Abstract
Despite major improvements in allogeneic hematopoietic cell transplantation over the last decades, corticosteroid-refractory (SR) acute (a) and chronic (c) graft-versus-host disease (GVHD) cause high mortality. Pre-clinical evidence indicates the potent anti-inflammatory properties of the JAK1/2 inhibitor ruxolitinib.
In this retrospective survey, 19 stem cell transplant centers in Europe and the United States reported outcome data from 95 patients who had received ruxolitinib as salvage-therapy for SR-GVHD. Patients were classified as having SR-aGVHD (n=54, all grade III or IV) or SR-cGVHD (n=41, all moderate or severe). The median number of previous GVHD-therapies was 3 for both SR-aGVHD (1–7) and SR-cGVHD (1–10).
The ORR was 81.5% (44/54) in SR-aGVHD including 25 CRs (46.3%), while for SR-cGVHD the ORR was 85.4% (35/41). Of those patients responding to ruxolitinib, the rate of GVHD-relapse was 6.8% (3/44) and 5.7% (2/35) for SR-aGVHD and SR-cGVHD, respectively. The 6-month-survival was 79% (67.3%–90.7%,95% CI) and 97.4% (92.3%–100%,95% CI) for SR-aGVHD and SR-cGVHD, respectively. Cytopenia and CMV-reactivation were observed during ruxolitinib-treatment in both SR-aGVHD (30/54, 55.6% and 18/54, 33.3%) and SR-cGVHD (7/41, 17.1% and 6/41, 14.6%) patients. Ruxolitinib may constitute a promising new treatment option for SR-aGVHD and SR-cGVHD that should be validated in a prospective trial.
Introduction
The curative potential of allogeneic hematopoietic stem cell transplantation (allo-HCT) is hampered by acute and chronic graft-versus-host disease (GVHD). Despite prophylactic treatment with immunosuppressive agents, 20% to 80% of recipients develop acute GVHD (aGVHD). Corticosteroid-refractory GVHD (SR-GVHD) is associated with a dismal outcome 1, 2, with only 5% to 30% long-term survival 3. Chronic GVHD (cGVHD) causes high morbidity, and is associated with a significantly higher risk of treatment-related mortality and inferior overall survival 4. Steroids currently represent the gold-standard treatment for aGVHD based on prospective randomized trials, while second-line therapy is based on data from retrospective analyses, one phase III trial and uncontrolled phase II trials 3. Available second-line therapy approaches such as cyclosporine A (CYA), sirolimus, tacrolimus, mycophenolate mofetil (MMF), pentostatin, infliximab, daclizumab, alemtuzumab, mesenchymal stroma cells (MSC), Antithymocyte globulin (ATG) or extracorporal photopheresis (ECP) have shown some activity, but none has been established as a standard salvage therapy for SR-aGVHD, which is reflected in the non-uniform strategies in SR-aGVHD applied by different transplant centers 5. For SR-cGVHD second-line therapies are CYA, sirolimus, tacrolimus, MMF, ECP or experimentally low dose IL-2 6, 7.
We previously reported that ruxolitinib, a selective Janus kinase (JAK) 1/2 inhibitor approved for the treatment of myelofibrosis 8, was effective for the treatment of GVHD in a murine aGVHD model 9. JAK1/2 signalling has been shown to be instrumental in multiple steps leading to inflammation and tissue damage in GVHD. A critical event involved in T cell activation, lineage commitment and survival is signaling through the common gamma chain, a constituent of the receptor complexes for six different interleukins (ILs): IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 10. Common gamma chain signaling occurs via JAK1 and we were recently able to identify the common gamma chain as a potent therapeutic target in aGVHD and cGVHD 11. Besides its role in adaptive immune responses, JAK1/2 signaling was also reported to play a central role in innate immunity, including activation of neutrophils 12. Our group and others have shown an important role for neutrophils in the pathogenesis of aGVHD 13, 14. Furthermore, dendritic cells (DC) were shown to depend on JAK1/2 activation during differentiation and maturation 15, which may also reduce priming of incoming donor T cells by recipient DC after allo-HCT. In addition to these preclinical findings, the JAK-inhibitor tofacitinib was shown to have clinical efficacy in rheumatoid arthritis 16 and ulcerative colitis 17, supporting the concept of a potent anti-inflammatory effect for JAK inhibition in patients. We here report safety and response data to ruxolitinib salvage-treatment in patients suffering from either SR-cGVHD or SR-aGVHD in a retrospective multicenter survey involving institutions in Europe and the United States. We observed high response rates (>80%) and 6-month survival rates in both disease entities, although the patients were heavily pretreated and all had either severe acute (grade III or IV) or chronic (moderate to severe) GVHD. These findings may pave the way for a novel targeted therapy approach in patients with this life-threatening complication after allo-HCT and sets the stage for future prospective testing of this approach against other therapeutic modalities in SR-GVDH in prospective clinical trials.
Patients and Methods
Patients
Patients were recruited at the University Medical Centers of Freiburg, Marburg, Basel, Munich, Essen, Bonn, Frankfurt, Cologne, Paris, Berlin, Hamburg, Düsseldorf, Dresden, Würzburg, Stanford, Gothenburg, Nijmegen, Utrecht and Patras between January 2012 and April 2015. This cohort was selected by including all patients that were reported to receive ruxolitinib for aGVHD or cGVHD by the different centers and no reported patient was excluded. In the different centers patients were informed of the off-label use of ruxolitinib and gave their informed consent. Consent to publish of patient photos was obtained. Serum sample collection and analysis were approved by the institutional Ethic committee review board of the Freiburg University Medical Center. Histological GVHD grading was performed on the basis of a published staging system 18 and clinical grading was according to criteria for aGVHD 19 or cGVHD 20. The patients’ characteristics are shown in Table 1. Transplant characteristics including donor type, conditioning regimen, and immunosuppressive regimen used as prophylaxis for GVHD or as treatment, are detailed for aGVHD or cGVHD in Suppl. Tables 1 and 2, respectively.
Table 1.
aGvHD and cGvHD patients characteristics
| Variable | aGvHD (n=54) | cGvHD (n=41) |
|---|---|---|
| Pt. age in years median (range) | 51 (21–75) | 55 (22–74) |
| Gender | ||
| female | 31.5 (17) | 29.3 (12) |
| male | 68.5 (37) | 70.7 (29) |
| % (absolute number) | % (absolute number) | |
| Disease | ||
|
| ||
| AML | 48.1 (26) | 51.2 (21) |
|
| ||
| ALL | 11.1 (6) | - |
|
| ||
| MDS | 9.2 (5) | 7.3 (3) |
|
| ||
| NHL | 7.4 (4) | 7.3 (3) |
|
| ||
| MM | 7.4 (4) | 2.4 (1) |
|
| ||
| CMMoL | 1.8 (1) | 4.9 (2) |
|
| ||
| PMF | 3.7 (2) | 12.2 (5) |
|
| ||
| CLL | 3.7 (2) | 12.2 (5) |
|
| ||
| M. Hodgkin | 1.8 (1) | - |
|
| ||
| CML | 1.8 (1) | - |
|
| ||
| T-PLL | 1.8 (1) | - |
|
| ||
| HLH | 1.8 (1) | - |
|
| ||
| MPN (nonPMF) | - | 2.4 (1) |
|
| ||
| CMV serostatus | ||
|
| ||
| R+/D− | 24.1 (13) | 21.9 (9) |
|
| ||
| R−/D− | 27.8 (15) | 41.5 (17) |
|
| ||
| All others | 48.1 (26) | 34.1 (14) (unknown in 1 pt) |
Abbreviations: AML = Acute myeloid leukemia, ALL = Acute lymphoblastic leukemia, MDS = Myelodysplastic syndrome, NHL = Non Hodgkins lymphoma, MM = Multiple myeloma, CMMoL = chronic myelomonocytic leukemia, PMF = Primary myelofibrosis, CLL = Chronic lymphoid leukemia, M. Hodgkin = Hodgkin lymphoma, CML = chronic myeloid leukemia, T-PLL = T-prolymphocytic leukemia, HLH = Hemophagocytic lymphohistiocytosis, MPN = Myeloproliferative neoplasms (excluding PMF), R+ recipient CMV positive, R- recipient CMV negative, D- donor CMV negative
Inclusion and exclusion criteria
Patients were included in this retrospective survey if they were treated with ruxolitinib for GVHD that was refractory to corticosteroids given for at least 1 week for aGVHD or 3 weeks for cGVHD based on previous definitions for SR-aGVHD 21 or SR-cGVHD 22. Initial treatment for cGVHD and aGVHD for the majority of patients was prednisone at 1 mg/kg/day. For cGVHD, the presence of at least one diagnostic clinical sign of cGVHD, or the presence of at least one distinctive manifestation confirmed by pertinent biopsy was used 20. aGVHD was defined according to previously published criteria 19.
Treatment and evaluation of response
The majority of patients was treated with ruxolitinib as an add-on immunosuppression therapy at a dose of 5–10 mg orally twice daily. Evaluation of response was done for aGVHD according to previously defined diagnostic criteria for aGVHD 19. In brief, treatment responses were categorized as complete response (CR), partial response (PR) or treatment failure. A CR to ruxolitinib was defined as the absence of any symptoms related to GVHD. A PR was defined as the improvement of at least one stage in the severity of aGVHD in one organ without deterioration in any other organ. A response had to last for at least 3 weeks. Treatment failure was defined by the absence of improvement of aGVHD, deterioration of aGVHD in any organ by at least one stage, the development of aGVHD manifestations in a previously unaffected organ, and the use of any additional agents to control the disease. Patients were scored for their best response at any time after starting treatment with ruxolitinib, with follow-up censored at the onset of any subsequent systemic immunosuppressive therapy.
In case of cGVHD, organ sites considered for GVHD scoring included skin, mouth, eyes, intestinal tract, liver, lungs, joints and fascia, and the genital tract. Each organ or site was scored according to a 4-point scale (0–3), with 0 representing no involvement and 3 reflecting severe impairment 20. A CR to ruxolitinib was defined as the absence of any symptoms related to cGVHD. PR of cGVHD was defined as the discontinuation or long-lasting (4 weeks) reduction of all systemic immunosuppressive therapy by at least 50%. Failure was defined as the use of any additional agents to control GVHD once treatment with ruxolitinib had started, including the resumption of agents used earlier or an increase in the dose of any immunosuppressive treatment. Discontinuation of treatment with ruxolitinib because of toxicity was not considered a treatment failure. The duration of response was calculated from the time of onset of response after initiation of treatment with ruxolitinib until the end of the follow-up, GVHD relapse, the development of new or the deterioration of pre-existing GVHD symptoms, or the reinstitution of any additional agents to control the disease.
Defining the severity of cytopenias
Cytopenias were defined according to NCI-CTCAE grades. Anemia: grade 1: Hb <LLN to 10.0 g/dL, grade 2: Hb <10.0 g/dL to 8.0 g/dL, grade 3: Hb <8.0 g/dL; transfusion indicated, grade 4: Life-threatening consequences; urgent intervention indicated. Neutropenia: grade 1: Neutrophils <LLN to 1,500/mm3, grade 2: Neutrophils <1,500 to 1,000/mm3, grade 3: Neutrophils <1,000 to 500/mm3, grade 4: Neutrophils <500/mm3. Thrombocytopenia: grade 1: Platelets <LLN to 75,000/mm3, grade 2: <75,000 to 50,000/mm3, grade 3: <50,000 to 25,000/mm3, grade 4: <25,000/mm3.
Cytokine measurements
The levels of IL-6, soluble IL-2R, and CD3+HLA-DR+ T cells were analyzed from peripheral blood on the day before or within 5 days after starting ruxolitinib in 10 random patients at Freiburg University Medical Center.
Mouse model of cGVHD
B10.BR recipients (male 6–12 weeks old) were conditioned with cyclophosphamide (Sigma, St. Louis, MO, USA) on days −3 and −2 (120 mg/kg/day i.p.) as previously described 23. On day −1, recipients received whole body gamma irradiation (8.3 Gy). B6 donor BM was T cell depleted with anti-Thy1.2 mAb followed by rabbit complement. T cells were purified from spleens by incubation with biotin-labeled anti-CD19 mAb (eBioscience, San Diego, USA), followed by EasySep streptavidin rapidspheres, and were then depleted on a magnetic column (StemCell Technologies, Vancouver, BC, Canada). On day 0, recipients received 1 × 107 T cell depleted BM cells with or without purified splenic T cells (1 × 105). Ruxolitinib (Novartis, Basel, Switzerland) was given on days 28–56 after transplantation. All experimental procedures were approved by and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Minnesota, the University
Statistics
Data were analysed using SAS statistical software version 9.2 (SAS Institute Inc, Cary, NC, USA). Overall survival (OS) was calculated as the time from start of treatment with ruxolitinib to death from any cause. Patients still alive at the last follow-up were treated as censored observations. OS rates were estimated and displayed using the Kaplan Meier method.
The duration of response was assessed for responders only by calculating the time from first observation of response to the first observation of GVHD relapse or progression. Death without prior observation of GVHD relapse or progression was considered to be a competing risk, and observations where neither death nor GVHD relapse or progression occurred were treated as censored observations. One patient’s observation time was censored when stopping ruxolitinib treatment due to allergic reactions. Cumulative incidence rates for GVHD relapse were estimated using the Aalen-Johanson estimator24.
Results
Patients with severe aGVHD respond to ruxolitinib
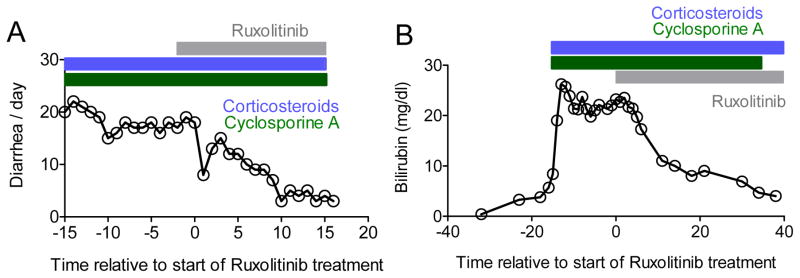
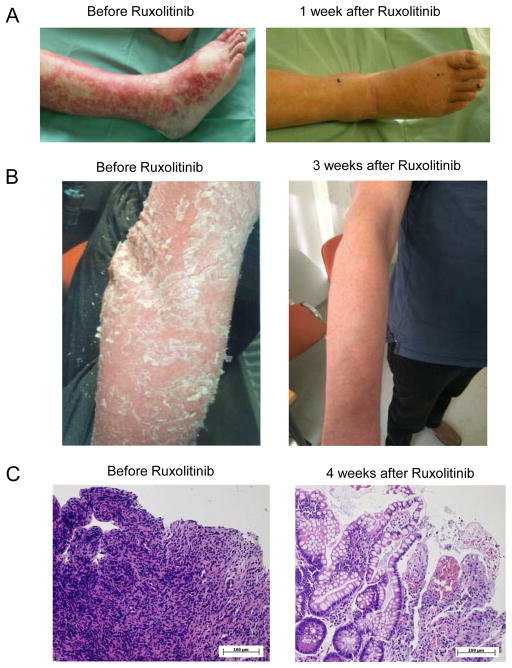
A total of 54 patients with aGVHD, with 34/54 (63%) patients having multiple organ involvement (Figure 1A), were treated with ruxolitinib. All patients had grade 3 or 4 aGVHD and 39/54 (72.2%) patients were beyond second line treatment for aGVHD (Suppl. Table 1). The median number of previous aGVHD-therapies was 3 (range: 1–7). The overall response rate (ORR) was 81.5% (44/54) including 25 CRs (46.3%). The median time to response was 1.5 (1–11) weeks after initiation of ruxolitinib treatment. Several patients with severe intestinal (Figure 1A), liver (Figure 1B) or skin GVHD (Figure 2A, B) showed impressive responses to ruxolitinib. One patient with grade 4 intestinal SR-aGVHD received sequential biopsies before and after response to ruxolitinib. Histological evaluation showed massive destruction of the intestinal epithelium before ruxolitinib treatment and significant epithelial regeneration of the intestine after ruxolitinib therapy was initiated (Figure 2C).
Figure 1. Representative responses in patients with acute GVHD of the liver and intestines.
A: One patient with histologically proven intestinal GVHD grade IV was treated with ruxolitinib as described in the Methods. No other immunosuppressive therapy was started at the same time-point. The patient also had corticosteroids (blue area) and cyclosporine A (green area) when ruxolitinib was started. The frequency of diarrhea decreased upon treatment with ruxolitinib and corticosteroids could be tapered.
B: One patient with clinically diagnosed liver GVHD grade IV was treated with ruxolitinib as described in the Methods. The bilirubin level decreased following ruxolitinib treatment. No other immunosuppressive therapy was started at the same time-point although corticosteroid treatment (blue area) was continued and reduced by 50% in the observation period. There was no change in treatment with regard to potential liver toxic agents throughout the entire time period displayed.
Figure 2. Skin and intestinal GvHD responds to ruxolitinib.
A: A representative patient with cutaneous acute GVHD is shown prior and 1 week after ruxolitinib.
B: A representative patient with cutaneous chronic GVHD is shown prior and 3 weeks after ruxolitinib.
C: Serial biopsies of the intestinal tract of a patient with GVHD is displayed. Biopsies were taken 1 day prior to start of ruxolitinib and 4 weeks after ruxolitinib had been started.
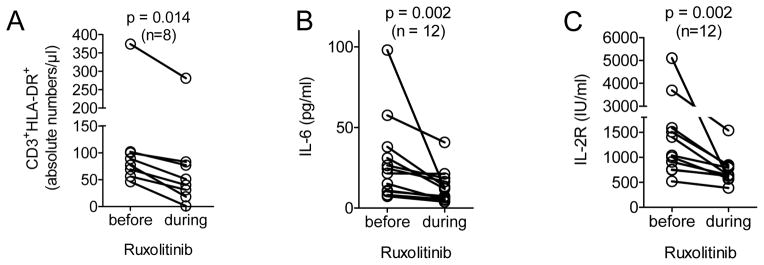
The absolute number of activated T cells (CD3+HLA-DR+) were measured in 8/25 patients and levels of IL-6 and the soluble IL-2 (R) receptor were determined in 12/25 patients treated at the Transplant Center Freiburg. We observed a significant decline in all three pro-inflammatory parameters during ruxolitinib treatment compared to the day before the drug was started (Figure 3A–C), and all of these patients responded to ruxolitinib treatment. These data indicate that ruxolitinib can induce significant clinical responses in a majority of patients suffering from SR-aGVHD going along with a marked reduction of pro-inflammatory cytokines.
Figure 3. Inflammation related markers in the blood decrease upon ruxolitinib treatment.
A–C: CD3+HLA-DR+ cells, IL-6 and soluble IL-2R were measured one day prior and 5 to 7 days after the start of ruxolitinib in the peripheral blood. The levels of these 3 parameters declined significantly after ruxolitinib treatment when analysed by the Wilcoxon matched-pairs signed rank test. The number of patients are indicated in each graph.
OS and GVHD-relapse after ruxolitinib treatment in patients with aGVHD
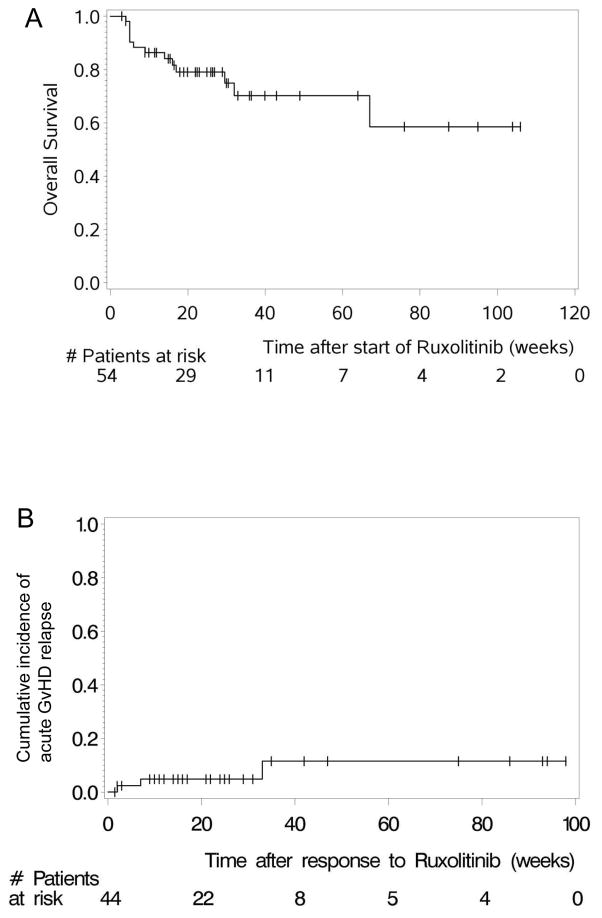
The 6-month survival estimate was 79% (67.3%–90.7%, 95% CI) in patients treated with ruxolitinib for SR-aGVHD (Figure 4A). The median follow-up time was 26.5 (3–106) weeks for SR-aGVHD patients. The GVHD relapse rate was analysed for patients who had achieved CR or PR. The cumulative incidence of aGVHD relapse was low (Figure 4B). Relapses in aGVHD occurred in 6.8% (3/44) of ruxolitinib-responsive patients (2 PR, 1 CR). Thus, ruxolitinib induces durable clinical responses in SR-aGVHD.
Figure 4. Acute GVHD-relapse free and overall survival.
A: The overall survival of all patients treated with ruxolitinib for acute GVHD is displayed.
B: The cumulative incidence of acute GVHD relapse is displayed.
Ruxolitinib improves cGVHD
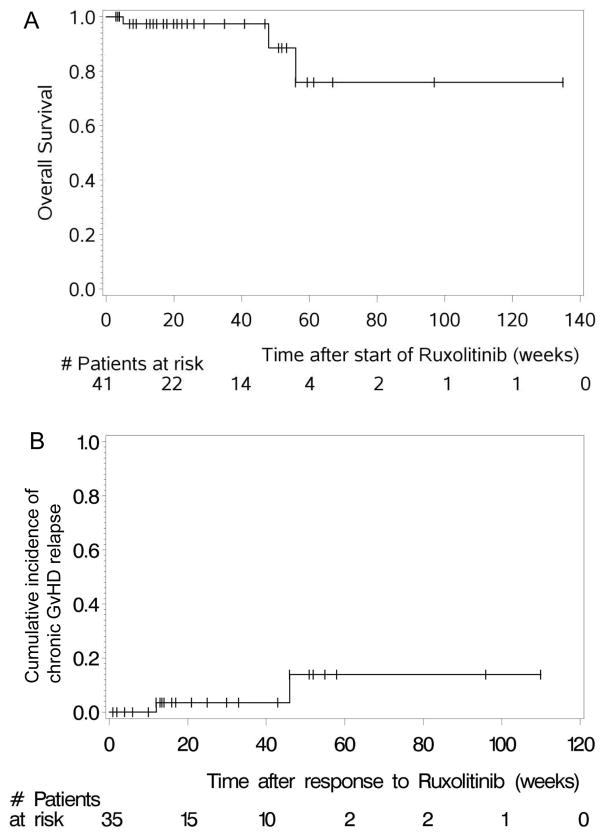
Preclinical efficacy of ruxolitinib has only been proven in a murine aGVHD model 9. Therefore, we evaluated the therapeutic potential of ruxolitinib in a murine cGVHD model 23. We observed significantly improved cGVHD in ruxolitinib versus vehicle-treated mice, as measured by pulmonary function and levels of collagen deposition (Suppl. Fig 1, 2). On the basis of these preclinical results, ruxolitinib was applied to 41 patients suffering from cGVHD (Table 1) involving the skin, the liver, intestinal tract, the lungs and musculoskeletal tissues. In 29/41 (70.7%) of patients more than one organ system was involved. All patients had moderate (n = 6/41, 14.6%) to severe (n = 35, 85.4%) cGVHD. Most patients were beyond second line treatment for cGVHD, with a median number of 3 prior treatments (range: 1–10) before ruxolitinib was administered (Suppl. Table 2). The ORR was 85.4% (35/41), with 78% (32/41) PR and 7.3% (3/41) CR. 14.6% (6/41) of the patients showed no response. Responses to ruxolitinib were not restricted to specific organ systems affected by SR-cGVHD. The median time to response was 3 (1–25) weeks after initiation of ruxolitinib treatment.
OS after ruxolitinib treatment for cGVHD
The 6-month survival estimate was 97.4% (92.3%–100%, 95% CI) for patients treated with ruxolitinib for SR-cGVHD (Figure 5A). The cumulative incidence of cGVHD relapse was low (Figure 5B). GVHD relapsed in 5.7% (2/35) of ruxolitinib-responsive (CR or PR) patients with cGVHD. The median follow-up was 22.4 (3–135) weeks for cGVHD patients. One of the patients with cGVHD who died developed metastatic squamous cell carcinoma of the lung and died of this disease.
Figure 5. Chronic GVHD-relapse free and overall survival.
A: The overall survival of all patients treated with ruxolitinib for chronic GVHD is displayed.
B: The cumulative incidence of chronic GVHD relapse is displayed.
Infections, toxicity and relapse under ruxolitinib treatment
Cytomegalovirus (CMV) reactivation was observed in both SR-aGVHD (18/54, 33.3%) and SR-cGVHD (6/41, 14.6%) patients (Table 2). CMV infection was controlled by antiviral therapy in all patients even though ruxolitinib therapy was continued, indicating that ruxolitinib treatment does not alleviate CMV treatment response. One patient was diagnosed with CMV retinitis that responded to valgancyclovir treatment. Overall, these findings indicate that in patients treated with ruxolitinib for GVHD, infectious surveillance is critical and particularly CMV needs to be monitored carefully.
Table 2.
Adverse events
| Variable | aGvHD (n=54) | cGvHD (n=41) |
|---|---|---|
| % (absolute number) | % (absolute number) | |
| CMV reactivation | 33.3 (18) | 14.6 (6) |
|
| ||
| Severe cytopenia (Grade 3 and 4) | 33.3 (18) | 7.3 (3) |
|
| ||
| Mild cytopenia (Grade 1 and 2) | 22.2 (12) | 9.7 (4) |
|
| ||
| Cytopenia before ruxolitinib | 51.8 (28) | 14.6 (6) |
|
| ||
| Malignancy relapse | 9.2 (5) | 2.4 (1) |
Cytopenias (anemia, leukopenia or thrombocytopenia) are a known side effect of ruxolitinib 8, 25 and were also observed in our SR-aGVHD (30/54, 55.5%) and SR-cGVHD (7/41, 17%) patients. Severe cytopenia (Grade 3 and 4) was found in 33.3% (18/54) and 7.3% (3/54) of patients (Table 2). However, cytopenias preceded ruxolitinib treatment in 51.8% (28/54) and 14.6% (6/41) of the patients with SR-aGVHD and SR-cGVHD, respectively (Table 2).
Relapse of the underlying malignancy occurred in 9.3% (5/54) and 2.4% (1/41) of the patients with SR-aGVHD or SR-cGVHD, respectively. One responding patient relapsed four weeks after ruxolitinib had been discontinued. One non-responder relapsed after ruxolitinib had been discontinued. The other four patients were responders and still on ruxolitinib at the time of relapse.
Discussion
During the last two decades, the number of patients undergoing allo-HCT has more than doubled worldwide. GVHD remains the major hurdle to improve allo-HCT outcome, and patients suffering from SR-aGVHD are very likely to die 1–3 while cGVHD is associated with a decreased quality of life. While corticosteroids are the established first-line treatment, there is no established second-line treatment and response rates beyond first line are unsatisfactory 3. Our previous data indicated that ruxolitinib improves murine aGVHD, and induced responses in six patients with SR-GVHD treated with ruxolitinib 9. Further, our current preclinical data indicate that cGVHD may be suppressed by ruxolinitib treatment. To understand if ruxolitinib could have a role as a salvage therapy for GVHD patients which failed to respond to corticosteroids, we collected the data from multiple Stem Cell Transplantation Centers in the US and Europe which had treated patients with ruxolitinib for GVHD.
Patients had a median of three immunosuppressive treatments prior to ruxolitinib for both acute and chronic GVHD. Despite this heavily pre-treated population, the ORR was 81.5% (44/54) in aGVHD including 25 CRs (46.3%). In cGVHD, the ORR was 85.4% (35/41) with the majority of patients achieving a PR (78%). The time to response was variable, with a maximum of 25 weeks in a patient with cGVHD and a minimum of 1 week, indicating the heterogeneous biology of SR-cGVHD in individual patients. Responses were durable as shown by the long GVHD-relapse free survival rates in both aGVHD and cGVHD patients.
While controlled trials on ruxolitinib in GVHD versus available second line therapies have yet to be performed, our data suggest that response rates of both SR-aGVHD and SR-cGVHD to ruxolitinib were favourable compared to other second line GVHD therapies reported. As the retrospective analyses from multiple centers could bias towards a selection of responding patients we asked the centers to include any patient irrespective of the response. The mTOR inhibitors sirolimus or everolimus are frequently used as second line treatment for aGVHD. Response rates to mTOR inhibitors of 24% to 72% were reported in a phase 2 trial (21 patients) and in 2 retrospective analyses (22 and 34 patients) 26–28. A frequently used agent for second-line treatment of SR-aGVHD is MMF 5. The reported ORR to MMF range between 15% and 31% 29, 30. The reported ORR of aGVHD to ECP range between 47% and 65% 31–34. Antibodies against TNF (etanercept, infliximab) are used as second-line agents for SR-aGVHD with a wide range of ORR reported 35. ATG yielded response rates ranging from 8% to 56% in SR-aGVHD 36, 37. A particular side effect of ATG was Epstein-Barr virus–associated post-transplant lymphoproliferative disorder 36–38, which we did not observe in patients treated with ruxolitinib. Reported ORR were 50% to 65% to alemtuzumab 39, 40 and 58% to methotrexate 41 in patients with SR-aGVHD. Given the limitation of a direct comparison from different studies, these ORR appear lower when compared to the ORR of 81% seen in our retrospective cohort of SR-aGVHD patients exposed to ruxolitinib. Intestinal SR-aGVHD might affect the bioavailability of ruxolinitib. The fact that 36/42 of the patients with intestinal SR-aGVHD responded suggest that ruxolitinib is resorbed at least partially. Serum levels of ruxolitinib need to be evaluated in patients with SR-aGVHD in a prospective trial.
The 6-month survival estimate was 79% (67.3%–90.7%, 95% CI) in our patients treated with ruxolitinib for SR-aGVHD. The weighted average 6-month survival estimate across 25 studies on SR-aGVHD was 49% 3. Within these retrospective or phase 2 trials, the largest study analysed the impact of horse ATG as a second line treatment and included 79 patients 42. The 6-month survival estimate for this study was 44% 42. In a different study, ECP was given to 23 patients with SR-aGVHD with a reported 6-month survival rate of 57% 32. In contrast to our survey, this study also analysed grade II aGVHD patients (n=10) while our survey included only grade III or IV aGVHD patients. Patients with SR-aGVHD treated with daclizumab and infliximab died at a median of 35 days from initiation of daclizumab/infliximab 43. The 6-month survival estimate ranged from 0% 44 to 61% 40 in SR-GVHD patients treated with alemtuzumab.
In our survey, the ORR of SR-cGVHD to ruxolitinib was 85.4%. Most studies including sirolimus, MMF, ECP, IL-2 or CYA reported ORR of 45–72% 6, 7, 45. However as for SR-aGVHD, a randomized phase-II or III trial will be necessary to draw definitive conclusions. Furthermore the confidence intervals of responses from previous trials are wide, making a direct comparison difficult.
The exact mechanism of how JAK1/2 inhibition could interfere with the allogeneic immune response while established immunosuppressive medications have failed remains unclear. However, it is likely that interfering with the immune response at multiple levels via JAK1/2 inhibition might be more potent than targeting individual pathways such as the calcineurin/NFAT or mTOR pathways. Cytokine signals in target cells are integrated via cytokine receptor mediated JAK1 and JAK2 phosphorylation as for example gamma chain cytokines that promote the pathogenesis of GVHD 11, 46–48. Furthermore, besides their role in T cells, JAK1 and 2 were shown to be required for the activation of neutrophils 12 and DCs 15, which both contribute to GVHD 13, 14.
The safety profile of ruxolitinib in our survey was rather favourable. Consistent with its side effect-profile in myeloproliferative neoplasms 8, 25, the drug induced cytopenias in both patient cohorts. This side effect is well described under ruxolitinib and reversible upon discontinuation of the drug. Cytopenias had preceded ruxolitinib treatment in 51.9% (28/54) and 14.6% (6/41) of the patients with SR-aGVHD or SR-cGVHD, respectively. The frequency of cytopenias was not unexpectedly high but requires regular monitoring of blood counts in patients receiving ruxolitinib as a salvage treatment for SR-GVHD.
CMV reactivation was observed during ruxolitinib treatment in both acute (33.3%) and chronic (14.6%) SR-GVHD patients, indicating that CMV copy numbers need to be closely monitored in patients receiving ruxolitinib as a salvage treatment for GVHD, and that antiviral therapy needs to be started rapidly when needed. A comparable frequency of CMV reactivation was reported in patients treated with other immunosuppressive drugs including steroids, infliximab, alemtuzumab, MMF or CYA 39, 40, 43. Positive CMV antigenemia was observed in 73% of MMF-treated 49 and 7/8 of pentostatin-treated 50 SR-aGVHD patients. GVHD severity was correlated with CMV reactivation in multiple reported studies 51. Therefore, better control of GVHD by ruxolitinib could in the long-term also improve CMV control as reported for MSC treatment 52.
An important concern in patients receiving novel immunosuppressive treatments is a potential loss of the graft-versus-leukemia (GVL) effect, finally leading to an increased relapse risk. Relapse of the underlying malignancy in ruxolitinib-exposed patients was low, as only 9.3% (5/54) and 2.4% (1/41) of the patients with acute or chronic GVHD, respectively had disease recurrence. This frequency is comparable to other studies and suggests that ruxolitinib treatment is not linked to a higher relapse risk when compared to other currently applied immunosuppressive drugs.
Overall, this retrospective survey of a large patient number treated with ruxolitinib due to SR-aGVHD and SR-cGVHD in various European and US transplant centers highlights the therapeutic potential of JAK-inhibitors in the treatment of SR-GVHD. JAK-inhibitor treatment was safe and well tolerated in this heavily pre-treated patient cohort. The high ORR above 80% in both SR-aGVHD and SR-cGVHD supports the potential clinical value of this compound as salvage therapy for SR-GVHD. Importantly, the 6-month overall survival for ruxolitinib-treated SR-aGVHD patients was higher than those previously reported for any other treatment modality in this particular scenario. Our results set the stage for future testing of ruxolitinib in acute and chronic SR-GVHD against best available therapy in prospective clinical trials.
Supplementary Material
Acknowledgments
Funding: DFG (RZ, ZE872/3-1), NIH (BRB, P01 CA142106), German José Carreras Leukemia Foundation (AN, AH06-01), DKTK (R.Z., N.v.B.).
Footnotes
Authors contribution: Authors contributed patient data and reviewed the manuscript. A.S.G. analysed the histopathology of the intestinal GVHD patient that was treated with ruxolitinib and provided the images. J.D., R.F. and B.R.B. contributed the data on murine cGVHD. G.I. helped the statistical analysis of the study. R.Z. and N.v.B. analysed the data and wrote the manuscript.
Supplementary information is available at Leukemia’s website.
Declaration of interests: F. Ayuk, U. Holtick, C. Scheid, G. Bug, J. Kuball, N. Kröger, N. von Bubnoff: Research support from Novartis, Bristol Meyer Squibb. D. Wolf: grants and personal fees from Novartis. R. Zeiser: Speakers fee from Bristol Meyer Squibb, travel grant from Gilead. All grants from Novartis were outside/unrelated to the submitted work. The other authors have no conflict of interest to disclose.
References
- 1.MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115:5412–5417. doi: 10.1182/blood-2009-12-258442. [DOI] [PubMed] [Google Scholar]
- 2.Westin JR, Saliba RM, De Lima M, Alousi A, Hosing C, Qazilbash MH, et al. Steroid-refractory acute GVHD: predictors and outcomes. Adv Hematol. 2011;2011:601953–601953. doi: 10.1155/2011/601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin P, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18:1150–1163. doi: 10.1016/j.bbmt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124:374–384. doi: 10.1182/blood-2014-01-514752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff D, Ayuk F, Elmaagacli A, Bertz H, Lawitschka A, Schleuning M, et al. Current practice in diagnosis and treatment of acute graft-versus-host disease: results from a survey among German-Austrian-Swiss hematopoietic stem cell transplant centers. Biol Blood Marrow Transplant. 2013;19:767–776. doi: 10.1016/j.bbmt.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greinix HT, Worel N, Just U, Knobler R. Extracorporeal photopheresis in acute and chronic graft-versus-host disease. Transfus Apher Sci. 2014;50:349–357. doi: 10.1016/j.transci.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 9.Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123:3832–3842. doi: 10.1182/blood-2013-12-543736. [DOI] [PubMed] [Google Scholar]
- 10.He YW, Adkins B, Furse RK, Malek TR. Expression and function of the gamma c subunit of the IL-2, IL-4, and IL-7 receptors. Distinct interaction of gamma c in the IL-4 receptor. J Immunol. 1995;154:1596–1606. [PubMed] [Google Scholar]
- 11.Hechinger AK, Smith BA, Flynn R, Hanke K, McDonald-Hyman C, Taylor PA, et al. Therapeutic activity of multiple common gamma chain cytokine inhibition in acute and chronic GVHD. Blood. 2015;125:570–580. doi: 10.1182/blood-2014-06-581793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson SE, Oates AC, Harpur AG, Ziemiecki A, Wilks AF, Layton JE. Tyrosine kinase JAK1 is associated with the granulocyte-colony-stimulating factor receptor and both become tyrosine-phosphorylated after receptor activation. PNAS. 1994;91:2985–2988. doi: 10.1073/pnas.91.8.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwab L, Goroncy L, Palaniyandi S, Gautam S, Triantafyllopoulou A, Mocsai A, et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance GVHD via tissue damage. Nat Med. 2014;20:648–654. doi: 10.1038/nm.3517. [DOI] [PubMed] [Google Scholar]
- 14.Giroux M, Delisle JS, Gauthier SD, Heinonen KM, Hinsinger J, Houde B, et al. SMAD3 prevents graft-versus-host disease by restraining Th1 differentiation and granulocyte-mediated tissue damage. Blood. 2011;117:1734–1744. doi: 10.1182/blood-2010-05-287649. [DOI] [PubMed] [Google Scholar]
- 15.Heine A, Held SA, Daecke SN, Wallner S, Yajnanarayana SP, Kurts C, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122:1192–1202. doi: 10.1182/blood-2013-03-484642. [DOI] [PubMed] [Google Scholar]
- 16.Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253–261. doi: 10.7326/0003-4819-159-4-201308200-00006. [DOI] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 18.Lerner K, Kao GF, Storb R, Buckner CD, Clift RA, Thomas ED. Histopathology of graft-vs. -host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant Proc. 1974;6:367–371. [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Greinix HT, Volc-Platzer B, Kalhs P, Fischer G, Rosenmayr A, Keil F, et al. Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood. 2000;96:2426–2431. [PubMed] [Google Scholar]
- 22.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn R, Du J, Veenstra RG, Reichenbach DK, Panoskaltsis-Mortari A, Taylor PA, et al. Increased T follicular helper cells and germinal center B cells are required for chronic GVHD (cGVHD) and bronchiolitis obliterans. Blood. 2014;123:3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012 Mar 1;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benito AI, Furlong T, Martin PJ, Anasetti C, Appelbaum FR, Doney K, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation. 2001;72:1924–1929. doi: 10.1097/00007890-200112270-00010. [DOI] [PubMed] [Google Scholar]
- 27.Ghez D, Rubio MT, Maillard N, Suarez F, Chandesris MO, Delarue R, et al. Rapamycin for refractory acute graft-versus-host disease. Transplantation. 2009;88:1081–1087. doi: 10.1097/TP.0b013e3181ba0a13. [DOI] [PubMed] [Google Scholar]
- 28.Hoda D, Pidala J, Salgado-Vila N, Kim J, Perkins J, Bookout R, et al. Sirolimus for treatment of steroid-refractory acute graft-versus-host disease. Bone Marrow Transplant. 2010;45:1347–1351. doi: 10.1038/bmt.2009.343. [DOI] [PubMed] [Google Scholar]
- 29.Furlong T, Martin P, Flowers ME, Carnevale-Schianca F, Yatscoff R, Chauncey T, et al. Therapy with mycophenolate mofetil for refractory acute and chronic GVHD. Bone Marrow Transplant. 2009;44:739–748. doi: 10.1038/bmt.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JG, Sohn SK, Kim DH, Lee NY, Suh J, Lee KS, et al. Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant. Eur J Haematol. 2004;73:56–61. doi: 10.1111/j.1600-0609.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 31.Greinix HT, Knobler RM, Worel N, Schneider B, Schneeberger A, Hoecker P, et al. The effect of intensified extracorporeal photochemotherapy on long-term survival in patients with severe acute graft-versus-host disease. Haematologica. 2006;91:405–408. [PubMed] [Google Scholar]
- 32.Perfetti P, Carlier P, Strada P, Gualandi F, Occhini D, Van Lint MT, et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant. 2008;42:609–617. doi: 10.1038/bmt.2008.221. [DOI] [PubMed] [Google Scholar]
- 33.Berger M, Albiani R, Sini B, Fagioli F. Extracorporeal photopheresis for graft-versus-host disease: the role of patient, transplant, and classification criteria and hematologic values on outcome-results from a large single-center study. Transfusion. 2014 Oct 29; doi: 10.1111/trf.12900. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hautmann AH, Wolff D, Hahn J, Edinger M, Schirmer N, Ammer J, et al. Extracorporeal photopheresis in 62 patients with acute and chronic GVHD: results of treatment with the COBE Spectra System. Bone Marrow Transplant. 2013;48:439–445. doi: 10.1038/bmt.2012.156. [DOI] [PubMed] [Google Scholar]
- 35.Busca A, Locatelli F, Marmont F, Ceretto C, Falda M, et al. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82:45–52. doi: 10.1002/ajh.20752. [DOI] [PubMed] [Google Scholar]
- 36.Arai S, Margolis J, Zahurak M, Anders V, Vogelsang GB. Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol Blood Marrow Transplant. 2002;8:155–160. doi: 10.1053/bbmt.2002.v8.pm11939605. [DOI] [PubMed] [Google Scholar]
- 37.Ballesteros M, Ferra C, Serrano D, Batlle M, Ribera JM, Díez-Martín JL. Antithymocyte globulin therapy for steroid-resistant acute graft versus host disease. Am J Hematol. 2008;83:824–825. doi: 10.1002/ajh.21251. [DOI] [PubMed] [Google Scholar]
- 38.Van Lint MT, Milone G, Leotta S, Uderzo C, Scimè R, Dallorso S, et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006;107:4177–4181. doi: 10.1182/blood-2005-12-4851. [DOI] [PubMed] [Google Scholar]
- 39.Schnitzler M, Hasskarl J, Egger M, Bertz H, Finke J. Successful treatment of severe acute intestinal graft-versus-host resistant to systemic and topical steroids with alemtuzumab. Biol Blood Marrow Transplant. 2009;15:910–918. doi: 10.1016/j.bbmt.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Schub N, Gunther A, Schrauder A, Claviez A, Ehlert C, Gramatzki M, et al. Therapy of steroid-refractory acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow Transplant. 2011;46:143–147. doi: 10.1038/bmt.2010.68. [DOI] [PubMed] [Google Scholar]
- 41.de Lavallade H, Mohty M, Faucher C, Fürst S, El-Cheikh J, Blaise D. Low-dose methotrexate as salvage therapy for refractory graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation. Haematologica. 2006;91:1438–1440. [PubMed] [Google Scholar]
- 42.MacMillan MLW, Davies SM, DeFor TE, Burns LJ, Ramsay NK, et al. Early antithymothyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:40–46. doi: 10.1053/bbmt.2002.v8.pm11858189. [DOI] [PubMed] [Google Scholar]
- 43.Rager A, Frey N, Goldstein SC, Reshef R, Hexner EO, Loren A, et al. Inflammatory cytokine inhibition with combination daclizumab and infliximab for steroid-refractory acute GVHD. Bone Marrow Transplant. 2011;46:430–435. doi: 10.1038/bmt.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez C, Solano C, Ferrá C, Sampol A, Valcárcel D, Pérez-Simón JA. Alemtuzumab as treatment of steroid-refractory acute graft-versus-host disease: results of a phase II study. Biol Blood Marrow Transplant. 2009;15:639–642. doi: 10.1016/j.bbmt.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Mielke S, Lutz M, Schmidhuber J, Kapp M, Ditz D, Ammer J, et al. Salvage therapy with everolimus reduces the severity of treatment-refractory chronic GVHD without impairing disease control: a dual center retrospective analysis. Bone Marrow Transplant. 2014;49:1412–1418. doi: 10.1038/bmt.2014.170. [DOI] [PubMed] [Google Scholar]
- 46.Dean RM, Fry T, Mackall C, Steinberg SM, Hakim F, Fowler D, et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol. 2008;26:5735–5741. doi: 10.1200/JCO.2008.17.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thiant S, Labalette M, Trauet J, Coiteux V, de Berranger E, Dessaint JP, et al. Plasma levels of IL-7 and IL-15 after reduced intensity conditioned allo-SCT and relationship to acute GVHD. Bone Marrow Transplant. 2011;46:1374–1381. doi: 10.1038/bmt.2010.300. [DOI] [PubMed] [Google Scholar]
- 48.Bucher C, Koch L, Vogtenhuber C, Goren E, Munger M, Panoskaltsis-Mortari A, et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood. 2009;114:5375–5384. doi: 10.1182/blood-2009-05-221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onishi C, Ohashi K, Sawada T, Nakano M, Kobayashi T, Yamashita T, et al. A high risk of life-threatening infectious complications in mycophenolate mofetil treatment for acute or chronic graft-versus-host disease. Int J Hematol. 2010;91:464–470. doi: 10.1007/s12185-010-0516-x. [DOI] [PubMed] [Google Scholar]
- 50.Poi MJ, Hofmeister CC, Johnston JS, Edwards RB, Jansak BS, Lucas DM, et al. Standard pentostatin dose reductions in renal insufficiency are not adequate: selected patients with steroid-refractory acute graft-versus-host disease. Clin Pharmacokinet. 2013;52:705–712. doi: 10.1007/s40262-013-0064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gor D, Sabin C, Prentice HG, Vyas N, Man S, Griffiths PD, et al. Longitudinal fluctuations in cytomegalovirus load in bone marrow transplant patients: relationship between peak virus load, donor/recipient serostatus, acute GVHD and CMV disease. Bone Marrow Transplant. 1998;21:597–605. doi: 10.1038/sj.bmt.1701139. [DOI] [PubMed] [Google Scholar]
- 52.Te Boome LC, Mansilla C, van der Wagen LE, Lindemans CA, Petersen EJ, Spierings E, et al. Biomarker profiling of steroid-resistant acute GVHD in patients after infusion of mesenchymal stromal cells. Leukemia. 2015 doi: 10.1038/leu.2015.89. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.