Cyclic electron flow is accelerated under heat stress in Symbiodinium to promote photoprotective thermal energy dissipation.
Abstract
Increases in seawater temperature impair photosynthesis (photoinhibition) in the symbiotic dinoflagellate Symbiodinium within cnidarian hosts, such as corals and sea anemones, and may destroy their symbiotic relationship. Although the degree of photoinhibition in Symbiodinium under heat stress differs among strains, the differences in their responses to increased temperatures, including cyclic electron flow (CEF), which sustains photoprotective thermal energy dissipation, have not been investigated. Here, we examined CEF in cultured Symbiodinium cells or those in an endosymbiotic relationship within a cnidarian host. The light-dependent reduction of the primary electron donor photosystem I, i.e. P700+, was enhanced in any Symbiodinium cell by increasing temperatures, indicating CEF was induced by heat, which was accompanied by thermal energy dissipation activation. The critical temperatures for inducing CEF were different among Symbiodinium strains. The clade A strains with greater susceptibility to photoinhibition, OTcH-1 and Y106, exhibited higher CEF activities under moderate heat stress than a more phototolerant clade B strain Mf1.05b, suggesting that the observed CEF induction was not a preventive measure but a stress response in Symbiodinium.
Dinoflagellate algae of the genus Symbiodinium form endosymbiotic relationships with marine cnidarian animals, such as corals, sea anemones, and jellyfish (Davy et al., 2012). In their symbiotic relationships, Symbiodinium cells provide photosynthetically produced energy, such as Glc or glycerol, to their host, and in return, they receive CO2 and inorganic nutrients, such as nitrogen and phosphate (Davy et al., 2012). Because the hosts rely on the symbionts for the majority of their energy, efficient photosynthesis is key to maintaining the symbiotic relationship. This symbiotic relationship can be disrupted due to elevated temperatures, which results in mortality in corals and eventually the destruction of coral reefs (Hoegh-Guldberg, 1999; Lesser, 2011). The frequency and scale of this phenomenon, which is known as coral bleaching, are increasing due to global warming, and coral bleaching is becoming a present danger for tropical ecosystems (Hoegh-Guldberg, 1999).
The photosystem II (PSII) activity is sensitive to elevated temperature in Symbiodinium (Iglesias-Prieto et al., 1992; Warner et al., 1996). This down-regulation of PSII is widely observed when an imbalance between the light energy absorption and its consumption occurs, collectively called photoinhibition (Aro et al., 1993). The damaged PSII is normally repaired in Symbiodinium, but this process is sensitive to heat (Takahashi et al., 2004), which gives rise to the loss of PSII and its light-harvesting antenna, namely, bleaching (Takahashi et al., 2008).
To avoid photoinhibition, photosynthetic organisms have developed diverse photoprotective mechanisms, such as scavenging systems for reactive oxygen species, the rerouting of reducing equivalents to oxygen via photorespiration or the Mehler reaction, and the thermal dissipation of excess energy (qE; Takahashi and Badger, 2011). Because Symbiodinium cells develop high levels of qE under heat stress conditions, qE is expected to help prevent heat stress-associated photoinhibition in Symbiodinium (Warner et al., 1996; Roth, 2014). Thus, the capacity of qE in each Symbiodinium strain may determine that strain’s sensitivity to photoinhibition under heat stress.
It is generally accepted that qE is activated by the generation of a proton gradient (∆pH) across the thylakoid membrane (Müller et al., 2001). In Symbiodinium, qE is fully relaxed upon dissipation of ∆pH by the uncoupler NH4Cl (Takahashi et al., 2009). Because the formation of ∆pH is accelerated by cyclic electron flow (CEF) around PSI (Shikanai, 2014), CEF has been proposed to be crucial for establishing high qE and alleviating photoinhibition in Symbiodinium under heat stress conditions. Symbiodinium is genetically diverse (Pochon and Gates, 2010), and the members of this genus exhibit different sensitivities to photoinhibition under heat stress (Warner et al., 1996). Reynolds et al. (2008) have characterized CEF in Symbiodinium with chlorophyll fluorescence transients and have demonstrated that CEF capacity is higher in the heat-tolerant strain (clade A) than in the heat-sensitive strains (clades B and C). Recently, however, Roberty et al. (2014) have suggested that the fluorescence signature reported by Reynolds et al. (2008) was not necessarily due to CEF and have claimed that the CEF capacity, as estimated on the basis of the electrochromic shift of carotenoids and the rereduction kinetics of the oxidized primary electron donor in PSI (P700+), is negligible in all four Symbiodinium strains. Therefore, the capacity and significance of CEF remain controversial in Symbiodinium.
CEF is induced under moderate heat stress in vascular plants, such as tobacco (Sazanov et al., 1998), spinach (Bukhov et al., 1999), maize (Egorova and Bukhov, 2002), and oat (Quiles, 2006), which presumably helps to temporally alleviate photoinhibition due to high temperature. Therefore, it is likely that CEF is also induced in Symbiodinium under high temperature conditions, and the extent of this induction may determine the heat sensitivities of the different strains. However, no report has examined the effects of heat stress on CEF in Symbiodinium. In this study, we examined the effects of increased temperatures on CEF in three cultured Symbiodinium strains (OTcH-1, Y106, and Mf1.05b). We demonstrated that CEF is indeed enhanced by increasing temperature in Symbiodinium, but the extent of this enhancement varies by strain. Possible mechanisms and the significance of CEF increases in Symbiodinium under moderate heat stress are discussed.
RESULTS
Acceleration of CEF around PSI in Symbiodinium under Moderate Heat Stress
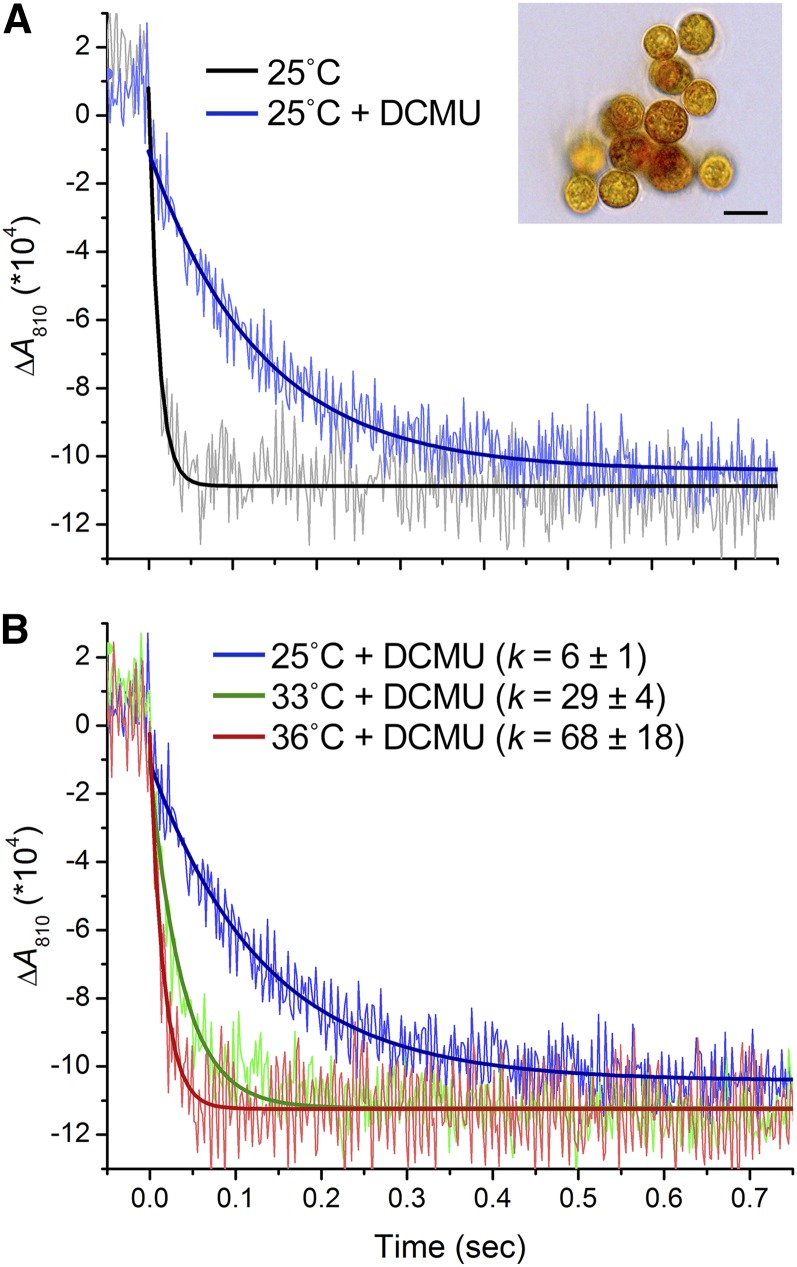
To measure the CEF around PSI in the clade A strain of Symbiodinium OTcH-1, we monitored the rereduction kinetics of P700 (Iwai et al., 2010; Supplemental Fig. S1). After exposure to a saturating pulse of white light (2,000 µmol photons m−2 s−1), absorption changes at 810 nm (∆A810) were monitored in the dark (Fig. 1). The rereduction kinetics of P700+ were fitted to a single-exponential-decay curve. The extent of CEF was examined in the presence of the PSII inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), which completely abolished the electron transfer on PSII at a concentration of >20 µm (Supplemental Fig. S2). Normally in oxic conditions, the reduction of P700+ still occurred in the presence of DCMU but at a considerably slower rate than in its absence (Fig. 1); however, this rate was accelerated in anoxic conditions because of the induction of CEF, much as it is in the green alga Chlamydomonas reinhardtii (Supplemental Fig. S3; Takahashi et al., 2013). We then examined the effects of high temperatures on CEF. Following incubation in the dark at 25°C, 33°C, or 36°C for 15 min, the P700+ reduction was monitored in the presence of DCMU in Symbiodinium OTcH-1 cells (Fig. 1B). The rate constants for P700+ reduction in the cells incubated at 33°C and 36°C were 5- and 10-fold greater, respectively, than that for the cells incubated at 25°C, which demonstrated that the levels of CEF were elevated upon incubation at higher temperatures in Symbiodinium OTcH-1.
Figure 1.
Dark reduction kinetics of photooxidized P700+ in Symbiodinium OTcH-1. Five measurements taken at 10-s intervals were averaged and fit with a single-exponential decay. A, Effect of DCMU. The cells were incubated at 25°C for 15 min and subsequently treated with or without DCMU (80 µm) for 5 min in the dark before measurement. Inset, A micrograph of Symbiodinium cells (bar = 10 µm). B, Effects of elevated temperatures. The cells were incubated at 25°C, 33°C, or 36°C for 15 min and subsequently treated with DCMU for 5 min in the dark before measurement. The reduction rate constants shown (k) are means ± sd from three independent experiments.
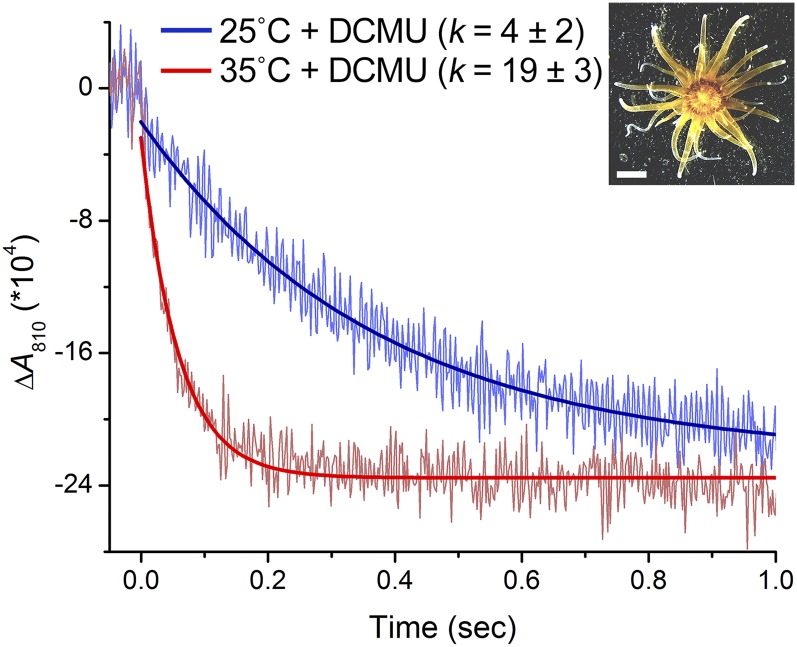
To examine whether the heat induction of CEF also occurs when Symbiodinium cells are in an endosymbiotic relationship within a cnidarian host, we tested the sea anemone Aiptasia sp. H2, which harbors a clade B Symbiodinium (Xiang et al., 2013). The P700+ reduction kinetics was monitored in the presence of DCMU in the Aiptasia polyps following dark incubation at 25°C or 35°C for 15 min (Fig. 2). The P700+ reduction in the polyps at 35°C was nearly 5-fold faster than that at 25°C. These results demonstrated that CEF was also accelerated in endosymbiotic Symbiodinium cells.
Figure 2.
Dark reduction kinetics of photooxidized P700+ in Aiptasia H2. Aiptasia polyps were incubated at 25°C in the presence of DCMU (200 µm) for 15 min in the dark, and the reduction kinetics of P700+ were measured (blue line). The polyps were subsequently incubated at 35°C for 15 min in the dark, and the reduction kinetics of P700+ were measured (red line). The reduction rate constants shown (k) are means ± sd from three independent experiments. Inset, Micrograph of an Aiptasia polyp (bar = 2 mm).
To further characterize the high temperature-induced CEF, we tested potential CEF inhibitors, including methyl viologen (MV) and antimycin A (AA). MV accepts electrons from PSI and limits the electron flow to CEF (Bukhov et al., 2001) in barley, whereas AA inhibits the PGR5-dependent CEF pathway in Arabidopsis (Munekage et al., 2002). To examine whether MV acts as an electron acceptor for PSI in Symbiodinium, we determined the effects of MV on the relative electron transport rate (rETR) in the presence of glycolaldehyde, which depletes the electron acceptor for PSI, i.e. NADP+. If MV accepted electrons from PSI, the inhibitory effect of glycolaldehyde on rETR would be abolished. However, there was no effect of MV on rETR in the presence of glycolaldehyde in Symbiodinium OTcH-1 (Supplemental Fig. S4A), suggesting that MV does not function as an electron acceptor for PSI in Symbiodinium OTcH-1. We then tested the effects of AA on CEF by measuring the nonphotochemical quenching of chlorophyll fluorescence (NPQ) to determine whether AA suppresses NPQ by impairing the generation of a ∆pH across the thylakoid membrane. NPQ development under strong light conditions was not inhibited by AA (Supplemental Fig. S4B), which suggests that AA does not function as a CEF inhibitor in Symbiodinium. Together, these results indicate that the characterization of electron transfer pathway of high temperature-induced CEF in Symbiodinium using these CEF inhibitors was unsuccessful.
In Symbiodinium, high temperatures have been demonstrated to inhibit the Calvin-Benson cycle (Jones et al., 1998; Lilley et al., 2010). As described above, we determined that CEF is enhanced under similar conditions in Symbiodinium. To examine whether the high temperature-induced CEF was due to the inhibition of the Calvin-Benson cycle, we examined the effects of inhibitors of the Calvin-Benson cycle, including glycolaldehyde and iodoacetamide, on the P700+ reduction rate in Symbiodinium OTcH-1 (Supplemental Fig. S5). Glycolaldehyde inhibits the Calvin-Benson cycle by inactivating phosphoribulokinase without any side effects on photosynthetic electron transfer (Takahashi and Murata, 2006). Iodoacetamide inhibits the Calvin-Benson cycle though inactivating phosphoribulokinase, Fru 1,6-bisphosphatase, sedoheptulose 1,7-bisphosphatase, and GAPDH (Ferri et al., 1981). When Symbiodinium cells were incubated with glycolaldehyde (120 mm) and iodoacetamide (5 mm), the net photosynthetic oxygen production rate dropped to 30% and 13% of the initial (control) levels, respectively (Supplemental Fig. S5A). Monitoring of the P700+ reduction revealed that both glycolaldehyde and iodoacetamide enhanced the P700+ reduction rate (glycolaldehyde and iodoacetamide increased the P700+ reduction rate to 127% and 208%, respectively, as compared with that in the absence of the inhibitor; Supplemental Fig. S5B). Thus, inhibition of the Calvin-Benson cycle enhances CEF in Symbiodinium. However, the effects of these inhibitors on the P700+ reduction rate were considerably smaller than that of high temperature. Therefore, it is unlikely that the high temperature-associated increase in CEF was primarily due to the inhibition of the Calvin-Benson cycle.
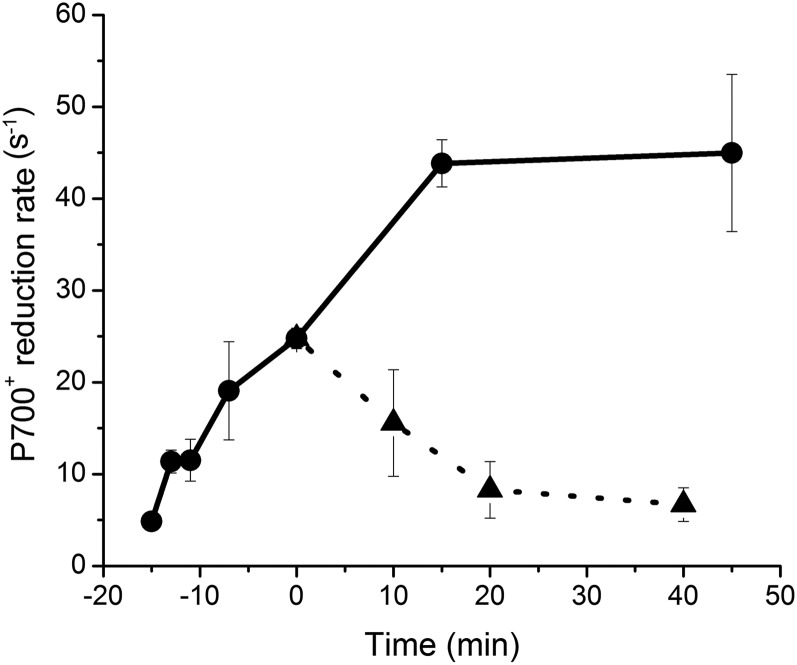
To examine whether the heat induction of CEF was reversible, we examined the time courses of P700+ reduction at 25°C and 33°C over 40 min following preincubation at 33°C for 15 min (Fig. 3). During the preincubation, the P700+ reduction rate gradually increased. The increased P700+ reduction rate was rapidly relaxed within 20 min of the transfer to 25°C, and it was further increased and saturated after 15 min at 33°C. Our results demonstrated that the heat-induced CEF is reversible. Symbiodinium might acclimatize to high temperature by up-regulating CEF.
Figure 3.
Time course of the P700+ reduction rate in Symbiodinium OTcH-1 after transfer from a high (33°C) temperature to the optimal growth (25°C) temperature. The P700+ reduction rate was monitored at 25°C (triangles) and 33°C (circles) following preincubation at 33°C for 15 min. The P700+ reduction rate was determined in the presence of DCMU. The values are presented as means ± sd from three independent experiments.
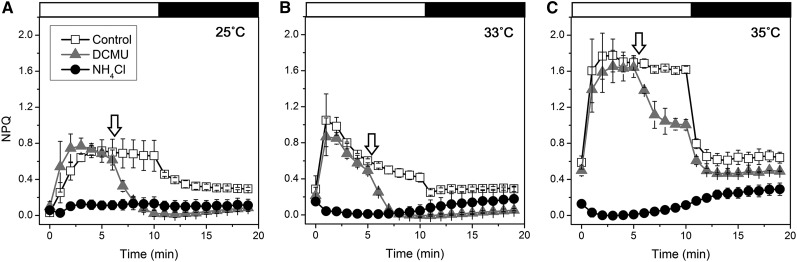
We next examined the effects of high temperature on the induction of NPQ (Fig. 4). Three factors could contribute to NPQ: qE, state transitions (qT), and photoinhibition (qI; Müller et al., 2001). Because qE is the only factor relevant to NPQ that is regulated by the ∆pH across the thylakoid membrane, the qE signal can be isolated by measuring the effect of the uncoupler NH4Cl. When Symbiodinium cells were exposed to high light at 25°C, 33°C, and 35°C, NPQ was significantly induced to 0.7, 1.0, and 1.6, respectively (Fig. 4). However, in the presence of NH4Cl, the induction of NPQ was nearly completely abolished at all temperatures tested (Fig. 4). These results demonstrate that the observed NPQ was due to qE and not to state transitions or photoinhibition. When DCMU was added during the light exposure, the induced NPQ was completely abolished at 25°C and 33°C (Fig. 4), which indicates that qE development was solely dependent on linear electron flow. However, at 35°C, NPQ was only partially suppressed by DCMU, which suggests that CEF as well as linear electron flow contributed to the qE development at 35°C (Fig. 4).
Figure 4.
Effects of DCMU and NH4Cl on NPQ at different temperatures in Symbiodinium OTcH-1. The cells were incubated at 25°C for 30 min in the dark with or without NH4Cl (100 mm). Next, the cells were incubated at 25°C (A), 33°C (B), or 35°C (C) for 15 min in the dark, and the NPQ development was monitored in the light at 240 µmol photons m−2 s−1 for 10 min. The relaxation of NPQ was monitored in the dark for another 10 min. DCMU was added to the cells 5 min after the onset of light exposure (arrows). The values are presented as means ± sd of two or three independent experiments.
Relationships between Photoinhibition Sensitivity and CEF Activity in Different Symbiodinium Strains
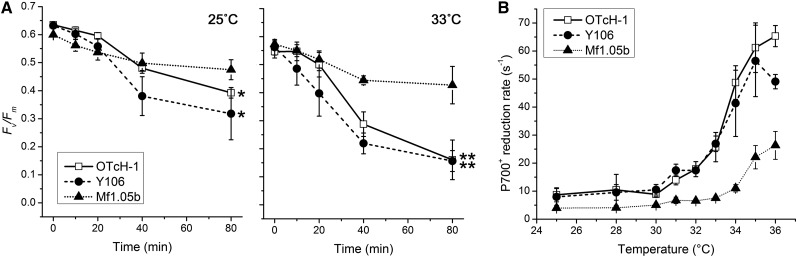
Symbiodinium strains are diverse, and their sensitivities to photoinhibition under heat stress vary considerably (Warner et al., 1996). We examined the relationships between photoinhibition sensitivity and CEF activity using three different Symbiodinium strains, i.e. OTcH-1 (clade A), Y106 (clade A), and Mf1.05b (clade B1), at 25°C and 33°C. The extent of photoinhibition was monitored on the basis of the decrease in the maximum quantum yield of PSII (Fv/Fm) during light exposure (Fig. 5A). Fv/Fm decreased slightly faster in OTcH-1 and Y106 than in Mf1.05b at 25°C (Fv/Fm declined to 62%, 50%, and 79% of the initial values after 80 min of light exposure in OTcH-1, Y106, and Mf1.05b, respectively). At 33°C, the Fv/Fm in OTcH-1 and Y106 decreased significantly faster than at 25°C (the Fv/Fm declined to 29% and 28% of the initial values at 33°C in OTcH-1 and Y106, respectively), but there was no difference in the Fv/Fm of Mf1.05b. These results indicate that Mf1.05b is less sensitive to heat-induced photoinhibition than OTcH-1 and Y106. We further examined the effects of high temperature on the P700+ reduction rate (Fig. 5B). At temperatures ranging from 25°C to 30°C, P700+ reduction was slow in all three Symbiodinium strains, which is consistent with the results of a previous report (Roberty et al., 2014). However, this rate was considerably higher in all three Symbiodinium strains at temperatures above 30°C; the rates started to increase at 31°C in OTcH-1 and Y106 and at 34°C in Mf1.05b (Fig. 5A). The P700+ reduction rate was considerably higher in OTcH-1 and Y106 than in Mf1.05b at all temperatures. The P700+ reduction rates at 34°C in OTcH-1 and Y106 were 4.6- and 3.9-fold greater than in Mf1.05b, respectively (Fig. 5A). Our results demonstrate that Symbiodinium strains commonly enhance CEF under high temperature conditions and that the extent of this enhancement differs among strains. Intriguingly, the clade A Symbiodinium strains (OTcH-1 and Y106), which are more sensitive to photoinhibition, exhibited greater CEFs than the clade B strain (Mf1.05b), which is less sensitive to photoinhibition at 34°C, thus suggesting that the extent of CEF does not correspond to the strains’ abilities to cope with photoinhibition under heat stress but rather reflects their stress responses.
Figure 5.
Relationships between the extent of CEF and the photoinhibition sensitivity in three different Symbiodinium strains. A, Effects of high temperature on PSII photoinhibition. The cells were preincubated at 25°C (left) and 33°C (right) for 1 h in the dark and were subsequently exposed to light at 500 µmol photons m−2 s−1. The maximum quantum yield of PSII, i.e. Fv/Fm, was measured at each time point after dark incubation for 10 to 15 min. The asterisks indicate statistically significant differences compared with the corresponding data from Mf1.05b (*P < 0.05 and **P < 0.01, Student’s t tests). B, Effects of elevated temperatures on the P700+ reduction rate. The cells were incubated at temperatures ranging from 25°C to 36°C for 15 min in the dark before the measurements. DCMU was subsequently added, and the rate constant of P700+ reduction was taken as demonstrated in Figure 1. The values are presented as means ± sd from three independent experiments.
DISCUSSION
High Temperature Activates CEF in Symbiodinium
Reynolds et al. (2008) have demonstrated that clade A Symbiodinium strains exhibit significant CEF activity. In contrast, Roberty et al. (2014) have reported that the CEF activities in four Symbiodinium strains, including clade A, are only marginal. Therefore, the significance of CEF and whether CEF is always active, always inactive, or active only under certain conditions in Symbiodinium have remained unclear. In our study, CEF activities were low in all three of the Symbiodinium strains we tested, including the clade A strains, when they were grown at the optimal growth temperatures. However, CEF activity was enhanced by increasing the temperature, and this enhancement was apparent at temperatures above 33°C (Fig. 1). Therefore, CEF can be induced by heat in Symbiodinium. The enhanced CEF activity after heat treatment was rapidly relaxed by the return to the optimal growth temperature (Fig. 3), which suggests that heat-induced CEF is a reversible process that responds to heat stress in Symbiodinium. Because similar phenomena have been observed in Chlamydomonas (Supplemental Fig. S6) and land plants (Sazanov et al., 1998; Bukhov et al., 1999; Egorova and Bukhov, 2002; Quiles, 2006), heat-induced CEF could be a common response of photosynthetic organisms, although its extent depends on the organism. Because such heat-induced CEF is more evident in Symbiodinium than in Chlamydomonas (Fig. 5; Supplemental Fig. S5), such acclimation of electron flow may be important for these tropical symbiotic algae to cope with the acute heat stress that they likely encounter frequently in their niche.
CEF is necessary to activate qE because it contributes to the generation of a ∆pH across the thylakoid membrane. Accordingly, mutant land plants deficient in CEF activity exhibit significantly lower qE capacities that lead to greater susceptibility to photoinhibition compared with that of wild-type plants (Munekage et al., 2002), which explains why Symbiodinium becomes highly susceptible to photoinhibition after treatment with the proton uncoupler NH4Cl (Takahashi et al., 2009). Thus, the induced CEF observed in this study could help alleviate photoinhibition under hot and/or excessive light conditions in Symbiodinium. Because photoinhibition causes the bleaching of the pigments in endosymbiotic Symbiodinium and eventually causes “coral bleaching” under high temperature conditions (Takahashi et al., 2004; Takahashi et al., 2008), the heat-induced CEF observed in this study (Fig. 2) might also help alleviate coral bleaching.
How Do Temperature Increases Enhance CEF in Symbiodinium?
In Symbiodinium, CEF was rapidly enhanced by increases in temperature (Fig. 3). In higher plants, the heat induction of CEF has been hypothesized to be caused by the accumulation of reductants, such as NADPH, in the chloroplast stroma (Bukhov and Carpentier, 2004; Livingston et al., 2010; Johnson, 2011). This hypothesis is based on findings that environmental stresses, including high temperatures, inhibit reactions in the Calvin-Benson cycle (Weis, 1981; Jones et al., 1998; Lilley et al., 2010), which increases the NADPH/NADP+ ratio and in turn up-regulates CEF (Endo et al., 2005; Breyton et al., 2006; Govindachary et al., 2007). However, in this study, we demonstrated that glycolaldehyde, which inhibits the Calvin-Benson cycle, only slightly enhanced CEF in Symbiodinium (Supplemental Fig. S5). Therefore, the heat-induced CEF was not primarily due to the inhibition of the Calvin-Benson cycle in Symbiodinium.
In land plants (Joët et al., 2002) and green algae (Takahashi et al., 2013), CEF has been demonstrated to be accelerated under anaerobic conditions. Because inhibitors of mitorespiration and chlororespiration accelerate CEF in tobacco leaves (Joët et al., 2002), the anaerobic acceleration of CEF is likely due to the accumulation of reductants in the stroma (Bulté et al., 1990; Joët et al., 2002; Takahashi et al., 2013). Our results demonstrated that CEF was accelerated under anaerobic conditions in Symbiodinium (Supplemental Fig. S3), which suggests that the accumulation of reductants could also accelerate CEF in this alga. It is also possible that the heat-induced CEF was caused by the inhibition of mitorespiration or chlororespiration in heat-treated in Symbiodinium. However, mitorespiration is generally less sensitive to increased temperature than photosynthesis in photosynthetic organisms, including Symbiodinium (Iglesias-Prieto et al., 1992). Furthermore, we cannot exclude other mechanisms, for example, the conformational changes in the cytochrome b6f complex that have previously been demonstrated in isolated chloroplasts from peas (Thomas et al., 1986).
Two possible CEF pathways have been proposed for CEF: an NAD(P)H dehydrogenase (NDH)-dependent pathway and an Fd-dependent pathway (Shikanai, 2007). In the former pathway, NDH mediates NADPH oxidation and the plastoquinone pool reduction in much the same manner as complex I does in the mitochondria (Ogawa, 1991). Some algae, such as Chlamydomonas, do not have type I NDH but have only a single subunit of NDH (Nda2) that is localized in the chloroplast (Jans et al., 2008). The genome of Symbiodinium Mf1.05b (Shoguchi et al., 2013) contains only type II NDH (Supplemental Table S1). In the latter pathway, two molecular models have been proposed: the CEF supercomplex comprising PSI and its own antenna system (the PSI-LHCI supercomplex), LHCIIs, the cytochrome b6f complex, FNR, and PGRL1 with equimolar amounts of the cytochrome b6f complex and PSI (Iwai et al., 2010), and the PGR5-PGRL1 heterocomplex (Hertle et al., 2013) in which the 25-kD single membrane-spanning protein PGRL1 is reduced by Fd when it forms a heterocomplex with PGR5, which is an 8-kD membrane peripheral polypeptide (Munekage et al., 2002). The reduced and monomerized PGRL1 in turn reduces a plastoquinone (Hertle et al., 2013). The Symbiodinium genome contains copies of type II NDH (Nda2), PGR5, and PGRL1 in addition to the authentic components for CEF, including the cytochrome b6f complex, PSI, and FNR (Supplemental Table S1; Shoguchi et al., 2013). Thus, Symbiodinium has at least one type of NDH and possible machinery for an Fd-dependent pathway. Because the inhibitor of the PGR5-PGRL1 heterocomplex, i.e. AA, did not inhibit CEF in Symbiodinium (Supplemental Fig. S4), the PGR5-PGRL1 heterocomplex may not operate in this organism, and the NDA2-dependent and/or CEF supercomplexes may be responsible for heat-induced CEF. However, AA may simply not be taken into Symbiodinium cells as has been commonly observed for various chemical compounds in marine algae. This CEF pathway issue must be further clarified in future studies.
Differences in the Extent of CEF among Symbiodinium Strains under High Temperature Conditions
Symbiodinium is genetically diverse and is grouped into nine clades from A to I (Pochon and Gates, 2010). Each clade contains multiple phylotypes (Correa and Baker, 2009). The physiological characteristics, such as photosynthetic activity (Hennige et al., 2009) and thermal sensitivity (Tchernov et al., 2004; Takahashi et al., 2008; Takahashi et al., 2009), have been demonstrated to differ among Symbiodinium strains. In this study, we used three different Symbiodinium strains and found that the extent of CEF at high temperatures differed among the strains (Fig. 5A). Because CEF contributes to the activation of qE and the synthesis of ATP, CEF efficiency might differ among Symbiodinium strains under heat stress. We initially expected that the Symbiodinium strains that exhibit greater CEF would be more tolerant to heat stress, as proposed previously (Reynolds et al., 2008). However, the Symbiodinium strains that exhibited greater CEF (Fig. 5B) were actually more susceptible to photoinhibition at both moderate and high temperatures (Fig. 5A). Thus, the thermal tolerances of Symbiodinium strains do not readily correlate with their CEF activities but are rather inversely correlated. Thus, CEF is activated as an acute response to heat stress, which explains why the “heat-sensitive” strains that sensed heat stress at a lower temperature (33°C) induced greater CEF at that temperature.
MATERIALS AND METHODS
Strains and Growth Conditions
Cultures of Symbiodinium sp. OTcH-1 (clade A) were obtained from the National Institute of Technology and Evaluation (Chiba, Japan). The Y106 strain (clade A) was originally maintained in Dr. Michio Hidaka’s laboratory (University of the Ryukyus, Okinawa, Japan) and was a gift from Dr. Eiichi Shoguchi (Okinawa Institute of Science and Technology Graduate University, Okinawa, Japan). The Mf1.05b strain (clade B) was a gift from Dr. Mary Alice Coffroth (State University of New York at Buffalo, Buffalo, NY). The clades of these Symbiodinium cultures were determined as previously described (Toller et al., 2001; Santos et al., 2003). The Symbiodinium cells were grown in artificial seawater (sea salts no. S-9883; Sigma-Aldrich) containing Daigo’s IMK medium for marine microalgae at 25°C under white fluorescent bulbs at 60 µmol photons m−2 s−1 with a light/dark cycle of 12 h/12 h. Chlamydomonas reinhardtii 137c cells were grown in a high-salt minimal medium (Sueoka, 1960) at 25°C under continuous irradiation from white fluorescent lights at 40 µmol photons m−2 s−1. The sea anemone Aiptasia sp. (H2 strain) was a gift from Dr. John Pringle (Stanford University, Stanford, CA) and was grown in artificial seawater (sea salts REI-SEA; IWAKI) under white fluorescent bulbs at 30 µmol photons m−2 s−1 with a light/dark cycle of 12 h/12 h at 25°C. Artemia was fed to Aiptasia once per week.
Sample Preparation
AA, DCMU, MV, glycolaldehyde, Glc oxidase, and catalase were obtained from Sigma-Aldrich. Iodoacetamide was obtained from Tokyo Chemical Industry. DCMU, AA, and iodoacetamide were made up as stock solutions in ethanol at concentrations of 40, 50, and 500 mm, respectively. Glycolaldehyde, MV, and NH4Cl were made up as stock solutions in water.
The Symbiodinium cells were collected by mild centrifugation (715g for 1 min) during their late-logarithmic growth phase and suspended in fresh growth medium. The total concentrations of chlorophylls a and c2 were measured as described previously (Jeffrey and Humphrey, 1975; Takahashi et al., 2008). The harvested cells were diluted to 3 to 5, 20, and 80 µg Chl/mL for the measurements of chlorophyll fluorescence, oxygen evolution, and P700 absorption changes, respectively. Anoxic conditions were induced by the addition of Glc oxidase (1 mg/mL), Glc (20 mm), and catalase (200 units/mL) and subsequent incubation for 30 min in the dark. AA, NH4Cl, MV, glycolaldehyde, or iodoacetamide was added to the sample 30 min before the measurements or temperature treatments. For the measurements of P700 absorption changes and NPQ, 20% (w/v) Ficoll PM 400 (Sigma-Aldrich) was added to prevent cell sedimentation.
Aiptasia polyps were paralyzed by the addition of 0.37 m MgCl2 in a 1-to-1 ratio to artificial seawater and then used for experiments.
Temperature Treatment
The Symbiodinium or Aiptasia polyps were placed in a glass vial or in a plastic cuvette and incubated at different temperatures using an aluminum gradient heat bar as previously described (Takahashi et al., 2008) or in a MiniT-H2C aluminum heat block (BMS). For the induction of photoinhibition, the Symbiodinium cells were exposed to halogen lamps from the top during the heat incubation. During the NPQ measurement, the cells were placed in a plastic cuvette, and the temperature was controlled with a cuvette holder connected to a water bath (UA-100; EYELA).
Spectrophotometry
Measurements of P700 absorption changes and chlorophyll fluorescence were performed with a custom-made spectrophotometer as described previously (Takizawa et al., 2009; Iwai et al., 2010). For the analysis of the photoinhibition of PSII, the Fv/Fm was measured after dark incubation for 10 min. The fluorescence transient parameters were calculated as follows: Fv/Fm = (Fm − Fo)/Fm; NPQ = (Fm − Fm′)/Fm′; and rETR = PAR × (Fm′ − F)/Fm′, where PAR is the photosynthetically active radiation (Ralph et al., 2002).
Photosynthetic Oxygen Production Rate Measurements
Light-dependent oxygen evolution was measured with a Clark-type oxygen electrode (Hansatech Instruments) in a closed cuvette in the light at 2,000 µmol photons m−2 s−1 at 25°C. The cells (20 µg Chl/mL, 1.5 mL) were preincubated in the dark for 3 min and then exposed to saturating light for 3 min. The light-dependent oxygen evolution rate was calculated as the sum of the oxygen consumption rate in the dark and the oxygen production rate in the light.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Kinetics of P700 oxidation/reduction in Symbiodinium OTcH-1 during light exposure.
Supplemental Figure S2. Effect of DCMU on electron transfer in PSII in Symbiodinium OTcH-1.
Supplemental Figure S3. Effects of anoxia on the rate of P700+ reduction in Symbiodinium OTcH-1 in the presence of DCMU at 25°C.
Supplemental Figure S4. MV and AA were not effective in Symbiodinium OTcH-1.
Supplemental Figure S5. Effects of glycolaldehyde and iodoacetamide on the reduction of P700+ in the presence of DCMU in Symbiodinium OTcH-1.
Supplemental Figure S6. Effects of elevated temperatures on the P700+ reduction rates in C. reinhardtii.
Supplemental Table S1. Possible CEF components in Symbiodinium.
Supplementary Material
Acknowledgments
We thank Dr. John Pringle for Aiptasia H2 strain and Drs. Eiichi Shoguchi and Mary Alice Coffroth for Symbiodinium strains Y105 and Mf1.05b, respectively. We also thank the Model Plant Research Facility, National Institute for Basic Biology BioResource Center, for their technical support.
Glossary
- CEF
cyclic electron flow
- qE
high-energy-dependent thermal dissipation
- ∆pH
proton gradient
- DCMU
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- MV
methyl viologen
- AA
antimycin A
- rETR
relative electron transport rate
- NPQ
nonphotochemical quenching
- NDH
NAD(P)H dehydrogenase
Footnotes
This work was supported in part by a cooperative program between the Japan Science and Technology Agency and the Fonds de la Recherche Scientifique; the Japan Society for the Promotion of Science through Grant-in-Aid for Scientific Research (A); the Ministry of Education, Culture, Sports, Science and Technology (through the Network of Centres of Carbon Dioxide Resource Studies in Plants); and by the research project on environmental response strategies of life at the National Institute for Basic Biology.
References
- Aro EM, Virgin I, Andersson B (1993) Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134 [DOI] [PubMed] [Google Scholar]
- Breyton C, Nandha B, Johnson GN, Joliot P, Finazzi G (2006) Redox modulation of cyclic electron flow around photosystem I in C3 plants. Biochemistry 45: 13465–13475 [DOI] [PubMed] [Google Scholar]
- Bukhov N, Carpentier R (2004) Alternative photosystem I-driven electron transport routes: mechanisms and functions. Photosynth Res 82: 17–33 [DOI] [PubMed] [Google Scholar]
- Bukhov N, Carpentier R, Samson G (2001) Heterogeneity of Photosystem I reaction centers in barley leaves as related to the donation from stromal reductants. Photosynth Res 70: 273–279 [DOI] [PubMed] [Google Scholar]
- Bukhov NG, Wiese C, Neimanis S, Heber U (1999) Heat sensitivity of chloroplasts and leaves: leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth Res 59: 81–93 [Google Scholar]
- Bulté L, Gans P, Rebéillé F, Wollman F-A (1990) ATP control on state transitions in vivo in Chlamydomonas reinhardtii. Biochim Biophys Acta 1020: 72–80 [Google Scholar]
- Correa AMS, Baker AC (2009) Understanding diversity in coral-algal symbiosis: a cluster-based approach to interpreting fine-scale genetic variation in the genus Symbiodinium. Coral Reefs 28: 81–93 [Google Scholar]
- Davy SK, Allemand D, Weis VM (2012) Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol Mol Biol Rev 76: 229–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova EA, Bukhov NG (2002) Effect of elevated temperatures on the activity of alternative pathways of photosynthetic electron transport in intact barley and maize leaves. Russ J Plant Physiol 49: 575–584 [Google Scholar]
- Endo T, Kawase D, Sato F (2005) Stromal over-reduction by high-light stress as measured by decreases in P700 oxidation by far-red light and its physiological relevance. Plant Cell Physiol 46: 775–781 [DOI] [PubMed] [Google Scholar]
- Ferri G, Iadarola P, Zapponi MC (1981) Chloroplast glyceraldehyde-3-phosphate dehydrogenase (NADP+). Reactivity of essential cysteine residues in holo- and apoenzyme. Biochim Biophys Acta 660: 325–332 [DOI] [PubMed] [Google Scholar]
- Govindachary S, Bigras C, Harnois J, Joly D, Carpentier R (2007) Changes in the mode of electron flow to photosystem I following chilling-induced photoinhibition in a C3 plant, Cucumis sativus L. Photosynth Res 94: 333–345 [DOI] [PubMed] [Google Scholar]
- Hennige SJ, Suggett DJ, Warner ME, McDougall KE, Smith DJ (2009) Photobiology of Symbiodinium revisited: bio-physical and bio-optical signatures. Coral Reefs 28: 179–195 [Google Scholar]
- Hertle AP, Blunder T, Wunder T, Pesaresi P, Pribil M, Armbruster U, Leister D (2013) PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol Cell 49: 511–523 [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O. (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50: 839–866 [Google Scholar]
- Iglesias-Prieto R, Matta JL, Robins WA, Trench RK (1992) Photosynthetic response to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proc Natl Acad Sci USA 89: 10302–10305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Takizawa K, Tokutsu R, Okamuro A, Takahashi Y, Minagawa J (2010) Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 464: 1210–1213 [DOI] [PubMed] [Google Scholar]
- Jans F, Mignolet E, Houyoux P-A, Cardol P, Ghysels B, Cuiné S, Cournac L, Peltier G, Remacle C, Franck F (2008) A type II NAD(P)H dehydrogenase mediates light-independent plastoquinone reduction in the chloroplast of Chlamydomonas. Proc Natl Acad Sci USA 105: 20546–20551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1, c2 in higher plants, algae, and natural phytoplankton. Biochem Physiol Pflanz 167: 191–194 [Google Scholar]
- Joët T, Cournac L, Peltier G, Havaux M (2002) Cyclic electron flow around photosystem I in C(3) plants. In vivo control by the redox state of chloroplasts and involvement of the NADH-dehydrogenase complex. Plant Physiol 128: 760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GN. (2011) Physiology of PSI cyclic electron transport in higher plants. Biochim Biophys Acta 1807: 384–389 [DOI] [PubMed] [Google Scholar]
- Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U (1998) Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ 21: 1219–1230 [Google Scholar]
- Lesser M. (2011) Coral bleaching: causes and mechanisms. In Dubinsky Z, Stambler N, eds, Coral Reefs: An Ecosystem in Transition. Springer, Dordrecht, The Netherlands, pp 405–419 [Google Scholar]
- Lilley RM, Ralph PJ, Larkum AW (2010) The determination of activity of the enzyme Rubisco in cell extracts of the dinoflagellate alga Symbiodinium sp. by manganese chemiluminescence and its response to short-term thermal stress of the alga. Plant Cell Environ 33: 995–1004 [DOI] [PubMed] [Google Scholar]
- Livingston AK, Kanazawa A, Cruz JA, Kramer DM (2010) Regulation of cyclic electron flow in C₃ plants: differential effects of limiting photosynthesis at ribulose-1,5-bisphosphate carboxylase/oxygenase and glyceraldehyde-3-phosphate dehydrogenase. Plant Cell Environ 33: 1779–1788 [DOI] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110: 361–371 [DOI] [PubMed] [Google Scholar]
- Ogawa T. (1991) A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon transport of Synechocystis PCC6803. Proc Natl Acad Sci USA 88: 4275–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon X, Gates RD (2010) A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai’i. Mol Phylogenet Evol 56: 492–497 [DOI] [PubMed] [Google Scholar]
- Quiles MJ. (2006) Stimulation of chlororespiration by heat and high light intensity in oat plants. Plant Cell Environ 29: 1463–1470 [DOI] [PubMed] [Google Scholar]
- Ralph PJ, Polk SM, Moore KA, Orth RJ, Smith WO Jr (2002) Operation of the xanthophyll cycle in the seagrass Zostera marina in response to variable irradiance. J Exp Mar Biol Ecol 271: 189–207 [Google Scholar]
- Reynolds JM, Bruns BU, Fitt WK, Schmidt GW (2008) Enhanced photoprotection pathways in symbiotic dinoflagellates of shallow-water corals and other cnidarians. Proc Natl Acad Sci USA 105: 13674–13678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberty S, Bailleul B, Berne N, Franck F, Cardol P (2014) PSI Mehler reaction is the main alternative photosynthetic electron pathway in Symbiodinium sp., symbiotic dinoflagellates of cnidarians. New Phytol 204: 81–91 [DOI] [PubMed] [Google Scholar]
- Roth MS. (2014) The engine of the reef: photobiology of the coral-algal symbiosis. Front Microbiol 5: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SR, Kinzie RA III, Sakai K, Coffroth MA (2003) Molecular characterization of nuclear small subunit (18S)-rDNA pseudogenes in a symbiotic dinoflagellate (Symbiodinium, Dinophyta). J Eukaryot Microbiol 50: 417–421 [DOI] [PubMed] [Google Scholar]
- Sazanov LA, Burrows PA, Nixon PJ (1998) The chloroplast Ndh complex mediates the dark reduction of the plastoquinone pool in response to heat stress in tobacco leaves. FEBS Lett 429: 115–118 [DOI] [PubMed] [Google Scholar]
- Shikanai T. (2007) Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 58: 199–217 [DOI] [PubMed] [Google Scholar]
- Shikanai T. (2014) Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Curr Opin Biotechnol 26: 25–30 [DOI] [PubMed] [Google Scholar]
- Shoguchi E, Shinzato C, Kawashima T, Gyoja F, Mungpakdee S, Koyanagi R, Takeuchi T, Hisata K, Tanaka M, Fujiwara M, et al. (2013) Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr Biol 23: 1399–1408 [DOI] [PubMed] [Google Scholar]
- Sueoka N. (1960) Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardi. Proc Natl Acad Sci USA 46: 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Clowez S, Wollman FA, Vallon O, Rappaport F (2013) Cyclic electron flow is redox-controlled but independent of state transition. Nat Commun 4: 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Badger MR (2011) Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16: 53–60 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Murata N (2006) Glycerate-3-phosphate, produced by CO2 fixation in the Calvin cycle, is critical for the synthesis of the D1 protein of photosystem II. Biochim Biophys Acta 1757: 198–205 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Nakamura T, Sakamizu M, van Woesik R, Yamasaki H (2004) Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Physiol 45: 251–255 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Whitney S, Itoh S, Maruyama T, Badger M (2008) Heat stress causes inhibition of the de novo synthesis of antenna proteins and photobleaching in cultured Symbiodinium. Proc Natl Acad Sci USA 105: 4203–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Whitney SM, Badger MR (2009) Different thermal sensitivity of the repair of photodamaged photosynthetic machinery in cultured Symbiodinium species. Proc Natl Acad Sci USA 106: 3237–3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa K, Takahashi S, Hüner NP, Minagawa J (2009) Salinity affects the photoacclimation of Chlamydomonas raudensis Ettl UWO241. Photosynth Res 99: 195–203 [DOI] [PubMed] [Google Scholar]
- Tchernov D, Gorbunov MY, de Vargas C, Narayan Yadav S, Milligan AJ, Häggblom M, Falkowski PG (2004) Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci USA 101: 13531–13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PG, Quinn PJ, Williams WP (1986) The origin of photosystem-I-mediated electron transport stimulation in heat-stressed chloroplasts. Planta 167: 133–139 [DOI] [PubMed] [Google Scholar]
- Toller WW, Rowan R, Knowlton N (2001) Zooxanthellae of the Montastraea annularis species complex: patterns of distribution of four taxa of Symbiodinium on different reefs and across depths. Biol Bull 201: 348–359 [DOI] [PubMed] [Google Scholar]
- Warner ME, Fitt WK, Schmidt GW (1996) The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: a novel approach. Plant Cell Environ 19: 291–299 [Google Scholar]
- Weis E. (1981) Reversible heat-inactivation of the calvin cycle: a possible mechanism of the temperature regulation of photosynthesis. Planta 151: 33–39 [DOI] [PubMed] [Google Scholar]
- Xiang T, Hambleton EA, DeNofrio JC, Pringle JR, Grossman AR (2013) Isolation of clonal axenic strains of the symbiotic dinoflagellate Symbiodinium and their growth and host specificity. J Phycol 49: 447–458 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.