Abstract
The primary aim of the epidemiologic study of one million U.S. radiation workers and veterans [the Million Worker Study (MWS)] is to provide scientifically valid information on the level of radiation risk when exposures are received gradually over time, and not within seconds as was the case for Japanese atomic-bomb survivors. The primary outcome of the epidemiologic study is cancer mortality but other causes of death such as cardiovascular disease and cerebrovascular disease will be evaluated. The success of the study is tied to the validity of the dose reconstruction approaches to provide realistic estimates of organ-specific radiation absorbed doses that are as accurate and precise as possible and to properly evaluate their accompanying uncertainties. The dosimetry aspects for the MWS are challenging in that they address diverse exposure scenarios for diverse occupational groups being studied over a period of up to 70 y. The dosimetric issues differ among the varied exposed populations that are considered: atomic veterans, U.S. Department of Energy workers exposed to both penetrating radiation and intakes of radionuclides, nuclear power plant workers, medical radiation workers, and industrial radiographers. While a major source of radiation exposure to the study population comes from external gamma- or x-ray sources, for some of the study groups there is a meaningful component of radionuclide intakes that require internal radiation dosimetry assessments.
Scientific Committee 6–9 has been established by the National Council on Radiation Protection and Measurements (NCRP) to produce a report on the comprehensive organ dose assessment (including uncertainty analysis) for the MWS. The NCRP dosimetry report will cover the specifics of practical dose reconstruction for the ongoing epidemiologic studies with uncertainty analysis discussions and will be a specific application of the guidance provided in NCRP Report Nos. 158, 163, 164, and 171. The main role of the Committee is to provide guidelines to the various groups of dosimetrists involved in the MWS to ensure that certain dosimetry criteria are considered: calculation of annual absorbed doses in the organs of interest, separation of low and high linear-energy transfer components, evaluation of uncertainties, and quality assurance and quality control. It is recognized that the MWS and its approaches to dosimetry are a work in progress and that there will be flexibility and changes in direction as new information is obtained, both with regard to dosimetry and with regard to the epidemiologic features of the study components.
This manuscript focuses on the description of the various components of the MWS, on the available dosimetry results, and on the challenges that have been encountered. It is expected that the Committee will complete its report in 2016.
Keywords: National Council on Radiation Protection and Measurements, dose reconstruction, occupational exposures, radiation workers, Million Worker Study
INTRODUCTION
The National Council on Radiation Protection and Measurements (NCRP) is coordinating an expansive epidemiologic effort entitled the One Million U.S. Radiation Workers and Veterans Study (Boice 2012). The primary aim of the Million Worker Study (MWS) is to provide scientifically valid information on the level of radiation risk when exposures are received gradually over time, and not acutely as was the case for Japanese atomic-bomb survivors. The major health outcomes of interest for the MWS are deaths from specific cancers and leukemia, but other causes of noncancer death such as cardiovascular disease and cerebrovascular disease will be evaluated. For the purposes of the MWS, namely to estimate health effects at low doses and dose rates, it is important to define what are considered to be “low doses” and “low dose rates” in the context of epidemiologic studies. Radiation dose rates can range from very large (e.g., in the case of the very rapid exposure occurring from a detonated nuclear weapon such as occurred at Hiroshima and Nagasaki) to very small (such as from environmental sources of natural background radiation). Upon a careful review of the literature, Wakeford and Tawn (2010) concluded: a low dose is <100 mGy delivered acutely and a low dose rate is <5 mGy h−1, which is consistent with the guidance provided by the U.K. National Radiological Protection Board (Muirhead et al. 1993) (now the U.K. Public Health England). These definitions are used in the MWS. Indirect support for these definitions comes from a study of nuclear industry workers with cumulative recorded doses of external radiation of up to 1 Gy, which found a linear dose response for stable chromosome aberrations (Tawn et al. 2004).
It is recognized that much of the statistical power of radiation worker studies will come from cumulative doses >100 mGy so it is important to confirm that such dose is accumulated over a prolonged period and not acutely. For the MWS, then, a high dose rate will be considered in excess of 5 mGy h−1 which would presumably be the result of a radiological incident. Persons experiencing such an exposure will not be included in the low dose-rate analyses. Any person who receives >50 mGy in a single reporting period will be individually evaluated to confirm whether the dose was received gradually over time. Any worker who received >250 mGy in a single reporting period will be considered an “accident victim” consistent with the Radiation Emergency Assistance Center/Training Site (REAC/TS) definitions (Ricks et al. 2000) and excluded.
NCRP set up Scientific Committee 6–9 to provide guidance in the derivation of annual organ absorbed doses and their associated uncertainty for epidemiologic studies in general, but with a focus on the diverse populations that make up the MWS, namely ~115,000 atomic veterans, 360,000 U.S. Department of Energy (DOE) workers, 330,000 nuclear power plant workers, 130,000 industrial radiographers, and 240,000 medical radiation workers. The preparation of the NCRP dosimetry report is ongoing. The dosimetric information currently available for each component of the study is presented in the first part of the paper, while the current thinking on the guidelines that could be recommended to implement the dosimetric aspects of the MWS is described in the second part.
DESCRIPTION OF THE COMPONENTS OF THE MILLION WORKER STUDY
To-date, dose assessments have been performed for part of the DOE workers, is well under way for the atomic veterans, has been undertaken for the nuclear power plant workers and the industrial radiographers, and is at the planning stage for the medical radiation workers.
Atomic veterans
The cohort of atomic veterans consists of ~115,000 military personnel who were mainly exposed at either:
the Nevada Test Site where they participated in military maneuvers, observed tests, or provided support during related operations that occurred from 1953–1957;
the Pacific Proving Grounds where personnel were aboard ships or stationed on islands in the area during and following the tests detonated from 1946–1958; or
the White Sands Missile Range in New Mexico, where the first nuclear test, TRINITY, occurred in July 1945 (Boice 2012, 2014a).
In addition to their exposure at one of the eight test series included in the study (Till et al. 2014), ~12,000 test participants were also exposed at other test, such as the 1952 TUMBLER-SNAPPER series and the 1955 TEAPOT series at the Nevada Test Site, and the 1952 IVY series at the Pacific Proving Grounds. Their exposures from these test series were also included. The selection of the series to be included for study was based on continuing the follow-up of three previously studied cohorts of nuclear weapons test participants (Watanabe et al. 1995; Johnson et al. 1996; Thaul et al. 2000) with only the TRINITY shot added for historical importance and long-term follow-up.
The initial focus of the atomic-veterans study is on leukemia and male breast cancer. Thus, the organs of interest are red bone marrow and male breast. Because of cost and time, it was found impractical to conduct historical dose reconstructions on all of the ~115,000 cohort members. Therefore, doses are estimated for 1,857 individuals, including all leukemia and male breast cancer cases and 1% random sample of the entire cohort, used for comparison in a case-cohort design (Wacholder 1991; Breslow et al. 2009). Primary emphasis is given to external exposure since almost all the exposure to breast and red bone marrow is known to be from penetrating external radiation. The Nuclear Test Program Review (NTPR) assembled a wealth of information from classified and unclassified historical records collected during the nuclear atmospheric tests and also records of military personnel that participated in the testing. The purpose of the NTPR program is to estimate doses to be used for compensation and these doses are deliberately estimated to be conservative (high-sided) in accordance with regulations NTPR is required to follow in order to be claimant friendly (NA/NRC 2003; Blake and Komp 2014). The distribution of the available NTPR doses among the ~115,000 cohort members is presented in Table 1. The dose estimation process performed in the MWS consists in refining and modifying, as necessary, the information available in the NTPR records for each veteran and producing a realistic dose estimate suitable for epidemiology with a corresponding uncertainty estimate. However, if it can be demonstrated that an individual’s dose from all sources is <5 mGy, then the limited resources available are not expended to calculate a best estimate of dose and uncertainty. Instead the NTPR estimate of dose is accepted with an estimated uncertainty that reflects the likely upper bound of these doses. Details about the dose assessment methods currently applied in the study of atomic veterans are reported in Till et al. (2014).
Table 1.
Distribution of the available Nuclear Test Personnel Review (NTPR) Program doses among the cohort of atomic veterans.
| NTPR dose (mSv)a | Number of workers | Percentage of workers |
|---|---|---|
| <5 | 63,192 | 55.6 |
| 5 – 9 | 19,970 | 17.6 |
| 10–24 | 18,791 | 16.6 |
| 25 – 49 | 9,658 | 9.4 |
| ≥50 | 1,969 | 1.7 |
| All | 113,580 | 100.0 |
The dose is essentially from low-LET radiation (external irradiation from photons).
Future follow-up investigations will include other cancers such as thyroid, salivary gland, liver, and bone and thus will also include estimates of doses from intakes of radioactive material.
DOE workers
Scores of facilities involved in the production of nuclear weapons, fuel rods, heat sources, or other devices containing large quantities of radioactive material have been operated in the United States since the 1940s. Workers at these facilities have the potential for elevated intake of radionuclides as well as exposure to external irradiation. Most of the populations that constitute the 360,000 DOE workers have been previously studied, but >20 y ago. The populations recently under investigation include the workers at Rocketdyne (Leggett et al. 2005; Boice et al. 2006, 2011), Mound (Boice et al. 2014), and Mallinckrodt (Dupree-Ellis et al. 2000). Populations that have just been or will soon be studied include workers at Los Alamos (Wiggs et al. 1994) and those in the 50 mSv study (Fry et al. 1996).
Two case studies are used in the NCRP dosimetry report to illustrate the typical issues encountered in a dose reconstruction for radiation workers at a production facility and to describe the common sources of uncertainty in the reconstructed doses:
The first case study (Boice et al. 2006) is a dose reconstruction for the 5,801 workers who were monitored for radiation exposure and employed between 1948 and 1999 at the Rocketdyne (formerly, Atomics International) site north of Los Angeles, California in the Simi Valley. Rocketdyne workers were involved in a wide range of radiological activities, including uranium and plutonium fuel fabrication, spent fuel evaluation, radiochemistry, plutonium fuel fabrication, and storage of nuclear material. Some workers were exposed to various radionuclides including isotopes of uranium, plutonium, thorium, cesium, strontium, cerium and promethium. Although previous dose reconstructions had been done for Rocketdyne workers, the study described in the report did not rely on products of those studies. The dose estimation process was based on the analysis of records available at Rocketdyne and on interviews with previous workers with good institutional memory. For the workers that spent some of their career in other radiation facilities, the relevant records from those facilities were recovered in order to estimate the entire radiation exposure history for all workers (Boice et al. 2006). Absorbed doses from low linear-energy transfer (LET) radiation (e.g., external doses, cesium, strontium) were separated from the absorbed doses from high-LET radiations (e.g., plutonium, uranium). As an example, the distribution of the doses to lung, cumulated over all modes of exposure and over the entire careers of the Rocketdyne workers under consideration, is presented in Table 2.
The second case study (Boice et al., 2014) is a dose reconstruction for the 4,977 workers at the Mound Site in Miamisburg, Ohio, who were monitored for radiation exposure and were first hired between 1944 and 1979. Polonium-210, in combination with beryllium, was used at the Mound Nuclear Facility as a source of neutrons for triggering nuclear weapons. Other exposures included external gamma radiation and to a lesser extent 238Pu, tritium, and neutrons. The Mound analysis was performed in a stepwise approach in conducting time- and cost-efficient epidemiologic studies of radiation workers at production facilities across the United States. The dose reconstruction for Mound generally followed the scheme laid out earlier for the Rocketdyne site but differed in some ways, primarily due to differences in the dominant internal emitters at the two sites and in the main sources of uncertainty in tissue dose estimates for the internal emitters. As an example, the distribution of the doses to lung, cumulated over all modes of exposure and over the entire careers of the Mound workers is presented in Table 3.
Table 2.
Distribution of the doses to lung, cumulated over all modes of exposure and the entire careers of the Rocketdyne workers monitored for radiation exposure (Boice et al. 2006).
| Career lung dose (mSv) | Number of workers | Percentage of workers |
|---|---|---|
| <5 | 3,683 | 64.0 |
| 5 – 9 | 609 | 10.6 |
| 10 – 49 | 1,039 | 18.0 |
| 50 – 99 | 203 | 3.5 |
| 100 – 199 | 113 | 2.0 |
| ≥200 | 109 | 1.9 |
| All | 5,756b | 100 |
The highest lung doses are mainly due to high-LET radiation (intakes of UAlx); most of the small doses are due to low-LET radiation (external irradiation from photons).
Excluding 45 workers who were not monitored for external irradiation and were subjected to very low levels of dose from internal irradiation.
Table 3.
Distribution of the doses to lung, cumulated over all modes of exposure and the entire careers of the Mound workers monitored for radiation (Boice et al. 2014).
| Career lung dose (mSv)a,b | Number of workers | Percentage of workers |
|---|---|---|
| <10 | 2,285 | 48.9 |
| 10 – 99 | 1,482 | 31.7 |
| 100 – 499 | 683 | 14.6 |
| 500 – 999 | 145 | 3.1 |
| ≥1,000 | 77 | 1.7 |
| All | 4,672c | 100 |
In this table a radiation-weighting factor of 1 was assumed for the alpha particles from 210Po and plutonium isotopes; a radiation-weighting factor of 10 was assumed for the neutrons. For the analyses other assumptions as to the relative biological effectiveness of alpha particles were made, i.e. weighting factors for the absorbed dose of 10 and of 20.
On average, about 75% of the lung dose is due to high-LET radiation (intakes of 210Po), the remainder of the lung dose being due to low-LET radiation (external irradiation from photons).
Excluding 305 workers who were not monitored for external irradiation and were subjected to very low levels of doses from internal irradiation.
The two cohorts are characterized by long and complete follow-up, beginning in the 1940s, and comprehensive organ-specific dose reconstructions. Nearly a half-million bioassays (primarily urine samples) were available to reconstruct organ doses from intakes of radionuclides including, 210Po, 239Pu, uranium aluminide (UAlx), and tritium. Linkages with national dosimetry data bases enabled the determination of career doses.
For a number of exposure situations encountered for both Rocketdyne and Mound, it was not found possible to simulate bioassay data for internally-deposited radionuclides using the International Commission on Radiological Protection’s (ICRP) recommended biokinetic models and parameter values. For example, a group of Rocketdyne workers were exposed to airborne UAlx during the fabrication of reactor fuel plates. In the early stages of the UAlx program the intake and lung retention of uranium were substantially underestimated by Rocketdyne health physicists because the inhaled material was thought to be moderately soluble, and the rates of urinary excretion of uranium were initially very low. As it was later discovered, the initially low excretion rates resulted from a long, unexpected delay in the dissolution of material deposited in the lungs. In workers who had been removed from UAlx work for various reasons, the rate of urinary excretion of uranium gradually increased, peaked after a few months, and then declined at a rate consistent with moderately soluble material. This pattern differs markedly from the monotonically decreasing rates of absorption to blood represented by the standard absorption types in the ICRP’s Human Respiratory Tract Model (HRTM). Thus, for purposes of the Rocketdyne epidemiologic study it was necessary to develop special parameter values for the HRTM to describe the apparent pattern of dissolution of UAlx in the respiratory tract. On the basis of the derived parameter values it was estimated that the UAlx workers received by far the highest radiation doses among Rocketdyne workers.
It was also necessary to develop special parameter values of the HRTM to reproduce urinary excretion data for Mound workers. ICRP Publication 68 recommends Type M, representing moderately soluble material, as a default absorption type for inhaled polonium, but application of Type M parameter values yielded a much more slowly declining polonium excretion pattern than observed in Mound workers. In dose reconstructions for inhaled polonium at Mound, the long-term biological half-time of ~140 d for polonium in the deep lungs specified for Type M material was replaced with a half-time of 30 d. These examples indicate the attention to detail that is taken for each of the components of the MWS. Further the ICRP models were used to compute organ specific absorbed doses per calendar year after intake up until the date of death or date last known to be alive (i.e., they were modified for the purposes of epidemiologic study).
Nuclear power plant workers
One of the largest groups occupationally exposed to low doses over a period of years consists of workers in the nuclear power industry who since the dawn of the nuclear age have been monitored with the use of personal dosimeters when potentially exposed to ionizing radiation, and the measurements have been maintained. Worker exposures in the nuclear industry have decreased over the years from >10 mSv y−1 down to <2 mSv y−1 (annual personal dose equivalent) on average (Blevins and Andersen 2011).
As part of the MWS, cancer mortality is specifically being examined among 330,000 workers at nuclear power plants first employed from 1957–1985. The cohort members were selected from databases available from the Radiation Exposure Information and Reporting System (REIRS) maintained by the U.S. Nuclear Regulatory Commission (REIRS 2011) and Landauer, Inc. The NRC system (REIRS) contains dose information since the late 1950s for employees of facilities licensed by NRC (and its predecessor organizations). Landauer, Inc. also collects individualized and annual dosimetry information in an electronic database since 1977 and on microfilm since the early 1960s. Dose monitoring records also were typically maintained by the utilities where the nuclear power plant workers were employed, especially records for doses received prior to 1979. In addition, the Radiation Exposure Monitoring System contains records for all DOE workers, again since the mid-1960s. In order to provide as complete a dose history as possible for those included in the cohort of nuclear power plant workers for the MWS, all sources (individual facilities, REIRS, the Radiation Exposure Monitoring System, dosimetry service providers, and military dosimetry systems when possible) are being utilized. The current distribution of the cumulated doses, as extracted from the REIRS and Landauer databases, over the 330,000 selected nuclear power plant workers, is presented in Table 4.
Table 4.
Distribution of the cumulated recorded doses, extracted from the REIRS and Landauer databases, among the cohort of nuclear power plant workers; the doses are expressed in terms of personal dose equivalent.
| Cumulated recorded dosea (mSv) | Number of workers | Percentage of workers |
|---|---|---|
| <10 | 181,948b | 74.2 |
| 10 – 49 | 77,383 | 17.7 |
| 50 – 99 | 21,578 | 4.5 |
| 100 – 499 | 18,846 | 3.5 |
| 500 – 999 | 322 | 0.02 |
| ≥1,000 | 22 | 0.002 |
| All | 330,099b | 100 |
The dose is essentially due to low-LET radiation (external irradiation from photons).
Similar to the study of atomic veterans where approximately 115,000 of the over 250,000 aboveground nuclear weapons test participants were selected for study on the basis of cost considerations, not all of the nuclear utility workers will be studied. A sample of workers with <5 mSv is being considered for inclusion and not the entire number of available workers such that the selected cohort of early nuclear power plant workers (1957–1985) will likely be of the order of 150,000.
Most radiation exposures received by nuclear utility workers were due to penetrating external gamma radiation with only a few neutron exposures or internal contamination exposures. Dose reconstructions for external exposures are less challenging than when meaningful intakes of radionuclides are involved (Boice 2014b).
Industrial radiographers
Shortly after Roentgen’s discovery of x rays in 1895, Pierre and Marie Curie discovered radium (in 1898). This discovery led to the use of the first sealed sources in industrial radiography, the radium industrial gamma-ray source. This was a pivotal step in industrial imaging, as it allowed industrial radiography to be performed at temporary job sites where electricity was not available. Gamma radiography allowed castings of up to 30 cm thick to be radiographed, and was used heavily during World War II for the U.S. Navy’s ship building program. Then in 1946, other gamma radiography sealed sources such as 60Co and 192Ir became available; these new sources had higher average energies than radium, and they were much less expensive to obtain. Accordingly, iridium and cobalt quickly replaced radium as the sources of choice for industrial radiographic nondestructive testing (NDTERC 2014). Industrial radiographers are only exposed to external radiation, generally in the anterior-posterior geometry.
To-date, information on annual personal dose equivalents has been collected as part of the MWS for 130,000 industrial radiographers. The main sources of information are the REIRS and the Landauer databases. The distribution of the results, accumulated over the career of the workers, is presented in Table 5.
Table 5.
Distribution of the cumulated recorded doses, extracted from the REIRS and Landauer databases, among a group of 130,000 industrial radiographers; the doses are expressed in terms of personal dose equivalent.
| Cumulated recorded dosea (mSv) | Number of workers | Percentage of workers |
|---|---|---|
| <5 | 89,021 | 67.6 |
| 5 – 9 | 10,021 | 7.7 |
| 10 – 24 | 12,101 | 9.2 |
| 25 – 49 | 8,187 | 6.2 |
| ≥50 | 12,248 | 9.3 |
| All | 131,578 | 100 |
The dose is essentially due to low-LET radiation (external irradiation from photons).
Sealed sources are also used in the well-logging industry, but it was decided not to include their exposure information in the NCRP report, because there is little chance for radiation exposure from the sources during normal operations, except during the time that the sources are transferred from the transport container (usually mounted onto the well-logging truck) to the logging tool, which carries the source or sources downhole. Further, well loggers are not included in the MWS, in part because the exposures have been measured to be very low (Inskip et al. 1991).
Medical workers
Radiation has been used in medical practice for more than a century. Initially, the use of ionizing radiation in medicine was mostly limited to radiologists, radiation oncologists, and associated technical personnel. Since the 1960s developments in imaging, radiotherapy, catheters and other devices used in fluoroscopically-guided interventional and nuclear medicine procedures have revolutionized medical practice and have increased the potential for radiation exposure to radiologists and nonradiologists alike. Historically, the average annual occupational equivalent dose estimates have trended downward for the medical radiation worker populations (Simon et al. 2006). Most present day medical radiation workers generally experience very low radiation exposures. Those individuals who perform certain fluoroscopically-guided interventional procedures and potentially those who prepare or administer radionuclides for nuclear medicine procedures, however, are an exception to this generalization.
Medical radiation workers represent a large fraction of radiation-exposed workers, with ~2.5 million monitored workers in 2006 (NCRP 2009a). Dose records for ~240,000 workers have been processed for the MWS from Landauer data (Table 6). The derivation of organ absorbed doses from monitoring data (measured in terms of exposure or of personal dose equivalent) poses some challenges because of the potential inhomogeneity of exposures over the body, the position of the personal dosimeter on the body (e.g., whether on the collar, belt, pocket, outside a lead apron or inside), whether or not individual protective devices (e.g., lead aprons) were used as well as the attenuation characteristics of those devices, the greater access to medical radiation for personal examination (whether for practicing technique or for medical reasons), and incomplete monitoring periods. Nonetheless, the selection of medical workers for study is based on personal dosimetry records of workers with long-term monitoring data which minimizes the assumptions that might be necessary in the absence of such robust data.
Table 6.
Distribution of the cumulated recorded doses, extracted from the Landauer database, among a group of 240,000 medical workers; the doses are expressed in terms of personal dose equivalent.
| Cumulated recorded dosea (mSv) | Number of workers | Percentage of workers |
|---|---|---|
| 10 – 49b | 182,794 | 75.1 |
| 50 – 99 | 36,403 | 15.0 |
| 100 – 499 | 23,041 | 9.4 |
| 500 – 999 | 910 | 0.4 |
| ≥1,000 | 259 | 0.1 |
| All | 243,407 | 100.0 |
The dose is essentially due to low-LET radiation (external irradiation from photons).
In addition, about 1.5 million medical workers have cumulated recorded doses of less than 10 mSv.
Landauer dosimetry measurements have followed stringent procedures to provide accurate dose information for workers, often to comply with legal requirements. The measurements have used a variety of techniques over the years, from film badges to thermoluminescent dosimeters to optical luminescence methods. For the medical workers in the MWS, the measurements are considered consistent and reproducible but converting these measurements into organ doses is the challenge ahead. Because selection of the population for study was based on long-term coverage within Landauer, there are unlikely to be serious gaps in monitoring. The approach to dose reconstruction will be similar to that used in the National Cancer Institute (NCI) radiological technologist study (Simon et al. 2006, 2014a; Simon 2011) but will differ in some aspects because all workers in the MWS have long-term personal dosimeter reading.
In summary, the approach to dose reconstruction will rely on the estimated four million film-badge and thermoluminescent-dosimeter measurements available for workers based on long-term monitoring histories, the additional information available from archived Landauer records which have recently been scanned, linkages with other dosimetry databases, and modeling that may be needed to account for different average energies of medical gamma rays and x rays (Simon 2011), use of protective aprons, placement of dosimeters when worn, and minimal detectable doses over calendar years (Gilbert et al. 1996; Simon et al. 2006, 2014b).
Dose estimation in populations that are not included in the MWS
For completion and general interest, the NCRP dosimetry report will include a brief description of other exposed population groups that incorporate substantial dose reconstruction methodologies: astronauts exposed to galactic cosmic rays (GCRs), Russian Mayak workers exposed to plutonium and external radiation, and subjects within specialized exposure registries, specifically the U.S. Transuranium and Uranium Registries and REAC/TS.
Astronauts
Humans in space are exposed to radiation doses that are typically modest, but that are associated with potential health hazards due to the unusual nature of the exposures (Cucinotta et al. 2012, 2013, 2014a, 2014b). Approximately 325 U.S. citizens have flown in space since the dawn of the Space Age, averaging 2.5 missions per astronaut over their careers. The radiation dose comes in the form of energetic charged particles and, in shielded environments, neutrons. In deep space, away from the influence of Earth’s magnetic field, the primary radiation is due to two distinct sources: continuous GCRs (e.g., Simpson 1983), and unpredictable, episodic solar particle events (SPEs) (e.g., Reames 1995). In shielded environments, secondary radiation is also important. In low-Earth orbit, significant dose contributions come from traversals of the South Atlantic Anomaly (SAA), a region of trapped protons and electrons through which orbiting spacecraft pass several times per day. Transits through the SAA typically take a few minutes, during which time the dose rate rises by roughly an order of magnitude. GCRs are mostly highly charged relativistic particles, with kinetic energies of hundreds of million electron volts per nucleon or more; they are therefore highly penetrating, and difficult to shield against. The lower-energy particles in the SAA and in SPEs are more readily shielded, both by the body, the hull of the spacecraft and equipment racks. Average dose rates in space have historically been in the range of 0.3–1.2 mGy d−1, depending on details of the mission (Benton and Benton 2001). Altitude and inclination of the orbit are key factors, as are the shielding of the vehicle and the state of the 11 y solar cycle at the time of a mission. The most important effects of the solar cycle are the varying probabilities of SPEs (more likely to occur near solar maximum) and the modulation of GCRs (fluxes are suppressed by solar activity). GCR dose rates can vary by as much as a factor of three over the course of the solar cycle. Doses received crossing the SAA typically account for about half the total for a low-Earth orbit mission. Mission duration is a critical variable and historically has varied from minutes in the earliest flights to 3 and 6 mo intervals on the International Space Station.
Mayak workers
Beginning in 1948, the Soviet Union initiated a program for production of nuclear materials for a weapons program. The first facility for production of plutonium, now known as the Mayak Production Association, was built to irradiate uranium in reactors, separate the resulting plutonium in reprocessing plants, and prepare plutonium metal in the metallurgical plant. The rush to production, coupled with inexperience in handling radioactive materials, led to high radiation exposures to the workers in the facilities. The Mayak workers have been exposed to high doses of low-LET external gammas and, for a large number of them, to additional high doses of high-LET internal plutonium alpha particles. Radiation doses to Mayak workers were much higher than those to workers from similar operations in other countries, especially during the early period of operations (1948–1958). Thus, study of these workers offers a unique opportunity to evaluate health effects from both protracted external exposure and plutonium intakes. The Mayak workers are the focus of dose reconstruction and epidemiologic studies funded by the United States, Russia, and European Union to address the same question as that for the MWS: “How does the level of risk from a low dose rate exposure (experienced over several months to years) compare to that from the same dose delivered all at once?”
The Mayak worker cohort includes 25,757 workers hired during the period from 1948–1982 in one of the main plants (nuclear reactors, radiochemical plant, plutonium production facility) or in auxiliary plants involved with water treatment or mechanical repair (Gilbert et al. 2013). Nearly all workers have dosimeter records of external exposure; workers in the radiochemical and plutonium production facilities had potential for plutonium exposure, which is mainly derived from bioassay measurements. Unfortunately bioassay measurements, specifically urine samples, were available only on about one-third of the population (Gilbert et al. 2013). Estimates of individual annual external and internal (plutonium) doses are being developed as a collaborative effort of Russian and U.S. dosimetrists (Napier 2014). The Mayak personnel department record archive contains information on staff work assignments at the Mayak facilities throughout their working career beginning in 1948 through the present. The archive contains information for ~100,000 Mayak workers, subcontractor institutions, and military commands that worked at the Mayak site. During the early period of Mayak operations from 1948–1953, workers were exposed to comparatively high doses, which were recorded daily. As doses to workers decreased with improvements in equipment, operational controls, etc., the film dosimeter monitoring period increased to monthly recording. External dose was primarily from whole-body gamma radiation and in most cases was protracted over many years. Neutron doses were estimated for certain work locations on the basis of gamma-to-neutron ratios that were estimated either via historical measurements or simulations. An early version of the external dose reconstruction system was named DOSES-2005 and is documented in a series of articles (Smetatin et al. 2007a, 2007b; Vasilenko et al. 2007a, 2007b). Newer versions continue to expand the background data and refine the interpretations; a Monte-Carlo approach is being taken to estimate uncertainty in annual organ dose.
Internal doses were caused by inhalation of mixtures of oxides and nitrates of plutonium. Plutonium content in the body was derived from autopsy measurements and/or from urine bioassay measurements. A limitation of the Mayak data is that the workers with bioassay data had only one, or a very limited number of, urinalysis measurements, usually late in the worker’s career. Thus many assumptions on the nature and timing of the exposures were required. The richness of the bioassay data for many of the U.S. radiation workers allows for much higher quality internal dosimetry than for the Mayak workers. In the Mayak study, the worker histories developed for the external dosimetry were used to create “inhalation exposure scenarios” to supplement the bioassay measurements. The current version of the internal dosimetry system, MWDS-2008, is described by Khokhryakov et al. (2013). An ongoing advance in the dosimetry that will provide estimates of uncertainty is underway, with scheduled completion in 2014, incorporating a two-dimension Monte-Carlo approach, which will allow dose estimates based upon simultaneous use of urinalysis and autopsy data.
U.S. Transuranium and Uranium Registries (USTUR)
Although the USTUR exposure registries are not suitable for epidemiologic studies per se, they are a unique source of data on worker intakes of uranium and transuranic radionuclides. A key function of the USTUR has been radiochemical analysis of tissue samples collected at autopsy from volunteer registrants who had known or suspected occupational intakes of uranium and/or transuranics. The intent is to compare tissue contents measured at autopsy with those predicted from the biokinetic models used to estimate internal radiation doses from bioassay measurements. As of March 2012, the USTUR had a total of 415 registrants, of whom 80 were living and 335 deceased. The National Human Radiobiology Tissue Resource had 7,340 tissue samples archived, collected from 31 whole- and 56 partial-body donations; dates of death for these donors ranged from 1984–2011 (Parker and Tolmachev 2013). Organ doses estimated for registrants range from a few milligray to 1 Gy or more.
Radiation Emergency Assistance Center/Training Site Accident Registry (REAC/TS)
Established in 1976, the REAC/TS accident registry is not an epidemiologic database, but rather a collection of case studies that is of use in determining the acute effects of radiation in humans and informing medical management of future incidents. REAC/TS maintains a radiation accident registry that contains data on 469 radiation accidents (as of June 2013) worldwide. Some of the better-known accidents include the 137Cs teletherapy source rupture in Goiânia, Brazil, and the Chernobyl Nuclear Power Plant accident. The criteria for including an accident in the registry have changed over the years, but basically consist of a dose to the victim exceeding five times the current U.S. dose limits for a radiation worker, based on the limits in ICRP Publication 26 (ICRP 1977) or ~250 mSv (Lushbaugh et al. 1987; Ricks et al. 2000). The accidents in the registry have resulted in 102 fatalities due to acute radiation syndrome plus eight trauma fatalities, although it is likely that all eight of those victims also received lethal radiation doses. Of the 26 deaths in the United States, 21 have occurred from medical misadministrations, mostly from improperly calibrated external beam radiotherapy devices.
GUIDELINES REGARDING THE ESTIMATION OF DOSES FOR AN EPIDEMIOLOGIC STUDY
Within the framework of an epidemiologic study evaluating the health effect attributed to radiation dose, the dosimetric quantity of interest is the annual absorbed dose to the organ or tissue that is assumed to be the origin of the subject’s cancer. For example, the dose to red bone marrow is the radiation dose of interest if leukemia is the disease being considered in the epidemiologic study. Because life tables based on annual data are generally used in the epidemiologic analysis (e.g., Cox proportional hazards modeling), the absorbed dose is calculated on an annual basis. The annual absorbed dose to the organ or tissue that is considered must be estimated for each year of the entire period of time over which the dose is delivered, beginning with the date of first exposure and ending with the date of cancer diagnosis, if available (which is infrequently known in a mortality investigation), and otherwise the date of death, or the date last known to be alive. The absorbed dose is a physical quantity that is expressed in grays and does not include factors that take into account the type of radiation (radiation weighting factor) or the specific organ or tissue (tissue weighting factor), as is done in the estimation of equivalent and effective dose — which are units of radiation protection and strictly suitable for the study of health effects. Although it is recognized that the dose distribution may not be uniform over all parts of the organ or tissue, it is assumed in the NCRP dosimetry report that the mean dose over the entire organ or tissue is the quantity of interest in the epidemiologic analysis.
The dose assessment is a process that begins with a defined purpose, which is the estimation of annual absorbed doses in the organs of interest in the case of the epidemiologic analyses related to the MWS, and is performed in a logical and orderly manner. The dose assessment process is described, for example, by the National Academies/National Research Council (NA/NRC 1995, 2003), the International Commission on Radiation Units and Measurements (ICRU 2002), and NCRP (2009b). The process shares several basic elements. These elements can be divided into: (1) the essential steps in the dose assessment process, and (2) the foundations of the entire dose reconstruction process that determine the methodology to perform each step. The essential steps and foundation elements of the dose reconstruction process are:
-
steps in the dose reconstruction process
definition of exposure scenarios
identification of exposure pathways
development and implementation of methods of estimating dose
evaluation of limitations and uncertainties in estimates of dose
-
foundation elements of the dose reconstruction process
data and other information
squality management (quality assurance and quality control).
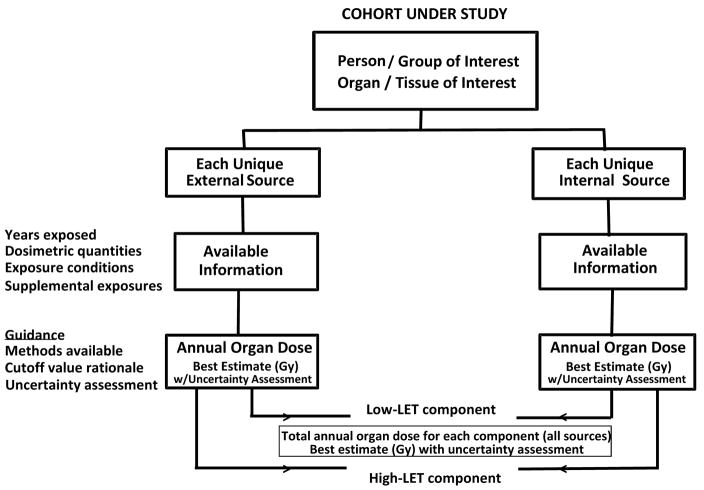
These steps and foundation elements are used to define the radiation field to which the individual was exposed, place the individual in time and space within that field, and estimate the uncertainty in the resulting dose reconstruction. Fig. 1 outlines how the guidance on deriving the same desired product (annual organ dose and its uncertainty) for each population will be described and presented in the final NCRP dosimetry report, so the reader can follow the different characteristics for each population. The flowchart applies to each individual or group of individuals similarly exposed. The objective is to obtain for that individual or group the total annual organ dose of interest (from all applicable external and internal radiation sources), with that total annual organ dose partitioned into its low- and high-LET components (both expressed in grays). For each partitioned sum, the associated uncertainty also should be derived. There are common aspects in all components of the MWS but the implementation of some of the elements of the generic flowchart may vary from study to study.
Fig 1.
Generic flow chart for the derivation of organ absorbed doses and their uncertainty.
For the purposes of the overall MWS, it was deemed helpful to provide a template for recording data in a comprehensive organ dose database of individual results that would apply to all members of the populations in the MWS. The template includes the relevant sources of exposure, the annual organ doses of interest, and some measure of uncertainty in the dose values. The results recorded in the template would derive from the flow charts prepared for each group of workers to achieve consistency in the overall approach used to estimate annual organ doses for very different populations of workers.
Estimation of absorbed doses from external irradiation
In the case of occupational exposure to external irradiation from photons, individual monitoring data, in terms of exposure (X) or of personal dose equivalent [Hp(10)], are often available. The main effort then consists in defining the exposure scenarios for the various tasks performed by the workers in order to determine the irradiation geometry and the energy spectrum of the incident photons on the body. A substantial part of the report is devoted to the estimation of the conversion coefficients relating the exposure and the personal dose equivalent to the absorbed doses in the organs of interest, given the photon energy spectrum and the irradiation geometry. It is important to note that the relative geometrical relationship between the dosimeter’s placement on the worker, the incident source direction, and the organ must be considered to estimate a mean organ absorbed dose. For many common irradiation geometries, namely anterior-posterior, posterior-anterior, lateral or rotational, tables and figures have been derived from data published by ICRP (2010) to relate the Hp(10) to the mean organ absorbed dose for male and female anatomies. For two irradiation geometries occasionally encountered in nuclear power plants (namely, cranial-caudal and caudal-cranial), conversion coefficients were calculated for the purposes of the NCRP dosimetry report. In the absence of detailed information on the irradiation geometry related to work activities, it is recommended to assume typical or representative geometries (e.g., 70% anterior-posterior and 30% rotational).
Another consideration is undetected dose, commonly referred to as missed dose, and unmonitored dose. Undetected dose is defined as the dose received that was not measured by the dosimeter, because it fell below the minimum detectable response of the dosimeter. Since the undetected dose may in reality range from zero to the minimum detectable or reportable dose, it is recommended to consider assigning some fraction of the minimum detectable dose for each monitoring period in which the dosimeter reads zero (i.e., less than the minimum detectable or reportable dose), although, ideally, the imputed dose should reflect historical expectations and experiences such as the frequency of doses being reported just above the minimum detectable dose for the individual or similarly exposed workers. For example, it is possible that a worker with an equal number of minimal readings and small positive readings to be receiving dose below the detectable limit and the fraction may be larger than an individual with an extended period of no doses above the detection limit where the record demonstrates a very low probability of exceeding the detection limit. It is also possible that the monitored individual never entered a radiation area and that the zero dose on record for extended reporting periods reflected this. Further, it seems unlikely whether any assignment should be made for internal radionuclides when multiple bioassays over time and whole body counting are all negative.
Unmonitored dose is that assumed to be received when a personal dosimeter was not worn, and often may be reconstructed from knowledge of workplace activities or from co-worker data when others in the same location did wear dosimeters. This latter approach is needed for some atomic-veteran scenarios, when one or two dosimeters were issued to a unit that may have totaled 40 or more individuals.
Additional complications are that (1) the recorded doses may include a mixture of radiations (e.g., photons and neutrons); (2) organs or tissues may be only partially irradiated (e.g., when medical personnel wear lead aprons); and (3) recorded doses from all facilities where the worker was exposed during his career, with possible differences regarding the photon energy spectra, the irradiation geometries and the measured dose quantities, must be processed. For some worker populations such as the nuclear utility workers these challenges are of less of a concern.
Estimation of absorbed doses from internal irradiation
In the case of occupational exposure from internal irradiation, the recorded dose, which is generally expressed in terms of committed effective dose or committed effective dose equivalent, is usually based on records of workplace air and surface monitoring, and individual bioassay data. The available information must be processed in order to (1) estimate the radionuclide intake and its characteristics, and (2) calculate the annual absorbed dose for each year of the entire period of time over which the dose is delivered, beginning with the date of first exposure and ending with the date of cancer diagnosis, if available, and otherwise the date of death or date last known to be alive. Guidelines for estimating internal doses in a large scale production facility are described in detail in the report and are summarized here as follows:
Characteristics of the intake
The development of exposure scenarios is generally required to characterize radionuclide intakes in a nuclear facility. These exposure scenarios include factors such as the route of intake, the time pattern of intake, the specific radionuclide(s) taken into the body, and the chemical and physical form of the deposited radionuclide(s). Typically the information used to build exposure scenarios comes from several sources:
A review of the site history including times and sites of elevated exposure and information pertinent to estimation of respiratory deposition fractions of clearance rates. Information from the site history pertinent to construction of exposure scenarios might include types of operations, specific tasks performed, types of radiation sources, and specific radionuclides present at the site, likely chemical and physical forms of airborne radionuclides, details of the monitoring program, radiological incidents, and periods and locations of prolonged above-normal exposures. Monitoring data for internal emitters usually consist primarily of measurements of radionuclides in air and in bioassay samples collected routinely at evenly spaced time intervals and more frequently following accidental intake of a radionuclide. The bioassay data usually consist primarily of measurements of specific radionuclides or groups of radionuclides (e.g., alpha emitters or mixed fission products) in urine but in some cases include fecal or blood measurements; external lung, thyroid, or total-body measurements of specific internal emitters; and measurement of activity in nasal swipes.
A review of work and exposure histories for individual radiation workers. Particularly useful information for purposes of building worker-specific or generic exposure scenarios often can be found in health physics records or “radiation folders” for the most extensively monitored workers who were involved in radiological incidents or prolonged elevated exposures in specific work areas. The monitoring data to be reviewed typically include area or personal air monitoring data and bioassay data.
A search for similarities among groups of workers in the time course and magnitude of positive bioassay data.
Interviews with long-time site workers; interviews with workers involved in different aspects of site operations can provide considerable insight into the nature of the operations and exposure situations. Ideally, interviews with a group of workers involved in different time periods and a range of job descriptions would be conducted at the start of the dose reconstruction process, and interviews with another group would be conducted later in the process, after the investigators have become familiar with the monitoring data and have identified many of the cases of elevated exposure.
Worker-specific exposure scenarios for internal emitters should be developed where feasible. Generic exposure scenarios should be developed for cases in which worker-specific data are sparse or missing.
Calculation of the annual absorbed doses
The annual absorbed doses are derived from the radionuclide intake, using (1) biokinetic models to estimate the variation with time of the radionuclide concentrations in the various organs and tissues of the body, and (2) dosimetric models to calculate the absorbed doses.
Biokinetic models used in an epidemiological study should describe typical rather than dosimetrically conservative behavior of radionuclides in the adult human body. The biokinetic models recommended in recent reports of ICRP often are the best available bioassay models. Use of alternate models may be justified in some cases. For application to poorly characterized exposures the default exposure mode generally is inhalation, and the default absorption type for an element is based on current ICRP recommendations. For a worker with sparse or missing internal monitoring data, data for a surrogate worker may be applied if a reasonably good case for surrogacy can be made. Suppose, for example, that records indicate that Workers A and B were involved in the same radiological incident, activity on nasal swabs taken immediately afterward the exposure was three times higher for A than for B, and A has long-term follow-up urinary excretion measurements but no such records are found for B. Then it seems reasonable to assume that B’s tissue doses associated with the incident are one-third the tissue doses calculated for A.
Dosimetric models are used to derive the organ absorbed doses from the temporal and spatial distribution of the activity of the radioactive material in the body; this is obtained using representations or models of human anatomy and composition appropriate for radiation transport. For the purposes of epidemiologic studies, the calculation of the absorbed dose is performed for each calendar year during and after the exposure. In practice, many of the radionuclides that are considered emit only low-LET radiations and have effective mean times of residence in the body that do not exceed several months, so that the numerical value of the absorbed dose per unit intake (in gray per becquerel) is equal to the committed equivalent dose coefficient (in sievert per becquerel) reported in ICRP publications. However, specific calculations have to be made if the radionuclide considered: (1) has a long effective mean time of residence in the body, as is the case for 90Sr or 239Pu, or (2) has a chemical form that requires the use of a biokinetic model more appropriate than those recommended by ICRP (e.g., UAlx), or (3) has a decay scheme that includes both low- and high-LET radiations.
Additional considerations
Detailed dose reconstructions for internal emitters often require considerable time and effort for purposes of a given epidemiological study, may not be justified below some level of intake of a given radionuclide. If comprehensive dose reconstructions for internal emitters would require prohibitive resources, it is useful to devise rapid screening techniques that yield conservative tissue dose estimates based on available monitoring data. Dose reconstructions for internal emitters can then be limited to workers whose initial dose estimates are above a criterion level representing insignificant tissue doses for purposes of the epidemiological study. The same observation applies to external doses that must be reconstructed on the basis of environmental measurements, as is the case for some of the atomic veterans.
Dose estimates for internal emitters should be radionuclide-specific (i.e., specific to each parent radionuclide taken into the body) and for each parent radionuclide should be broken down into low-LET absorbed dose and high-LET absorbed dose. Tissue-specific absorbed dose estimates for external irradiation and all addressed internal emitters should be added to get total absorbed dose estimates to tissues for individual workers. A template showing how the annual doses should be reported for each component of the MWS is included in the NCRP dosimetry report.
Assessment of uncertainties
All estimates of dose obtained in a dose reconstruction have limitations and are uncertain. Limitations and lack of certainty in estimated doses can result from such factors as:
lack of complete knowledge of an exposure scenario;
variability in relevant measurements;
lack of relevant data at locations and times of exposure;
lack of knowledge of relevant processes;
uncertainty in internal dosimetry; and
conversion of externally measured quantities to organ doses.
All uncertainties, including uncertainties in exposure scenarios and uncertainties in data and models used to estimate dose, should be considered and taken into account in an appropriate manner in a dose reconstruction. As discussed in the NCRP report as well as in previous NCRP (2007, 2009a, 2009b, 2012) reports, the uncertainties are often classified as:
aleatory or epistemic;
classical or Berkson;
random or systematic;
shared or unshared.
In the context of epidemiological studies, knowledge of the existence and likely magnitude of correlations arising from shared parameters is essential to proper interpretation and use of the dose estimates (e.g., Stram and Kopecky 2003; Li et al. 2007; NCRP 2009a).
There are three commonly used ways in which to represent uncertainty in dosimetry numerically. If almost all uncertainty is due to unshared uncertainty, due for example to imperfections in individual input data (location, shielding, etc.) that can be treated as independent from individual to individual then it is reasonable to attach to each dose estimate an uncertainty estimate applicable to only that dose.
Providing single uncertainty estimates can be improved if shared uncertainty can be quantified. In the NCRP dosimetry report, the calculation of what is essentially a covariance matrix of dose uncertainties for one of the atomic-veterans studies, those taking part in the eight-series detonations in the 1940s and 1950s, will be discussed. This idea of providing both dose estimates for N study participants and a covariance matrix (representing both shared and unshared uncertainty) was described in Stram and Kopecky (2003).
A third approach to representing uncertainty which is increasingly popular for complex exposure situations has been to represent the shared/unshared (and aleatory/epistemic) uncertainties as repeated draws from a complex dosimetry system that provides many as opposed to just one sample of dose. By appropriate Monte-Carlo sampling methods the repeated draws (or realizations) are designed to incorporate sharing of uncertainties, so that if two individuals share important uncertain dose determinants, they will tend to have a high correlation of doses between each other over all dose realizations that are sampled, whereas study participants exposed by entirely different pathways may have much less correlation in their doses over the sampled dose realizations. As pointed out by Stram and Kopecky (2003) it is most useful for epidemiologic analysis if each realization from the complex dosimetry system can be thought of as a possible value of true dose, sampled from the conditional distribution of true dose given all that is known about exposure determinants. The use of Monte-Carlo methods to evaluate the shared and unshared uncertainties in the framework of a complex dosimetry system requires much care and investigator effort, and should arguably only be produced when it is likely to represent an improvement over simpler procedures.
SUMMARY
The current draft of the NCRP dosimetry report provides guidance in the derivation of annual organ absorbed doses and their associated uncertainty for epidemiologic studies in general, but with a focus on the very diverse populations that make up the One Million U.S. Radiation Workers and Veterans Study, which include ~115,000 atomic veterans, 360,000 DOE workers, 330,000 nuclear power plant workers, 130,000 industrial radiographers, and 240,000 medical radiation workers.
The recommended guidelines can be summarized as follows:
The dosimetric quantity of interest is usually the annual absorbed dose to the organ or tissue that is assumed to be the origin of the subject’s cancer. For example, the dose to red bone marrow will be the radiation dose of interest if leukemia is the disease being considered in the epidemiologic study. Because life tables based on annual data are generally used in the epidemiologic analysis (e.g. Cox proportional hazard models), the absorbed dose will be calculated on an annual basis. The annual absorbed dose to the organ or tissue that is considered must be estimated for each year of the entire period of time over which the dose is delivered, beginning with the date of first exposure and ending with the date of cancer diagnosis, if available, and otherwise the date of death or the date last known to be alive. Although it is recognized that the dose distribution may not be uniform over all parts of the organ or tissue, it is assumed in the current draft of the NCRP dosimetry report that the mean dose over the entire organ or tissue is the quantity of interest in the epidemiologic analysis.
In the calculation of the annual absorbed doses, realistic models, assumptions, and parameter values will be used, the dose contribution from the high-LET components will be separated from the contribution from the low-LET components, and the uncertainties attached to the dose estimates will be evaluated.
At the planning stage of the study, all relevant information has to be collected and scenarios of exposure to radiation have to be established on the basis of that information, which includes all available dose records and monitoring data, reviews of the documents from the site files, and interviews of long-time site workers. For the workers that spent their career in several radiation facilities, the relevant records from those facilities have to be recovered in order to estimate the entire radiation exposure history for those workers (e.g. Boice et al. 2006). It is useful to prepare a flowchart identifying all steps of the dose assessment for all categories of study subjects.
At the implementation stage of the study. It is essential to create a database in which all relevant information and all parameter values used to assess the doses to each study subject and documented.
For most components of the MWS, the absorbed doses are due to external irradiation from photons of different energies under geometries that may differ from one group of workers to another. Strict rules will be followed to convert the dose record, which is usually expressed in terms of exposure or of personal dose equivalent, into the quantity of interest, which is the organ absorbed dose. It is important to note that the relative geometrical relationship between the dosimeter’s placement on the worker, the incident source direction, and the organ must be considered to estimate an average organ absorbed dose. Additional complications are that (1) the recorded doses may include a mixture of radiations (e.g., photons and neutrons); (2) organs or tissues may be only partially irradiated (e.g., when medical personnel wear lead aprons); and (3) recorded doses from all facilities where the worker was exposed during his career, with possible differences regarding the photon energy spectra, the irradiation geometries and the measured dose quantities, must be processed.
When processing dose records from external irradiation, another consideration is undetected dose, commonly referred to as missed dose, and unmonitored dose. Undetected dose is defined as the dose received that was not measured by the dosimeter, because it fell below the minimum detectable response of the dosimeter. Since the undetected dose may in reality range from zero to the minimum detectable, it assigning some fraction of the minimum detectable dose for each monitoring period in which the dosimeter read zero (i.e., less than the minimum detectable) should be considered. Unmonitored dose is that assumed to be received when a personal dosimeter was not worn, and often may be reconstructed from knowledge of workplace activities or from co-worker data when others in the same location did wear dosimeters. This latter approach is typically applied to atomic veterans in those cases when one or two dosimeters were issued to a unit that may have totaled 40 or more individuals.
In case of internal irradiation, the calculation of the absorbed dose will be performed for each calendar year during and after the exposure. In practice, many of the radionuclides that are considered emit only low-LET radiations and have effective mean times of residence in the body that do not exceed several months, so that the numerical value of the absorbed dose per unit intake (in gray per becquerel) is equal to the committed equivalent dose coefficient (in sievert per becquerel) reported in ICRP publications. However, specific calculations have to be made if the radionuclide considered: (1) has a long effective mean time of residence in the body, as is the case for 90Sr or 239Pu, or (2) has a chemical form that requires the use of a biokinetic model more appropriate than those recommended by ICRP (e.g., UAlx), or (3) has a decay scheme that includes both low- and high-LET radiations.
Detailed dose reconstructions for internal emitters often require considerable time and effort for purposes of a given epidemiological study, may not be justified below some level of intake of a given radionuclide. If comprehensive dose reconstructions for internal emitters would require prohibitive resources, it is useful to devise rapid screening techniques that yield conservative tissue dose estimates based on available monitoring data. Dose reconstructions for internal emitters can then be limited to workers whose initial dose estimates are above a criterion level representing insignificant tissue doses for purposes of the epidemiological study. The same observation applies to external doses that must be reconstructed on the basis of environmental measurements, as is the case for some of the atomic veterans.
All estimates of dose obtained in a dose reconstruction have limitations and are uncertain. All uncertainties, including uncertainties in exposure scenarios and uncertainties in data and models used to estimate dose, should be considered and taken into account in an appropriate manner in a dose reconstruction. In the context of epidemiologic studies, knowledge of the existence and likely magnitude of correlations arising from shared parameters is essential to proper interpretation and use of the dose estimates.
Table 7.
Main sources of radiation exposure for categories included in the MWS.
| Worker category | Main sources of radiation exposure |
|---|---|
| Atomic veterans | Gamma-emitting fission products (140Ba-La, 95Zr-Nb, etc.) and some intakes of radionuclides |
| Rocketdyne workers | Gamma -emitting radioactive materials and intakes of uranium and plutonium isotopes |
| Mound workers | Gamma -emitting radioactive materials and intakes of 210Po, 239Pu, 3H |
| Nuclear power plant workers | Gamma -emitting fission products (137Cs) and activation products (58Co, 60Co) |
| Industrial radiographers | 192Ir, 60Co, 75Se, x-ray units |
| Medical workers | Diagnostic x-ray production devices, x-ray or gamma-ray teletherapy units, 99mTc and other medical radiopharmaceuticals, brachytherapy sources such as radium. |
Acknowledgments
This research was supported in part by contracts and grants from the U.S. Department of Energy (Grant No. DE-SC0008944 awarded to NCRP) which included interagency support from the U.S. Nuclear Regulatory Commission, the U.S. Environmental Protection Agency and the National Aeronautics and Space Administration; the National Cancer Institute (Grant No. U01 CA137026); and a Discovery Grant from the Vanderbilt-Ingram Cancer Center (Center no. 404-357-9682). We also acknowledge Dr. Paul Blake, Director, Chief, Nuclear Test Personnel Review, Defense Threat Reduction Agency, Department of Defense and his staff for their technical support of the atomic-veterans project. Similarly, Han Kang and Tim Bullman, Environmental Epidemiology Service, U.S. Department of Veterans Affairs were instrumental in providing support and assistance throughout the conduct of the atomic-veteran study.
References
- Benton ER, Benton EV. Space radiation dosimetry in low-Earth orbit and beyond. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 2001;184(1):255–294. doi: 10.1016/s0168-583x(01)00748-0. [DOI] [PubMed] [Google Scholar]
- Blake PK1, Komp GR. Radiation exposure of U.S. military individuals. Health Phys. 2014;106(2):272–278. doi: 10.1097/HP.0000000000000032. [DOI] [PubMed] [Google Scholar]
- Blevins MR, Andersen RL. Radiation protection at U.S. nuclear power plants--today and tomorrow. Health Phys. 2011;100(1):35–38. doi: 10.1097/HP.0b013e3181f6590d. [DOI] [PubMed] [Google Scholar]
- Boice JD., Jr Study of atomic veterans who participated at U.S. aboveground atmospheric nuclear weapons tests, 1945–1962. [Accessed 28 September 2014];Health Phys News. 2012 Dec; Available at: http://www.ncrponline.org/PDFs/BOICE-HPnews/Dec-2012_AtomicVeterans.pdf.
- Boice JD., Jr Atomic bombs, asbestos and healthy warriors. [Accessed 28 September 2014];Health Phys News. 2014a Jan; Available at: http://www.ncrponline.org/PDFs/BOICE-HPnews/20_Atomic_Veterans_Jan2014.pdf.
- Boice JD., Jr The importance of radiation worker studies (editorial) J Radiol Prot. 2014b;34:E7–E12. doi: 10.1088/0952-4746/34/3/E7. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Mandel JS, Doody MM, Yoder RC, McGowan R. A health survey of radiologic technologists. Cancer. 1992;69:586–598. doi: 10.1002/1097-0142(19920115)69:2<586::aid-cncr2820690251>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Leggett RW, Ellis ED, Wallace PW, Mumma M, Cohen SS, Brill AB, Chadda B, Boecker BB, Yoder RC, Eckerman KF. A comprehensive dose reconstruction methodology for former Rocketdyne/Atomics International radiation workers. Health Phys. 2006;90:409–430. doi: 10.1097/01.HP.0000183763.02247.7e. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Cohen SS, Mumma MT, Ellis ED, Eckerman KF, Leggett RW, Boecker BB, Brill AB, Henderson BE. Updated mortality analysis of radiation workers at Rocketdyne (Atomics International), 1948–2008. Radiat Res. 2011;176:244–258. doi: 10.1667/RR2487.1. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Cohen SS, Mumma MT, Ellis ED, Cragle DL, Eckerman KF, Wallace PW, Chadda B, Sonderman JS, Wiggs LD, Richter BS, Leggett RW. Mortality among Mound workers exposed to Polonium-210 and other sources of radiation, 1944–1979. Radiat Res. 2014;181:208–228. doi: 10.1667/RR13395.1. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Lumley T, Ballantyne CM, Chambless LE, Kulich M. Using the whole cohort in the analysis of case-cohort data. Am J Epidemiol. 2009;169:1398–1405. doi: 10.1093/aje/kwp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta FA, Kim MH, Chappell L. Space radiation cancer risk projections and uncertainties. Hanover, MD: Center for AeroSpace Information; 2012. NASA/TP-2013–217375. [Google Scholar]
- Cucinotta FA, Kim MH, Chappell L, Huff JL. How safe is safe enough? Radiation risk for a human mission to Mars. PloS One. 2013;8(10):e74988. doi: 10.1371/journal.pone.0074988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta FA. Space radiation risks for astronauts on multiple International Space Station missions. PLoS One. 2014a Apr 23;9(4):e96099. doi: 10.1371/journal.pone.0096099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta FA, Alp M, Sulzman FM, Wang Space radiation risks to the central nervous system. Life Sciences in Space Reasearch. 2014b Jul;2:54–69. [Google Scholar]
- Dupree-Ellis E, Watkins J, Ingle JN, Phillips J. External radiation exposure and mortality in a cohort of uranium processing workers. Am J Epidemiol. 2000;152(1):91–95. doi: 10.1093/aje/152.1.91. [DOI] [PubMed] [Google Scholar]
- Fry SA, Dupree EA, Sipe AH, Seiler DL, Wallace PW. A study of mortality and morbidity among persons occupationally exposed to ≥50 mSv in a year: Phase I, mortality through 1984. Appl Occup Environ Hyg. 1996;11(4):334–343. [Google Scholar]
- Gilbert ES, Fix JJ, Baumgartner WV. An approach to evaluating bias and uncertainty in estimates of external dose obtained from personal dosimeters. Health Phys. 1996;70:336–345. doi: 10.1097/00004032-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Gilbert ES, Sokolnikov ME, Preston DL, Schonfeld SJ, Schadilov AE, Vasilenko EK, Koshurnikova NA. Lung cancer risks from plutonium: an updated analysis of data from the Mayak Worker Cohort. Radiat Res. 2013;179(3):332–342. doi: 10.1667/RR3054.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskip PD, Wang ZY, Fen YS. Suitability of Chinese oil well loggers for an epidemiologic study of the carcinogenic effects of neutrons. Health Phys. 1991;61(5):637–640. doi: 10.1097/00004032-199111000-00007. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiation Units and Measurements. Retrospective assessment of exposures to ionizing radiation. Vol. 68. Bethesda, MD: ICRU; 2002. Report. [Google Scholar]
- International Commission on Radiological Protection. Ann ICRP. 3. Vol. 1. Thousand Oaks, CA: Sage Publications; 1977. Recommendations of the ICRP. ICRP Publication 26. [Google Scholar]
- International Commission on Radiological Protection. Ann ICRP. 2–5. Vol. 40. Thousand Oaks, CA: Sage Publications; 2010. Conversion coefficients for radiological protection quantities for external radiation exposures. ICRP Publication 116. [DOI] [PubMed] [Google Scholar]
- Johnson JC, Thaul S, Page WF, Crawford H. Mortality of veteran participants in the CROSSROADS nuclear test. Washington DC: National Academies Press; 1996. [PubMed] [Google Scholar]
- Khokhryakov VV, Khokhryakov VF, Suslova KG, Vostrotin VV, Vvedensky VE, Sokolova AB, Krahenbuhl MP, Birchall A, Miller SC, Schadilov AE, Ephimov AV. Mayak Worker Dosimetry System 2008 (MWDS-2008): assessment of internal dose from measurement results of plutonium activity in urine. Health Physics. 2013;104:366–378. doi: 10.1097/HP.0b013e31827dbf60. [DOI] [PubMed] [Google Scholar]
- Leggett RW, Eckerman KF, Boice JD., Jr A respiratory model for uranium aluminide based on occupational data. J Radiol Prot. 2005;25:405–416. doi: 10.1088/0952-4746/25/4/004. [DOI] [PubMed] [Google Scholar]
- Li Y, Guolo A, Hoffman FO, Carroll RJ. Shared uncertainty in measurement error problems with application to Nevada Test Site fallout data. Biometrics. 2007;63:1226–1234. doi: 10.1111/j.1541-0420.2007.00810.x. [DOI] [PubMed] [Google Scholar]
- Lushbaugh CC, Fry SA, Ricks RC. Medical and radiobiological basis of radiation accident management. Br J Radiol. 1987;60(720):1159–1163. doi: 10.1259/0007-1285-60-720-1159. [DOI] [PubMed] [Google Scholar]
- Muirhead CR, Cox R, Stather JW, MacGibbon BH, Edwards AA, Haylock RGE. Estimates of late radiation risks to the UK population London: Health Protection Agency; Doc. NRPB. 1993;4:13–157. [Google Scholar]
- Napier BA. Joint U.S./Russian studies of population exposures resulting from nuclear production activities in the Southern Urals. Health Phys. 2014;106(2):294–304. doi: 10.1097/HP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- National Academies/National Research Council. Radiation dose reconstruction for epidemiologic uses. Washington, DC: National Academies Press; 1995. [Google Scholar]
- National Academies/National Research Council. A review of the dose reconstruction program of the Defense Threat Reduction Agency. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- National Council on Radiation Protection and Measurements. Uncertainties in the measurement and dosimetry of external radiation. Bethesda, MD: NCRP; 2007. Report No. 158. [Google Scholar]
- National Council on Radiation Protection and Measurements. Uncertainties in internal radiation dose assessment. Bethesda, MD: NCRP; 2009a. Report No. 164. [Google Scholar]
- National Council on Radiation Protection and Measurements. Radiation dose reconstruction: principles and practices. Bethesda, MD: NCRP; 2009b. Report No. 163. [Google Scholar]
- National Council on Radiation Protection and Measurements. Uncertainties in the estimation of radiation risks and probability of disease causation. Bethesda, MD: NCRP; 2012. Report No. 171. [Google Scholar]
- Nondestructive Testing Education Resource Center (2001–2014) The collaboration for NDT education [online] Iowa: NDTERC, Iowa State University; [Accessed 28 September 2014]. 2014. Available at: https://www.nde-ed.org/index_flash.htm. [Google Scholar]
- Parker MD, Tolmachev SY. Annual report of the United States Transuranium and Uranium Registries: October 2010–March 2012. Richland, WA: Washington State University; 2013. USTUR-0344-12. [Google Scholar]
- Radiation Exposure Information and Reporting System (REIRS) for Radiation Workers [online] Washington, DC: U.S. Nuclear Regulatory Commission; [Accessed 28 September 2014]. 2011. Available at: http://www.reirs.com/ [Google Scholar]
- Reames DV. Solar energetic particles: A paradigm shift. Reviews of Geophysics. 1995;33(S1):585–589. [Google Scholar]
- Ricks RC, Berger ME, Holloway EC, Goans RE. REAC/TS Radiation Accident Registry: Update of accidents in the United States. 10th International Congress of the International Radiation Protection Association (IRPA) [online]; International Radiation Protection Association; [Accessed 28 September 2014]. 2000. Available at: http://www.irpa.net/irpa10/cdrom/00325.pdf. T-21-2, P-11-238. [Google Scholar]
- Simon SL. Organ-specific external dose coefficients and protective apron transmission factors for historical dose reconstruction for medical personnel. Health Phys. 2011;101(1):13–27. doi: 10.1097/HP.0b013e318204a60a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Weinstock RM, Doody MM, Neton J, Wenzl T, Stewart P, Mohan AK, Yoder RC, Hauptmann M, Freedman DM, Cardarelli J, Feng HA, Bouville A, Linet M. Estimating historical radiation doses to a cohort of U.S. radiologic technologists. Radiat Res. 2006;166:174–192. doi: 10.1667/RR3433.1. [DOI] [PubMed] [Google Scholar]
- Simon SL, Preston DL, Linet MS, Miller JS, Sigurdson AJ, Alexander BH, Kwon D, Yoder RC, Bhatti P, Little MP, Rajaraman P, Melo DM, Drozdovitch V, Weinstock RM, Doody MM. Radiation organ doses received in a nationwide cohort of U.S. radiologic technologists: methods and findings. Radiat Res. 2014a doi: 10.1667/RR13542.1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Hoffman FO, Hofer E. The two-dimensional Monte Carlo: a new methodologic paradigm for dose reconstruction for epidemiological studies. Radiat Res. 2014b doi: 10.1667/RR13729.1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JA. Elemental and isotopic composition of the galactic cosmic rays. Ann Rev Nucl Sci. 1983;33(1):323–382. [Google Scholar]
- Smetanin M, Vasilenko EK, Lyubarskaya I, Knyazev V, Gorelov M, Scherpelz RI, Fix JJ. Mayak film dosimeter response studies, part II: response models. Health Phys. 2007a;93:231–238. doi: 10.1097/01.HP.0000268729.56660.b9. [DOI] [PubMed] [Google Scholar]
- Smetanin M, Vasilenko EK, Scherpelz RI. Mayak film dosimeter response studies, part III: application to worker dose assessment. Health Phys. 2007b;93:239–244. doi: 10.1097/01.HP.0000268727.68079.d7. [DOI] [PubMed] [Google Scholar]
- Stram DO, Kopecky KJ. Power analysis of epidemiological studies of radiation-related disease risk when dose estimates are based on a complex dosimetry system with an application to the Hanford Thyroid Disease Study. Radiat Res. 2003:408–417. doi: 10.1667/3046. [DOI] [PubMed] [Google Scholar]
- Tawn EJ, Whitehouse CA, Tarone RE. FISH chromosome aberration analysis on retired radiation workers from the Sellafield nuclear facility. Radiat Res. 2004;162:249–256. doi: 10.1667/rr3214. [DOI] [PubMed] [Google Scholar]
- Thaul S, Page WF, Crawford H, O’Maonaigh H. The five series study: the mortality of military participants in U.S. nuclear weapons tests. Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- Till JE, Beck HB, Aanenson JW, Grogan HA, Mohler HJ, Mohler SS, Voillequé PG. Military participants at the U.S. atmospheric nuclear weapons testing–methodology for estimating dose and uncertainty. Radiat Res. 2014;181:471–484. doi: 10.1667/RR13597.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilenko EK, Khokhryakov VF, Miller SC, Fix JJ, Eckerman KF, Choe DO, Gorelov M, Khokhryakov VV, Knyazev V, Krahenbuhl MP, Scherpelz RI, Smetanin M, Suslova KG, Vostrotin V. Mayak worker dosimetry study: an overview. Health Phys. 2007a;93:190–206. doi: 10.1097/01.HP.0000266071.43137.0e. [DOI] [PubMed] [Google Scholar]
- Vasilenko EK, Knyazev V, Gorelov M, Smetanin M, Scherpelz RI, Fix JJ. Mayak film dosimeter response studies, part I: measurements. Health Phys. 2007b;93:220–230. doi: 10.1097/01.HP.0000267861.63767.a7. [DOI] [PubMed] [Google Scholar]
- Wacholder S. Practical considerations in choosing between the case-cohort and nested case-control designs. Epidemiology. 1991;2:155–158. doi: 10.1097/00001648-199103000-00013. [DOI] [PubMed] [Google Scholar]
- Wakeford R, Tawn EJ. Editorial: The meaning of low dose and low dose-rate. J Radiol Protect. 2010;30(1):1–3. doi: 10.1088/0952-4746/30/1/E02. [DOI] [PubMed] [Google Scholar]
- Watanabe KK, Kang HK, Dalager NA. Cancer mortality risk among military participants of a 1958 atmospheric nuclear weapons test. Am J Public Health. 1995;85:523–527. doi: 10.2105/ajph.85.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs LD, Johnson ER, Cox-DeVore CA, Voelz GL. Mortality through 1990 among white male workers at the Los Alamos National Laboratory: considering exposures to plutonium and external ionizing radiation. Health Phys. 1994;67(6):577–588. doi: 10.1097/00004032-199412000-00001. [DOI] [PubMed] [Google Scholar]