined by CD14 and CD16 expression but otherwise are often assumed to be relatively homogeneous. However, we observed unexpected heterogeneity in other properties of monocytes from healthy adults without clinical immunological abnormalities.
Methods
Blood samples from 200 healthy adults were examined by flow cytometry (FCM) with antibodies to monocyte-associated molecules including CD4, CD14, CD16, CD33, CD38, CD80, CD86, CD91, CD163, CD244, CD274, TIA-1, Toll-like receptor 1 (TLR1), TLR2, TLR4, HLA-DQ and HLA-DR.
Results
The monocyte subsets defined by CD14 and CD16 and the levels of CD16 expression differed among subjects. Unexpectedly, expression of the monocyte lineage marker CD33 also varied among subjects as did that of CD38. TLR2 expression was variable but we were unable to detect B7-H1 (PD-L1 or CD274), TLR1 and TLR4 whereas CD80 was barely detectable. HLA-DR and HLA-DQ expression varied to a similar extent among individuals but there was substantially greater heterogeneity of HLA-DR within individuals. CD163 and CD86 varied among subjects with a modest reciprocal relationship. In contrast to this overall pattern of variability, the expression of CD4, CD244 and TIA-1 were more consistently expressed.
Conclusions
Contrary to many assumptions, human blood monocytes are heterogeneous within and among individuals. The pattern of HLA-DR expression within an individual may be related to the timing of interferon gamma elevations. Finally, expression of CD86 and CD163 may indicate whether circulating monocytes are tending toward M1 or M2 polarization.
Keywords: Monocytes, CD14, CD16, CD33, CD91, CD163, HLA-DR, HLA-DQ, heterogeneity
Controlled Key Words: Cytometry, Flow Cytometry, fluorescence cytometry, immunology, Immunophenotyping, PBMC, peripheral blood
Introduction
Blood monocytes can enter tissues and differentiate into immunologically relevant macrophages (1) and dendritic cells (2) and can also participate in the formation of atherosclerotic lesions (3). More than 20 years ago, it was established that elevated numbers of CD14Lo/NegCD16+ “non-classical” (4) monocytes can be of prognostic importance, e.g., (5). More recently, it has been clinically recognized that elevated expression of CD64 on myeloid cells is consistent with activation resulting from an infection ((6); BD Test #340768) (7). In acute myeloid leukemia (AML), characterization of tumor monocytes is of therapeutic significance. In the context of HIV/AIDS, monocytes are of interest since they express CD4, can be infected with HIV and can serve as a reservoir of virus, e.g., (8, 9, 10, 11). Aside from these situations, there appears to be a tacit (mis)understanding that monocytes are relatively homogeneous and that there is little to be learned from detailed studies of their phenotypic properties. In this study, we have used flow cytometry (FCM) to examine the phenotypic variability of monocytes from 200 healthy subjects without clinical immunological abnormalities. The expression of CD33, CD14, CD16, HLA-DQ, HLA-DR, CD86, Toll-like receptor 2 (TLR2), CD38 and CD163 on monocytes varied substantially among individuals. In contrast, CD4, CD244 and TIA-1 varied relatively little among individuals. Our results suggest that detailed characterization of monocytes in health and disease could provide useful information concerning immune states and predisposition to diseases.
Materials and Methods
The methods used in these studies were described in detail in “Properties of human blood monocytes I. CD91 expression and log orthogonal light scatter provide a robust method to identify monocytes that is more accurate than CD14 expression” (Hudig et al., submitted). The procedures are briefly summarized below with additional details included in the supplement.
Blood Collection and Processing
Venous blood samples were collected from 200 subjects (142 female, 58 male) who provided informed consent in accordance with University of Nevada Institutional Review Board Protocol Approval #B02/03-34.
Antibodies & Labeling
Antibodies (listed in supplement table ST1) were combined into mixtures as indicated in the legends to the figures and tables. Antibody quantities were used as recommended by the suppliers. After adding the antibodies to aliquots of whole blood, the samples were treated with FACSLysing solution, washed and resuspended for FCM. In some cases, combined surface and intracellular labeling was performed using IntraPrep reagents (#IM2389, Immunotech, a subsidiary of Beckman-Coulter, Fullerton, CA).
Instrumentation & Flow Cytometry
FCM examinations were performed with a Coulter Epics XL/MCL cytometer with optical filters modified to measure fluorescein, PE, PC5 and PC7 as depicted in the supplement. Sufficient total cells were examined such that the data files typically included 3,000 to 5,000 monocytes.
Analyses of Data
Data files were analyzed with the Mac versions of FlowJo (TreeStar, Ashland, OR). Fluorescence intensity values are reported as the “median fluorescence intensity” (MFI) as determined in FlowJo. In some cases, such as the expression of CD163 on monocytes, there was no clear distinction between “positive” and “negative” cells. In order to estimate the “per cent positive” cells, we used the population comparison in FlowJo that is based on the Overton subtraction method (12) as further modified by Bagwell (13). In those cases, we also report the MFI value for the total population, e.g., the expression of CD163 and that of HLA-DR. Tabular data were transferred to Excel where the built-in statistical functions were used to determine means and other values such as correlations.
Results
Variable CD16 expression
Monocytes can be divided into subsets based on their expression of CD14 and CD16 (4) and as illustrated in Figure 3 of the previous paper in this series (“Properties of human blood monocytes I. CD91 expression and log orthogonal light scatter provide a robust method to identify monocytes that is more accurate than CD14 expression”, Hudig et al., submitted). The “non-classical” monocytes are CD14Lo/NegCD16+ whereas the “classical” monocytes are CD14Br+ but CD16Neg and a third “intermediate” population is CD14Br+CD16+. The non-classical monocytes can be readily distinguished by CD14Lo/Neg expression but the intermediate subset cannot be reliably defined without a CD16 “fluorescence minus one (FMO)” control. Therefore, we divided the monocytes into two subsets, i.e., the CD14Br+ cells (including both classical and intermediate cells) and the CD14Lo/Neg (non-classical) cells. Seventy-nine subjects were examined with PE-anti-CD14 and PC7-anti-CD16. Among those subjects, 11.8±3.58% (range 4.71% – 20.8%) of the monocytes were non-classical (CD14Lo/Neg) with widely variable CD16 expression (MFI = 3.84±4.50, range 0.10 – 22.70). The CD14Br+ population had much lower CD16 expression (MFI = 0.24±0.15, range 0.10 – 0.85) with the variability reflecting varying proportions of classical and intermediate monocytes. Thus, not only did the fraction of non-classical monocytes vary among subjects, but the amount of CD16 expressed on these cells was also heterogeneous.
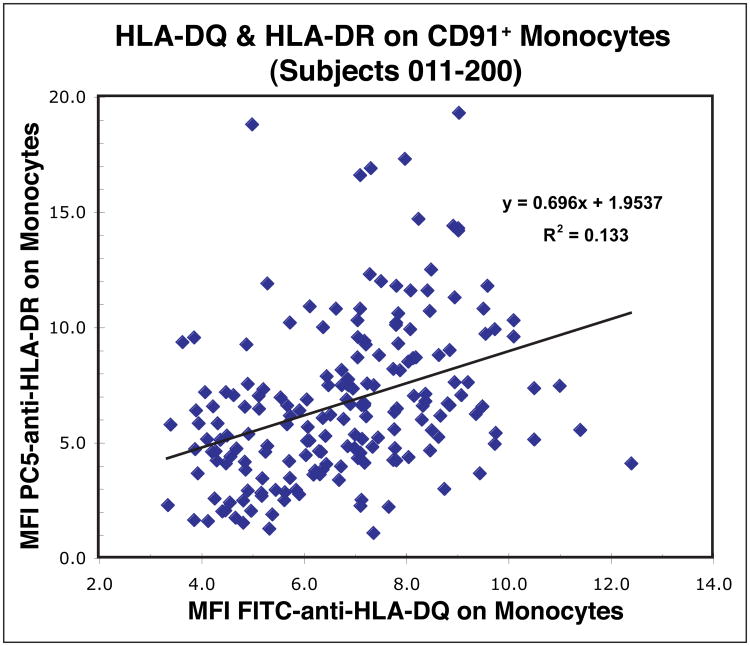
Figure 3. The expression levels of HLA-DQ and HLA-DR vary among individuals.
Aliquots of blood from 190 subjects (#011-#200) were labeled and the monocytes identified as illustrated in Figure 2. The MFI values for FITC-anti-HLA-DQ and PC5-anti-HLA-DR on monocytes were determined and plotted as shown. There was a weak association between the levels of expression of HLA-DQ and HLA-DR on monocytes.
Variable CD33 expression
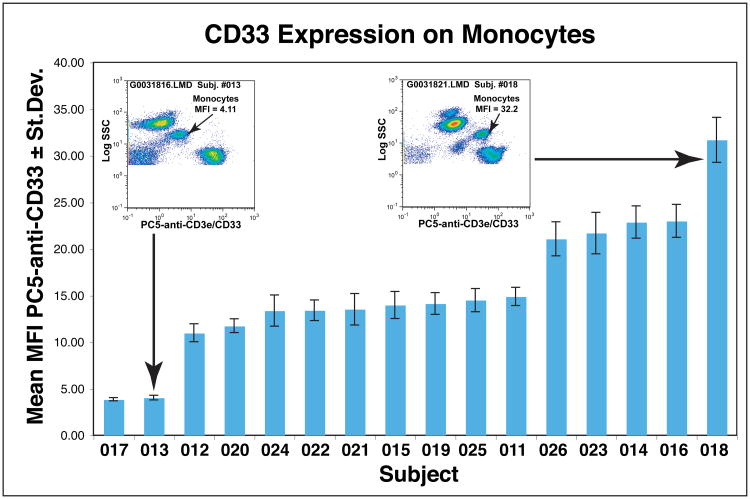
In these studies, we examined multiple aliquots of blood from each subject with different combinations of antibodies in order to measure the properties of several populations of cells, e.g., monocytes, B cells, T cells, etc. Where necessary, we used antibody to CD91 and/or to CD33 to identify the monocytes. For example, blood samples from one group of 16 subjects were tested with four combinations of antibodies that each included PC5-anti-CD33 to identify monocytes. As we reported in the previous paper, tests of single samples labeled simultaneously with antibodies to CD91 and to CD33 showed much more variability among subjects in CD33 expression. In order to verify that the apparent variability among subjects was not simply sample to sample to variation, we measured CD33 expression among multiple samples from each individual in the group that contained four combinations that each included anti-CD33. The mean±Stdev PC5-anti-CD33 MFI values for each subject are depicted in Figure 1. These data illustrate that the variation in CD33 MFI among samples from each subject was much smaller than the variation among subjects. In this group, there were two subjects with low CD33 and one with high CD33 expression as depicted in the inset CD33 vs. SSC bivariate plots. The other 13 subjects had levels of CD33 expression that fell into two intermediate groups. Comparable results were obtained with another group of subjects that was similarly examined with multiple combinations of antibodies that all contained PC5-anti-CD33. Altogether, all 200 subjects were tested with either clone WM53 or clone D3HL60.251 PC5-anti-CD33 with similar results. Among the 84 subjects tested with clone WM53, the mean MFI was 17.4±7.55 (range 3.42 – 34.0) whereas among the 116 subjects tested with D3HL60.251 the mean MFI was 17.3±7.82 (range 2.28–39.0). The results for all the subjects are depicted in Supplemental Figure S2. There was no association between the expression of CD33 and the age (Figure S2) or gender of the subjects (not shown). The possible explanations for this variability and the potential implications will be discussed.
Figure 1. CD33 is expressed at variable levels on blood monocytes.
Four different aliquots of single blood samples from subjects 011-026 were each labeled with 1 of 4 different antibody combinations that included PC5-anti-CD33. For each individual, the means of the four PC5-anti-CD33 MFI values ± standard deviations were determined. These mean MFI values are plotted in the order of low to high expression of CD33. The inset plots depict samples labeled with FITC-anti-CD80, PE-anti-TLR2, PC5-anti-CD3e/CD33 and PC7-anti-CD19 from the two subjects with highest and lowest levels of CD33 expression in this group.
Variable CD38 expression
The amount of CD38 expressed on CD8 T lymphocytes is prognostic for AIDS in patients infected with HIV, e.g., (14, 15). However, less is known about the significance of monocyte CD38 expression. Supplemental Figure Figure S3 illustrates the MFI values of CD38 expression on monocytes from 97 of our healthy subjects (mean MFI = 16.9±2.80, range 4.76 – 28.6). Although CD38 expression can be increased by IFNγ (16), we observed no association between CD38 expression and that of HLA-DR which is also elevated in response to IFNγ (not shown). CD38 expression In combination with SSC can be used to identify monocytes, but CD38 also occurs on granulocytes and some lymphocytes (not shown).
Variable expression of MHC class II proteins
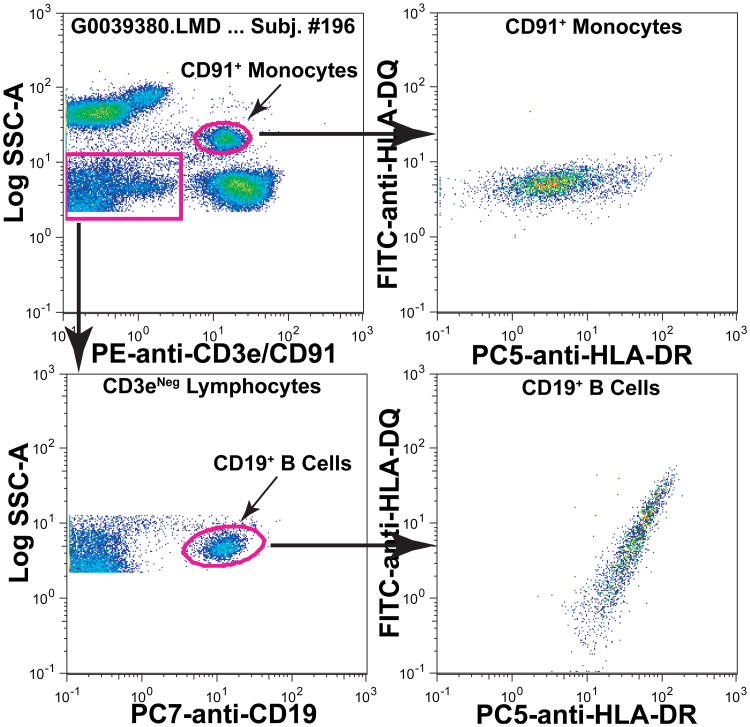
MHC Class II proteins, e.g., HLA-DQ and HLA-DR, are expressed on both B cells and monocytes. When we examined the expression of these MHC II proteins on B cells and monocytes within and among individuals we made four major observations. First, within individuals, HLA-DQ and HLA-DR appeared to be coordinately expressed on B cells whereas on monocytes HLA-DR expression varied independently of HLA-DQ and to a greater extent. These differences are illustrated for the B cells and monocytes of one typical individual in Figure 2. Second, among all the individuals, expression of both MHC II loci on monocytes varied to a similar extent (mean HLA-DQ MFI = 6.53±2.09, range 1.23–12.4; mean HLA-DR MFI = 6.55±3.40, range 1.15 – 19.3). Third, although the overall ranges of variation in expression of HLA-DQ and HLA-DR were similar, there was only a modest association among individuals as illustrated in Figure 3. Fourth, as illustrated for one subject in Figure 2, the variation in HLA-DR expression was substantially greater than that of HLA-DQ within an individual. The robust CV is one measure of dispersion in a distribution and that of HLA-DR (mean Robust CV = 157.5±33.7, range 84.3 – 306) was approximately four fold greater than that for HLA-DQ (mean Robust CV = 40.5±14.7, range 24.8 – 111). These differences are graphically illustrated for one group of 17 subjects in Supplemental Figure S5. These univariate distributions illustrate that the HLA-DR expression was not only more variable within a subject, but the distributions tended to be asymmetrical and skewed. Taken together, these data suggest that the levels of expression of HLA-DQ and HLA-DR are under separate mechanisms of control in monocytes. Although an association with age has been reported (17), when we compared expression of HLA-DR or HLA-DQ with the ages of subjects (21 to 67 years with one 82 year old subject), we noted no association (not shown). We also noted no differences in HLA-DQ or HLA-DR expression related to gender (not shown). Although it has been known for more than 30 years that HLA-DR occurs on monocytes, e.g., (18), we believe there is additional new information that can be gained as will be discussed.
Figure 2. HLA-DQ and HLA-DR are expressed differently on blood monocytes and B cells.
An aliquot of blood from one subject (#196) was labeled with FITC-anti-HLA-DQ, PE-anti-CD3e/CD91, PC5-anti-HLA-DR and PC7-anti-CD19. Monocytes and B cells were identified as shown and the expression of HLA-DQ and HLA-DR are depicted. On monocytes, the expression of HLA-DR varied considerably more than that of HLA-DQ (upper right panel). In contrast, on B cells the expression of both MHC II products varied in a more coordinated manner (lower right panel) with variation in both HLA-DQ and HLA-DR expression per cell.
Expression of Toll-like receptors (TLR) TLR 1, TLR2 and TLR4
A total of 190 subjects were examined for TLR2 and TLR4 expression and 84 subjects for TLR1 expression on monocytes. Neither TLR1 nor TLR4 could be reliably detected (not shown). On the other hand, TLR2 was detectable at levels varying over a 4-5 fold range (mean MFI = 7.14±1.74, range 3.86 to 14.4). Cellular levels of TLR2 varied independently of CD86 expression (Supplemental Figure S6), of MHC II proteins, of age or of gender (not shown).
CD4, TIA-1 and CD244 Expression
We observed much less variability among individuals in expression of these molecules. One combination of antibodies (FITC-anti-CD3e, PE-anti-CD4, PC5-anti-CD8a plus PC7-anti-CD2) was examined with samples from 103 subjects. In those samples, the monocytes and CD4+ T (CD3e+) lymphocytes were identified and the MFI values of PE-anti-CD4 expression were determined for both monocytes and T lymphocytes. The mean MFI value for T lymphocytes was 46.9±5.06 and that for monocytes was 6.86±1.11. Thus, there was a similar range of variation for CD4 expression on the two cell types (± ∼11-14%) with no significant correlation of CD4 expression on these two cell types within individuals (not shown). TIA-1 (also known as GMP-17, (19)) is an intracellular product that is known to occur in blood monocytes (20). We examined blood samples from 38 subjects that were surface labeled with PC5-anti-CD33, PC5-anti-CD3e, and PC7-anti-CD8a and labeled intracellularly with FITC-anti-PRF1 and PE-anti-TIA-1. Supplemental Figure S7 illustrates typical data from two of these subjects showing that TIA-1 occurred in monocytes (Mean MFI = 9.79±2.41; Range 6.79 to18.1) and was most highly expressed in neutrophils (Mean MFI = 109±36.8; Range 57.0 to 206). (In Supplemental Figure S7 it appears that TIA-1 is present in eosinophils but it is autofluorescence.) We noted that CD244 occurred on monocytes and eosinophils as had been previously observed (21, 22, 23, 24). In total, we examined CD244 expression in blood samples from 127 subjects and all of them had similar amounts of CD244 on their monocytes (Mean MFI = 4.85±0.73; Range 2.75 to 7.06).
Monocyte Polarization: B7 molecules (CD80 & CD86) and the scavenger receptor CD163
On fully differentiated macrophages, the co-stimulatory B7 molecules CD80 and CD86 are elevated on pro-inflammatory or M1 cells (25) while the scavenger receptor CD163 is elevated on alternatively activated M2 cells (26). We examined the expression of B7 molecules and that of CD163, compared them with the expression of other monocyte molecules and then compared them with each other.
B7 Molecules
Monocytes from 200 subjects had variable levels of CD86 (B7.2, mean MFI 2.66 ± 0.71, range 0.98 – 6.68). Eighty-four subjects were characterized for both B7.1 & B7.2 (CD80 & CD86). In those subjects, the expression of CD80 was barely detectable (mean MFI = 0.87±0.24, range 0.56 – 1.63). Comparisons of the expression of these co-stimulatory molecules showed modest association (CD86 vs. CD80; slope = 0.22, R2 = 0.450; Supplemental Figure S6). In contrast, there was no detectable association between the expression of these B7 molecules and MHC Class II proteins (not shown). Another member of the B7 family, namely B7-H1 (PD-L1 or CD274), could not be reliably detected on the monocytes of any of 74 subjects examined (not shown).
CD163
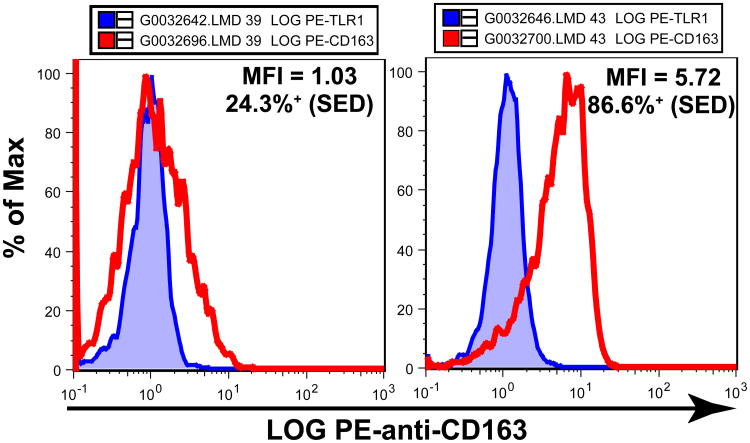
CD163 is a scavenger receptor for hemoglobin-haptoglobin complexes (27, 28) that is selectively expressed on “alternatively activated” or M2 macrophages and monocytes (Review, 29). We examined the expression of CD163 on monocytes from 74 subjects and found that it was highly variable. The monocytes from the tested subjects ranged from 24.3% to 95.1% CD163+ (mean = 76.0% SED) with PE-anti-CD163 MFI values from 1.03 to 5.72 (mean = 3.52). Figure 4 depicts the cells from the subject with the lowest frequency of CD163+ cells (Subject #039) and from the subject with the highest PE-anti-CD163 MFI value (Subject #043). These data illustrate that although expression of CD163 was variable, there were at least some monocytes from all the tested healthy subjects that expressed this scavenger receptor. There was no detectable relationship between the levels of expression of CD163 and CD33 on monocytes (not shown). We also examined possible relationships between CD163 and HLA-DR expression and found no associations (not shown). However, there was a modest association between the expression of HLA-DQ and that of CD163 on monocytes (HLA-DQ vs. CD163; slope = 0.358, R2 = 0.346; Supplemental Fig S8).
Figure 4. CD163 is variably expressed on blood monocytes.
Aliquots of blood from 74 subjects (#011–#084) were labeled with antibody mixtures containing PC5-anti-CD33 plus PE-anti-TLR1 or PE-anti-CD163 and the CD33+ monocytes were identified. CD163 did not occur on a clearly defined subset of monocytes so CD163 expression was determined in two ways. First, PE-anti-CD163 MFI values for the monocytes were determined and ranged from 1.03 to 5.72 with a mean of 3.52. Second, the “Population Comparison” platform of FlowJo was used to estimate the fraction of CD163+ monocytes. This method of analysis is based on the Overton subtraction technique with the Bagwell modification to produce “SED” values of the estimated per cent “positive” relative to the control sample. There was no detectable expression of TLR1 so the CD33+ monocytes from the aliquots labeled with PE-anti-TLR1 were used as “negative controls” since both anti-TLR1 and anti-CD163 were PE-labeled mouse IgG1 monoclonals. By this method, the monocytes from the tested subjects ranged from 24.3% to 95.1% CD163+ (mean = 76.0% SED CD163+). The two panels in the figure depict results from the subject with the lowest frequency of CD163+ cells (left, #039) and from the subject with the highest PE-anti-CD163 MFI value (right, #043). In both cases, the fluorescence intensity histogram of monocytes labeled with PE-anti-CD163 (red line) is superimposed on the comparable histogram of monocytes from the same subject labeled with PE-anti-TLR1 (blue shaded).
CD163 vs. CD86
Of greatest interest, there was a modest reciprocal association between the MFI values of CD163 and those of CD86 (Supplemental Figure S9). The expression levels of CD86 and CD163 were not examined on the same cells so we could not determine if this reciprocal relationship existed at the level of individual monocytes. However, overall, individuals with monocytes that had higher levels of CD86 tended to have monocytes with lower levels of CD163 and vice versa.
Discussion
The present studies demonstrate that there is considerable heterogeneity in the monocytes within and among healthy subjects who were neither acutely ill nor had known immunological abnormalities. For example, it has been known for more than two decades, e.g., (4, 30), that human monocytes could be divided into subsets based on expression of CD14 and CD16. We found that the proportions of those subsets varied among individuals and we also found that the amount of CD16 expressed on the “non-classical” CD14Lo/NegCD16+ cells was widely variable among subjects. It has already been established that elevated numbers of these non-classical monocytes can be of prognostic significance, e.g., (5, 31, 32, 33, 34). The possible significance of variable expression of CD16 on these non-classical monocytes is not currently known but would be worthy of further study.
The variability in CD33 expression among individuals (Figure 1 and Supplemental Figure S2) was unexpected and was of both practical and theoretical interest. At a practical level, the expression of CD33 is a known lineage marker for monocytes (35) but the variation in expression could complicate the use of anti-CD33 as a lineage identifier. Of potentially greater theoretical significance, variations in CD33 expression may alter the function of monocytes. CD33 is a sialic acid-binding Ig-like lectin (SIGLEC) with an extracellular V domain containing the lectin-like region that binds sialic acid groups, a second extracellular C domain not directly involved in binding sialic acid groups and an intracellular domain with two potential immunoreceptor tyrosine inhibitory motif (ITIM) sites (36). Thus, engagement of CD33 can deliver an inhibitory signal (37), particularly for activation mediated through the Fc-receptor (36). The variation we observed could indicate that individuals simply express different amounts of CD33 as controlled by unknown mechanism(s) with unknown effects on monocytes. Alternatively, different individuals could express different proportions of the CD33M (both V and C domains) and CD33m (C domain only) isoforms (38). The antibodies we used (WM53 and D3HL60.251) both recognize epitopes in the extracellular V domain which includes the lectin-like site and is absent on the smaller CD33m isoform (39). Finally, binding of sialo-proteins to the lectin-like V domain can partially block the binding of these antibodies so that different degrees of labeling could reflect variable ligand binding. In studies currently underway, we are comparing labeling with antibodies to V-domain epitopes such as those we used here and clone HIM3-4 which is an antibody to a C-domain epitope (39). The results will assist in resolving the basis for the variability in CD33 labeling we have observed.
CD38, a type II transmembrane protein with extracellular NAD'ase activity, e.g., (40, 41), could be involved in at least three functional activities on monocytes. First, CD31 (42) is one defined ligand of CD38 so it could participate in the adherence of CD38-bearing blood monocytes to vascular endothelial cells that express high levels of CD31. Second, CD38 may deliver activation signals to monocytes, e.g., (42, 43). Third, although the enzymatic activity of CD38 is extracellular, it can participate in regulating intracellular NAD levels in monocytes (44). The variations in CD38 expression we have observed on monocytes (Figure S4) could affect any or all these functional activities. At a practical level, variation in CD38 expression on monocytes could affect CD38 assays that utilize monocytes as a biological reference value for measuring CD38 on CD8 T cells (45). Using cells from a single donor could provide a stable reference value but the variability among subjects depicted in Figure S4 (mean MFI = 16.9±2.80, range 4.76 – 28.6) indicates that the donor to be used should be selected with care.
Although we were unable to detect TLR1 or TLR4, it is well established that LPS can stimulate human blood monocytes, e.g., (46), via TLR4 (47). We could readily detect TLR2 expression and found that it varied over more than a three-fold range. We did not test whether these variations affected function, but others have demonstrated that over-expression of TLR2 does increase responsiveness (48). Clinically, Kuwahata, et al. (49) demonstrated that patients with atherosclerosis had elevated levels of TLR2 on their monocytes. This group used beads with recombinant TLR2 as a reference material and had found in longitudinal studies that TLR2 elevations were associated with bacterial (50) and viral (51) diseases. More recently, another group (52) has found that cardiac allograft rejection is accompanied by elevated TLR2 on blood monocytes. Thus, it seems likely that variations in TLR2 expression could affect monocyte function and/or could reflect underlying disease processes.
It has been known for more than 30 years that products of MHC II loci, such as HLA-DR, are expressed on human blood monocytes, e.g., (18). We examined expression of both HLA-DQ and HLA-DR on monocytes and observed that both varied to a similar extent among individuals as reported above. However, there was only a modest association between HLA-DQ and HLA-DR expression among subjects (Figure 3). Within individuals, HLA-DR expression varied much more widely, i.e., about fourfold, than did HLA-DQ expression (Figure 2 and Supplemental Figure S5). The differences in variability within individuals and the modest association between the levels of expression of these products among individuals (Figure 3) are consistent with reports that HLA-DQ expression on monocytes can be controlled by mechanism(s) independent of Class II transactivator (CIITA) (53) that can regulate the products of both loci. In monocytes, CIITA is stimulated in response to IFN-γ (54, 55) and reviewed by (56) and has a short half-life (57). Human blood monocytes have also been reported to have a half-life of only 1-3 days in circulation (58). Thus, the wider range of variation of HLA-DR expression within an individual likely reflects mixtures of monocytes that have or have not been exposed to elevated IFN-γ. The shape of the distribution of HLA-DR expression could also reveal information about the length of time since the last exposure to elevated IFN-γ. In Supplemental Figure S5, one can see that in most individuals, there were relatively small numbers of cells with high amounts of HLA-DR. Acutely ill subjects were excluded from our studies so these patterns of expression would be consistent with some time having elapsed since the last exposure to increased amounts of IFN-γ.
In contrast to the overall patterns of heterogeneity we observed among subjects, the expression of CD4 and CD244 on the surface of monocytes and the expression of TIA-1 within monocytes varied much less. TIA-1 can sequester mRNA molecules that contain AU-rich elements as are found in many cytokine mRNA's (59, 60). The identities of mRNA's that may be bound to TIA-1 in monocytes remain to be determined. CD4 on monocytes is of clinical interest because it can allow HIV to infect monocytes, e.g., (8, 9, 61, 62). However, the real biological function of CD4 on monocytes remains to be determined. CD244 occurs on NK cells and on T cells that contain perforin. CD244 binds CD48 (63, 64) and this interaction on NK and T cells can enhance cytolytic activity by those cells e.g., (65, 66, 67, 68). It has been known for more than 10 years that CD244 also occurs on monocytes (21, 22, 23, 24), but its function on monocytes has not been determined. The expression of these molecules on monocytes appears to be very closely regulated suggesting that they may have significant functions which could be elucidated by additional basic studies.
It is now known that monocyte/macrophage type cells can differentiate through two pathways to become pro- or anti-inflammatory cells, e.g., (69); review by (29). The pro-inflammatory “classically activated” macrophages typically develop under Th1 conditions with IFN-γ and are also known as M1 macrophages. On the other hand, “alternatively activated” or anti-inflammatory macrophages are associated with Th2 conditions in which IL-4 is predominant and are also known as M2 macrophages (70, 71). When M1 macrophages are activated, e.g., via TLR stimulation, they produce nitric oxide via inducible nitric oxide synthetase (iNOS) and inflammatory cytokines such as TNF-α. On the other hand, activated M2 macrophages produce arginase and anti-inflammatory cytokines such as IL-10. M1 macrophages support the induction of immune responses whereas M2 macrophages are important in tissue differentiation since they efficiently phagocytose apoptotic cells via receptors for the phosphatidylserine (PS) that is present on the surface of apoptotic cells (72). M1 and M2 macrophages express characteristic patterns of gene products. M1 cells have elevated B7 molecules (25) whereas M2 macrophages express the mannose receptor (CD206) and/or the scavenger receptor (CD163) (29). We tested 74 subjects with antibodies to CD86 and CD163 and found that all those subjects had monocytes with CD163. The frequency of CD163+ monocytes was variable among subjects and ranged from ∼24-95%. with a mean of ∼76%. Thus, it appeared that substantial numbers of blood monocytes were on the pathway to becoming alternatively activated or M2 cells. We also noted a modest negative association between the levels of CD86 and CD163 (Supplemental Figure S9) as one might expect since these molecules tend to be elevated on M1 and M2 monocytes/macrophages, respectively. Expression of CD163 and CD86 on human blood monocytes has been examined previously, but to our knowledge this report is the first in which a cohort of healthy subjects has been examined in this way.
In summary, we have found considerable heterogeneity in the properties of blood monocytes from healthy subjects in addition to the long-known subsets defined by differential expression of CD14 and CD16. In order to identify these CD14/CD16 subsets, monocytes should be identified by SSC and a molecule expressed on all monocytes such as CD91 or CD33, bearing in mind that CD33 itself is variably expressed among individuals as we have shown. It is also helpful to display SSC on a log scale, especially if one intends to perform leukocyte differential counts on CD45+ lymphocytes, monocytes and granulocytes. In addition to defining the CD14/CD16 subsets, there may be further clinically relevant information derived from expression of TLR2, HLA-DR and a combination of CD86 and CD163 as described above. In examining many of these molecules on monocytes, it is not a matter of simply defining “positive” or “negative” cells but rather monitoring the relative levels of expression. Therefore, additional quality control steps may be required to make longitudinal comparisons as others have described for measuring TLR2 on monocytes (50) or CD38 on T cells (15, 45).
The following. a more detailed description of the procedures used in these studies; is excerpted from the previous paper in this series, namely, “Properties of human blood monocytes I. CD91 expression and log orthogonal light scatter provide a robust method to identify monocytes that is more accurate than CD14 expression” (Hudig et al.).
Materials and Methods
Blood Collection, Processing & Hematology Analyses
Subjects (142 female, 58 male) were selected by one of the authors (WJD) from his general medical practice. Dr. Diamond excluded children and adolescents under age 20, individuals over age 70 (with one elderly exception), pregnant women and patients with known immunological abnormalities such as HIV infection or autoimmune conditions including multiple sclerosis, rheumatoid arthritis, etc. Participants provided informed consent and the study was approved by the University of Nevada Institutional Review Board (Approval #B02/03-34).
Peripheral blood samples were collected from 12 groups of participants, typically consisting of 15-20 subjects, at the site of WJD's medical practice. Blood samples (EDTA Vacutainer Tube #367861 & #367863, BD BioSciences, San Jose, CA) were collected between 7 and 9 am and transported to the University of Nevada laboratories. Blood samples were mixed by inversion and aliquots of 50 μl were dispensed for labeling with antibodies. A portion of each blood sample was taken to the local LabCorp® clinical laboratory for differential leukocyte and CBC determinations. (At the time of these studies, the LabCorp® facility was using a Beckman-Coulter LH750 hematology analyzer.) Other portions of the blood samples were centrifuged and aliquots of plasma were collected and frozen (for cytokine and other analyses), after which the leukocyte-enriched buffy coats were collected and frozen (for genetic analyses). The processing of the blood samples, including labeling with antibodies, was typically completed shortly after noon on the day of collection.
Antibodies & Labeling
The antibodies used for this report are listed in supplement Table ST1. The volumes of antibodies used for labeling were based on the amounts recommended by the suppliers as scaled to the volumes of blood that were used. (Separate titrations had verified that the recommended volumes of antibodies were sufficient to produce maximal labeling.) Antibodies to different antigens were premixed and added to blood samples in a single pipetting step (except for antibodies to intracellular components, i.e., perforin, granzyme B and TIA-1). Aliquots of blood plus antibodies were mixed and incubated in the dark at room temperature for 30-45 min at which time 1ml of diluted FACSLysing Solution (#349202, BDBioSciences) was added. After the erythrocytes were lysed (∼10 min), 3 ml of PBS was added, the samples were centrifuged and the pellets resuspended in 1 ml of staining buffer (sheath solution as described below supplemented with 1% fetal bovine serum and 0.09% sodium azide, 0.22 μm filtered). Labeled samples were kept refrigerated until they were run on the cytometer. Separate tests showed that surface labeled samples produced comparable FCM results (cell frequency and MFI values) for as long as ∼72 hours after labeling, indicating that large groups of samples could be run without losses of fluorescence as reflected in the MFIs of each sample.
Samples to be examined for both surface and intracellular components were processed differently, according the procedures recommended for the IntraPrep kit (#IM2389, Immunotech, a subsidiary of Beckman-Coulter, Fullerton, CA). The antibodies to surface components were first added as described above. After the initial incubation, the labeled samples were treated with the fixation reagent from the IntraPrep kit, diluted with PBS, centrifuged and then rendered permeable with the IntraPrep perm reagent at which time the antibodies to the intracellular components were added. The most critical step in this procedure was vortexing to ensure that the pellets of fixed cells were thoroughly resuspended before adding the perm reagent and the antibody(ies) to intracellular components. After labeling intracellular component(s), these samples were washed by centrifugation and resuspended in PBS with 1% formalin. We found that the intracellular labels tended to “leak” upon overnight storage so these samples were run on the cytometer as soon as possible after labeling, typically within a few hours.
Flow Cytometry
All samples in these studies were run on a Beckman-Coulter Epics XL/MCL using the carousel with BioSure sheath solution (BioSure, Grass Valley, CA, #1023, 8× concentrate properly diluted with deionized water). The optical filters in the instrument were originally configured to detect fluorescein (FITC), phycoerythrin (PE), PE-Texas Red tandem (ECD) and PE-cyanine 5 tandem (PC5). In order to reduce the amounts of fluorescence spillover, we modified the configuration (see supplement figure S1) in order to detect FITC, PE, PC5 and PE-cyanine 7 tandem (PC7) labeled antibodies. PMT detector voltages were set to optimize the signal to background ratio and were kept constant throughout the study. Beads (9.2 μm pink fluorescent beads, Polymer Laboratories, a division of Varian, purchased by Agilent) were run each day to verify proper cytometer operation and the data files from these beads demonstrated that the instrument performed comparably throughout the study. The XL generates considerable heat and we found previously that elevations in room temperature could reduce the PMT outputs. Therefore, the room temperature was kept at 65-70°F during these studies.
The XL is a fully digital instrument that collects data with 20 bit resolution but stores data in files as 10 bit unsigned integers. Therefore, fluorescence compensation was performed during data collection in order to utilize the full 20 bit instrument resolution. In order to set the online compensation, antibody capture beads (made in house with biotin-anti-mouse Ig from Jackson Immunoresearch, West Grove, PA, bound to 6.0 μm streptavidin beads, Polysciences, Warrington, PA) were loaded with FITC, PE, PC5 or PC7 antibodies and run with the standard PMT voltages and without compensation. These files were processed using FlowJo software to determine a compensation matrix, the values of which were then loaded into the XL software (MS-DOS based System II). The unsigned integers stored in the data files could not record the negative values that can be produced from compensation. Therefore, cells with negative values accumulated in the zero channel and were included in analyses. The quality of some tandem fluorochromes, particularly those with PC7, varied and some had considerable residual PE signal. These residual PE signals were left without “compensation”. However, antibody combinations were designed so that residual PE signals from PC7 were less than any PE signals to be measured on cells that were labeled with PC7 antibodies.
Data files were collected with linear forward scatter (FSC) set with gain 2 and logarithmic amplification on orthogonal scatter (SSC) and on all fluorescence detectors. FSC signals on the XL can be attenuated by sliding a neutral density filter in place. The data in these studies were collected without that filter in place. Also, all signals were collected as pulse areas, i.e., the normal mode on the XL. The elliptical laser spot on the XL is ∼ 9μm high which is comparable to the size of monocytes. Therefore, collecting fluorescence signals as pulse areas instead of pulse heights more accurately reflects the fluorescence signal from the cell. The small size of the laser spot can enable one to do effective doublet discrimination by measuring both pulse height and area in applications in which it is critical, e.g., cell cycle studies, but we did not include doublet discrimination in these studies. With regard to SSC, it is somewhat unconventional to collect and display log SSC signals but we wanted to examine both myeloid cells (neutrophils as well as monocytes) and lymphocytes in order to determine leukocyte differential frequencies in this comprehensive study of healthy donors. There is nearly an order of magnitude (7-8 fold) difference between the median SSC signals for lymphocytes and neutrophils so the only practical way to keep all cells on scale is to collect and display log SSC signals as we have done here. Some antibody combinations were “multiplexed” with the same fluorochrome on more than one antibody, e.g., (13, 14). In these cases, the populations labeled with different antibodies with the same fluorochrome could be distinguished by SSC differences, by differences in the fluorescence intensity of various populations and/or by differential labeling with another antibody. Examples of multiplexed labeling are depicted and described in Figure 1 and in supplemental Figure S3.
Data files were collected with a threshold set on FSC or on CD45 fluorescence (Figure S3). When a threshold was set on FSC, an additional fluorescence and/or SSC “live gate” was set to eliminate debris. Samples were collected with the XL set on “high” with stop conditions based on regions that were set around populations of interest to ensure that adequate numbers of relevant cells were included in the data files. There were no limits placed on the total events collected and the data files typically contained 50,000 to 200,000 total events. The studies here focused on monocytes so that stop conditions were set to include thousands of monocytes per file.
Analyses of Data
Data files were analyzed with FlowJo (TreeStar, Ashland, OR) using the Mac versions of the software. Fluorescence intensity values are reported as the “median fluorescence intensity” (MFI) as determined by FlowJo. Tabular data were transferred to Excel spreadsheets where the built-in statistical functions were used to determine means and other values such as standard deviations and correlations.
Supplementary Material
Supplemental Figure S1. Optical filter configurations for the XL/MCL cytometer, The diagram above depicts the modified arrangements of optical filters that were evaluated in order to measure fluorescein, PE, PC5 and PC7. The numbers correspond to the numbered filter slots in the XL cytometer. The standard optical filters in the Coulter Epics XL/MCL cytometer were configured to detect fluorescein, PE, PE-TxRed (“ECD”) and PC5. The filters supplied for that original configuration as labeled as “Slot n” with “n” equal to the original position of that filter. Since there was considerable fluorescence spillover with the original arrangement, the optical filters were altered to detect fluorescein, PE, PC5 and PC7 as illustrated
Supplemental Figure S2. Gating used to determine flow cytometric (FCM) differential leukocyte frequencies. Aliquots of blood were labeled with FITC-anti-CD3e, FITC-anti-CD15, PE-anti-CD20, PE-anti-CD91, PC5-anti-CD56 and PC7-anti-CD45, treated with FACSLysing solution, washed and resuspended for analysis. Data files were collected with linear forward scatter (FSC, gain 2) and log amplification for side scatter (SSC) and all fluorescence parameters using a CD45 threshold (as indicated). All parameters were collected using the Beckman-Coulter standard pulse area signals. The SSC signals for granulocytes were nearly an order of magnitude greater than that for lymphocytes so log amplification was used in order to keep all CD45+ blood leukocytes on scale. Antibody to CD56 was included since we initially planned to identify natural killer (NK) cells as CD56+ lymphocytes that expressed neither CD3e nor CD20 (or CD19). However, we found that CD56 expression consistently underestimated the number of CD3eNeg lymphocytes that expressed perforin (PRF1) so we ultimately did not use CD56 expression to identify NK cells.
Supplemental Figure S3. Expression of CD33 on monocytes from 199 subjects. Aliquots of blood from the subjects were labeled with antibody combinations including either clone WM53 or clone D3HL60.251 PC5-anti-CD33. The CD33+ monocytes were identified and the PC5 MFI values were determined and plotted vs. subject age as shown above. There was considerable heterogeneity in the amounts of CD33 expressed per monocyte among these subjects as depicted here and described elsewhere but there was no apparent association between the age of the subject and amount of CD33 expressed as illustrated. Likewise, there were no systematic differences between these two cross-competing clones reactive with the lectin-like V domain of CD33.
Supplemental Figure S4. Expression of CD38 on blood monocytes from subjects #104 - #200. Aliquots of blood from subjects #104 - #200 were surface labeled with antibody combinations including PC5-anti-CD38, fixed, rendered permeable and then labeled with FITC-anti-PRF1. The primary goal with these antibody combinations was to examine CD38 on T lymphocytes and natural killer (NK) cells. However, monocytes could be identified by a combination of CD38 expression and SSC so that the the MFI of PC5-anti-CD38 could be determined. As shown in the figure, the MFI values mostly varied by ±∼16% and there was no apparent association between the amount of CD38 detected and the age of the subjects that were examined.
Supplemental Figure S5. Expression of HLA-DQ and HLA-DR on CD91+ Monocytes, Blood samples from a group of 17 subjects (158 – 174) were labeled with a mixture of FITC-anti-HLA-DQ, PE-anti-CD91, PE-anti-CD3e, PC5-anti-HLA-DR and PC7-anti-CD19. The CD91+ monocytes were identified as depicted in Figure 2 and the univariate distributions of HLA-DQ and HLA-DR expression are depicted above.
Supplemental Figure S6. Expression of CD80 (B7.1) and CD86 (B7.2) on CD33+ monocytes. Replicate aliquots of blood from subjects #001 - #084 were labeled with a mixture of 1) FITC-anti-CD25 (or anti-PD-1), PE-anti-CD86, PC5-anti-CD3e/CD33 and PC7-anti-CD19 or of 2) FITC-anti-CD80, PE-anti-TLR1, PC5-anti-CD3e/CD33 and PC7-anti-CD19. The CD33+ monocytes were identified and the MFI values for FITC-anti-CD80 or for PE-anti-CD86 were determined from each labeled aliquot and plotted as depicted. CD80 was expressed at low levels on the monocytes from these subjects but there was a modest association between the amounts of CD80 and CD86 detected on these monocytes.
Supplemental Figure S7. The expression of CD86 and Toll-like Receptor 2 (TLR2) vary independently on monocytes, Replicate aliquots of blood from all 200 subjects were labeled with a mixture of 1) FITC-anti-TLR2, PE-anti-TLR4, PC5-anti-CD3e/CD33 and PC7-anti-CD4 or of 2) FITC-anti-CD25 or FITC-anti-PD-1, PE-anti-CD86, PC5-anti-CD3e/CD33 and PC7-anti-CD19. The CD33+ monocytes were identified and the MFI values for FITC-anti-TLR2 and PE-anti-CD86 were determined and plotted as shown. The expression of both molecules varied among individuals but there was no detectable association as indicated.
Supplemental Figure S8. HLA-DQ and CD163 expression on monocytes are modestly associated. Replicate aliquots of blood from 74 subjects (#011 - #084) were labeled with antibody mixtures of 1) FITC-anti-HLA-DQ, PE-anti-CD3e/CD91, PC5-anti-HLA-DR and PC7-anti-CD19 and of 2) FITC-anti-CD57, PE-anti-CD163, PC5-anti-CD3e/CD33 and PC7-anti-CD8a. The CD91+ or CD33+ monocytes were identified and the MFI values for FITC-anti-HLA-DQ and PE-anti-CD163, respectively, were determined and plotted as shown. There was a modest association between the levels of HLA-DQ and CD163 expressed per cell as indicated.
Supplemental Figure FS9. Expression of CD163 and CD86 on monocytes is negatively associated. Replicate aliquots of blood from 74 subjects (#011 - #084) were labeled with antibody mixtures containing FITC-anti-CD57, PE-anti-CD163, PC5-anti-CD3e/CD33 and PC7-anti-CD8a and with with other mixtures containing FITC-anti-PD-1, PE-anti-CD86, PC5-anti-CD3e/CD33 and PC7-anti-CD19. CD33+ monocytes were identified and the MFI values for PE-anti-CD163 and for PE-anti-CD86 were determined and plotted as shown. There was a modest negative association such that monocytes from subjects with higher levels of CD163 tended to have lower levels of CD86.
Supplemental Table ST1. Antibodies used in flow cytometric examinations, Antibodies listed above were used in the present studies. The exact combinations are described in the relevant legends.
Acknowledgments
We thank the subjects who participated in the study and David Berner for his expert phlebotomy services. We also thank Mike Bardsley, David Berner and Sally DuPre for assistance in processing and labeling blood samples. These studies were supported in part by a research contract from the Office of Naval Research and by the Nevada BRIN/INBRE grants (NIH P20 RR016464).
Abbreviations
- FCM
flow cytometry
- FITC
fluorescein isothiocyanate
- MFI
median fluorescence intensity
- MHC
major histocompatibility complex
- PE
phycoerythrin
- PC5
phycoerythrin-cyanine 5 tandem fluorochrome
- PC7
phycoerythrin-cyanine 7 tandem fluorochrome
Footnotes
The authors lack commercial or proprietary interests with the companies cited and with the information of this publication and thus have no conflicts of interest to declare.
Literature Cited
- 1.Kennedy DW, Abkowitz JL. Mature monocytic cells enter tissues and engraft. Proc Natl Acad Sci U S A. 1998;95(25):14944–9. doi: 10.1073/pnas.95.25.14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72(4):621–7. [PubMed] [Google Scholar]
- 3.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9(1):61–7. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 4.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74(7):2527–34. [PubMed] [Google Scholar]
- 5.Ziegler-Heitbrock HW, Strobel M, Fingerle G, Schlunck T, Pforte A, Blumenstein M, Haas JG. Small (CD14+/CD16+) monocytes and regular monocytes in human blood. Pathobiology. 1991;59(3):127–30. doi: 10.1159/000163629. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi SS, Lewis SM, Gant VA, Treacher D, Davis BH, Brown KA. Increased distribution and expression of CD64 on blood polymorphonuclear cells from patients with the systemic inflammatory response syndrome (SIRS) Clin Exp Immunol. 2001;125(2):258–65. doi: 10.1046/j.1365-2249.2001.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Lee PY, Kellner ES, Paulus M, Switanek J, Xu Y, Zhuang H, Sobel ES, Segal MS, Satoh M, et al. Monocyte surface expression of Fcgamma receptor RI (CD64), a biomarker reflecting type-I interferon levels in systemic lupus erythematosus. Arthritis Res Ther. 2010;12(3):R90. doi: 10.1186/ar3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnizlein-Bick CT, Sherman MR, Boggs DL, Leemhuis TB, Fife KH. Incidence of HIV infection in monocyte subpopulations characterized by CD4 and HLA-DR surface density. Aids. 1992;6(2):151–6. doi: 10.1097/00002030-199202000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Dudhane A, Wang ZQ, Orlikowsky T, Gupta A, Wormser GP, Horowitz H, Kufer P, Hoffmann MK. AIDS patient monocytes target CD4 T cells for cellular conjugate formation and deletion through the membrane expression of HIV-1 envelope molecules. AIDS Res Hum Retroviruses. 1996;12(10):893–9. doi: 10.1089/aid.1996.12.893. [DOI] [PubMed] [Google Scholar]
- 10.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349(9053):692–5. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 11.Zembala M, Bach S, Szczepanek A, Mancino G, Colizzi V. Phenotypic changes of monocytes induced by HIV-1 gp120 molecule and its fragments. Immunobiology. 1997;197(1):110–21. doi: 10.1016/S0171-2985(97)80061-7. [DOI] [PubMed] [Google Scholar]
- 12.Overton WR. Modified histogram subtraction technique for analysis of flow cytometry data. Cytometry. 1988;9(6):619–26. doi: 10.1002/cyto.990090617. [DOI] [PubMed] [Google Scholar]
- 13.Bagwell B. A journey through flow cytometric immunofluorescence analyses – Finding accurate and robust algorithms that estimate positive fraction distributions. Clin Immunol Newsletter. 1996;16(3):33–37. [Google Scholar]
- 14.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(2):83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Iyer SB, Hultin LE, Zawadzki JA, Davis KA, Giorgi JV. Quantitation of CD38 expression using QuantiBRITE beads. Cytometry. 1998;33(2):206–12. doi: 10.1002/(sici)1097-0320(19981001)33:2<206::aid-cyto15>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 16.Musso T, Deaglio S, Franco L, Calosso L, Badolato R, Garbarino G, Dianzani U, Malavasi F. CD38 expression and functional activities are up-regulated by IFN-gamma on human monocytes and monocytic cell lines. J Leukoc Biol. 2001;69(4):605–12. [PubMed] [Google Scholar]
- 17.Villanueva JL, Solana R, Alonso MC, Pena J. Changes in the expression of HLA-class II antigens on peripheral blood monocytes from aged humans. Dis Markers. 1990;8(2):85–91. [PubMed] [Google Scholar]
- 18.Colbaugh P, Stastny P. Antigens in human monocytes. III. Use of monocytes in typing for HLA-D related (DR) antigens. Transplant Proc. 1978;10(4):871–4. [PubMed] [Google Scholar]
- 19.Medley QG, Kedersha N, O'Brien S, Tian Q, Schlossman SF, Streuli M, Anderson P. Characterization of GMP-17, a granule membrane protein that moves to the plasma membrane of natural killer cells following target cell recognition. Proc Natl Acad Sci U S A. 1996;93(2):685–9. doi: 10.1073/pnas.93.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meehan SM, McCluskey RT, Pascual M, Preffer FI, Anderson P, Schlossman SF, Colvin RB. Cytotoxicity and apoptosis in human renal allografts: identification, distribution, and quantitation of cells with a cytotoxic granule protein GMP-17 (TIA-1) and cells with fragmented nuclear DNA. Lab Invest. 1997;76(5):639–49. [PubMed] [Google Scholar]
- 21.Chuang SS, Kumaresan PR, Mathew PA. 2B4 (CD244)-mediated activation of cytotoxicity and IFN-gamma release in human NK cells involves distinct pathways. J Immunol. 2001;167(11):6210–6. doi: 10.4049/jimmunol.167.11.6210. [DOI] [PubMed] [Google Scholar]
- 22.Bai Y, Fu S, Honig S, Wang Y, Qin L, Chen D, Bromberg JS. CD2 is a dominant target for allogeneic responses. Am J Transplant. 2002;2(7):618–26. doi: 10.1034/j.1600-6143.2002.20706.x. [DOI] [PubMed] [Google Scholar]
- 23.Romero X, Benitez D, March S, Vilella R, Miralpeix M, Engel P. Differential expression of SAP and EAT-2-binding leukocyte cell-surface molecules CD84, CD150 (SLAM), CD229 (Ly9) and CD244 (2B4) Tissue Antigens. 2004;64(2):132–44. doi: 10.1111/j.1399-0039.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 24.Mathew SO, Vaidya SV, Kim JR, Mathew PA. Human natural killer cell receptor 2B4 (CD244) down-regulates its own expression by reduced promoter activity at an Ets element. Biochem Biophys Res Commun. 2007;355(2):483–7. doi: 10.1016/j.bbrc.2007.01.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orlikowsky T, Dannecker GE, Wang Z, Horowitz H, Niethammer D, Hoffmann MK. Activation or destruction of T cells via macrophages. Pathobiology. 1999;67(5-6):298–301. doi: 10.1159/000028084. [DOI] [PubMed] [Google Scholar]
- 26.Rae F, Woods K, Sasmono T, Campanale N, Taylor D, Ovchinnikov DA, Grimmond SM, Hume DA, Ricardo SD, Little MH. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol. 2007;308(1):232–46. doi: 10.1016/j.ydbio.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Gronlund J, Vitved L, Lausen M, Skjodt K, Holmskov U. Cloning of a novel scavenger receptor cysteine-rich type I transmembrane molecule (M160) expressed by human macrophages. J Immunol. 2000;165(11):6406–15. doi: 10.4049/jimmunol.165.11.6406. [DOI] [PubMed] [Google Scholar]
- 28.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409(6817):198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 30.Zembala M, Uracz W, Ruggiero I, Mytar B, Pryjma J. Isolation and functional characteristics of FcR+ and FcR- human monocyte subsets. J Immunol. 1984;133(3):1293–9. [PubMed] [Google Scholar]
- 31.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82(10):3170–6. [PubMed] [Google Scholar]
- 32.Rothe G, Gabriel H, Kovacs E, Klucken J, Stohr J, Kindermann W, Schmitz G. Peripheral blood mononuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1996;16(12):1437–47. doi: 10.1161/01.atv.16.12.1437. [DOI] [PubMed] [Google Scholar]
- 33.Scherberich JE, Nockher WA. CD14++ monocytes, CD14+/CD16+ subset and soluble CD14 as biological markers of inflammatory systemic diseases and monitoring immunosuppressive therapy. Clin Chem Lab Med. 1999;37(3):209–13. doi: 10.1515/CCLM.1999.039. [DOI] [PubMed] [Google Scholar]
- 34.Nockher WA, Wiemer J, Scherberich JE. Haemodialysis monocytopenia: differential sequestration kinetics of CD14+CD16+ and CD14++ blood monocyte subsets. Clin Exp Immunol. 2001;123(1):49–55. doi: 10.1046/j.1365-2249.2001.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terstappen LW, Hollander Z, Meiners H, Loken MR. Quantitative comparison of myeloid antigens on five lineages of mature peripheral blood cells. J Leukoc Biol. 1990;48(2):138–48. doi: 10.1002/jlb.48.2.138. [DOI] [PubMed] [Google Scholar]
- 36.Ulyanova T, Blasioli J, Woodford-Thomas TA, Thomas ML. The sialoadhesin CD33 is a myeloid-specific inhibitory receptor. Eur J Immunol. 1999;29(11):3440–9. doi: 10.1002/(SICI)1521-4141(199911)29:11<3440::AID-IMMU3440>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Mingari MC, Vitale C, Romagnani C, Falco M, Moretta L. p75/AIRM1 and CD33, two sialoadhesin receptors that regulate the proliferation or the survival of normal and leukemic myeloid cells. Immunol Rev. 2001;181:260–8. doi: 10.1034/j.1600-065x.2001.1810122.x. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez-Caselles T, Martinez-Esparza M, Perez-Oliva AB, Quintanilla-Cecconi AM, Garcia-Alonso A, Alvarez-Lopez DM, Garcia-Penarrubia P. A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: two isoforms of CD33 are generated by alternative splicing. J Leukoc Biol. 2006;79(1):46–58. doi: 10.1189/jlb.0205096. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Oliva AB, Martinez-Esparza M, Vicente-Fernandez JJ, Corral-San Miguel R, Garcia-Penarrubia P, Hernandez-Caselles T. Epitope mapping, expression and post-translational modifications of two isoforms of CD33 (CD33M and CD33m) on lymphoid and myeloid human cells. Glycobiology. 2011;21(6):757–70. doi: 10.1093/glycob/cwq220. [DOI] [PubMed] [Google Scholar]
- 40.Lund FE, Cockayne DA, Randall TD, Solvason N, Schuber F, Howard MC. CD38: a new paradigm in lymphocyte activation and signal transduction. Immunol Rev. 1998;161:79–93. doi: 10.1111/j.1600-065x.1998.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 41.Funaro A, Malavasi F. Human CD38, a surface receptor, an enzyme, an adhesion molecule and not a simple marker. J Biol Regul Homeost Agents. 1999;13(1):54–61. [PubMed] [Google Scholar]
- 42.Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, Dianzani U, Stockinger H, Malavasi F. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol. 1998;160(1):395–402. [PubMed] [Google Scholar]
- 43.Lande R, Urbani F, Di Carlo B, Sconocchia G, Deaglio S, Funaro A, Malavasi F, Ausiello CM. CD38 ligation plays a direct role in the induction of IL-1beta, IL-6, and IL-10 secretion in resting human monocytes. Cell Immunol. 2002;220(1):30–8. doi: 10.1016/s0008-8749(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 44.Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2006;345(4):1386–92. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 45.Coetzee LM, Tay SS, Lawrie D, Janossy G, Glencross DK. From research tool to routine test: CD38 monitoring in HIV patients. Cytometry B Clin Cytom. 2009;76(6):375–84. doi: 10.1002/cyto.b.20478. [DOI] [PubMed] [Google Scholar]
- 46.Kornbluth RS, Edgington TS. Tumor necrosis factor production by human monocytes is a regulated event: induction of TNF-alpha-mediated cellular cytotoxicity by endotoxin. J Immunol. 1986;137(8):2585–91. [PubMed] [Google Scholar]
- 47.Beutler B. Endotoxin, toll-like receptor 4, and the afferent limb of innate immunity. Curr Opin Microbiol. 2000;3(1):23–8. doi: 10.1016/s1369-5274(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 48.Means TK, Lien E, Yoshimura A, Wang S, Golenbock DT, Fenton MJ. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163(12):6748–55. [PubMed] [Google Scholar]
- 49.Kuwahata S, Fujita S, Orihara K, Hamasaki S, Oba R, Hirai H, Nagata K, Ishida S, Kataoka T, Oketani N, et al. High expression level of Toll-like receptor 2 on monocytes is an important risk factor for arteriosclerotic disease. Atherosclerosis. 2010;209(1):248–54. doi: 10.1016/j.atherosclerosis.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 50.Orihara K, Nagata K, Hamasaki S, Oba R, Hirai H, Ishida S, Kataoka T, Oketani N, Ogawa M, Mizoguchi E, et al. Time-course of Toll-like receptor 2 expression, as a predictor of recurrence in patients with bacterial infectious diseases. Clin Exp Immunol. 2007;148(2):260–70. doi: 10.1111/j.1365-2249.2007.03352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kajiya T, Orihara K, Hamasaki S, Oba R, Hirai H, Nagata K, Kumagai T, Ishida S, Oketani N, Ichiki H, et al. Toll-like receptor 2 expression level on monocytes in patients with viral infections: monitoring infection severity. J Infect. 2008;57(3):249–59. doi: 10.1016/j.jinf.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 52.McDaniel DO, Zhou X, Moore CK, Aru G. Cardiac allograft rejection correlates with increased expressions of Toll-like receptors 2 and 4 and allograft inflammatory factor 1. Transplant Proc. 2010;42(10):4235–7. doi: 10.1016/j.transproceed.2010.09.091. [DOI] [PubMed] [Google Scholar]
- 53.Zhou H, Su HS, Zhang X, Douhan J, 3rd, Glimcher LH. CIITA-dependent and -independent class II MHC expression revealed by a dominant negative mutant. J Immunol. 1997;158(10):4741–9. [PubMed] [Google Scholar]
- 54.de Preval C, Lisowska-Grospierre B, Loche M, Griscelli C, Mach B. A trans-acting class II regulatory gene unlinked to the MHC controls expression of HLA class II genes. Nature. 1985;318(6043):291–3. doi: 10.1038/318291a0. [DOI] [PubMed] [Google Scholar]
- 55.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75(1):135–46. [PubMed] [Google Scholar]
- 56.Fontes JD, Kanazawa S, Nekrep N, Peterlin BM. The class II transactivator CIITA is a transcriptional integrator. Microbes Infect. 1999;1(11):863–9. doi: 10.1016/s1286-4579(99)00232-4. [DOI] [PubMed] [Google Scholar]
- 57.Schnappauf F, Hake SB, Camacho Carvajal MM, Bontron S, Lisowska-Grospierre B, Steimle V. N-terminal destruction signals lead to rapid degradation of the major histocompatibility complex class II transactivator CIITA. Eur J Immunol. 2003;33(8):2337–47. doi: 10.1002/eji.200323490. [DOI] [PubMed] [Google Scholar]
- 58.Ziegler-Heitbrock HW. Definition of human blood monocytes. J Leukoc Biol. 2000;67(5):603–6. doi: 10.1002/jlb.67.5.603. [DOI] [PubMed] [Google Scholar]
- 59.Forch P, Puig O, Kedersha N, Martinez C, Granneman S, Seraphin B, Anderson P, Valcarcel J. The apoptosis-promoting factor TIA-1 is a regulator of alternative pre-mRNA splicing. Mol Cell. 2000;6(5):1089–98. doi: 10.1016/s1097-2765(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 60.Anderson P, Phillips K, Stoecklin G, Kedersha N. Post-transcriptional regulation of proinflammatory proteins. J Leukoc Biol. 2004;76(1):42–7. doi: 10.1189/jlb.1103536. [DOI] [PubMed] [Google Scholar]
- 61.Riera N, Galassi N, Felippo M, Ruibal-Ares B, Perez Bianco R, de Bracco M. Increased CD4-positive monocytes in HIV-infected haemophilic patients. Haemophilia. 1998;4(5):725–30. doi: 10.1046/j.1365-2516.1998.00159.x. [DOI] [PubMed] [Google Scholar]
- 62.Tuttle DL, Harrison JK, Anders C, Sleasman JW, Goodenow MM. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol. 1998;72(6):4962–9. doi: 10.1128/jvi.72.6.4962-4969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J Exp Med. 1998;188(11):2083–90. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Latchman Y, McKay PF, Reiser H. Identification of the 2B4 molecule as a counter-receptor for CD48. J Immunol. 1998;161(11):5809–12. [PubMed] [Google Scholar]
- 65.Valiante NM, Trinchieri G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J Exp Med. 1993;178(4):1397–406. doi: 10.1084/jem.178.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tangye SG, Lazetic S, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. Cutting edge: human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J Immunol. 1999;162(12):6981–5. [PubMed] [Google Scholar]
- 67.Nakajima H, Cella M, Langen H, Friedlein A, Colonna M. Activating interactions in human NK cell recognition: the role of 2B4-CD48. Eur J Immunol. 1999;29(5):1676–83. doi: 10.1002/(SICI)1521-4141(199905)29:05<1676::AID-IMMU1676>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 68.Dong Z, Davidson D, Perez-Quintero LA, Kurosaki T, Swat W, Veillette A. The Adaptor SAP Controls NK Cell Activation by Regulating the Enzymes Vav-1 and SHIP-1 and by Enhancing Conjugates with Target Cells. Immunity. 2012;36(6):974–85. doi: 10.1016/j.immuni.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 69.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 70.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176(1):287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10(2):137–42. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 72.Park SY, Jung MY, Lee SJ, Kang KB, Gratchev A, Riabov V, Kzhyshkowska J, Kim IS. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J Cell Sci. 2009;122(Pt 18):3365–73. doi: 10.1242/jcs.049569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Optical filter configurations for the XL/MCL cytometer, The diagram above depicts the modified arrangements of optical filters that were evaluated in order to measure fluorescein, PE, PC5 and PC7. The numbers correspond to the numbered filter slots in the XL cytometer. The standard optical filters in the Coulter Epics XL/MCL cytometer were configured to detect fluorescein, PE, PE-TxRed (“ECD”) and PC5. The filters supplied for that original configuration as labeled as “Slot n” with “n” equal to the original position of that filter. Since there was considerable fluorescence spillover with the original arrangement, the optical filters were altered to detect fluorescein, PE, PC5 and PC7 as illustrated
Supplemental Figure S2. Gating used to determine flow cytometric (FCM) differential leukocyte frequencies. Aliquots of blood were labeled with FITC-anti-CD3e, FITC-anti-CD15, PE-anti-CD20, PE-anti-CD91, PC5-anti-CD56 and PC7-anti-CD45, treated with FACSLysing solution, washed and resuspended for analysis. Data files were collected with linear forward scatter (FSC, gain 2) and log amplification for side scatter (SSC) and all fluorescence parameters using a CD45 threshold (as indicated). All parameters were collected using the Beckman-Coulter standard pulse area signals. The SSC signals for granulocytes were nearly an order of magnitude greater than that for lymphocytes so log amplification was used in order to keep all CD45+ blood leukocytes on scale. Antibody to CD56 was included since we initially planned to identify natural killer (NK) cells as CD56+ lymphocytes that expressed neither CD3e nor CD20 (or CD19). However, we found that CD56 expression consistently underestimated the number of CD3eNeg lymphocytes that expressed perforin (PRF1) so we ultimately did not use CD56 expression to identify NK cells.
Supplemental Figure S3. Expression of CD33 on monocytes from 199 subjects. Aliquots of blood from the subjects were labeled with antibody combinations including either clone WM53 or clone D3HL60.251 PC5-anti-CD33. The CD33+ monocytes were identified and the PC5 MFI values were determined and plotted vs. subject age as shown above. There was considerable heterogeneity in the amounts of CD33 expressed per monocyte among these subjects as depicted here and described elsewhere but there was no apparent association between the age of the subject and amount of CD33 expressed as illustrated. Likewise, there were no systematic differences between these two cross-competing clones reactive with the lectin-like V domain of CD33.
Supplemental Figure S4. Expression of CD38 on blood monocytes from subjects #104 - #200. Aliquots of blood from subjects #104 - #200 were surface labeled with antibody combinations including PC5-anti-CD38, fixed, rendered permeable and then labeled with FITC-anti-PRF1. The primary goal with these antibody combinations was to examine CD38 on T lymphocytes and natural killer (NK) cells. However, monocytes could be identified by a combination of CD38 expression and SSC so that the the MFI of PC5-anti-CD38 could be determined. As shown in the figure, the MFI values mostly varied by ±∼16% and there was no apparent association between the amount of CD38 detected and the age of the subjects that were examined.
Supplemental Figure S5. Expression of HLA-DQ and HLA-DR on CD91+ Monocytes, Blood samples from a group of 17 subjects (158 – 174) were labeled with a mixture of FITC-anti-HLA-DQ, PE-anti-CD91, PE-anti-CD3e, PC5-anti-HLA-DR and PC7-anti-CD19. The CD91+ monocytes were identified as depicted in Figure 2 and the univariate distributions of HLA-DQ and HLA-DR expression are depicted above.
Supplemental Figure S6. Expression of CD80 (B7.1) and CD86 (B7.2) on CD33+ monocytes. Replicate aliquots of blood from subjects #001 - #084 were labeled with a mixture of 1) FITC-anti-CD25 (or anti-PD-1), PE-anti-CD86, PC5-anti-CD3e/CD33 and PC7-anti-CD19 or of 2) FITC-anti-CD80, PE-anti-TLR1, PC5-anti-CD3e/CD33 and PC7-anti-CD19. The CD33+ monocytes were identified and the MFI values for FITC-anti-CD80 or for PE-anti-CD86 were determined from each labeled aliquot and plotted as depicted. CD80 was expressed at low levels on the monocytes from these subjects but there was a modest association between the amounts of CD80 and CD86 detected on these monocytes.
Supplemental Figure S7. The expression of CD86 and Toll-like Receptor 2 (TLR2) vary independently on monocytes, Replicate aliquots of blood from all 200 subjects were labeled with a mixture of 1) FITC-anti-TLR2, PE-anti-TLR4, PC5-anti-CD3e/CD33 and PC7-anti-CD4 or of 2) FITC-anti-CD25 or FITC-anti-PD-1, PE-anti-CD86, PC5-anti-CD3e/CD33 and PC7-anti-CD19. The CD33+ monocytes were identified and the MFI values for FITC-anti-TLR2 and PE-anti-CD86 were determined and plotted as shown. The expression of both molecules varied among individuals but there was no detectable association as indicated.
Supplemental Figure S8. HLA-DQ and CD163 expression on monocytes are modestly associated. Replicate aliquots of blood from 74 subjects (#011 - #084) were labeled with antibody mixtures of 1) FITC-anti-HLA-DQ, PE-anti-CD3e/CD91, PC5-anti-HLA-DR and PC7-anti-CD19 and of 2) FITC-anti-CD57, PE-anti-CD163, PC5-anti-CD3e/CD33 and PC7-anti-CD8a. The CD91+ or CD33+ monocytes were identified and the MFI values for FITC-anti-HLA-DQ and PE-anti-CD163, respectively, were determined and plotted as shown. There was a modest association between the levels of HLA-DQ and CD163 expressed per cell as indicated.
Supplemental Figure FS9. Expression of CD163 and CD86 on monocytes is negatively associated. Replicate aliquots of blood from 74 subjects (#011 - #084) were labeled with antibody mixtures containing FITC-anti-CD57, PE-anti-CD163, PC5-anti-CD3e/CD33 and PC7-anti-CD8a and with with other mixtures containing FITC-anti-PD-1, PE-anti-CD86, PC5-anti-CD3e/CD33 and PC7-anti-CD19. CD33+ monocytes were identified and the MFI values for PE-anti-CD163 and for PE-anti-CD86 were determined and plotted as shown. There was a modest negative association such that monocytes from subjects with higher levels of CD163 tended to have lower levels of CD86.
Supplemental Table ST1. Antibodies used in flow cytometric examinations, Antibodies listed above were used in the present studies. The exact combinations are described in the relevant legends.