The N-terminal domain of the Stt7/STN7 protein kinase is essential for its activity and interacts directly with the Rieske protein of the cytochrome b6f complex.
Abstract
Photosynthetic organisms have the ability to adapt to changes in light quality by readjusting the cross sections of the light-harvesting systems of photosystem II (PSII) and photosystem I (PSI). This process, called state transitions, maintains the redox poise of the photosynthetic electron transfer chain and ensures a high photosynthetic yield when light is limiting. It is mediated by the Stt7/STN7 protein kinase, which is activated through the cytochrome b6f complex upon reduction of the plastoquinone pool. Its probable major substrate, the light-harvesting complex of PSII, once phosphorylated, dissociates from PSII and docks to PSI, thereby restoring the balance of absorbed light excitation energy between the two photosystems. Although the kinase is known to be inactivated under high-light intensities, the molecular mechanisms governing its regulation remain unknown. In this study we monitored the redox state of a conserved and essential Cys pair of the Stt7/STN7 kinase and show that it forms a disulfide bridge. We could not detect any change in the redox state of these Cys during state transitions and high-light treatment. It is only after prolonged anaerobiosis that this disulfide bridge is reduced. It is likely to be mainly intramolecular, although kinase activation may involve a transient covalently linked kinase dimer with two intermolecular disulfide bonds. Using the yeast two-hybrid system, we have mapped one interaction site of the kinase on the Rieske protein of the cytochrome b6f complex.
Photosynthetic organisms are subjected to constant changes in light quality and quantity and need to adapt to these changes in order to optimize, on the one hand, their photosynthetic yield, and to minimize photo-oxidative damage on the other. The photosynthetic electron transfer chain consists of photosystem II (PSII), the plastoquinone (PQ) pool, the cytochrome b6f complex (Cyt b6f), plastocyanin, and photosystem I (PSI). All of these complexes and components are integrated or closely associated with the thylakoid membrane. The two antenna systems of PSII and PSI capture and direct the light excitation energy to the corresponding reaction centers in which a chlorophyll dimer is oxidized and charge separation occurs across the thylakoid membrane. These processes lead to the onset of electron flow from water on the donor side of PSII to ferredoxin on the acceptor side of PSI coupled with proton translocation across the thylakoid membrane. In order to sustain optimal electron flow along this electron transfer chain, the redox poise needs to be maintained under changing environmental conditions. Several mechanisms have evolved for the maintenance of this redox balance. In the case of over-reduction of the acceptor side of PSI, excess electrons can reduce molecular oxygen through the Mehler reaction to superoxide, which is then converted to hydrogen peroxide by a plastid superoxide dismutase and ultimately to water by a peroxidase (Asada, 2000). Over-reduction of the PQ pool can be alleviated by PTOX, the plastid terminal oxidase responsible for oxidizing PQH2 to form hydrogen peroxide, which is subsequently converted to water (Carol et al., 1999; Cournac et al., 2000; Wu et al., 1999).
In addition to these electron sinks that prevent the over-reduction of the electron transfer chain, the photosynthetic apparatus is able to maintain the redox poise of the PQ pool by readjusting the relative cross sections of the light harvesting systems of PSII and PSI upon unequal excitation of the two photosystems. This readjustment can occur both in the short term through state transitions and in the long term by changing the stoichiometry between PSII and PSI (Bonaventura and Myers, 1969; Murata, 1969; Pfannschmidt, 2003). State transitions occur because of perturbations of the redox state of the PQ pool due to unequal excitation of PSII and PSI, limitations in electron acceptors downstream of PSI, and/or in CO2 availability. Excess excitation of PSII relative to PSI leads to reduction of the PQ pool and thus favors the docking of PQH2 to the Qo site of the Cyt b6f complex. This process activates the Stt7/STN7 protein kinase (Vener et al., 1997; Zito et al., 1999), which is closely associated with this complex and leads to the phosphorylation of some LHCII proteins and to their detachment from PSII and binding to PSI (Depège et al., 2003; Lemeille et al., 2009). Although both Lhcb1 and Lhcb2 are phosphorylated, only the phosphorylated form of Lhcb2 is associated with PSI whereas phosphorylated Lhcb1 is excluded from this complex (Longoni et al., 2015). This state corresponds to state 2. In this way the change in the relative antenna sizes of the two photosystems restores the redox poise of the PQ pool. The process is reversible as over-excitation of PSI relative to PSII leads to the oxidation of the PQ pool and to the inactivation of the kinase. Under these conditions, phosphorylated LHCII associated with PSI is dephosphorylated by the PPH1/TAP38 phosphatase (Pribil et al., 2010; Shapiguzov et al., 2010) and returns to PSII (state 1). It should be noted, however, that a strict causal link between LHCII phosphorylation and its migration from PSII to PSI has been questioned recently by the finding that some phosphorylated LHCII remains associated with PSII supercomplexes and that LHCII serves as antenna for both photosystems under most natural light conditions (Drop et al., 2014; Wientjes et al., 2013).
State transitions are important at low light but do not occur under high light because the LHCII kinase is inactivated under these conditions (Schuster et al., 1986). It was proposed that inactivation of the kinase is mediated by the ferredoxin-thioredoxin system and that a disulfide bond in the kinase rather than in the substrate may be the target site of thioredoxin (Rintamäki et al., 1997, 2000). Analysis of the Stt7/STN7 protein sequences indeed reveals the presence of two conserved Cys residues close to the N-terminal end of this kinase, which are conserved in all species examined and both are essential for kinase activity although they are located outside of the kinase catalytic domain (Fig. 1) (Depège et al., 2003; Lemeille et al., 2009). Based on protease protection studies, this model of the Stt7/STN7 kinase proposes that the N-terminal end of the kinase is on the lumen side of the thylakoid membrane separated from the catalytic domain on the stromal side by an unusual transmembrane domain containing several Pro residues (Lemeille et al., 2009). This configuration of the kinase allows its catalytic domain to act on the substrate sites of the LHCII proteins, which are exposed to the stroma. Although in this model the conserved Cys residues in the lumen are on the opposite side from the stromal thioredoxins, it is possible that thiol-reducing equivalents are transferred across the thylakoid membrane through the CcdA and Hcf164 proteins, which have been shown to operate in this way during heme and Cyt b6f assembly (Lennartz et al., 2001; Page et al., 2004) or through the LTO1 protein (Du et al., 2015; Karamoko et al., 2011).
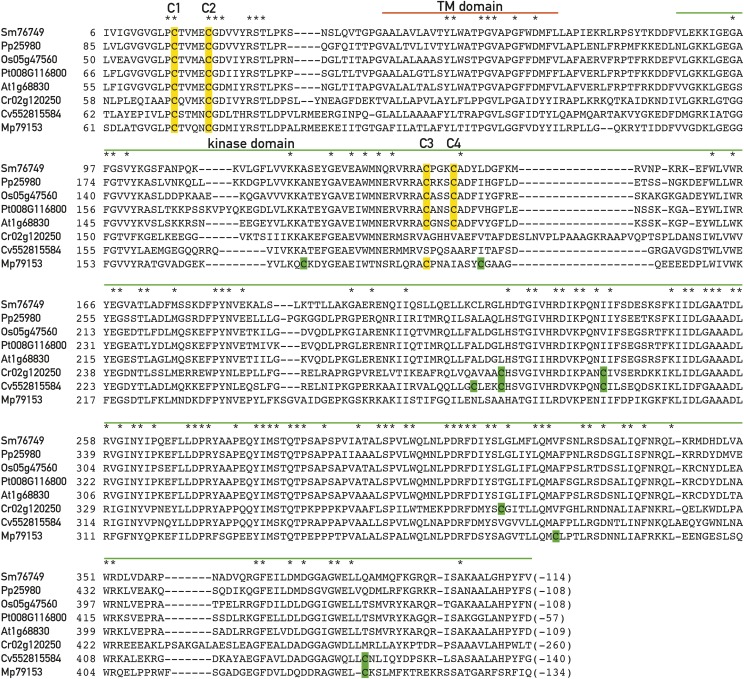
Figure 1.
Conserved Cys in the Stt7/STN7 kinase. Alignment of the sequences of the Stt7/STN protein kinase from Selaginella moelendorffii (Sm), Physcomitrella patens (Pp), Oryza sativa (Os), Populus trichocarpa (Pt), Arabidopsis thaliana (At), Chlamydomonas reinhardtii (Cr), Chlorella variabilis (Cv), and Micromonas pusilla (Mp). The positions of the conserved C1, C2, C3, and C4 Cys are highlighted in yellow. Note that C3 and C4 are not conserved in C. reinhardtii. Additional Cys residues are highlighted in green. Regions corresponding to the putative transmembrane (TM) and kinase domains are indicated, and invariant residues are marked with an asterisk.
Here we have examined the redox state of the Stt7/STN7 kinase during state transitions and after illumination with high light to test the proposed model. We find that the Stt7/STN7 kinase contains a disulfide bridge that appears to be intramolecular and maintained not only during state transitions but also in high light when the kinase is inactive. Although these results suggest at first sight that the disulfide bridge of Stt7/STN7 is maintained during its activation and inactivation, we propose that a transient opening of this bridge occurs during the activation process followed by the formation of an intermolecular disulfide bridge and the appearance of a short-lived, covalently linked kinase dimer.
RESULTS
No Detectable Change in Redox State of the Stt7 Kinase during State Transitions
Comparison of the Stt7/STN7 protein kinase sequences from different eukaryotic photosynthetic organisms reveals that among the five Cys residues in Chlamydomonas and land plants, only the two N-terminal Cys residues outside of the catalytic kinase domain are highly conserved (Depège et al., 2003). In the following text, these two Cys will be called C1 and C2 (Fig. 1); they correspond to C68 and C73 in Chlamydomonas reinhardtii and to C65 and C70 in Arabidopsis. The remaining Cys are not conserved among plants and algae. However, two other Cys within the kinase catalytic domain, called C3 and C4 corresponding to C187 and C191 in Arabidopsis, are conserved in land plants but not in C. reinhardtii and Chlorella and only one of these Cys is present in Micromonas (Fig. 1). Thus, C1 and C2 are the most likely candidates to be subjected to redox control by the ferredoxin-thioredoxin system.
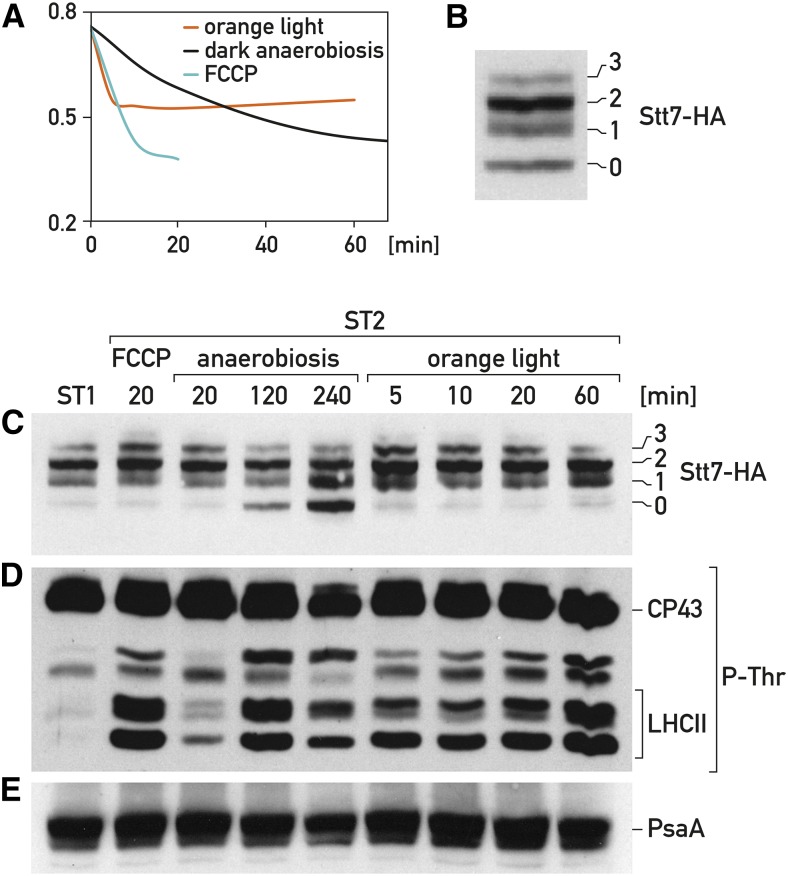
We first tested whether any change in the redox state of the Cys occurs during state transitions in Chlamydomonas. Cells expressing HA-tagged Stt7 protein were grown under aerobic conditions in low light that promote state 1. Transition from state 1 to state 2 was achieved in three different ways: first by adding FCCP (carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone), an uncoupler, to the cell culture, which leads to depletion of ATP and induction of state 2 (Bulté et al., 1990); second, by illuminating the culture with orange light, which is preferentially absorbed by PSII and leads to state 2; and third, through anaerobiosis in the dark (Bulté et al., 1990). In each of these three cases the transition to state 2 was monitored by observing the decrease of fluorescence at room temperature (Fig. 2A). The redox state of the Cys residues was determined using the following strategy. Cell cultures were lysed in presence of iodoacetamide to alkylate the free Cys. After washing off unbound iodoacetamide, the protein extract was treated with tributylphosphine to reduce all existing disulfide bonds and ultimately with mPEG-MAL (methoxy polyethylene glycol maleimide), which reacted with the newly available Cys after opening of the disulfide bonds (Wobbe et al., 2009). In this way, each reactive Cys gave rise to a discrete increase in molecular mass, which could be assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with an HA antiserum. A ladder of signals corresponding to the coupling of 0, 1, 2, and 3 mPEG-MAL molecules to Stt7-HA could be detected (Fig. 2B). A fifth weak band was observed in some experiments but it was not always reproducible and will not be considered further. Band 0 corresponds to fully reduced Stt7; band 1 corresponds to one intermolecular disulfide bridge, although an intramolecular disulfide bridge with partial mPEG-MAL coupling cannot be excluded; band 2 corresponds to one intramolecular or to two intermolecular disulfide bridges; and band 3 corresponds to one inter- and one intramolecular bond or to three intermolecular bonds. The mPEG-MAL treatment was performed during transition from state 1 to state 2 using the three different methods mentioned above. In the case of anaerobiosis in the dark and illumination with orange light, different time points were taken (Fig. 2C). No significant change in the redox state of Stt7 was observed when state 2 was induced by FCCP or orange light; in particular the disulfide bridge was maintained. In the case of anaerobiosis, a disulfide bond was opened as seen by the appearance of the fully reduced form of Stt7, but only after 120 min in contrast to transition to state 2, which occurred with a half-time of 20 min (Fig. 2A). To verify that LHCII phosphorylation takes place under the conditions used for the mPEG-MAL treatment, we determined the phosphorylation status of LHCII by using the same protein extracts for immunoblotting with antiphospho-Thr antiserum. It can be seen that phosphorylation of LHCII occurred under all three conditions in state 2 and that in the case of anaerobiosis, it was observed significantly earlier than the reduction of the disulfide bridge (compare immuno-blots with anti-HA and antiphospho-Thr antiserum in Fig. 2, C and D). Thus, no Cys redox change of Stt7 is detectable during state transitions, although a disulfide bridge is opened after prolonged anaerobic treatment.
Figure 2.
Changes in redox state of the Stt7 kinase and LHCII phosphorylation do not correlate during state transitions. A, Chlamydomonas cells were gown under low light (20 μmol photons m−2 s−1) with strong aeration. At time 0 transition from state 1 to state 2, monitored by measuring the decrease in fluorescence, was induced in three different ways: illumination of cells with orange light (red curve), anaerobiosis in the dark (black curve), and treatment with 5 μm FCCP (blue curve). B, Redox state of Stt7 kinase tagged with HA during state transitions. Total cell extract was treated with iodoacetamide to block free Cys, subsequently with tributylphosphine to reduce disulfide bonds and then with mPEG-MAL to alkylate the reduced Cys (for details, see “Materials and Methods”). The protein extract was fractionated by SDS-PAGE and immunoblotted with HA antiserum. The number of Cys that reacted with mPEG-MAL is indicated on the right. C, Redox state of Stt7 kinase tagged with HA during state transitions. Extracts from cells collected at the indicated times (min) after a transition from state 1 to state 2 as in (A) were processed as described in (B). The protein extract was fractionated by SDS-PAGE and immunoblotted with HA antiserum. The number of Cys that reacted with mPEG-MAL is indicated on the right. D, Immunoblots of total protein as in (C) (not labeled with mPEG-MAL) with antiphospho-Thr antiserum. E, Immunoblots of total protein as in (C) (not labeled with mPEG-MAL) with PsaA antiserum.
Effect of High Light on the Redox State of the Stt7/STN7 Kinase
We next examined whether high-light treatment leads to a change in the Cys redox state of the Stt7 kinase as predicted by earlier studies, which suggested that the kinase is inactivated through reduction by the ferredoxin-thioredoxin system (Rintamäki et al., 2000, 1997). Redox changes were monitored as described above at different time points of high-light irradiation (950 μmol photons m−2 s−1). However, no significant change could be observed, in particular disulfide bonds were maintained (Fig. 3A).
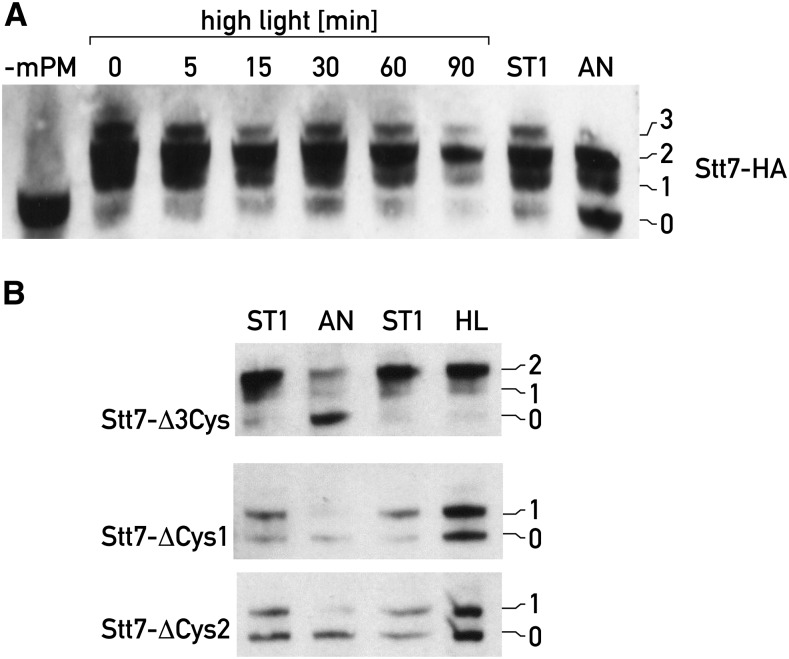
Figure 3.
Redox state of the Stt7 kinase does not change under high light. A, The redox state of Stt7 of Chlamydomonas cells transferred from low light to high light (950 µmol photons m-2s−1) was determined using mPEG-MAL at different time points as described in Fig. 2B. Cells in state 1 (ST1) and under prolonged anaerobiosis (AN, 60 min) were used as control. The number of Cys that reacted with mPEG-MAL is indicated on the right; mPM-, mPEG-MAL treatment was omitted. B, Same as A except that cells from three mutant Stt7 kinases, Stt7-Δ3Cys, Stt7- Δ Cys1 (Cys68Ala), Stt7- Δ Cys2 (Cys70Ala), were used. In Stt7- D3Cys the three non-conserved Cys within the catalytic domain were changed: C293L, C308I and C387I. HL, high light treatment.
To ascertain that bands 0, 1, and 2 obtained after mPEG-MAL treatment reflect the redox state of C1 and C2 of Stt7, the three remaining Cys within the catalytic kinase domain were mutated to C293L, C308I, and C387I (Stt7-Δ3Cys). The amino acids were changed to those found at the corresponding positions in the STN7 kinase of Arabidopsis. The stt7 mutant was transformed with this construct and shown to express the mutant kinase (Supplemental Fig. S1A). This mutant was locked in state 1 as revealed by the low temperature fluorescence emission spectra under conditions inducing state 1 and state 2 and therefore its Stt7 kinase was inactive (Supplemental Fig. S1B). For this mutant kinase, band 0, band 1, and band 2 arising from mPEG-MAL coupling could still be detected, but not band 3, indicating that band 1 and band 2 are due to C1 and C2. No change in the redox state was detected after high-light treatment (Fig. 3B), confirming the absence of redox change of the C1-C2 pair under high light. Moreover, opening of the disulfide bridge still occurred in this mutant after prolonged anaerobiosis, confirming that this redox change occurs independently of state transitions (Fig. 3B). Because the three nonconserved Cys of Stt7 on the stromal side are within the catalytic kinase domain, it is possible that the change of these residues in Stt7-Δ3Cys affects the proper folding of the kinase and its activity. In particular, it is noticeable that Cys-308 is located in a conserved region of the Stt7 kinase and might be essential for kinase activity.
We also examined mutants of Chlamydomonas in which Cys C1 was changed to Ala (Cys-68Ala and Cys-73Ala) (Lemeille et al., 2009). These mutants revealed only bands 0 and 1 after mPEG-MAL treatment (Fig. 3B). Taken together, these results indicate that the thiol bridges revealed by band 1 and band 2 in Figure 3, A and B, involve C1 and C2 in the N-terminal domain and suggest that band 3 arises from the stromal kinase domain. Band 1 is indicative of an intermolecular disulfide bridge and could reflect the existence of an Stt7 dimer in which the two monomers are connected by a single disulfide bridge in the case of the Cys C1 mutant.
A similar approach was used for examining the redox state of the Cys residues of STN7 of Arabidopsis subjected to high light (2200 μmol photons m−2 s−1; Supplemental Fig. S2A). Besides the 0 band, two bands were detectable after mPEG-MAL treatment. These bands were absent when the tributylphosphine treatment was omitted, indicating that these bands are specifically generated by free sulfhydryl groups (Supplemental Fig. S2A). As in the case of Chlamydomonas, no change in redox state could be detected after 90 min of high-light treatment of the Arabidopsis wild type (Col-0). However, the level of the STN7 kinase decreased during the high-light treatment (Supplemental Fig. S2B), a feature that was not observed with Stt7 from Chlamydomonas.
As in the case of Chlamydomonas, the dominant doubly labeled band corresponds to one intramolecular or to two intermolecular thiol bridges. It is interesting that the existence of STN7 dimers has recently been reported in Arabidopsis (Wunder et al., 2013). However, dimerization was undetectable in the wild type and could only be observed when the kinase was overexpressed or in the STN7-C1 or -C2 mutants.
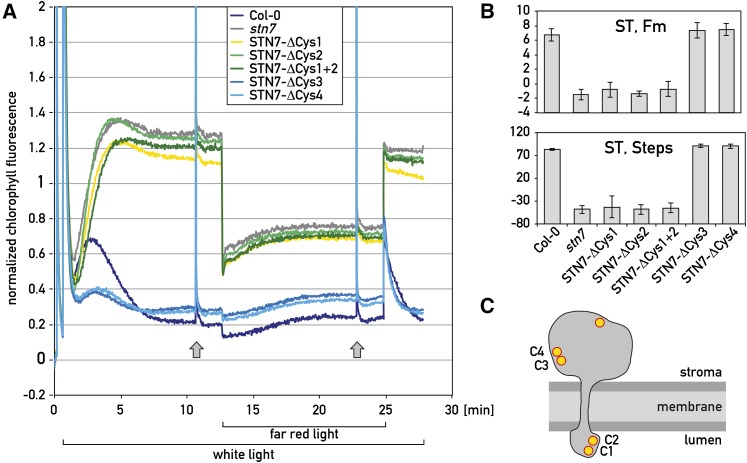
It was recently proposed that in land plants, the ferredoxin-thioredoxin system could act on Cys C3 and C4 on the stromal side, rather than on Cys C1 and C2 on the lumen side of the thylakoid membrane (Puthiyaveetil, 2011). Although C3 and C4 are not conserved in Chlamydomonas and Chlorella, they are present in all land plants tested (Fig. 1). To determine whether these Cys play any role in the activity of the STN7 kinase, C3 and C4 were changed to Ala and the corresponding mutant STN7 genes were introduced into the stn7 mutant of Arabidopsis. Similar mutations were created with C1 and C2 and these were combined in the double mutant C1C2. The level of the STN7 kinase was decreased in the C1 but not in the C2 mutant, and significantly increased in the C3 and C4 mutants compared to the wild type (Supplemental Fig. S3A). State transitions were measured by first illuminating three-week-old Arabidopsis seedlings with white light and recording their fluorescence corresponding to state 2. To induce transition to state 1, far-red light was superimposed on the white light as described (Bellafiore et al., 2005) (Fig. 4A). Saturating flashes were fired under both state 1 and state 2 conditions to estimate the maximum fluorescence. Turning off the far-red light led to a transition from state 1 to state 2. Under these conditions, the stn7 mutant can easily be distinguished from the wild type. In particular when the far-red light is turned on, the drop in fluorescence is significantly more pronounced in stn7 compared to wild type. Upon switching off far-red light, the fluorescence decreases in wild type but stays high in the mutant because it is locked in state 1. State transitions in wild type and in the different mutants were quantified (Fig. 4B). These results were further confirmed by determining the phosphorylation of Lhcb1 and Lhcb2, the major substrates of the kinase under state 1 and state 2 conditions and by recording the low temperature fluorescence emission spectra (Supplemental Fig. S3, B and C). As expected, LHCII phosphorylation was only observed under state 2 conditions induced by blue light illumination in the wild type, STN7-ΔCys-3, and STN7-ΔCys-4 mutants but not in the STN7-ΔCys-1, STN7-ΔCys-2, and STN7-ΔCys-1+2 mutants. State transitions were also monitored by measuring the fluorescence emission spectra at low temperature, which confirmed that the rise in the PSI peak at 730 nm characteristic for state 2 is significantly decreased in the STN7-ΔCys-1 and STN7-ΔCys-2 but not in the STN7-ΔCys-3 and STN7-ΔCys-4 mutants (Supplemental Fig. S3C). The fact that Cys C3 and C4 are located in a region, which is not well conserved (Fig. 1), could explain why their changes do not affect the activity of the kinase. We conclude from these results that Cys C3 and C4 are not required for STN7 kinase activity and state transitions. Moreover, comparison of the LHCII phosphorylation patterns under high light in the wild type and STN7-ΔCys-3 and STN7-ΔCys-4 mutants did not reveal any significant difference in the decline of kinase activity (Supplemental Fig. S3D). Taken together, these results indicate that Cys C3 and C4 cannot mediate the proposed shut-off of the kinase through the ferredoxin-thioredoxin system.
Figure 4.
Changes in the conserved stromal Cys of STN7 do not impair state transitions in Arabidopsis. A, Three-week-old wild-type (Col-0) and Cys mutant seedlings of Arabidopsis were subjected to state transitions. In these mutants the conserved Cys C1, C2, C3, and C4 of STN7 (see Fig. 1) were changed to Ala and are labeled as Stn7-ΔCys-1, -2, -3, -4; Stn7-ΔCys-1+2 refers to the C1C2 double mutant. Before the fluorescence measurements, seedlings were transferred to the dark for 30 min. They were then subjected to white light (state 2). After 12 min, far-red light was superimposed for 12 min (state 1). Fs levels were continuously monitored and Fm was determined by using saturating flashes as indicated with arrows (off-scale). All curves were normalized to (F−F0)/F0. B, Quantification of state transitions by the “ST, Fm” and “ST, Steps” methods (for details, see “Materials and Methods”). C, Schematic view of the STN7 kinase of Arabidopsis with the Cys residues.
Interaction between Stt7/STN7 and the Cytb6f Complex
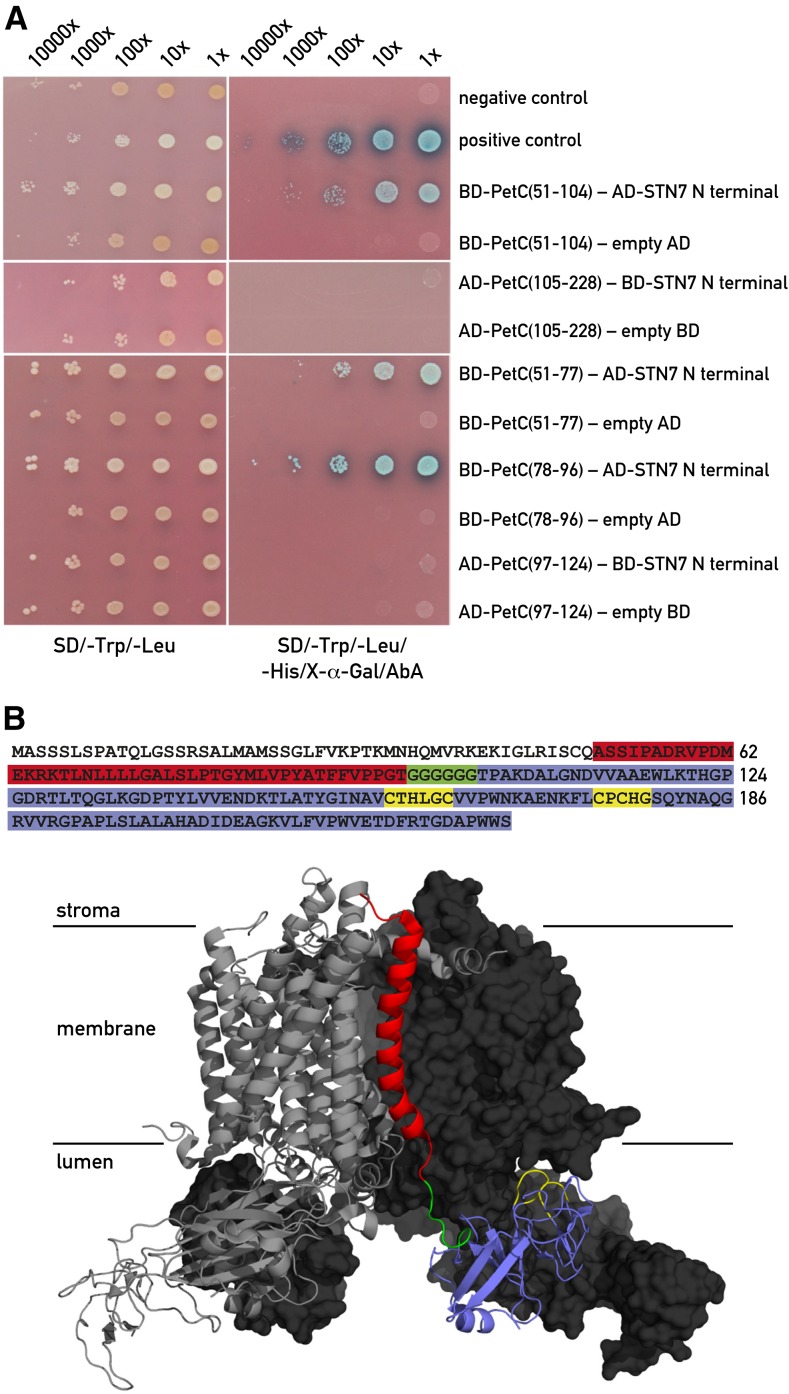
It is well established that the Stt7/STN7 kinase is closely associated with the Cytb6f complex (Lemeille et al., 2009) and that its activation is critically dependent on this association (Vener et al., 1997; Zito et al., 1999). Additional investigations using pull-down assays revealed that the interaction between this kinase and Cytb6f occurs through the Rieske protein (Lemeille et al., 2009). To further delineate the interaction site between the kinase and the Rieske protein, we performed yeast two-hybrid assays. Because most of the Rieske protein and its Fe-S center are localized on the lumen side of the thylakoid membrane and the N-terminal region of the kinase containing the two conserved Cys is on the same side (Lemeille et al., 2009), we tested the interaction between this part (STN7 residues 44–91) and different domains of the Rieske protein (PetC). As shown in Figure 5A, interactions were detected with the region between residues 51 and 104 of PetC but not with the domain containing the 2Fe-2S cluster (residues 105–228). Further analysis revealed that both PetC residues 51 to 77 and PetC residues 78 to 96 interact with the N-terminal part of the STN7 kinase. The first span covers 19 residues of the stromal region and part of the transmembrane region of PetC whereas the second span precedes a poly-Gly stretch, which acts as a hinge and separates the transmembrane portion from the lumenal domain of the Rieske protein. This indicates that the interaction site is mainly located near the hinge on the membrane side (Fig. 5B). PetC (97–124) including 22 residues downstream of the hinge did not interact with STN7. Similar results were obtained with yeast two-hybrid assays using the Stt7 N-terminal domain from Chlamydomonas and the same PetC fragments (Supplemental Fig. S4). We conclude that the region between residues 51 and 97 of PetC comprises the site of interaction with Stt7/STN7.
Figure 5.
Interaction between STN7 and Rieske protein. A, Yeast two-hybrid screens with the N-terminal domain of STN7 (residues 44–91) and subdomains of PetC (Rieske protein of the Cytb6f complex). The STN7 and Rieske fragments were either used as bait (BD) fused to the GAL4-DNA binding domain or as prey (AD) fused to the GAL4 activation domain in the yeast two-hybrid assays. This switch between prey and bait had to be performed because of autoactivation of the reporter gene by some of the constructs. Left panel shows the growth test on permissive medium lacking Trp and Leu. Right panel shows the same clones on selective medium lacking Trp, Leu, and His in the presence of aureobasidin A (AbA). The PetC regions are indicated by the residue numbers. Negative and positive controls are described in “Materials and Methods”. B, Summary of the yeast two-hybrid results. The domain of PetC interacting with the N-terminal end of STN7 is highlighted in red. PetC domains giving negative results in the two-hybrid system are highlighted in blue, the hinge region of PetC is in green, and the Cys ligands of the 2Fe-2S center are in yellow. The transit peptide of Pet C is not colored. The structure of the PetC dimer within the Cytb6f complex from C. reinhardtii (Stroebel et al., 2003) is shown in the lower part. The interacting domain is shown in red in the right monomer and the different regions of PetC are shown with the same colors as in the PetC sequence above.
DISCUSSION
Understanding the activation of the Stt7/STN7 protein kinase remains an important and challenging task in chloroplast biology because this enzyme plays a key role both in short-term and long-term acclimation to changes in the environment and in the cellular metabolic state. Under these conditions the redox state of the photosynthetic PQ pool is altered and the Stt7/STN7 is instrumental in maintaining a proper plastid redox poise. Moreover, this kinase appears to be the major regulator of the phosphorylation of the LHCII proteins because there is no evidence that the activity of the PPH1/TAP38 phosphatase, the counteractor of STN7, is regulated, suggesting that it is constitutively active (Shapiguzov et al., 2010; Wei et al., 2015). Our study has revealed new features of the Stt7/STN7 protein kinase that have to be taken into account for modeling this activation process.
Redox Status of the Stt7/STN7 Kinase during State Transitions
The classical view of state transitions is that upon increased reduction of the PQ pool, plastoquinol docks to the Qo site of the Cytb6f complex and thereby activates the Stt7/STN7 kinase. This protein acts as a sensor of the redox state of the photosynthetic electron transfer chain not only at the level of the PQ pool but probably also at the level of the PSI acceptor side. The presence of two conserved Cys residues, C1 and C2, in the N-terminal domain of the kinase raised the possibility that the redox state of these two Cys would change during state transitions. The C1 and C2 Cys are clearly essential for the activity of the kinase, because changes of either of these two Cys by site-directed mutagenesis abolish the activity of the kinase (Lemeille et al., 2009; Wunder et al., 2013). In our study no change in the redox status of the conserved C1 and C2 of Stt7/STN7 could be detected during state transitions in Chlamydomonas. Our mPEG-MAL analysis revealed four bands labeled 0 to 3 in Figures 2 and 3. Based on our analyses, C1 and C2 appear to form an intramolecular disulfide bridge under most conditions examined. An intermolecular disulfide bridge between two Stt7 monomers would give rise to a dimer that is undetectable under similar conditions as those used in this study (Wunder et al., 2013). However, it is important to note that our results do not exclude the possibility of rapid and transient changes in the redox state of these two Cys. In fact it is likely that such changes occur as explained by the model we propose, which takes into account all the known features of the Stt7/STN7 kinase.
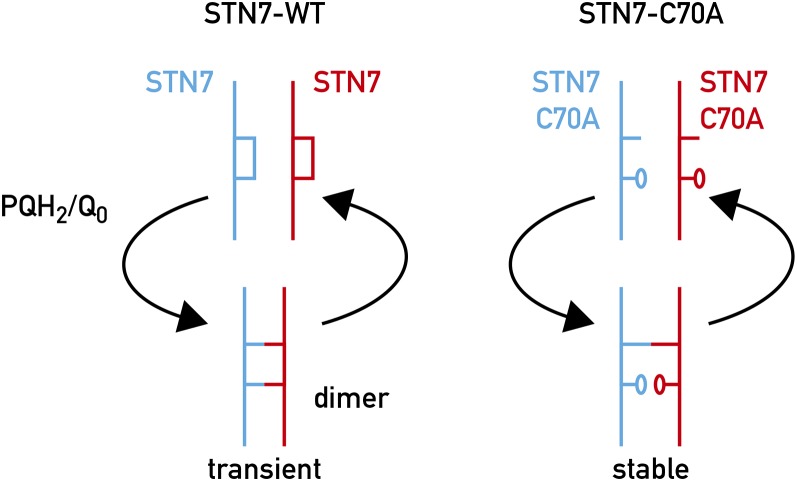
Based on the results obtained in this study, we postulate that the two lumenal Cys residues form a stable intramolecular disulfide bridge required for the proper folding of the kinase and/or for maintaining a configuration suitable for responding to changes in the redox state of the PQ pool. In this respect, based on our previous work (Lemeille et al., 2009) and on the yeast two-hybrid analysis (Fig. 5A), it is interesting to note that the interaction site of the Stt7/STN7 kinase with the Rieske protein is near the flexible Gly-rich hinge connecting the membrane anchor to the large head of this protein in the lumen. The crystal structure of the Cytb6f complex of C. reinhardtii (Stroebel et al., 2003) and of the thermophilic cyanobacterium Mastigocladus laminosus (Kurisu et al., 2003) reveals an open space on the lumen side in which the N-terminal domain of the kinase could fit (Fig. 5B). While the region upstream of the hinge is believed to be stable, the region downstream comprising the 2Fe-2S cluster is predicted to move significantly during the proximal/distal orientation shifts, which allow the Rieske protein to transfer electrons from plastoquinol at the Qo site to cytochrome f (for review, see Cramer et al., 2006; Darrouzet et al., 2001; de Vitry et al., 2004; Yan and Cramer, 2003). It is possible that this large-scale protein domain movement could transduce conformational changes to the kinase. In this respect it makes sense that the kinase would interact with the immobile Rieske domain and its conformation could be changed by the movement of the adjacent lumenal Rieske domain. We envisage that two Stt7/STN7 kinase monomers are associated with the Cytb6f dimer with weak interactions between the monomers because the dimer is undetectable in wild-type Arabidopsis plants (Wunder et al., 2013). As the kinase could also pass over the Qo site of the adjacent Cytb6f monomer, the quinol occupancy may create a better interaction surface between the kinase and the Rieske protein in a similar way to that described for the auxin receptor where the hormone acts as a molecular glue to enhance the interaction between the receptor and its substrate (Tan et al., 2007). It is also possible that the kinase senses PQH2 binding to the Qo site through the single chlorophyll a molecule in the Cytb6f complex whose phytyl tail is close to the Qo site (Stroebel et al., 2003). A specific role of the chlorophyll-binding niche in transmembrane signaling was suggested by the observation that insertion of an α-helix proximal to the chlorophyll-binding site inhibits state transitions (Zito et al., 2002). Based on site-directed mutagenesis of the chlorophyll a binding site, it was proposed that chlorophyll may play a role in the activation of the Stt7/STN7 kinase (de Lacroix de Lavalette et al., 2008; Hasan et al., 2013). It was further proposed that several lipid sites on the surface of Cytb6f complex close to the chlorophyll could form an adaptable surface for interaction with Stt7/STN7 kinase through lipid-mediated contacts (Hasan and Cramer, 2014). The activation of the kinase would be triggered through the transient formation of a STN7 dimer with two intermolecular disulfide bridges, which would transduce the signal to the catalytic domain on the stromal side of the thylakoid membrane. This proposal is based on the observation that although a STN7 dimer is undetectable in vivo in Arabidopsis, such a dimer could be detected in small amounts when STN7 was overexpressed and it could be clearly detected in the STN7-Cys mutants in which C1 or C2 was changed to Ala, presumably because the single disulfide bridge intermediate is more stable (Wunder et al., 2013). Such features of disulfide bridges are well established and have been used to trap substrates of thioredoxins (Balmer et al., 2003). We therefore propose that the dimer with two intermolecular disulfide bridges is required for the activation but it is rather unstable and reverts rapidly to the intramolecular disulfide bridge in each monomer when the Qo site is unoccupied (Fig. 6). In this dynamic model with a switch from intra- to intermolecular disulfide bonds, the kinase would need to be constantly reactivated though plastoquinol docking to the Qo site and eventually by the movement of the Rieske protein from its proximal to its distal position within the Cytb6f complex, and the kinase would be stably associated with the Cytb6f complex during phosphorylation of LHCII. This model would also explain why the kinase is rapidly inactivated upon oxidation of the PQ pool.
Figure 6.
Model for activation of the Stt7/STN7 kinase. Left: Upon docking of PQH2 to the Qo site of the Cytb6f complex and movement of the Rieske protein from the proximal to distal position, the two intramolecular disulfide bridges in the two adjacent wild-type Stt7/STN7 monomers associated with the Cytb6f complex are converted to two intermolecular disulfide bridges leading to a conformational change that activates the kinase. This state is unstable, and reverts to the intramolecular disulfide bridge configuration. Right: In the Cys-70Ala (C70A) mutant, the unique intermolecular disulfide bridge is stabilized but the kinase cannot be activated.
The only condition in which a change in the redox state of the Stt7/STN7 kinase could be detected was when the cells were subjected to prolonged anaerobiosis (Fig. 2). Under these conditions the disulfide bond was reduced, leading presumably to monomer formation. However, this process is clearly unrelated to state transitions because the time course of this change is significantly slower than state transitions and it occurs in a mutant deficient in state transitions (Fig. 3B). The physiological significance of this redox change of the kinase remains to be determined. It may allow the kinase to dissociate from the Cytb6f complex and perhaps to engage in other functions under prolonged anaerobic conditions.
How Is the Stt7/STN7 Inactivated by High Light?
Previous studies have shown that the LHCII kinase is inactivated by high-light irradiance (Schuster et al., 1986) and it was proposed that this inactivation could be mediated by the ferredoxin-thioredoxin system (Rintamäki et al., 2000). Obvious targets within Stt7/STN7 are the two conserved lumenal Cys C1 and C2. However, the analysis of the redox state of these residues did not reveal any opening of the disulfide bridge under high light (Fig. 3). Because two stromal-exposed Cys C3 and C4 located within the kinase catalytic domain are conserved in land plants although not in Chlamydomonas, it was proposed that they may act as alternative substrates for the ferredoxin-thioredoxin system (Puthiyaveetil, 2011). However, since changes of either Cys to Ala do not affect the activity of the kinase and state transitions, these residues are not essential, and their redox state cannot influence the activity of the Stt7/STN7 kinase (Fig. 4).
Moreover, when Arabidopsis plants are subjected to alternate low-light and high-light irradiance, phosphorylation of LHCII proteins occurs in low but not in high light, suggesting that the kinase is active in low but not in high light (Tikkanen et al., 2010). High light may induce a conformational change of the kinase possibly through changes in the redox state of the electron transfer chain and /or membrane potential, which would block its activity. Studies on isolated thylakoids have revealed that the Stt7 kinase is highly sensitive even to mild detergent treatment, at least in Chlamydomonas (W. Lu and J. D. Rochaix, unpublished results). Taken together, these findings raise the possibility that the folding of the kinase in the membrane is compromised by adverse environmental conditions, in particular by reactive oxygen species generated by the high-light treatment. In this respect it is interesting to note that singlet oxygen generated by PSII can oxidize plastoquinol with concomitant production of hydrogen peroxide in the thylakoid membranes (Khorobrykh et al., 2015). This finding led to the proposal that hydrogen peroxide may be involved in the activation of the Stt7/STN7 kinase by oxidizing the lumenal C1 and C2 to form intra- and/or intermolecular disulfide bridges (Khorobrykh et al., 2015). However, this proposal is difficult to reconcile with the observation that these Cys exist mostly in the oxidized form, and the conversion from intra- to intermolecular disulfide bridges appears to be only transient.
MATERIALS AND METHODS
Chlamydomonas Strains and Growth Conditions
The Chlamydomonas reinhardtii strains used in this study were maintained on Tris-acetate phosphate plates supplemented with 1.5% (w/v) Bacto-agar (Harris, 1989) at 25°C under constant light (60–40 μmol photons m−2 s−1) and grown in liquid Tris-acetate P medium under the same conditions.
Plant Material and Growth Conditions
Arabidopsis wild-type and mutant plants (all of Columbia-0 ecotype) were grown on Murashige-Skoog (MS) agar supplemented with 1.5% Suc in a growth chamber (Percival no. CU36L5; Percival Scientific, Perry, IA) in long days (16 h light/8 h dark) at 22°C under fluorescent white light (50 μmol photons m−2 s−1). Plants on soil were cultivated in growth chambers at 24°C under white light (100 μmol photons m−2 s−1) in long days. Plants for chlorophyll fluorescence measurements were grown in short days (8 h light/16 h dark). The stn7 mutant was described in Bellafiore et al. (2005). Agrobacterium transformations of the stn7 mutant (SALK 073254) were performed with the modified versions of the STN7 kinase as described in Clough and Bent (1998). Transformants were selected for Basta resistance and T3 plants were used for the experiments.
Site-Directed Mutagenesis
Site-directed mutagenesis of the Stt7 and STN7 cDNAs was performed as described in Willig et al. (2011) using primers described in Supplemental Table S1. Ultimately the resulting PCR fragment was cut with BglII and BamHI and inserted into the pPZmidiAt1HA plasmid containing the STN7 midi-gene digested with the same enzymes.
Thiol-Specific Labeling with mPEG-MAL
To determine the redox states of Stt7, Chlamydomonas cell cultures were lysed in the buffer containing 100 mm iodoacetamide, 2% SDS, and 50 mm Tris HCl pH 7.8, for 20 min at 60°C. The alkylated protein extracts were then precipitated with equal volumes of 20% trichloroacetic acid in acetone and intensively washed with 80% acetone. The samples were resuspended in a buffer containing 2% SDS and 50 mm Tris HCl pH 7.8, and proteins were reduced by incubation in the presence of 2 mm tributylphosphine for 20 min at room temperature. Then mPEG-MAL of Mr 5 kD was added to a final concentration of 10 mm (Wobbe et al., 2009) and the samples were incubated for 4 h at 28°C. Finally, the protein was again precipitated with trichloroacetic acid in acetone, resuspended, and used for immunoblotting. To determine the redox status of STN7, plant material was snap-frozen in liquid nitrogen, after which it was lysed/alkylated in presence of iodoacetamide and labeled as indicated above.
Immunoblotting
Immunoblotting was performed as described previously in Ingelsson et al. (2009) and Shapiguzov et al., (2010) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 10% acrylamide gels. Antibodies against HA and P-Thr were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against STN7 were generated using the full-size recombinant protein (with Professor Roberto Bassi, University of Verona, Verona, Italy).
Chlorophyll Fluorescence Measurements
Room temperature chlorophyll fluorescence was measured with a FluorCam 800MF kinetic imaging fluorimeter (Photon Systems Instruments, Albuquerque, NM) as described in Willig et al. (2011). Fourteen-to-18-day-old seedlings grown on MS agar were dark-adapted for 30 min. White actinic light (8%) was switched on for 12 min to induce state 2. Far-red light (100%) was then turned on against the background of continuous white light for 12 min to promote transition from state 2 to state 1. Finally, far red light was turned off to initiate the reverse transition. Measuring flashes were applied throughout the protocol to record Fs. Saturating light flashes were used at different times to measure Fm. Quantification of state transitions was performed in two ways. In the first method (“ST, Fm”), Fm is defined as (Fm1-Fm2)/Fm1 in % where Fm1 and Fm2 represent maximum fluorescence obtained with a saturating flash in states 1 and 2, respectively (Bellafiore et al., 2005). The second method (“ST, Steps”) uses the same parameters as defined by Lunde et al. (2000), i.e. [(Fi′–Fi) – (Fii′–Fii)]/(Fi′–Fi), where Fi and Fii designate fluorescence in the presence of PSI light in states 1 and 2, respectively, while Fi′ and Fii′ designate fluorescence in the absence of PSI light in state 1 and state 2, respectively. Detached rosette leaves were prepared for low temperature fluorescence measurements as described in Shapiguzov et al. (2010). Chlorophyll fluorescence at 77 K was measured with a model no. FP-750 spectrofluorometer (JASCO, Easton, MD). Excitation was at 480 nm (slit width 5 nm) and emission was recorded in the 600–800 nm range (slit width 5 nm). The curves were normalized for the PSII peak at 685 nm.
Chlamydomonas low temperature fluorescence spectra were recorded as described in Lemeille et al. (2009).
Yeast Two-Hybrid Screen
The Matchmaker Gold Yeast Two-Hybrid System (Clontech Laboratories, Madison, WI) was used as recommended by the manufacturer. Selection of positive clones was performed on medium lacking Leu, Trp, and His and containing aureobasidin A. For the positive control, pGBKT7-53 encoding the GAL4 DNA-binding domain fused with murine p53 was coexpressed with pGADT7-T encoding the GAL4 activation domain fused with SV40 large T-antigen, because p53 and large T-antigen are known to interact in a yeast two-hybrid assay (Li and Fields, 1993). For the negative control, pGBKT7-Lam encoding the GAL4 DNA-binding domain fused with lamin was coexpressed with pGADT7-T.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers XP_002961793 (Selaginella moelendorffii), XP_001773197 (Physcomitrella patens), NP_001056233 (Oryza sativa), XP_002312380 (Populus trichocarpa), NP_564946 (Arabidopsis thaliana), AAO63768 (Chlamydomonas reinhardtii), XP_005844483 (Chlorella variabilis), XP_002499717 (Micromonas pusilla).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Loss of the three non-conserved Cys in the catalytic domain of Stt7 inactivates the kinase.
Supplemental Figure S2. Redox state of the STN7 kinase does not change under high light.
Supplemental Figure S3. Fluorescence and phosphorylation patterns in different Arabidopsis STN7 Cys mutants.
Supplemental Figure S4. Interaction between Stt7 and Rieske protein.
Supplemental Table S1. List of primers.
Supplementary Material
Acknowledgments
We thank Michèle Rahire for technical assistance, Nicolas Roggli for preparing the figures, and Michel Goldschmidt-Clermont for critical reading of the manuscript. This work was supported by grant no. 31003A-133089/1 from the Swiss National Foundation.
Glossary
- LHCII
light-harvesting complex of PSII
- PQ
plastoquinone
- PSI
photosystem I
- PSII
photosystem II
Footnotes
Articles can be viewed without a subscription.
References
- Asada K. (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond B Biol Sci 355: 1419–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer Y, Koller A, del Val G, Manieri W, Schürmann P, Buchanan BB (2003) Proteomics gives insight into the regulatory function of chloroplast thioredoxins. Proc Natl Acad Sci USA 100: 370–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433: 892–895 [DOI] [PubMed] [Google Scholar]
- Bonaventura C, Myers J (1969) Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta 189: 366–383 [DOI] [PubMed] [Google Scholar]
- Bulté L, Gans P, Rebeille F, Wollman FA (1990) ATP control on state transitions in Chlamydomonas. Biochim Biophys Acta 1020: 72–80 [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M (1999) Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11: 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cournac L, Redding K, Ravenel J, Rumeau D, Josse EM, Kuntz M, Peltier G (2000) Electron flow between photosystem II and oxygen in chloroplasts of photosystem I-deficient algae is mediated by a quinol oxidase involved in chlororespiration. J Biol Chem 275: 17256–17262 [DOI] [PubMed] [Google Scholar]
- Cramer WA, Zhang H, Yan J, Kurisu G, Smith JL (2006) Transmembrane traffic in the cytochrome b6f complex. Annu Rev Biochem 75: 769–790 [DOI] [PubMed] [Google Scholar]
- Darrouzet E, Moser CC, Dutton PL, Daldal F (2001) Large scale domain movement in cytochrome bc1: a new device for electron transfer in proteins. Trends Biochem Sci 26: 445–451 [DOI] [PubMed] [Google Scholar]
- de Lacroix de Lavalette A, Finazzi G, Zito F (2008) b6f-Associated chlorophyll: structural and dynamic contribution to the different cytochrome functions. Biochemistry 47: 5259–5265 [DOI] [PubMed] [Google Scholar]
- Depège N, Bellafiore S, Rochaix JD (2003) Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299: 1572–1575 [DOI] [PubMed] [Google Scholar]
- de Vitry C, Ouyang Y, Finazzi G, Wollman FA, Kallas T (2004) The chloroplast Rieske iron-sulfur protein. At the crossroad of electron transport and signal transduction. J Biol Chem 279: 44621–44627 [DOI] [PubMed] [Google Scholar]
- Drop B, Webber-Birungi M, Yadav SK, Filipowicz-Szymanska A, Fusetti F, Boekema EJ, Croce R (2014) Light-harvesting complex II (LHCII) and its supramolecular organization in Chlamydomonas reinhardtii. Biochim Biophys Acta 1837: 63–72 [DOI] [PubMed] [Google Scholar]
- Du JJ, Zhan CY, Lu Y, Cui HR, Wang XY (2015) The conservative cysteines in transmembrane domain of AtVKOR/LTO1 are critical for photosynthetic growth and photosystem II activity in Arabidopsis. Front Plant Sci 6: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EH. (1989). The Chlamydomonas Source Book: a Comprehensive Guide to Biology and Laboratory Use. Academic Press, San Diego, CA: [DOI] [PubMed] [Google Scholar]
- Hasan SS, Cramer WA (2014) Internal lipid architecture of the hetero-oligomeric cytochrome b6f complex. Structure 22: 1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan SS, Stofleth JT, Yamashita E, Cramer WA (2013) Lipid-induced conformational changes within the cytochrome b6f complex of oxygenic photosynthesis. Biochemistry 52: 2649–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelsson B, Shapiguzov A, Kieselbach T, Vener AV (2009) Peptidyl-prolyl isomerase activity in chloroplast thylakoid lumen is a dispensable function of immunophilins in Arabidopsis thaliana. Plant Cell Physiol 50: 1801–1814 [DOI] [PubMed] [Google Scholar]
- Karamoko M, Cline S, Redding K, Ruiz N, Hamel PP (2011) Lumen Thiol Oxidoreductase1, a disulfide bond-forming catalyst, is required for the assembly of photosystem II in Arabidopsis. Plant Cell 23: 4462–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorobrykh SA, Karonen M, Tyystjärvi E (2015) Experimental evidence suggesting that H2O2 is produced within the thylakoid membrane in a reaction between plastoquinol and singlet oxygen. FEBS Lett 589: 779–786 [DOI] [PubMed] [Google Scholar]
- Kurisu G, Zhang H, Smith JL and Cramer WA (2003) Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302: 1009–1014. [DOI] [PubMed] [Google Scholar]
- Lemeille S, Willig A, Depège-Fargeix N, Delessert C, Bassi R, Rochaix JD (2009) Analysis of the chloroplast protein kinase Stt7 during state transitions. PLoS Biol 7: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartz K, Plücken H, Seidler A, Westhoff P, Bechtold N, Meierhoff K (2001) HCF164 encodes a thioredoxin-like protein involved in the biogenesis of the cytochrome b6f complex in Arabidopsis. Plant Cell 13: 2539–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Fields S (1993) Identification of mutations in p53 that affect its binding to SV40 large T antigen by using the yeast two-hybrid system. FASEB J 7: 957–963 [DOI] [PubMed] [Google Scholar]
- Longoni P, Douchi D, Cariti F, Fucile G, Goldschmidt-Clermont M (2015) Phosphorylation of the Lhcb2 isoform of Light Harvesting Complex II isoform Lhcb2 is central to state transitions. Plant Physiol 169: 2874–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde C, Jensen PE, Haldrup A, Knoetzel J, Scheller HV (2000) The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408: 613–615 [DOI] [PubMed] [Google Scholar]
- Murata N. (1969) Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta 172: 242–251 [DOI] [PubMed] [Google Scholar]
- Page ML, Hamel PP, Gabilly ST, Zegzouti H, Perea JV, Alonso JM, Ecker JR, Theg SM, Christensen SK, Merchant S (2004) A homolog of prokaryotic thiol disulfide transporter CcdA is required for the assembly of the cytochrome b6f complex in Arabidopsis chloroplasts. J Biol Chem 279: 32474–32482. [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T. (2003) Chloroplast redox signals: how photosynthesis controls its own genes. Trends Plant Sci 8: 33–41 [DOI] [PubMed] [Google Scholar]
- Pribil M, Pesaresi P, Hertle A, Barbato R, Leister D (2010) Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol 8: e1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthiyaveetil S. (2011) A mechanism for regulation of chloroplast LHC II kinase by plastoquinol and thioredoxin. FEBS Lett 585: 1717–1721 [DOI] [PubMed] [Google Scholar]
- Rintamäki E, Martinsuo P, Pursiheimo S, Aro EM (2000) Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin-thioredoxin system in chloroplasts. Proc Natl Acad Sci USA 97: 11644–11649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintamäki E, Salonen M, Suoranta UM, Carlberg I, Andersson B, Aro EM (1997) Phosphorylation of light-harvesting complex II and photosystem II core proteins shows different irradiance-dependent regulation in vivo. Application of phosphothreonine antibodies to analysis of thylakoid phosphoproteins. J Biol Chem 272: 30476–30482 [DOI] [PubMed] [Google Scholar]
- Schuster G, Dewit M, Staehelin LA, Ohad I (1986) Transient inactivation of the thylakoid photosystem II light-harvesting protein kinase system and concomitant changes in intramembrane particle size during photoinhibition of Chlamydomonas reinhardtii. J Cell Biol 103: 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiguzov A, Ingelsson B, Samol I, Andres C, Kessler F, Rochaix JD, Vener AV, Goldschmidt-Clermont M (2010) The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc Natl Acad Sci USA 107: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebel D, Choquet Y, Popot JL, Picot D (2003) An atypical haem in the cytochrome b6f complex. Nature 426: 413–418 [DOI] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446: 640–645 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Grieco M, Kangasjärvi S, Aro EM (2010) Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol 152: 723–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vener AV, van Kan PJ, Rich PR, Ohad I, Andersson B (1997) Plastoquinol at the quinol oxidation site of reduced cytochrome bf mediates signal transduction between light and protein phosphorylation: thylakoid protein kinase deactivation by a single-turnover flash. Proc Natl Acad Sci USA 94: 1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Guo J, Li M, Liu Z (2015) Structural mechanism underlying the specific recognition between the Arabidopsis state-transition phosphatase TAP38/PPH1 and phosphorylated light-harvesting complex protein Lhcb1. Plant Cell 27: 1113–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wientjes E, Drop B, Kouřil R, Boekema EJ, Croce R (2013) During state 1 to state 2 transition in Arabidopsis thaliana, the photosystem II supercomplex gets phosphorylated but does not disassemble. J Biol Chem 288: 32821–32826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willig A, Shapiguzov A, Goldschmidt-Clermont M, Rochaix JD (2011) The phosphorylation status of the chloroplast protein kinase STN7 of Arabidopsis affects its turnover. Plant Physiol 157: 2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobbe L, Blifernez O, Schwarz C, Mussgnug JH, Nickelsen J, Kruse O (2009) Cysteine modification of a specific repressor protein controls the translational status of nucleus-encoded LHCII mRNAs in Chlamydomonas. Proc Natl Acad Sci USA 106: 13290–13295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Wright DA, Wetzel C, Voytas DF, Rodermel S (1999) The IMMUTANS variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell 11: 43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunder T, Liu Q, Aseeva E, Bonardi V, Leister D, Pribil M (2013) Control of STN7 transcript abundance and transient STN7 dimerisation are involved in the regulation of STN7 activity. Planta 237: 541–558 [DOI] [PubMed] [Google Scholar]
- Yan J, Cramer WA (2003) Functional insensitivity of the cytochrome b6f complex to structure changes in the hinge region of the Rieske iron-sulfur protein. J Biol Chem 278: 20925–20933 [DOI] [PubMed] [Google Scholar]
- Zito F, Finazzi G, Delosme R, Nitschke W, Picot D, Wollman FA (1999) The Qo site of cytochrome b6f complexes controls the activation of the LHCII kinase. EMBO J 18: 2961–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito F, Vinh J, Popot JL, Finazzi G (2002) Chimeric fusions of subunit IV and PetL in the b6f complex of Chlamydomonas reinhardtii: structural implications and consequences on state transitions. J Biol Chem 277: 12446–12455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.