Maize chitinases, Pr4 and Endochitinase A, in fall armyworm frass suppress herbivore-induced defenses in maize.
Abstract
The perception of herbivory by plants is known to be triggered by the deposition of insect-derived factors such as saliva and oral secretions, oviposition materials, and even feces. Such insect-derived materials harbor chemical cues that may elicit herbivore and/or pathogen-induced defenses in plants. Several insect-derived molecules that trigger herbivore-induced defenses in plants are known; however, insect-derived molecules suppressing them are largely unknown. In this study, we identified two plant chitinases from fall armyworm (Spodoptera frugiperda) larval frass that suppress herbivore defenses while simultaneously inducing pathogen defenses in maize (Zea mays). Fall armyworm larvae feed in enclosed whorls of maize plants, where frass accumulates over extended periods of time in close proximity to damaged leaf tissue. Our study shows that maize chitinases, Pr4 and Endochitinase A, are induced during herbivory and subsequently deposited on the host with the feces. These plant chitinases mediate the suppression of herbivore-induced defenses, thereby increasing the performance of the insect on the host. Pr4 and Endochitinase A also trigger the antagonistic pathogen defense pathway in maize and suppress fungal pathogen growth on maize leaves. Frass-induced suppression of herbivore defenses by deposition of the plant-derived chitinases Pr4 and Endochitinase A is a unique way an insect can co-opt the plant’s defense proteins for its own benefit. It is also a phenomenon unlike the induction of herbivore defenses by insect oral secretions in most host-herbivore systems.
Insects are the most speciose group of organisms on the planet, more than half of which are phytophagous (Schoonhoven et al., 2005). Plants respond to insect herbivory with an arsenal of defenses, both direct defenses, when plants synthesize molecules that are detrimental to the insect pests, and indirect defenses, when plants emit volatiles after insect attack that recruit natural enemies of the pests (Chen, 2008). The recognition of herbivory by plants has been attributed to different insect behaviors, such as feeding, crawling, oviposition, and even defecation (Pechan et al., 2002; Felton and Tumlinson, 2008; Peiffer et al., 2009; Hilfiker et al., 2014; Ray et al., 2015). Herbivory not only mechanically damages the host tissue but also includes secretions such as insect saliva, regurgitant, or frass (Felton and Tumlinson, 2008; Ray et al., 2015). The molecules deposited from these secretions that trigger herbivore defenses have been referred to as elicitors or herbivore-associated molecular patterns (HAMPs) that are analogous to pathogen-associated molecular patterns (Bent and Mackey, 2007; Felton and Tumlinson, 2008; Acevedo et al., 2015; Schmelz, 2015). Plants may also recognize fragments of self molecules that have been referred to as damage-associated molecular patterns (Karban and Shiojiri, 2009; Acevedo et al., 2015). Several studies have identified some of these HAMPs and damage-associated molecular patterns from insect regurgitant and saliva (Acevedo et al., 2015; Schmelz, 2015). Similar to the model of pathogen-induced defense responses in plants, HAMP-mediated plant defenses may be suppressed by the other molecules in the herbivore secretions known as effectors (Felton and Tumlinson, 2008).
A strong antagonism exists between the pathogen and herbivore defense pathways in most plant species (Thaler et al., 2012). The induction of the herbivore defense pathway is often associated with a simultaneous suppression of the pathogen defense pathway and vice versa. While the jasmonic acid (JA) signaling pathway primarily mediates resistance to herbivores, the salicylic acid (SA) signaling pathway primarily mediates resistance against biotrophic pathogens (Glazebrook, 2005; Thaler et al., 2012). Although herbivory and insect-associated elicitors of plant defenses typically trigger the JA pathway (Alborn et al., 1997, 2007; Musser et al., 2002; Schmelz et al., 2007; Tian et al., 2012; Chuang et al., 2014b), insect secretions may suppress the herbivore defense pathway while simultaneously triggering the pathogen defense pathway (Zarate et al., 2007; Chung et al., 2013). For example, silverleaf whitefly (Bemisia tabaci) herbivory induces pathogen defenses in tomato (Solanum lycopersicum; Zarate et al., 2007), and Colorado potato beetle (Leptinotarsa decemlineata) oral secretions harbor microbes from the beetle gut that suppress JA-induced defenses in tomato (Chung et al., 2013). These studies strongly imply that insect herbivores can harbor effector molecules that not only suppress herbivore-induced defenses in plants but also trigger pathogen-induced defenses.
Although considerable work has been done on understanding plant responses to insect oral secretions, saliva, and oviposition, relatively little is known about how insect feces induces plant defense responses. In a recent work, it was shown that pea aphid honeydew contains SA that suppresses herbivore-induced defenses (Schwartzberg and Tumlinson, 2014). We recently showed that frass from the chewing herbivore fall armyworm (FAW; Spodoptera frugiperda) can suppress herbivore defenses in maize (Zea mays) over time, which leads to increased herbivory on the plant (Ray et al., 2015). This finding was specifically important in host-herbivore systems such as maize and FAW, where frass accumulates in the enclosed feeding site of the maize whorl and remains near the damaged areas for extended periods of time. This also strongly suggested the presence of effector molecule(s) in FAW frass that suppress herbivore defenses triggered by insect feeding. We also observed that proteins in FAW frass were responsible for inducing pathogen defenses in maize, thereby suppressing herbivore defenses (Ray et al., 2015). Therefore, in this study, we fractionated the proteins from FAW frass in an effort to identify potential effector molecules that are responsible for this suppression. Since insect frass is composed of proteins derived from the insect, plant, or microbes present in the frass, we performed MudPIT (Multidimensional Protein Identification Technology) analysis on the frass protein fractions that induced pathogen defenses and matched the peptides to protein databases from maize, lepidopterans, and microbes. Our search identified maize chitinases as possible effector molecules that induce pathogen defenses while suppressing herbivore defenses on the host. These maize chitinases are induced in response to insect herbivory and pass through the insect gut to be deposited back onto the host tissue (Rodríguez et al., 2012).
RESULTS
Proteins with Basic and Acidic pI Values in FAW Frass Induced Pathogen Defenses in Maize
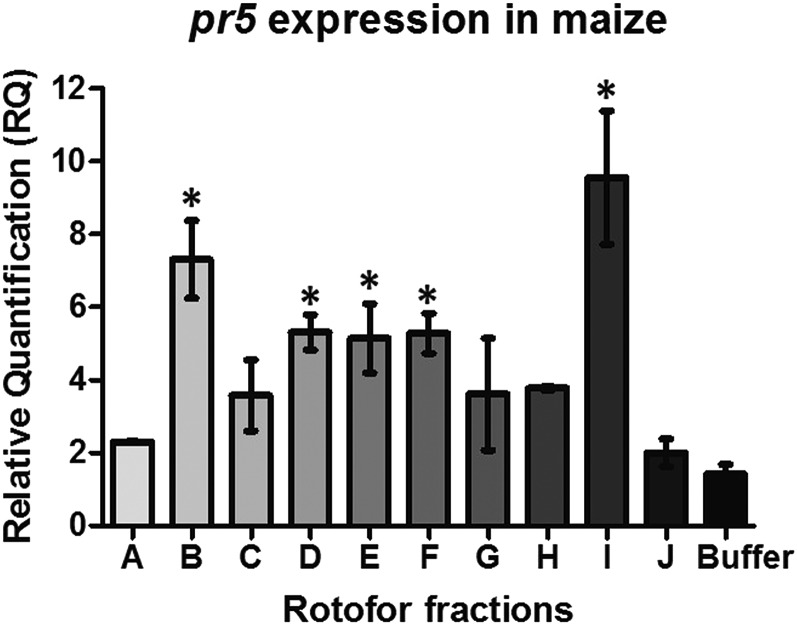
In a previous study, we showed that proteins from FAW frass triggered a temporal shift in plant defenses from herbivore to pathogen defenses in maize (Ray et al., 2015). In an attempt to identify the protein molecule(s) in frass that trigger the pathogen defense pathway, we fractionated frass proteins into 10 fractions based on their pI values that successively increased from pH 3 to 3.5 to pH 9.5 to 10. Each fraction was applied to maize plants, and the transcript abundance of the pathogen defense gene marker pathogenesis-related protein5 (pr5) was measured after 24 h. We observed that fractions containing proteins with acidic pI values (fraction B with pH range of 3.5 to 4), mildly acidic pI values (fraction D with pH ranging from 5.5 to 6 and fraction E with pH ranging from 6.5 to 7), mildly basic pI values (fraction F with pH of 7.5), and a fraction with proteins having a basic pI (fraction I with pH range of 8.5 to 9) induced higher pr5 transcript abundance compared with the buffer-treated plants (Fig. 1). This suggests that frass proteins responsible for inducing pathogen defenses in maize were in fractions B, D, E, F, and I.
Figure 1.
Maize pathogen defense gene expression in response to protein fractions from isoelectric separation of frass proteins. Maize leaves were wounded and treated with 10 protein fractions (A–J) or PBS buffer for 24 h, and the pathogen defense gene marker pr5 transcript abundance was measured by quantitative real-time (qRT)-PCR. Transcript abundance of pr5 was normalized to that of the reference gene actin and expressed as relative quantification (RQ). Data were analyzed by one-factor ANOVA, and mean separation was calculated by multiple comparison Tukey’s test. RQ values of frass-treated gene expression marked with asterisk are significantly different from buffer-treated expression (P < 0.05). Four plants were used as biological replicates for each treatment, and error bars indicate se.
Maize Chitinases Are in the Frass Fractions That Induce Pathogen Defenses
Frass protein fractions B and I induced the highest levels of pr5 RNA compared with buffer-treated plants (Fig. 1). Although fraction I showed the highest amount of pr5 induction, the number of proteins that we observed in fraction B were much higher than the ones we found in fraction I (Supplemental Fig. S1); hence, for this study, we chose to perform MudPIT analysis of fraction B only. We also identified proteins in fraction A that failed to induce pathogen defense marker pr5 expression but were adjacent to fraction B. We speculated that there could be an overlap of proteins in these two fractions that would help us identify unique proteins responsible for defense induction. Fraction B contained several maize chitinases, including Pr4 (gi|226501166), Prm3 (gi|226501166), and Endochitinase A (gi|226506778), as shown in Table I. β-Glucosidase (gi|162464369), maize lipoxygenases (gi|162463394), and maize protease inhibitors (gi|226496089 and gi|162459393) also were abundant in this fraction, but they were also quite abundant in fraction A that did not induce pathogen defenses (Table II). This suggested that chitinases in FAW frass could be involved in triggering the pathogen defenses.
Table I. MudPIT analysis of Rotofor fraction B.
| Protein Name | Accession No. | Source Species | Peptide Count (95%) |
|---|---|---|---|
| Endochitinase PR4 precursor | gi|226501166 | Zea mays | 15 |
| β-d-Glucosidase precursor | gi|162464369 | Zea mays | 15 |
| Cys protease1 precursor | gi|226496089 | Zea mays | 13 |
| Uncharacterized protein LOC100272880 precursor | gi|226508498 | Zea mays | 12 |
| Cys protease component of protease inhibitor complex precursor | gi|162459393 | Zea mays | 10 |
| Cys proteinase Mir3 precursor | gi|162463464 | Zea mays | 10 |
| Cystatin-1 precursor | gi|162463594 | Zea mays | 10 |
| Uncharacterized protein LOC100192522 | gi|212721998 | Zea mays | 9 |
| Lipoxygenase6 | gi|162463394 | Zea mays | 9 |
| Cystatin-1 precursor | gi|162463594 | Zea mays | 8 |
| Uncharacterized protein LOC100272379 precursor | gi|226510209 | Zea mays | 8 |
| Glucan endo-1,3-β-glucosidase, acidic isoform precursor | gi|162460244 | Zea mays | 8 |
| Uncharacterized protein LOC100273479 precursor | gi|226496763 | Zea mays | 7 |
| Bowman-Birk-type bran trypsin inhibitor precursor | gi|226492872 | Zea mays | 7 |
| Prm3 precursor | gi|525344103 | Zea mays | 6 |
| β-Galactosidase precursor | gi|226503159 | Zea mays | 5 |
| Uncharacterized protein LOC100383323 precursor | gi|293335795 | Zea mays | 5 |
| Phycocyanin | gi|428316123 | Oscillatoria nigro-viridis | 5 |
| Phycobilisome protein | gi|218203940 | Cyanothece spp. | 5 |
| Glucan endo-1,3-β-glucosidase homolog1 | gi|162464228 | Zea mays | 5 |
| Uncharacterized protein LOC100282665 | gi|226531488 | Zea mays | 4 |
| Chitinase2 precursor | gi|226528230 | Zea mays | 4 |
| Ferredoxin-1 | gi|259489872 | Zea mays | 4 |
| Pathogenesis-related protein5 precursor | gi|162462963 | Zea mays | 4 |
| Uncharacterized protein KIAA1211 homolog | gi|512887433 | Bombyx mori | 4 |
| Chitinase chem5 precursor | gi|162462924 | Zea mays | 4 |
| Peroxidase42 precursor | gi|363543419 | Zea mays | 3 |
| Prm3 | gi|7687414 | Zea mays | 3 |
| Endochitinase A precursor | gi|259490577 | Zea mays | 3 |
| Ferredoxin-3 | gi|226493434 | Zea mays | 3 |
| Endochitinase A precursor | gi|226506778 | Zea mays | 3 |
| Uncharacterized protein LOC100283021 precursor | gi|226532850 | Zea mays | 2 |
| Trehalase precursor | gi|112984260 | Bombyx mori | 2 |
| Uncharacterized protein LOC100279499 precursor | gi|226508174 | Zea mays | 2 |
| Ferredoxin 2Fe-2S | gi|428779889 | Dactylococcopsis salina | 2 |
| Ferredoxin 2Fe-2S | gi|428217589 | Pseudanabaena spp. | 2 |
| Ferredoxin 2Fe-2S | gi|172036521 | Cyanothece spp. | 2 |
| Phycobilisome rod-core linker polypeptide, phycocyanin associated | gi|495511152 | Richelia intracellularis | 2 |
| Ferredoxin | gi|497230147 | Cyanothece | 1 |
| Putative signal recognition particle 68-kD protein | gi|148298713 | Bombyx mori | 1 |
| Uncharacterized protein LOC100382901 | gi|293331667 | Zea mays | 1 |
| Uncharacterized protein LOC100193626 | gi|212723010 | Zea mays | 1 |
| Cys protease2 precursor | gi|162460343 | Zea mays | 1 |
| Hypothetical protein | gi|226502602 | Zea mays | 1 |
| Uncharacterized protein LOC100192567 | gi|212722994 | Zea mays | 1 |
| Bowman-Birk-type wound-induced proteinase inhibitor WIP1 precursor | gi|226502534 | Zea mays | 1 |
| Jasmonate-induced protein | gi|226504114 | Zea mays | 1 |
| Uncharacterized protein LOC100272602 | gi|226503962 | Zea mays | 1 |
| Signal recognition particle subunit SRP68-like, partial | gi|512939773 | Bombyx mori | 1 |
| Uncharacterized protein LOC100277189 | gi|226505282 | Zea mays | 1 |
| Uncharacterized protein LOC100274838 | gi|226501686 | Zea mays | 1 |
| Transposase, Mutator family protein, partial | gi|497570136 | Brevibacterium linens | 1 |
| Reversed plastidic ATP/ADP transporter | gi|226494716 | Zea mays | 1 |
| Membrane protein | gi|490259763 | Erwinia amylovora | 1 |
| Hypothetical protein EAMY_138 | gi|292487869 | Erwinia amylovora | 1 |
Table II. MudPIT analysis of Rotofor fraction A.
| Protein Name | Accession No. | Source Species | Peptide Count (95%) |
|---|---|---|---|
| β-d-Glucosidase precursor | gi|162464369 | Zea mays | 52 |
| Prm3 precursor | gi|525344103 | Zea mays | 23 |
| Glucan endo-1,3-β-glucosidase homolog1 precursor | gi|162464228 | Zea mays | 29 |
| Uncharacterized protein LOC100272880 precursor | gi|226508498 | Zea mays | 16 |
| Lipoxygenase6 | gi|162463394 | Zea mays | 14 |
| Uncharacterized protein LOC100272933 precursor | gi|226504688 | Zea mays | 19 |
| Plastocyanin | gi|226500192 | Zea mays | 13 |
| Uncharacterized protein LOC100192779 | gi|212722124 | Zea mays | 13 |
| Ferredoxin-1 | gi|259489872 | Zea mays | 8 |
| Uncharacterized protein LOC100193626 | gi|212723010 | Zea mays | 7 |
| LOC100282665 | gi|226531488 | Zea mays | 7 |
| Uncharacterized protein LOC100279566 | gi|226497580 | Zea mays | 5 |
| Allene oxide synthase1 | gi|162460508 | Zea mays | 5 |
| Uncharacterized protein LOC100273479 precursor | gi|226496763 | Zea mays | 4 |
| Cys protease1 precursor | gi|226496089 | Zea mays | 5 |
| Uncharacterized protein LOC100192522 | gi|212721998 | Zea mays | 5 |
| Exoglucanase1 precursor | gi|212274863 | Zea mays | 6 |
| Coproporphyrinogen III oxidase | gi|308081433 | Zea mays | 5 |
| Uncharacterized protein LOC100500945 | gi|308081534 | Zea mays | 5 |
| Trypsin, alkaline C-like | gi|512920011 | Bombyx mori | 5 |
| Uncharacterized protein LOC100136885 | gi|167860184 | Zea mays | 5 |
| Putative signal recognition particle 68-kD protein | gi|148298713 | Bombyx mori | 5 |
| Signal recognition particle subunit SRP68-like, partial | gi|512939773 | Bombyx mori | 5 |
| Endochitinase A precursor | gi|259490577 | Zea mays | 4 |
| Endochitinase A precursor | gi|226506778 | Zea mays | 4 |
| Subtilisin-chymotrypsin inhibitor homolog1 | gi|162460972 | Zea mays | 4 |
| Subtilisin-chymotrypsin inhibitor CI-1B | gi|350536017 | Zea mays | 4 |
| Uncharacterized protein KIAA1211 homolog | gi|512887433 | Bombyx mori | 4 |
| Uncharacterized protein LOC100273659 | gi|226510596 | Zea mays | 3 |
| Uncharacterized protein LOC100273401 | gi|226531398 | Zea mays | 3 |
| Prolyl carboxypeptidase-like protein precursor | gi|226506854 | Zea mays | 2 |
| Bowman-Birk-type wound-induced proteinase inhibitor WIP1 precursor | gi|226502534 | Zea mays | 2 |
| White cap1 | gi|162461079 | Zea mays | 2 |
| Carotenoid cleavage dioxygenase | gi|162461068 | Zea mays | 2 |
| Jasmonate-induced protein | gi|226504114 | Zea mays | 2 |
| Bowman-Birk-type wound-induced proteinase inhibitor WIP1 precursor | gi|162464148 | Zea mays | 2 |
| Brassinosteroid insensitive1-associated receptor kinase1 precursor | gi|226500148 | Zea mays | 2 |
| Cystatin-1 precursor | gi|162463594 | Zea mays | 2 |
| Wound/stress protein precursor | gi|226505912 | Zea mays | 2 |
| Bowman-Birk-type bran trypsin inhibitor precursor | gi|226492872 | Zea mays | 2 |
| Uncharacterized protein LOC100192066 | gi|212276291 | Zea mays | 2 |
| Ribosome-inactivating protein2 | gi|162461916 | Zea mays | 2 |
| Uncharacterized LOC100273935 precursor | gi|226500034 | Zea mays | 2 |
| Uncharacterized protein LOC100383323 precursor | gi|293335795 | Zea mays | 2 |
| β-Glucosidase aggregating factor precursor | gi|162461751 | Zea mays | 1 |
| Cystatin-2 precursor | gi|162459564 | Zea mays | 1 |
| Endochitinase PR4 precursor | gi|226501166 | Zea mays | 1 |
| Ser carboxypeptidase1 precursor | gi|226500142 | Zea mays | 1 |
| γ-Glutamyl hydrolase A-like | gi|512927972 | Bombyx mori | 1 |
| Splicing factor 3B subunit 1-like | gi|512900193 | Bombyx mori | 1 |
| Uncharacterized protein LOC100275569 | gi|226498070 | Zea mays | 1 |
| Tubulin-folding cofactor B-like isoform X1 | gi|512902838 | Bombyx mori | 1 |
| Uncharacterized protein LOC100217090 | gi|219362883 | Zea mays | 1 |
| Trehalase precursor | gi|112984260 | Bombyx mori | 1 |
| Rhicadhesin receptor precursor | gi|525344355 | Zea mays | 1 |
| Agmatine deiminase | gi|226508546 | Zea mays | 1 |
| Cortical cell-delineating protein precursor | gi|226494937 | Zea mays | 1 |
| Uncharacterized protein LOC100272963 | gi|226530914 | Zea mays | 1 |
Chitin-Binding Proteins in FAW Frass Induced Pathogen Defenses in Maize
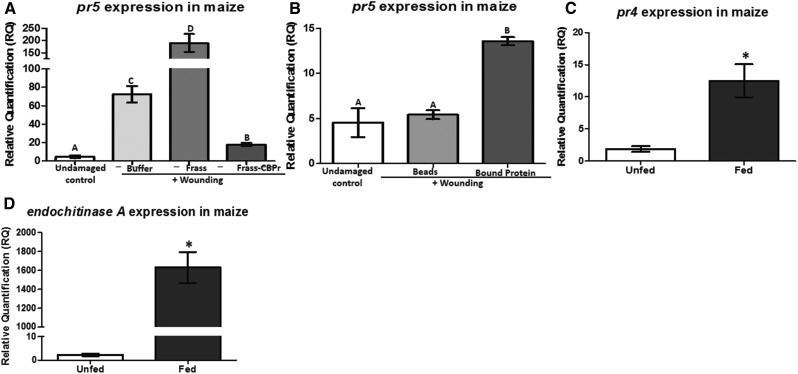
Proteomic analysis of frass proteins identified maize chitinases as possible candidates for elicitors of pathogen defense present in caterpillar frass. To better understand if FAW frass proteins with chitin-binding domains such as chitinases (Kasprzewska, 2003) induce pathogen defenses, we removed the chitin-binding proteins from frass proteins and applied this fraction to maize leaves and measured pathogen defenses. FAW frass depleted of chitin-binding proteins failed to induce pr5 defense transcript when compared with frass proteins containing chitin-binding proteins or buffer (Fig. 2A). Notably, the frass protein fraction depleted of chitin-binding proteins reduced pr5 expression compared with buffer (Fig. 2A). Furthermore, when beads containing the chitin-binding proteins from the frass were applied to the plants, pr5 transcript abundance was significantly higher than in plants treated with the beads alone (Fig. 2B). This strongly suggested that chitin-binding proteins present in the FAW frass were responsible for inducing pathogen defenses in maize.
Figure 2.
Pathogen defense gene expression in response to the absence and presence of chitin-binding proteins. A and B, Maize leaves were wounded and treated with frass protein, frass protein devoid of chitin-binding proteins (Frass-CBPr), or PBS buffer (A) or chitin-binding beads (Beads) in PBS buffer, beads with the chitin-binding proteins attached (bound protein), or left undamaged (B) for 24 h, and the pathogen defense gene marker pr5 transcript abundance was measured by qRT-PCR. Transcript abundance of pr5 was normalized to that of the reference gene actin and expressed as relative quantification (RQ). Data were analyzed by one-factor ANOVA, and mean separation was calculated by multiple comparison Tukey’s test. RQ values with different letters are significantly different from each other (P < 0.05). Four plants were used as biological replicates for each treatment, and error bars indicate se. C and D, Maize chitinase pr4 and endochitinase A transcript abundance in plants fed with FAW. Maize leaves were infested with FAW caterpillars for 24 h, and the abundance of pr4 (C) and endochitinase A (D) transcripts was measured by qRT-PCR. Transcript abundances were normalized to that of the reference gene actin and expressed as RQ. Data were analyzed by one-factor ANOVA, and mean separation was calculated by multiple comparison Tukey’s test. RQ values marked with asterisks indicate significantly different transcript abundance between fed and unfed plants (P < 0.05). Four plants were used as biological replicates for each treatment, and error bars indicate se.
Proteomic Analysis of Chitin-Binding Proteins in FAW Frass Showed the Presence of Two Maize Chitinases, Pr4 and Endochitinase A
The chitin-binding proteins from FAW frass that were bound to the beads in the previous experiment were boiled in 1× sample buffer (Laemmli, 1970), and a denaturing protein gel was run. We observed two major proteins with molecular masses of approximately 36 and 28 kD (Supplemental Fig. S2). Proteomic analysis of the proteins bound to these beads showed the following maize chitinases: Pr4 (gi|226501166), Endochitinase A (gi|259490577), chitinase precursor (gi|169234932), and Chitinase 2 (gi|226528230; Table III). Chitinases are known to be induced in plants in response to caterpillar herbivory and serve as a line of defense against insect pests by digesting the chitin in the gut (Hamid et al., 2013). Studies reporting the induction of chitinases in response to fungal attack in plants also are widely known (Schlumbaum et al., 1986). Maize contains a large chitinase gene family, and some of these are induced by FAW herbivory (Rodríguez et al., 2012). Since Pr4 and Endochitinase A were two of the most abundant proteins with a chitin-binding domain detected in FAW frass (Table III), we measured the transcript abundance of pr4 and endochitinase A in response to caterpillar herbivory. Transcripts of pr4 and endochitinase A in plants fed with caterpillars for 24 h were higher than in control undamaged plants (Fig. 2, C and D). Therefore, it appears that FAW herbivory in maize results in the foliar accumulation of chitinases Pr4 and Endochitinase A that are consumed by the caterpillars and deposited back on the plant in the insect frass. The plants could then recognize frass chitinases and induce pathogen defenses.
Table III. MudPIT analysis of chitin-binding proteins in FAW frass.
| Protein Name | Accession No. | Source Species | Peptide Count (95%) |
|---|---|---|---|
| Endochitinase A precursor | gi|259490577 | Zea mays | 12 |
| Thiol peroxiredoxin | gi|112982996 | Bombyx mori | 6 |
| β-d-Glucosidase precursor | gi|162464369 | Zea mays | 4 |
| Phosphoglycerate kinase-like | gi|512916352 | Bombyx mori | 3 |
| Uncharacterized protein LOC100282665 | gi|226531488 | Zea mays | 3 |
| Glucan endo-1,3-β-glucosidase homolog1 precursor | gi|162464228 | Zea mays | 3 |
| Endochitinase PR4 precursor | gi|226501166 | Zea mays | 3 |
| Glucan endo-1,3-β-glucosidase, acidic isoform precursor | gi|162460244 | Zea mays | 2 |
| Chitinase precursor | gi|169234932 | Bombyx mori | 2 |
| Uncharacterized protein LOC100384565 | gi|293336861 | Zea mays | 1 |
| Uncharacterized protein LOC100280398 | gi|226532106 | Zea mays | 1 |
| Uncharacterized protein LOC100280135 | gi|226498514 | Zea mays | 1 |
| Uncharacterized protein LOC100276660 | gi|226500234 | Zea mays | 1 |
| Ubiquitin and ribosomal protein s27a | gi|148298787 | Bombyx mori | 1 |
| Sedoheptulose bisphosphatase1 | gi|226506366 | Zea mays | 1 |
| Uncharacterized protein LOC100382381 | gi|293335659 | Zea mays | 1 |
| Spermatogenesis-associated protein6-like isoform X1 | gi|512930147 | Bombyx mori | 1 |
| Uncharacterized protein LOC101739495 | gi|512897697 | Bombyx mori | 1 |
| Trypsin, alkaline C-like | gi|512920011 | Bombyx mori | 1 |
| Transmembrane protease Ser9-like | gi|512905537 | Bombyx mori | 1 |
| Polyubiquitin C-like isoform X2 | gi|512920634 | Bombyx mori | 1 |
| Polyubiquitin C-like isoform X1 | gi|512920630 | Bombyx mori | 1 |
| Vacuolar protein-sorting-associated protein18 homolog | gi|512932595 | Bombyx mori | 1 |
| Collagen α-2(IV) chain-like | gi|512912209 | Bombyx mori | 1 |
| Leu-rich repeat Ser/Thr-protein kinase1-like | gi|512901468 | Bombyx mori | 1 |
| Chymotrypsin 2-like | gi|512905471 | Bombyx mori | 1 |
| Chymotrypsin 1-like | gi|512905475 | Bombyx mori | 1 |
| Polyubiquitin | gi|112983984 | Bombyx mori | 1 |
| Lipoxygenase6 | gi|162463394 | Zea mays | 1 |
| Knotted1-induced1 precursor | gi|162464233 | Zea mays | 1 |
| Hypothetical protein | gi|308080354 | Zea mays | 1 |
| Cyclin A2 | gi|226506380 | Zea mays | 1 |
| Chitinase 2 precursor | gi|226528230 | Zea mays | 1 |
| α-Amylase precursor | gi|290560875 | Bombyx mori | 1 |
Pr4 and Endochitinase A Induce Pathogen Defenses in Maize
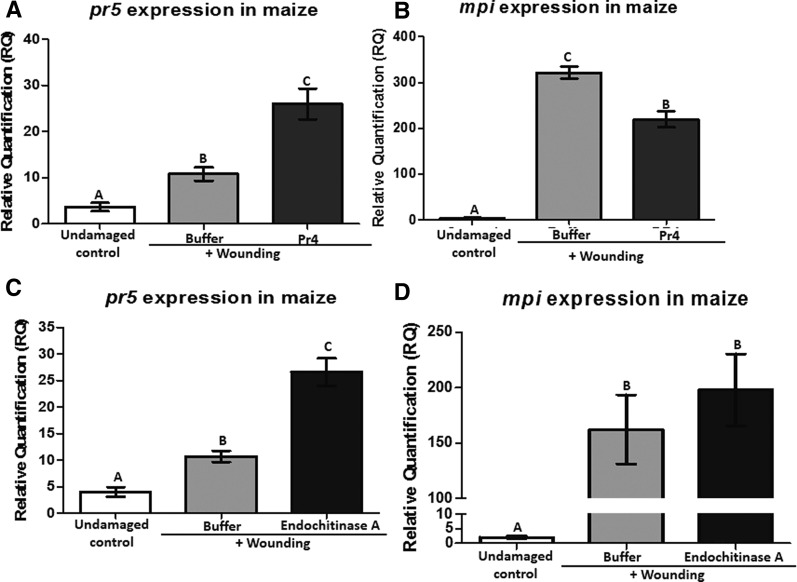
Maize chitinases Pr4 and Endochitinase A were expressed and purified in Escherichia coli, and both exhibited chitinase activity (Supplemental Fig. S3, A and B). We applied the purified Pr4 to maize leaves and measured the expression of pr5 and the herbivore defense marker maize protease inhibitor (mpi). Pr4 induced the expression of pr5 higher than the buffer-treated control, while it suppressed the expression of mpi in maize leaves (Fig. 3, A and B). This is another classic example of the antagonism between these two defense pathways present in plants. mpi is controlled by the JA pathway, while pr5 induction is mediated by the SA pathway (Supplemental Fig. S4, A and B). Notably, Endochitinase A application also induced pr5 transcripts (Fig. 3C), but it failed to suppress mpi RNA levels (Fig. 3D). These results indicate that maize chitinases Pr4 and Endochitinase A are proteins present in the FAW frass that can induce the SA defense pathway in maize, while Pr4 simultaneously suppresses herbivore defenses downstream of the JA defense pathway.
Figure 3.
Transcript abundance of maize defense genes in response to recombinant Pr4 and Endochitinase A application. Maize leaves were wounded and treated with recombinant Pr4, Endochitinase A, or buffer for 24 h. Maize pathogen defense-related pr5 (A and C) and herbivore defense-related mpi (B and D) transcript abundance was measured by qRT-PCR. The transcript abundance of pr5 and mpi was normalized to that of the reference gene actin and expressed as relative quantification (RQ). Data were analyzed by one-factor ANOVA, and mean separation was calculated by multiple comparison Tukey’s test. RQ values marked with different letters are significantly different from buffer-treated values (P < 0.05). Six plants were used as biological replicates for each treatment, and error bars indicate se.
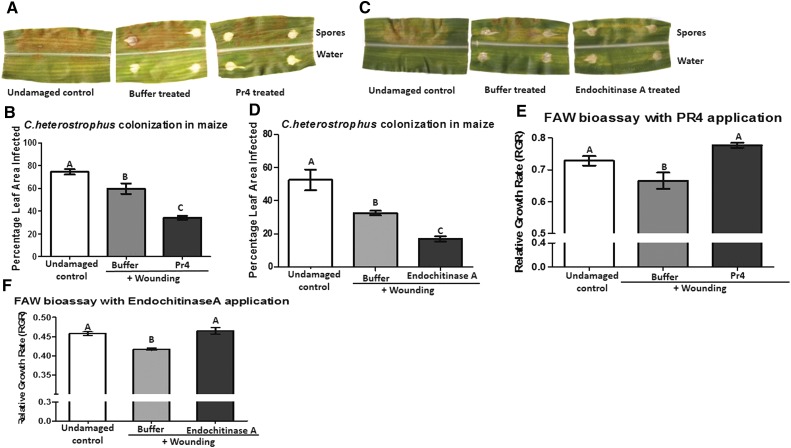
Pr4 and Endochitinase A Suppress Fungal Pathogen Growth While Increasing Caterpillar Growth
Since we observed the induction of pr5 in maize leaves in response to PR4 and Endochitinase A application, the growth of the fungal pathogen Cochliobolus heterostrophus on leaves treated with Pr4 and Endochitinase A was measured. C. heterostrophus is the maize pathogen that causes southern leaf blight. Plants treated with Pr4 showed much lower fungal colonization (only 34% of leaf area colonized) compared with buffer-treated plants (nearly 60% of leaf area colonized; Fig. 4, A and B). Similarly, C. heterostrophus colonization in maize leaves treated with Endochitinase A was significantly lower than in leaves treated with buffer (Fig. 4, C and D). Fungal growth was more than twice as high in undamaged leaves when compared with those treated with Pr4 and Endochitinase A. Therefore, we conclude that the application of Pr4 or Endochitinase A to maize leaves not only induces pathogen defense gene expression but also retards the growth of the fungal pathogen.
Figure 4.
A to D, Effects of Pr4- or Endochitinase A-treated maize leaves on the growth of C. heterostrophus. Maize leaves were wounded and treated with recombinant Pr4, Endochitinase A, or buffer or left undamaged (control) for 24 h and treated with equal amounts of C. heterostrophus spore inoculum on the upper half of the midrib or with water on the lower half of the midrib (A and C). Fungal growth was measured as a percentage of the leaf area infected after 60 h by determining the area of infected lesions (B and D). Six plants were used as biological replicates for each treatment. Percentage leaf area values with different letters are significantly different from each other according to one-way ANOVA and mean separation by Tukey’s test (P < 0.05). Error bars indicate se. E and F, Effects of recombinant Pr4- or Endochitinase A-treated maize leaves on the growth rate of naïve FAW caterpillars. Maize leaves were wounded and treated with recombinant Pr4 or Endochitinase A protein or buffer or left undamaged (control) for 24 h. Leaf tissue from 18 plants from each treatment was fed to naïve FAW caterpillars, and their relative growth rate (RGR) was measured after 3 d. RGR values with different letters are significantly different from each other according to one-way ANOVA and Tukey’s mean separation (P < 0.05). Error bars indicate se.
Pr4 application on maize leaves also suppressed the expression of herbivore/wound-induced mpi transcript abundance (Fig. 3B). Therefore, we fed naïve FAW caterpillars leaves treated with Pr4 and compared their growth rate with those fed buffer-treated or undamaged leaves. Caterpillars reared on Pr4-treated leaves had a growth rate similar to those fed on undamaged leaves (Fig. 4E). However, the larvae that consumed the buffer-treated tissue showed a significantly lower growth rate compared with those that fed on Pr4-treated or undamaged leaves. Similarly, FAW larvae performed better on leaves treated with Endochitinase A for 24 h compared with those treated with buffer (Fig. 4F). Therefore, it is clear that maize chitinases Pr4 and Endochitinase A act as effectors and suppress wound-induced defenses in maize while increasing the performance of the herbivore on the plant.
Pr4 and Endochitinase A Suppress Herbivore-Induced Defenses in Maize
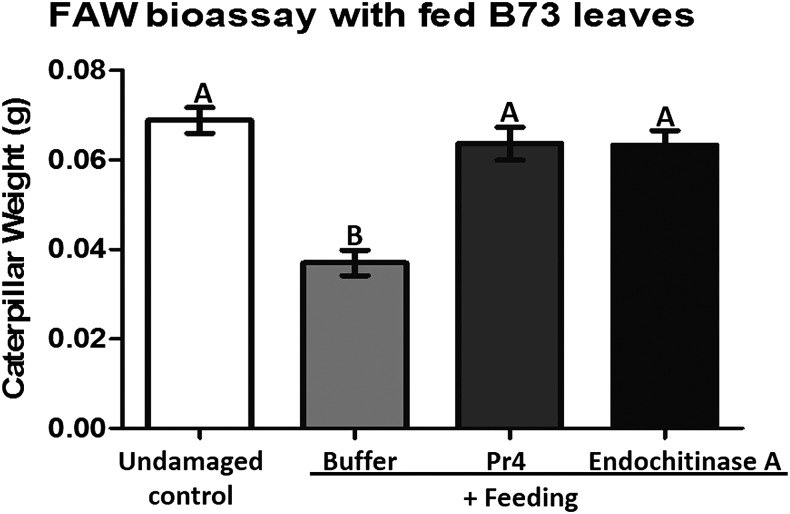
Caterpillar herbivory is associated with the deposition of oral secretions and/or saliva on the feeding site, which induces herbivore defenses in the host plant (Louis et al., 2013; Chuang et al., 2014b; Acevedo et al., 2015; Schmelz, 2015). Since frass is likely to come in contact with such fed tissue, we fed caged FAW larvae in inverted cages where frass from the larvae does not come in contact with the host tissue (Ray et al., 2015). Following FAW feeding for 3 h, we applied the recombinant frass protein Pr4 or Endochitinase A or buffer to the feeding site for 24 h. Then, these plants were used for bioassays with FAW neonates. The neonate larvae grew faster on leaf tissue treated with Pr4 or Endochitinase A after the initial feeding compared with those treated with buffer (Fig. 5). Notably, caterpillars feeding on plant tissue treated with either chitinase grew at the same pace compared with those that consumed undamaged maize leaves (Fig. 5). This strongly suggests that maize chitinases Pr4 and Endochitinase A are indeed effectors of herbivore defenses present in the caterpillar frass, attenuating herbivore-induced plant defenses in the host and facilitating a better performance of the herbivore on the plant.
Figure 5.
Growth of FAW neonates on maize leaves that were infested with FAW larvae prior to treatment with Pr4 or Endochitinase A. Maize leaves were fed with FAW larvae in inverted clip cages and treated with recombinant Pr4 or Endochitinase A protein or buffer or left undamaged for 24 h. Treated leaves were collected and fed to FAW neonates, and their weights were measured after 7 d. Each treatment consisted of 15 individual plants. Treatments with different letters are significantly different from each other according to Tukey’s mean separation in a one-way ANOVA (P < 0.05). Error bars indicate se.
Pr4 in the FAW Diet Transiently Retards FAW Growth, While Endochitinase A Transiently Promotes FAW Growth
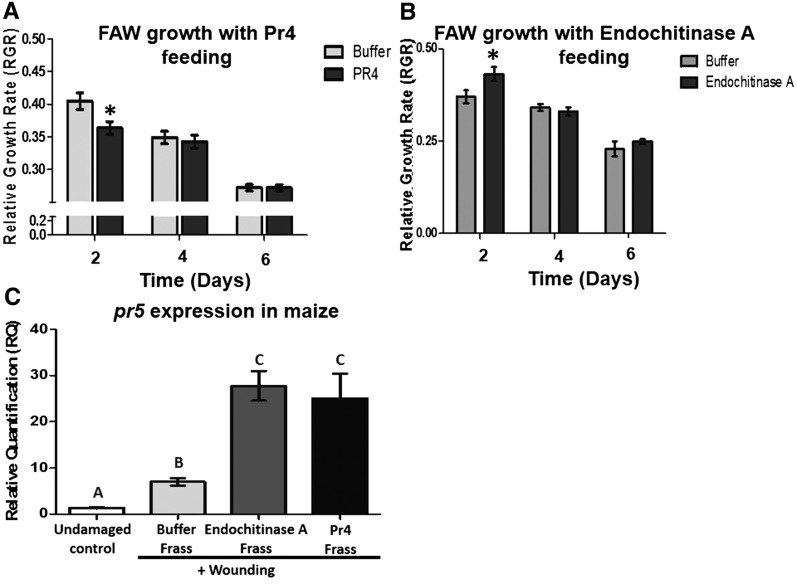
Thus far, we have observed that caterpillar feeding induces the accumulation of chitinases such as Pr4 and Endochitinase A in maize leaves. These chitinases then pass through the caterpillar gut and are deposited in the frass onto the leaves, where they induce pathogen defenses. To understand if the herbivore-induced chitinases directly affect caterpillar growth, we mixed the recombinant enzymes with artificial diet, fed them to naïve caterpillars, and measured their growth over 6 d. FAW larvae showed a slower growth rate when fed Pr4-containing diet for 2 d (Fig. 6A). However, as the bioassay time increased, larvae fed Pr4-treated diet showed no change in growth rate compared with buffer-treated controls at 4 and 6 d (Fig. 6A). On the other hand, FAW larvae performed marginally better when fed Endochitinase A-infused diet compared with one infused with buffer after 2 d (Fig. 6B). Similar to the trend observed with Pr4 feeding to FAW larvae, Endochitinase A feeding did not affect the growth of the larvae after 2 d (Fig. 6B). This strongly suggests that the FAW larvae may be largely unaffected by the direct ingestion of Pr4 and Endochitinase A accumulating in leaves in response to herbivory.
Figure 6.
A and B, Effects of recombinant Pr4 or Endochitinase A on the growth rate of FAW caterpillars. Naïve first instar FAW caterpillars were fed artificial diet infused with recombinant Pr4 (A), Endochitinase A (B), or buffer, and relative growth rate (RGR) of the caterpillars was measured at 2, 4, and 6 d. Asterisks indicate differences in RGR among the treatments at each time point according to ANOVA (P < 0.05). Thirty caterpillars were used as biological replicates for each treatment, and error bars indicate se. C, Effects of frass from FAW larvae fed on diet infused with recombinant Pr4 or Endochitinase A. Maize leaves were treated with FAW frass collected from larvae fed on diet infused with buffer, recombinant Pr4, or Endochitinase A for 24 h. Maize pathogen defense-related pr5 transcript abundance was measured by qRT-PCR, normalized to that of the reference gene actin, and expressed as relative quantification (RQ). Four biological replicates were used for each treatment, data were analyzed by one-factor ANOVA, and mean separation was calculated by a multiple comparison Tukey’s test. RQ values with different letters are significantly different from each other (P < 0.05). Error bars indicate se.
We collected frass from third instar FAW larvae fed on artificial diet infused with buffer, recombinant Pr4, or Endochitinase A and applied it to maize leaves for 24 h. Frass collected from larvae that ingested either Pr4 or Endochitinase A showed higher chitinase activity than frass collected from buffer-treated diet (Supplemental Fig. S5). Plants treated with frass from caterpillars that consumed Pr4 or Endochitinase A showed higher abundance of pr5 transcripts compared with the buffer-only diet (Fig. 6C). Therefore, it is apparent that maize chitinases Pr4 and Endochitinase A are plant defense proteins induced in response to caterpillar attack in maize and that the insect then defecates on the leaf, triggering pathogen defenses, thereby increasing herbivore performance.
DISCUSSION
Insect secretions such as saliva or regurgitant may harbor several elicitors that can trigger herbivore defenses in plants. Insect-derived elicitors of herbivore defenses, such as volicitin and caeliferin that were isolated from insect oral secretions and bruchins that were identified in the oviposition secretion of adult moths, are all fatty acid conjugates that induce herbivore defenses in plants (Alborn et al., 1997, 2007; Doss et al., 2000). On the other hand, elicitors such as inceptin, a fragment of plant ATP synthase, and β-glucosidase are proteinaceous in nature and were isolated from insect oral secretions (Mattiacci et al., 1995; Schmelz et al., 2006, 2007). Glc oxidase is another protein that is abundant in the saliva of tomato fruitworm (Helicoverpa zea) that induces herbivore defenses in tomato and suppresses them in other plants such as tobacco (Nicotiana tabacum; Musser et al., 2002; Tian et al., 2012). We observed that proteins from FAW frass induced the pathogen defense pathway over time (Ray et al., 2015). Since frass is one of the most abundant cues that herbivores leave on their host, we fractionated the FAW frass proteins based on their pI values and applied them to maize leaves. We found two fractions that had the greatest effect in increasing the expression of the pathogen defense marker pr5 in maize (Fig. 1).
Proteomic analysis of one of these fractions led us to infer that maize chitinases are FAW frass proteins that may be involved in inducing pathogen defenses in maize (Table I). Since the FAW frass fraction that induced pr5 contained proteins that bind chitin, we removed these proteins from the caterpillar frass. Application of chitin-binding protein-depleted frass to maize leaves resulted in the attenuation of pathogen defenses (Fig. 2A), while the beads with bound protein were able to induce the defense (Fig. 2B). Proteomic analysis of the chitin-binding beads after incubation with FAW frass indicated that Pr4 and Endochitinase A were abundant in this fraction.
Chitinases are enzymes that degrade chitin either to its oligomers (endochitinases) or to dimers and monomers (exochitinases). All chitinases belong to family 18 and 19 glycosyl hydrolases, and plant chitinases have been classified into six classes (Hamid et al., 2013). Plant chitinases have been shown to accumulate in response to pathogen attack as well as by herbivory (Kasprzewska, 2003; Rodríguez et al., 2012). When we applied the maize chitinases Pr4 and Endochitinase A, which were both present in fraction B and were attached to the chitin-binding beads, to maize leaves, it induced the expression of the pathogen defense gene marker pr5 (Fig. 3, A and C). While Pr4 simultaneously suppressed the expression of wound-induced mpi transcript abundance (Fig. 3B), Endochitinase A induced pr5 but did not suppress mpi. Consistent with the transcript abundances, the fungal pathogen C. heterostrophus showed lower growth on the Pr4- and Endochitinase A-treated plants compared with the control (Fig. 4, A–D). Furthermore, naïve FAW caterpillars grew faster on plants treated with recombinant Pr4 or Endochitinase A compared with the controls (Fig. 4, E and F), suggesting that these plant chitinases present in the FAW frass can mediate a suppression of the wound-induced defense response. More importantly, these chitinases could suppress herbivore-induced defenses in the maize leaves to the level of undamaged plants after FAW herbivory (Fig. 5). Therefore, in reference to our previous study (Ray et al., 2015), it can be said that the FAW frass proteins that suppress herbivore defenses and activate the pathogen defenses in maize are, in fact, the maize chitinases Pr4 and Endochitinase A. This also is direct evidence of the typical antagonism between plant defense pathways mediated by JA and SA (Zarate et al., 2007; Thaler et al., 2012; Chung et al., 2013). Such suppression of herbivore defenses and concomitant activation of pathogen defenses by herbivory were shown previously in tomato by whiteflies and Colorado potato beetles (Zarate et al., 2007; Chung et al., 2013). Pr4 and Endochitinase A also join the list of other proteins, such as apyrases and Glc oxidase, that are deposited by the herbivore on the host plant and suppress the plant’s herbivore defenses (Musser et al., 2002; Wu et al., 2012).
Notably, chitin oligomers are known elicitors and pathogen-associated molecular patterns involved in triggering the pathogen defense pathway in plants (Kombrink et al., 2011). The receptors that bind chitin oligomers and induce pathogen defenses in rice (Oryza sativa) and Arabidopsis (Arabidopsis thaliana) also have been identified (Wan et al., 2012; Tanaka et al., 2013; Kouzai et al., 2014). However, to our knowledge, there has been no previous report that chitinases may be involved in eliciting plant defenses. For the first time, we show that a plant chitinase can act as an effector of herbivore-induced defenses when deposited on the plant in the insect frass. The mechanism by which Pr4 or Endochitinase A induces pathogen defenses and suppresses the herbivore defenses in plants is beyond the scope of this article. However, we speculate that plants may perceive these chitinases as a self protein and induce defenses in response. Recognition of self from nonself has been known to play a role in plant reproduction and development (Gruntman and Novoplansky, 2004; Williams et al., 2015). Self recognition by damage associated with caterpillar herbivory also has been shown in plants (Heil, 2009; Karban and Shiojiri, 2009). Pr4 and Endochitinase A from caterpillar frass may be identified as self by the maize plant and thereby trigger its pathogen defenses.
Maize and its herbivore pests such as FAW have coevolved for thousands of years. The deposition of frass on damaged host tissue by the herbivore to suppress the wound-induced defenses could be a strategy that the herbivore has developed during the course of evolution. We observed that pr4 and endochitinase A transcripts were induced in response to caterpillar herbivory in an effort to suppress herbivore growth. However, the herbivore had only transiently reduced growth after feeding on the Pr4-infused diet, after which Pr4 had no effect on growth rate (Fig. 6A), while the FAW larvae grew marginally faster on Endochitinase A-infused diet for 2 d (Fig. 6B). Furthermore, frass collected from caterpillars that were fed on artificial diet containing Pr4 and Endochitinase A was able to trigger pathogen-induced pr5 expression in maize leaves (Fig. 6C). Taken together, these results suggest that, during evolution, FAW caterpillars developed a mechanism to cope with the host chitinases Pr4 and Endochitinase A and accumulate them in frass. In turn, Pr4 and Endochitinase A suppress the damage-associated defenses in the host, induce pathogen defense, and increase the long-term performance of the herbivore on the host.
MATERIALS AND METHODS
Plant and Insect Materials
Maize (Zea mays var B73) plants were grown in glasshouse conditions with a 16-h-light/8-h-dark cycle in Hagerstown loam until they reached the midwhorl (V7–V8) development stage (Ritchie et al., 1998). FAW (Spodoptera frugiperda) eggs were obtained from the U.S. Department of Agriculture-Agricultural Research Service at Mississippi State University and reared on maize plants to collect frass. Frass was collected every 24 h from caterpillars fed on V8 stage maize leaves. The collected frass was then homogenized in 1× phosphate-buffered saline (PBS) buffer (137 mm sodium chloride, 2.7 mm potassium chloride, 10 mm disodium hydrogen phosphate, and 1.8 mm potassium dihydrogen phosphate), filter sterilized, and concentrated using centrifugal columns with a 3-kD molecular mass cutoff (Pall Life Sciences) as described earlier (Ray et al., 2015). The protein concentration in the frass extract was quantified by the Bradford assay (Bradford, 1976) using 500 ng mL−1 to 5 mg mL−1 bovine serum albumin as a standard.
Fractionation of Frass Proteins by Isoelectric Focusing
Fractionation of frass proteins by isoelectric focusing was performed in the standard Rotofor chamber after assembly of the apparatus as suggested by the manufacturer (Bio-Rad). Five milligrams of frass protein in 1× PBS was loaded into the Rotofor chamber with 2% (w/v) ampholytes (pH 3–10; Bio-Rad) and 1.5 m urea. The electrolytes used were 0.1 n sodium hydroxide and 0.1 n phosphoric acid for the anion- and cation-exchange membranes, respectively. Fractionation was performed at 4°C at constant power of 12 W until the voltage was stable for 1 h. Twenty fractions were collected and pooled to obtain 10 fractions as follows: the first two acidic fractions were pooled to form fraction A, the next two formed fraction B, and so on. Fractions A to J were then concentrated using 3-kD centrifugal columns (Pall Life Sciences) and resuspended in 1× PBS. The protein concentration in each fraction was measured by the Bradford assay (Bradford, 1976), and 1 μg of protein was applied to each wound site on maize leaves.
MudPIT Analysis of Frass Protein Fractions
Proteins from Rotofor fractions that induced plant defenses were identified by gel-free MudPIT analysis. Proteins were alkylated with 10% (v/v) acetonitrile in 50 mm ammonium bicarbonate and digested with 1:20 (trypsin:protein) sequencing-grade trypsin (Promega) for 3 h at 48°C. Digested peptides were dried in a SpeedVac (Thermo Fisher Scientific) to completely remove acetonitrile and ammonium bicarbonate, and the reaction was stopped with 0.1% (v/v) trifluoroacetic acid. To identify the proteins bound to the chitin-binding beads, the beads with bound proteins were boiled in 1× sample buffer (Laemmli, 1970) for 10 min and loaded on a denaturing polyacrylamide gel (Laemmli, 1970). The gel was stained with Simply Blue Safe stain (Life Technologies) overnight and destained in deionized water. The bands from the gel were excised and treated with 200 µL of 200 mm ammonium bicarbonate in 50% (v/v) acetonitrile for 45 min at 37°C. The gel pieces were then reduced in 100 µL of 2 mm Tris-2-caboxymethylphosphine (Sigma-Aldrich) for 15 min at 37°C and subsequently alkylated in 100 µL of 20 mm iodoacetamide in 25 mm ammonium bicarbonate (pH 8) for 25 min at 37°C in the dark. They were then washed three times in 200 µL of 25 mm ammonium bicarbonate (pH 8) for 15 min. This was followed by drying in a SpeedVac and incubation in 70 µL of 0.02 µg µL−1 sequencing-grade trypsin (Promega) in 10% (v/v) acetonitrile, 40 mm ammonium bicarbonate (pH 8), and 0.1% (w/v) noctylglucoside (1-O-n-octyl-β-d-glucopyranoside; Sigma-Aldrich) at 48°C for 3 h. After incubation, the supernatant was removed and the reaction was stopped with 0.1% (v/v) trifluoroacetic acid. Peptides obtained from both the gel-free and in-gel digests were analyzed by electrospray in liquid chromatography-tandem mass spectrometry (ABSciex 5600 TripleTOF; Sciex). Data were analyzed using Protein Pilot 5.0 (Sciex), and peptides were matched against maize (National Center for Biotechnology Information [NCBI] taxid identifier 4577), lepidoptera (NCBI taxid identifier 7088), fungi (NCBI taxid identifier 4751), and bacteria (NCBI taxid identifier 2). The strength of matching the peptide fingerprint to a particular database was measured by the Mascot score (Perkins et al., 1999; Koenig et al., 2008; Tang et al., 2008), and only matches with 95% or higher confidence for a peptide having an unused score of 1.3 or higher are reported in Tables I to III.
Removal of Chitin-Binding Proteins from Frass
Frass proteins were incubated with chitin-binding magnetic beads (New England Biolabs) in a ratio of 5:1 (w/v) overnight at 4°C with constant shaking. The chitin-binding beads were removed with a magnet as described by the manufacturer, and the fraction containing frass proteins devoid of chitin-binding proteins was collected with a pipette. Frass proteins containing chitin-binding domains were attached to the remaining beads. The beads were washed five times in 1× PBS. Twenty micrograms of frass protein, an equivalent amount of frass proteins devoid of chitin-binding proteins, and 1× PBS were applied to maize leaves. Similarly, beads containing chitin-binding proteins from 20 µg of the total frass protein and an equivalent amount of beads without bound protein in 1× PBS were applied to wound sites on the leaves. One hundred milligrams of leaf tissue surrounding the wound site was collected for RNA extraction and qRT-PCR.
RNA Extraction, Complementary DNA Preparation, and Real-Time PCR
One hundred milligrams of leaf tissue was homogenized in liquid nitrogen using the GenoGrinder 2000 (OPS Diagnostics), and RNA was extracted with 1 mL of TRIzol (Life Technologies) according to the manufacturer’s protocol. Genomic DNA was removed from the RNA by treatment with 2 units of DNaseI enzyme (New England Biolabs). The genomic DNA-free RNA was quantified using Nanodrop (Thermo-Fisher Scientific), and 1 µg of total RNA was used to make complementary DNA (cDNA) using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) and oligo(dT). Then, qRT-PCR was performed with Fast Start Universal SYBR Green Master Mix (Roche Applied Science) with actin as a reference gene. Gene-specific primers were designed with Primer Express 3.0 (Life Technologies; details in Supplemental Table S1). RNA extraction, cDNA synthesis, and qRT-PCR were performed for each biological replicate separately, and each treatment had four biological replicates for the Rotofor frass fractions or chitin bead treatments. Six plants per treatment were used when purified Pr4 or Endochitinase A was applied.
Pr4 and Endochitinase A Purification and Activity Assay
Transcripts of maize chitinases Pr4 (NP_001150754.1) and Endochitinase A (NP_001158904.1) were amplified from RNA pools of maize leaves fed with FAW larvae for 24 h and cloned into the Champion expression vector pET102 (Life Technologies) using the primers listed in Supplemental Table S1. Escherichia coli cells (BL21 DE3) were transformed with pET102 vector containing the cloned Pr4 and Endochitinase A sequences separately. Transformed E. coli cells were then induced with 2 mm isopropyl β-d-1-thiogalactopyranoside as suggested by the manufacturer, and cells were harvested and lysed with 1 mg mL−1 lysozyme (Sigma-Aldrich). The expression vector added 6× His tags to the expressed Pr4 and Endochitinase A. Then, cell lysates were incubated with nickel-nitrilotriacetic acid agarose beads (Qiagen) overnight at 4°C, and the expressed proteins were eluted with the elution buffer containing 250 mm imidazole, 300 mm sodium chloride, and 50 mm sodium dihydrogen phosphate. The chitinase enzyme assay was performed with the eluted proteins as described earlier (Peethambaran et al., 2009). First, 100 µL of a mixture containing 1 mg mL−1 glycol chitosan, 12% acrylamide:bisacrylamide (37:1, v/v), 0.05% (w/v) ammonium persulfate, and 375 mm Tris (pH 8.8) was pipetted onto 96-well plates, and the mixture was solidified with 10 µL of N,N,N′,N′-tetramethylethylenediamine. One hundred microliters of 0.05, 0.1 0.15, 0.2, 0.25, and 0.3 mg mL−1 purified Pr4 and Endochitinase A were added to the wells containing glycol chitosan gels and incubated overnight. The wells were washed with water once the following day, incubated with 100 µL of 3 µg mL−1 Calcofluor (Sigma-Aldrich) for 30 min, and washed again with water. Fluorescence was measured in SpectraMax M5 (Molecular Devices) with an excitation wavelength of 356 nm and an emission wavelength of 435 nm for 1 s. For each concentration of Pr4 and Endochitinase A tested, four technical replicates were measured.
FAW Bioassays
The first three leaves of a maize whorl in a V8 stage plant were wounded twice using a wounding tool (Ray et al., 2015). Each wound site was treated with 20 µg of purified Pr4 or Endochitinase A protein or an equivalent volume of the elution buffer or left undamaged. We used 18 biological replicates for each treatment, each of which was fed to 18 naïve FAW caterpillars for 3 d. Caterpillar mass was measured at the beginning and the end of the bioassay and used to calculate relative growth rates as described earlier (Hoffmann and Poorter, 2002; Mohan et al., 2008). Caterpillar feeding on plants is associated with the deposition of insect oral secretions and saliva, which can trigger herbivore-induced defenses in plants (Louis et al., 2013; Chuang et al., 2014a; Acevedo et al., 2015). To understand the effect of maize chitinases Pr4 and Endochitinase A on such plant defenses triggered by caterpillar herbivory, we fed fifth instar FAW larvae in inverted clip cages for 3 h as described in our previous study (Ray et al., 2015). Following herbivory, we applied 50 µg of purified Pr4 or Endochitinase A protein or an equivalent volume of elution buffer and harvested the leaves after 24 h. Tissues were harvested from 15 plants for each treatment (fed with Pr4 or Endochitinase A protein or buffer, or undamaged control) and fed to FAW neonates. Plant tissue from each biological replicate was fed to three neonates for 7 d, after which the weight of caterpillars was measured. The average of three caterpillars fed on each plant was treated as one biological replicate, and one-way ANOVA was performed with Tukey’s mean separation (P < 0.05) to ascertain differences among treatments. To observe the direct effect of Pr4 or Endochitinase A on FAW larval growth, 0.2 g of artificial diet was infused with 60 µg of purified Pr4 or Endochitinase A protein or an equivalent volume of buffer and fed to 30 caterpillars. Diet pieces infused with Pr4, Endochitinase A, or buffer were replaced every 2 d, the larval weights were measured after 2, 4, and 6 d, and the relative growth rates were calculated. One-way ANOVA was performed to determine significance among treatments in all bioassays, and means separation was done with Tukey’s test (P < 0.05) using SAS 9.2 (SAS).
Collection of Frass from FAW Larvae Fed on Recombinant Pr4 and Endochitinase A
To determine if ingested recombinant Pr4 and Endochitinase A in FAW frass affect defenses, larvae were fed with artificial diet until the third instar. The third instar larvae were then fed on 0.2 g of the same diet infused with 50 µg of Pr4 or Endochitinase A or elution buffer containing 250 mm imidazole, 300 mm sodium chloride, and 50 mm sodium dihydrogen phosphate for 2 d, and their frass was collected. Fresh chitinases or buffer were added to the diet each day, and frass was removed. The collected frass was then homogenized in 1× PBS buffer in a 1:1 ratio (w/v) and centrifuged to remove insoluble debris, and the supernatant was collected. Total protein content in the frass extract was measured by the Bradford assay (Bradford, 1976). Two micrograms of total frass protein in 1× PBS buffer was then applied to maize leaves, and the transcript abundance of the pathogen defense marker pr5 was measured after 24 h. Four biological replicates were used for each treatment, and one-way ANOVA was performed to determine if the treatments differed from each other. Means separation was done by Tukey’s test (P < 0.05) using SAS 9.2 (SAS).
Fungal Bioassay
Cochliobolus heterostrophus was cultured on potato dextrose agar medium for 2 weeks under lights. Fresh leaves from B73 maize at the V8 stage were treated with 20 µg of purified Pr4 protein, an equivalent volume of buffer, or left undamaged and harvested after 24 h. Harvested tissue was placed on a 150-mm petri dish with a 1% (w/v) agar base with 0.01% (w/v) kinetin. C. heterostrophus spores (100 µL at a concentration of 104 spores mL−1) were applied to one side of the leaf between two wound sites, and the other side was treated with water (Fig. 4A). Inoculated leaves were incubated at 26°C for 60 h in a 16-h-light/8-h-dark cycle. The area infected by fungi was measured using Sigma Scan Pro 5.0 (SPSS) at 60 h after inoculation. Two leaves per biological replicate were analyzed for the infected area, with six biological replicates per treatment. The amount of fungal colonization on the leaf was measured as a function of the percentage of leaf area showing lesions. The significance of infection was measured by one-way ANOVA for the percentage of area infected by the pathogen, and means separation was done using Tukey’s test (P < 0.05).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers provided in the text.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Denaturing polyacrylamide gel showing proteins in fractions A to J after isoelectric focusing of frass proteins.
Supplemental Figure S2. Denaturing polyacrylamide gel showing chitin-binding proteins in FAW frass.
Supplemental Figure S3. Standard curve showing Pr4 and Endochitinase A chitinase activity.
Supplemental Figure S4. Expression of maize herbivore defense genes mpi and pr5 in response to 10 mm methyl jasmonate or 10 mm methyl salicylate spray.
Supplemental Figure S5. Chitinase activity in FAW frass fed on diet infused with recombinant Pr4, Endochitinase A, or buffer.
Supplemental Table S1. List of primers.
Supplementary Material
Acknowledgments
We thank Dr. Anne Stanley and Dr. Bruce Stanley for their help in running the MudPIT assays at the Proteomics Core Facility of the Hershey Medical Center at Pennsylvania State University; Dr. Kelli Hoover’s lab and Dr. Liwang Cui’s lab at the Department of Entomology at Pennsylvania State University for their generosity in lending the Nanodrop and the sonicator whenever they were needed; and Ruth Nissly at the Flow Cytometry Core Facility of the Huck Institutes of Life Sciences at Pennsylvania State University for help in performing the chitinase enzyme assays. We would like to thank Nick Sloff at the Department of Entomology at Pennsylvania State University for drawing the figure for the Table of Contents.
Glossary
- HAMP
herbivore-associated molecular pattern
- JA
jasmonic acid
- SA
salicylic acid
- FAW
fall armyworm
- PBS
phosphate-buffered saline
- NCBI
National Center for Biotechnology Information
- qRT
quantitative real-time
- cDNA
complementary DNA
Footnotes
This work was supported by the U.S. Department of Agriculture National Institute of Food and Agriculture (grant no. 2011–67013–30352 to D.S.L. and G.W.F.), the National Science Foundation (grant no. IOS–1256326 to G.W.F.), and the College of Agricultural Science at Pennsylvania State University (graduate student competitive grant to S.R.).
Articles can be viewed without a subscription.
References
- Acevedo FE, Rivera-Vega LJ, Chung SH, Ray S, Felton GW (2015) Cues from chewing insects: the intersection of DAMPs, HAMPs, MAMPs and effectors. Curr Opin Plant Biol 26: 80–86 [DOI] [PubMed] [Google Scholar]
- Alborn HT, Hansen TV, Jones TH, Bennett DC, Tumlinson JH, Schmelz EA, Teal PEA (2007) Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc Natl Acad Sci USA 104: 12976–12981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alborn HT, Turlings TC, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276: 945–949 [Google Scholar]
- Bent AF, Mackey D (2007) Elicitors, effectors, and R genes: the new paradigm and a lifetime supply of questions. Annu Rev Phytopathol 45: 399–436 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Chen MS. (2008) Inducible direct plant defense against insect herbivores: a review. Insect Sci 15: 101–114 [DOI] [PubMed] [Google Scholar]
- Chuang WP, Herde M, Ray S, Castano-Duque L, Howe GA, Luthe DS (2014a) Caterpillar attack triggers accumulation of the toxic maize protein RIP2. New Phytol 201: 928–939 [DOI] [PubMed] [Google Scholar]
- Chuang WP, Ray S, Acevedo FE, Peiffer M, Felton GW, Luthe DS (2014b) Herbivore cues from the fall armyworm (Spodoptera frugiperda) larvae trigger direct defenses in maize. Mol Plant Microbe Interact 27: 461–470 [DOI] [PubMed] [Google Scholar]
- Chung SH, Rosa C, Scully ED, Peiffer M, Tooker JF, Hoover K, Luthe DS, Felton GW (2013) Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc Natl Acad Sci USA 110: 15728–15733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss RP, Oliver JE, Proebsting WM, Potter SW, Kuy S, Clement SL, Williamson RT, Carney JR, DeVilbiss ED (2000) Bruchins: insect-derived plant regulators that stimulate neoplasm formation. Proc Natl Acad Sci USA 97: 6218–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton GW, Tumlinson JH (2008) Plant-insect dialogs: complex interactions at the plant-insect interface. Curr Opin Plant Biol 11: 457–463 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Gruntman M, Novoplansky A (2004) Physiologically mediated self/non-self discrimination in roots. Proc Natl Acad Sci USA 101: 3863–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid R, Khan MA, Ahmad M, Ahmad MM, Abdin MZ, Musarrat J, Javed S (2013) Chitinases: an update. J Pharm Bioallied Sci 5: 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M. (2009) Damaged-self recognition in plant herbivore defence. Trends Plant Sci 14: 356–363 [DOI] [PubMed] [Google Scholar]
- Hilfiker O, Groux R, Bruessow F, Kiefer K, Zeier J, Reymond P (2014) Insect eggs induce a systemic acquired resistance in Arabidopsis. Plant J 80: 1085–1094 [DOI] [PubMed] [Google Scholar]
- Hoffmann WA, Poorter H (2002) Avoiding bias in calculations of relative growth rate. Ann Bot (Lond) 90: 37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karban R, Shiojiri K (2009) Self-recognition affects plant communication and defense. Ecol Lett 12: 502–506 [DOI] [PubMed] [Google Scholar]
- Kasprzewska A. (2003) Plant chitinases: regulation and function. Cell Mol Biol Lett 8: 809–824 [PubMed] [Google Scholar]
- Koenig T, Menze BH, Kirchner M, Monigatti F, Parker KC, Patterson T, Steen JJ, Hamprecht FA, Steen H (2008) Robust prediction of the MASCOT score for an improved quality assessment in mass spectrometric proteomics. J Proteome Res 7: 3708–3717 [DOI] [PubMed] [Google Scholar]
- Kombrink A, Sánchez-Vallet A, Thomma BPHJ (2011) The role of chitin detection in plant-pathogen interactions. Microbes Infect 13: 1168–1176 [DOI] [PubMed] [Google Scholar]
- Kouzai Y, Nakajima K, Hayafune M, Ozawa K, Kaku H, Shibuya N, Minami E, Nishizawa Y (2014) CEBiP is the major chitin oligomer-binding protein in rice and plays a main role in the perception of chitin oligomers. Plant Mol Biol 84: 519–528 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Louis J, Peiffer M, Ray S, Luthe DS, Felton GW (2013) Host-specific salivary elicitor(s) of European corn borer induce defenses in tomato and maize. New Phytol 199: 66–73 [DOI] [PubMed] [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA (1995) β-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA 92: 2036–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Ma PWK, Williams WP, Luthe DS (2008) A naturally occurring plant cysteine protease possesses remarkable toxicity against insect pests and synergizes Bacillus thuringiensis toxin. PLoS ONE 3: e1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW (2002) Herbivory: caterpillar saliva beats plant defences. Nature 416: 599–600 [DOI] [PubMed] [Google Scholar]
- Pechan T, Cohen A, Williams WP, Luthe DS (2002) Insect feeding mobilizes a unique plant defense protease that disrupts the peritrophic matrix of caterpillars. Proc Natl Acad Sci USA 99: 13319–13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peethambaran B, Hawkins L, Windham GL, Williams WP, Luthe DS (2009) Anti-fungal activity of maize silk proteins and role of chitinases in Aspergillus flavus resistance. Toxin Rev 29: 27–39 [Google Scholar]
- Peiffer M, Tooker JF, Luthe DS, Felton GW (2009) Plants on early alert: glandular trichomes as sensors for insect herbivores. New Phytol 184: 644–656 [DOI] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20: 3551–3567 [DOI] [PubMed] [Google Scholar]
- Ray S, Gaffor I, Acevedo FE, Helms A, Chuang WP, Tooker J, Felton GW, Luthe DS (2015) Maize plants recognize herbivore-associated cues from caterpillar frass. J Chem Ecol 41: 781–792 [DOI] [PubMed] [Google Scholar]
- Ritchie JT, Singh U, Godwin DC, Bowen WT (1998) Cereal growth, development and yield. In Tsuji G, Hoogenboom G, Thornton P, eds, Understanding Options for Agricultural Production. Springer, Dordrecht, The Netherlands, pp 79–98 [Google Scholar]
- Rodríguez VM, Santiago R, Malvar RA, Butrón A (2012) Inducible maize defense mechanisms against the corn borer Sesamia nonagrioides: a transcriptome and biochemical approach. Mol Plant Microbe Interact 25: 61–68 [DOI] [PubMed] [Google Scholar]
- Schlumbaum A, Mauch F, Vögeli U, Boller T (1986) Plant chitinases are potent inhibitors of fungal growth. Nature 324: 365–367 [Google Scholar]
- Schmelz EA. (2015) Impacts of insect oral secretions on defoliation-induced plant defense. Curr Opin Insect Sci 9: 7–15 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Carroll MJ, LeClere S, Phipps SM, Meredith J, Chourey PS, Alborn HT, Teal PEA (2006) Fragments of ATP synthase mediate plant perception of insect attack. Proc Natl Acad Sci USA 103: 8894–8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, LeClere S, Carroll MJ, Alborn HT, Teal PE (2007) Cowpea chloroplastic ATP synthase is the source of multiple plant defense elicitors during insect herbivory. Plant Physiol 144: 793–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonhoven LM, Dicke M, van Loon JAJ (2005) Insect-Plant Biology. Oxford Biology, New York [Google Scholar]
- Schwartzberg EG, Tumlinson JH (2014) Aphid honeydew alters plant defence responses. Funct Ecol 28: 386–394 [Google Scholar]
- Tanaka K, Nguyen CT, Liang Y, Cao Y, Stacey G (2013) Role of LysM receptors in chitin-triggered plant innate immunity. Plant Signal Behav 8: e22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH, Shilov IV, Seymour SL (2008) Nonlinear fitting method for determining local false discovery rates from decoy database searches. J Proteome Res 7: 3661–3667 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17: 260–270 [DOI] [PubMed] [Google Scholar]
- Tian D, Peiffer M, Shoemaker E, Tooker J, Haubruge E, Francis F, Luthe DS, Felton GW (2012) Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. PLoS ONE 7: e36168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Tanaka K, Zhang XC, Son GH, Brechenmacher L, Nguyen THN, Stacey G (2012) LYK4, a lysin motif receptor-like kinase, is important for chitin signaling and plant innate immunity in Arabidopsis. Plant Physiol 160: 396–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JS, Wu L, Li S, Sun P, Kao TH (2015) Insight into S-RNase-based self-incompatibility in Petunia: recent findings and future directions. Front Plant Sci 6: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Peiffer M, Luthe DS, Felton GW (2012) ATP hydrolyzing salivary enzymes of caterpillars suppress plant defenses. PLoS ONE 7: e41947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.