ADP-ribosyl cyclase activity in Arabidopsis is up-regulated by nitric oxide to increase the cellular concentration of cyclic ADP ribose and free Ca2+.
Abstract
Cyclic ADP ribose (cADPR) is a Ca2+-mobilizing intracellular second messenger synthesized from NAD by ADP-ribosyl cyclases (ADPR cyclases). In animals, cADPR targets the ryanodine receptor present in the sarcoplasmic/endoplasmic reticulum to promote Ca2+ release from intracellular stores to increase the concentration of cytosolic free Ca2+ in Arabidopsis (Arabidopsis thaliana), and cADPR has been proposed to play a central role in signal transduction pathways evoked by the drought and stress hormone, abscisic acid, and the circadian clock. Despite evidence for the action of cADPR in Arabidopsis, no predicted proteins with significant similarity to the known ADPR cyclases have been reported in any plant genome database, suggesting either that there is a unique route for cADPR synthesis or that a homolog of ADPR cyclase with low similarity might exist in plants. We sought to determine whether the low levels of ADPR cyclase activity reported in Arabidopsis are indicative of a bona fide activity that can be associated with the regulation of Ca2+ signaling. We adapted two different fluorescence-based assays to measure ADPR cyclase activity in Arabidopsis and found that this activity has the characteristics of a nucleotide cyclase that is activated by nitric oxide to increase cADPR and mobilize Ca2+.
Cyclic ADP ribose (cADPR) is a signaling molecule that can evoke increases in the concentration of cytosolic free Ca2+ ([Ca2+]cyt) in plant and animal cells (Hetherington and Brownlee, 2004; Zhang and Li, 2006). In animals, cADPR is synthesized by a class of NADases called the ADP-ribosyl cyclases (ADPR cyclases). Metabolites of ADPR cyclase, including ADP-ribose (ADPR), cADPR, and nicotinic acid adenine dinucleotide phosphate, are all signaling molecules involved in Ca2+ signaling (Lee, 2001, 2006; Guse and Lee, 2008). In animals, both ADPR and cADPR stimulate Ca2+ influx through plasma membrane transient receptor potential channels (Perraud et al., 2001; Sano et al., 2001; Kraft et al., 2004). cADPR also mobilizes Ca2+ from the endoplasmic reticulum (ER) through an inositol 1,4,5-trisphosphate-independent mechanism (Galione et al., 1991; Lee and Aarhus, 1991, 1993; Galione, 1993, 1994; Lee, 1993), which most likely involves the modulation of ryanodine receptors (Li et al., 2001; Ozawa, 2001; Thomas et al., 2001). Nicotinic acid adenine dinucleotide phosphate mobilizes intracellular Ca2+ from lysosomal and/or acidic stores and is active in a variety of mammalian cell types (Lee, 2005).
In plants, neither ADPR cyclase nor an equivalent of the ryanodine receptor has been identified in genomic databases, even though ADPR cyclase activity and cADPR-evoked Ca2+ release from vacuoles and ER have been reported (Allen et al., 1995; Muir and Sanders, 1997; Leckie et al., 1998; Navazio et al., 2000; Sánchez et al., 2004). cADPR injected into guard cells causes stomatal closure (Leckie et al., 1998), and cADPR has been proposed to be involved in abscisic acid (ABA)-induced stomatal closure because 8-NH2-cADPR, a competitor of cADPR signaling, and nicotinamide, an inhibitor of ADPR cyclase activity, both reduced ABA-induced stomatal closure (Leckie et al., 1998). The role of cADPR in ABA signaling also is supported by the statistically significant intersection between the sets of transcripts induced by ABA and cADPR (Sánchez et al., 2004). There is a similar intersection between transcript populations that are regulated by cADPR and the circadian clock, and together with circadian oscillations in the concentration of cADPR and an increased circadian period in the presence of nicotinamide, these data have led to the proposal that cADPR forms a feedback loop in the Arabidopsis (Arabidopsis thaliana) circadian oscillator (Dodd et al., 2007).
The lack of orthologs for ADPR cyclase and RYR, and the limited characterization of their activities, have led to uncertainty concerning whether plants have a bona fide ADPR cyclase activity associated with Ca2+ signaling (Dodd et al., 2010). We sought to establish whether the reported ADPR cyclase-like activity in Arabidopsis has functional characteristics of an enzyme involved in the generation of cADPR to mobilize Ca2+ in plant signaling networks; specifically, we investigated if the enzyme activity was correlated with stimulus-induced increases in cADPR and also [Ca2+]cyt. We investigated the potential role of ADPR cyclase activity in nitric oxide (NO) signaling, because NO is a known regulator of the cADPR signaling pathway in animals (Galione et al., 1993; Willmott et al., 1996; Yu et al., 2000; Zhang and Li, 2006) and pharmacology suggests that NO-mediated increases in [Ca2+]cyt are cADPR dependent in Vicia faba (Garcia-Mata et al., 2003). We reasoned that if cADPR is associated with NO signaling, as predicted by pharmacological studies, there might be NO-induced increases in Arabidopsis ADPR cyclase activity and NO-induced increases in the concentration of cADPR.
RESULTS
Pharmacological Identification of cADPR-Dependent Signaling Pathways in Arabidopsis
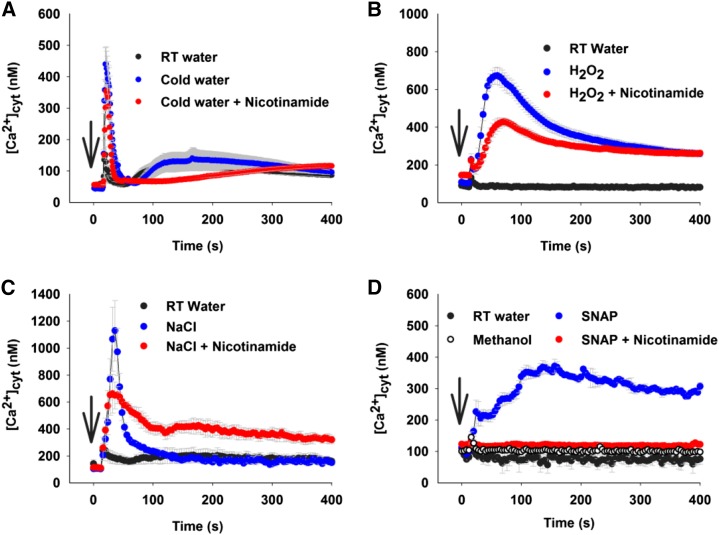
To investigate potential roles for cADPR in signaling in Arabidopsis, we investigated the effects of an antagonist of cADPR signaling on stimulus-induced increases of [Ca2+]cyt in response to cold, NaCl, hydrogen peroxide (H2O2), and NO. We selected nicotinamide as a suitable antagonist because it is a metabolic by-product of cADPR production that acts as an inhibitor through product inhibition and enzyme reversal described by basic Michaelis-Menten kinetics. This simple pharmacology is easier to interpret than that based on analog compound chemistry, and we previously demonstrated dose-dependent inhibition of Arabidopsis ADPR cyclase activity by nicotinamide (Dodd et al., 2007). Nicotinamide also inhibits other NADases, including poly-ADP ribose polymerases and SIRTUINS, through the same product inhibition; however, neither of those enzymes has a known role in Ca2+ signaling, so an effect of nicotinamide on stimulus-induced [Ca2+]cyt increases is indicative of ADPR cyclase activity (Galione, 1994). Cold treatment induced a transient increase of [Ca2+]cyt in Arabidopsis that reached a peak of 440 ± 60 nm (mean ± se; Fig. 1A), almost 3 times higher than the touch response evoked by room temperature water (152 ± 9 nm; Fig. 1A). In the presence of 50 mm nicotinamide, the cold-induced increase in [Ca2+]cyt was slightly smaller, with the highest [Ca2+]cyt peak of 358 ± 72 nm (Fig. 1A). A transient increase of [Ca2+]cyt was detected in response to 10 mm H2O2 (peak [Ca2+]cyt of 673 ± 45 nm; Fig. 1B). Preincubation with nicotinamide (50 mm) for 2 h reduced and slightly delayed the H2O2-induced [Ca2+]cyt increase (peak [Ca2+]cyt of 429 ± 20 nm; Fig. 1B). NaCl at 150 mm induced a large, rapid increase in [Ca2+]cyt to a peak of 981 ± 229 nm (Fig. 1C), which was higher than cold water- and H2O2-mediated [Ca2+]cyt responses. A partial reduction of the NaCl-induced [Ca2+]cyt response was found when plants were incubated with nicotinamide (50 mm; peak [Ca2+]cyt of 662 ± 144 nm; Fig. 1C). S-Nitroso-N-acetylpenicillamine (SNAP) acts as an NO donor and triggers increases in [Ca2+]cyt in Arabidopsis (Neill et al., 2002). SNAP at 300 μm elevated [Ca2+]cyt, and the increase was stable for 400 s, which was more prolonged than those induced by cold water, H2O2, and NaCl. The peak for SNAP-mediated [Ca2+]cyt increase was 368 ± 18 nm (Fig. 1D), which was achieved 160 s after SNAP treatment, compared with the rapid responses to cold water, H2O2, and NaCl, in which the peak of [Ca2+]cyt was induced within 15 to 30 s. Nicotinamide (50 mm) completely abolished SNAP-induced [Ca2+]cyt increases (peak [Ca2+]cyt of 123 ± 4 nm; Fig. 1D).
Figure 1.
Nicotinamide abolished NO-induced [Ca2+]cyt increases in Arabidopsis. A, Effect of cold water (4°C) on [Ca2+]cyt in the absence or presence of nicotinamide (50 mm). B, Effect of H2O2 on [Ca2+]cyt in the absence or presence of nicotinamide (50 mm). C, Effect of NaCl on [Ca2+]cyt in the absence or presence of nicotinamide (50 mm). D, Effect of NO (SNAP) on [Ca2+]cyt in the absence or presence of nicotinamide (50 mm). Twelve-day-old aequorin-expressing individual seedlings were incubated for 2 h with nicotinamide (50 mm). Cold water (4°C), 10 mm H2O2, 150 mm NaCl, and 300 μm SNAP (NO donor) were added at 15 s, and luminescence was measured for 896 s in a luminometer or multifunctional microplate reader. Data are presented as means of 12 biological replicates from three independent experiments (n = 12), and error bars represent se. Arrows indicate the time of stimulation. RT, Room temperature.
[Ca2+]cyt Increases Induced by NO Are ADPR Cyclase Dependent
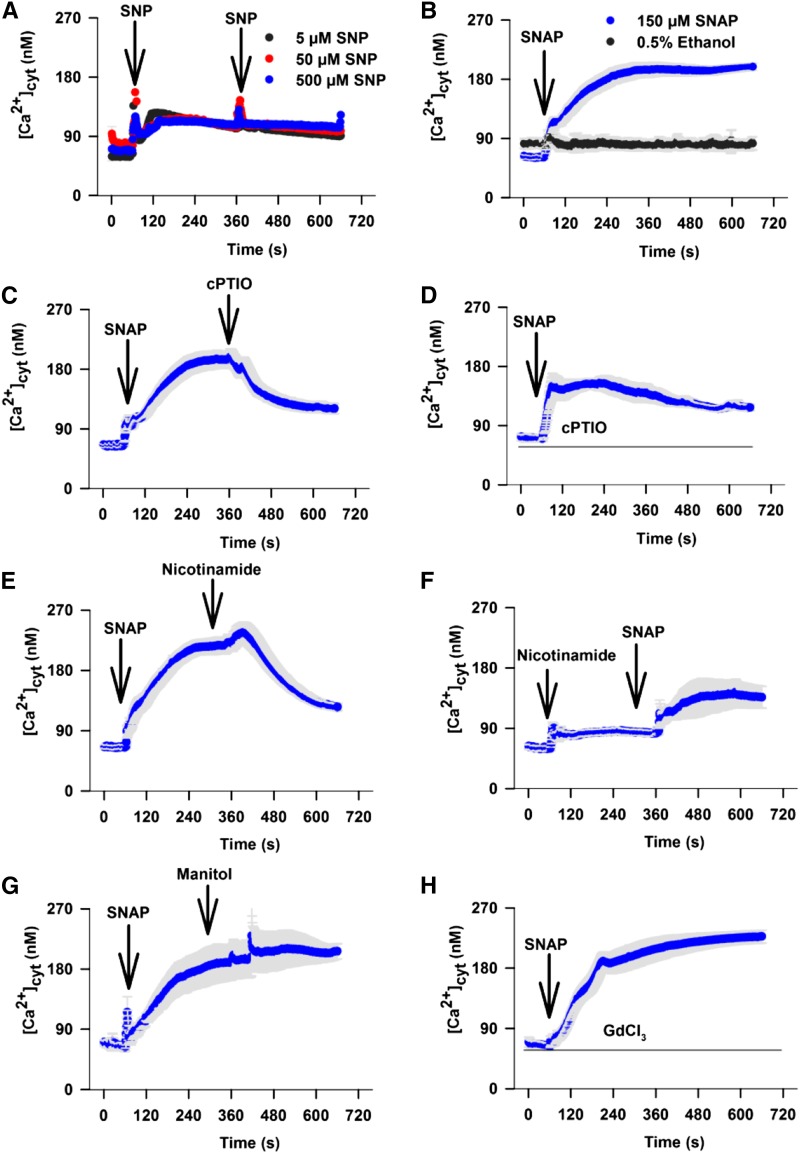
The inhibition of SNAP-induced increases in [Ca2+]cyt by nicotinamide was suggestive of a role for ADPR cyclase in the elevation of [Ca2+]cyt by NO. We performed a further set of experiments to confirm that the effects of SNAP were linked to NO production and not to an unintended side effect. First, we tested the effect of an alternative NO donor, sodium nitroprusside (SNP; Neill et al., 2002). At 5 μm, SNP induced sustained [Ca2+]cyt increases that reached a plateau at 131 ± 6.6 nm (Fig. 2A). Increasing the concentration of SNP to 50 or 500 μm SNP had no further effect on [Ca2+]cyt, possibly because the experiment was performed in the dark, which limits the effectiveness of SNP (Rico-Lemus and Rodríguez-Garay, 2014). At 150 μm, SNAP induced sustained increases in [Ca2+]cyt for 4 to 5 min until it reached a plateau at 195.1 ± 11.4 nm (Fig. 2B). Elevation of [Ca2+]cyt by these two donors suggested that the effects were due to NO synthesis. This was confirmed by testing the effects of the NO scavenger 2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO; Neill et al., 2002). Addition of 300 μm cPTIO 300 s after the addition of 150 μm SNAP decreased the [Ca2+]cyt levels from the elevated value of 192.2 ± 13.6 nm to 115.3 ± 7.6 nm (Fig. 2C). Preincubation with 300 μm cPTIO reduced the [Ca2+]cyt increase evoked by 150 μm SNAP to 145.3 ± 13.3 nm (Student’s t test against 150 μm SNAP without 300 μm cPTIO, P < 0.01; Fig. 2D). Nicotinamide was equally effective in inhibiting NO-mediated increases in [Ca2+]cyt if added before or after the NO donor SNAP (Fig. 2, E and F). Addition of 50 mm nicotinamide 300 s after the addition of SNAP reduced [Ca2+]cyt levels from 215.8 ± 11.7 nm to 121.6 ± 5.6 nm; however, there was a long delay of over 60 s after the addition of nicotinamide before [Ca2+]cyt decreased (Fig. 2E). This is supportive of the proposed role of nicotinamide in inhibiting the production of cADPR and possibly contributing to cADPR degradation by reversing the catalytic activity of ADPR cyclase to one of cADPR catalysis. SNAP addition after a prolonged incubation with nicotinamide resulted in a residual increase in [Ca2+]cyt only to 139.1 ± 19.1 nm (Fig. 2F), demonstrating that the NO-induced increase in [Ca2+]cyt might be almost completely dependent on cADPR. Osmotic effects of nicotinamide can be discounted, since an equimolar concentration of mannitol was without effect (Fig. 2G). Preincubation for 300 s with GdCl3 (the most effective blocker of Arabidopsis plasma membrane Ca2+ influx channels; Demidchik et al., 2002) at 1 mm, 10 times higher than required to inhibit NaCl-induced increases in [Ca2+]cyt in the same assay (Tracy et al., 2008), did not reduce the [Ca2+]cyt increase induced by 150 μm SNAP, which peaked at 198.7 ± 17.2 nm (Student’s t test against 150 μm SNAP without 1 mm GdCl3, P = 0.81; Fig. 2H), suggesting that plasma membrane influx of Ca2+ might not contribute to the response.
Figure 2.
NO evokes short-term [Ca2+]cyt increases. A, SNP was added at 60 and 360 s (n = 5 for each treatment) after the start of the experiment, and [Ca2+]cyt levels were measured for 600 s. B, SNAP was added to provide a final concentration of 150 μm or 0.5% ethanol control was added 60 s after the start of the experiment, and [Ca2+]cyt levels were measured for 600 s (n = 19). C, SNAP to a final concentration of 150 μm was added at 60 s, and 300 μm cPTIO was added at 360 s (n = 21). D, Seedlings were incubated with 300 μm cPTIO for 300 s before the start of the experiment, when 150 μm SNAP was added at 60 s (n = 10). E, SNAP at a final concentration of 150 μm was added 60 s after the start of the experiment, and 50 mm nicotinamide was added 300 s later (n = 20). F, Nicotinamide (50 mm) was added 60 s after the start of the experiment, and 150 μm SNAP was added 300 s later (n = 8). G, SNAP at a final concentration of 150 μm was added 60 s after the start of the experiment, and 50 mm mannitol was added 300 s later (n = 5). H, Seedlings were incubated for 300 s in 1 mm GdCl3 before the start of the experiment. SNAP at a final concentration of 150 μm was added after 60 s (360 s; n = 13). Arrows indicate the time of each drug addition. Error bars represent se.
Nicotinamide Guanine Dinucleotide- and Nicotinamide Hypoxanthine Dinucleotide-Based Fluorescence Spectrometry Assays of Arabidopsis ADPR Cyclase Activity
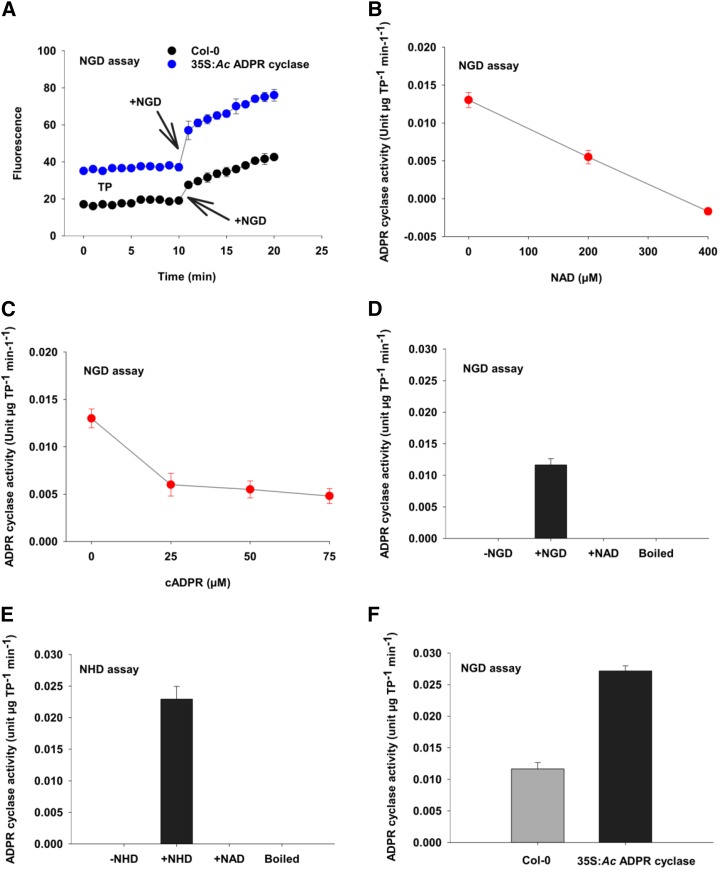
The pharmacological manipulation of [Ca2+]cyt is strongly indicative of a nicotinamide-sensitive component being required for NO-induced increases in [Ca2+]cyt in Arabidopsis. To test if this increase is mediated by the activation of an ADPR cyclase-like activity, we assayed for ADPR cyclase activity based on the conversion of nonfluorescent nucleotide analogs of NAD into fluorescent cyclic nucleotides. Soluble total protein extracts of Arabidopsis have an enzymatic activity capable of converting the nonfluorescent NAD analog, nicotinamide guanine dinucleotide (NGD), to the fluorescent cyclic GDP-ribose (cGDPR; Fig. 3A). The synthesis of the cGDPR was dependent on the presence of NGD in both ecotype Columbia-0 (Col-0) and plants heterologously expressing ADPR cyclase from the sea slug A. californica (35S:Ac ADPR cyclase; Dodd et al., 2007). Furthermore, the rate of fluorescence increase was higher in protein extracted from the 35S:Ac ADPR cyclase plants (Fig. 3A).
Figure 3.
Identification of ADPR cyclase activity in Arabidopsis. A, Time course of ADPR cyclase activity in soluble protein extracts of leaves of Col-0 or plants transformed with 35S:Aplysia californica ADPR cyclase (35S:Ac ADPR cyclase). All components except substrate were added to the cuvette, then 200 μm NGD was added at 10 min, and fluorescence intensity was measured for another 10 min. B, ADPR cyclase activity of extracts of Col-0 leaves in the presence of NAD. NAD reduced the activity in a concentration-dependent manner. C, ADPR cyclase activity of Col-0 leaf extracts measured using NGD as a substrate (200 μm) in the presence of cADPR. D, Estimated ADPR cyclase activity in extracts of Col-0 based on the cyclization of NGD in the absence or presence of 200 μm NGD, 200 μm NAD as an alternative substrate, and when enzymatic activity had been inhibited by boiling for 10 min. E, Estimated ADPR cyclase activity in extracts of Col-0 based on the cyclization of NHD in the absence or presence of 200 μm NHD, 200 μm NAD as an alternative substrate, and when enzymatic activity had been inhibited by boiling for 10 min. F, Activity of ADPR cyclase in extracts of Col-0 and 35S:Ac ADPR cyclase plants calculated from a standard curve derived from A. californica ADPR cyclase (Supplemental Fig. S1). Fluorescence is in arbitrary units. Data are presented as means of three biological replicates of three independent experiments, and error bars represent se. TP, Total protein.
The conversion of NGD to cGDPR was inhibited by NAD. In our assay, equal concentrations of NGD and NAD reduced the activity to 0.5-fold; however, an excess of NAD completely abolished the cyclization of NGD (Fig. 3B). While it is possible that NAD acts as a noncompetitive inhibitor, the reduction of the conversion of NGD to cGDPR by NAD is an expected characteristic of a nucleotide cyclase activity that favors NAD as a substrate to generate cADPR as a product. Animal ADPR cyclase is reversible under standard conditions, so we tested whether the inclusion of cADPR in the assay would inhibit the production of cGDPR from NGD. Addition of 25 μm cADPR reduced ADPR cyclase activity significantly (P < 0.001); however, higher concentrations of cADPR (up to 75 μm) did not cause any further changes in activity (Fig. 3C). The ADPR cyclase-like activity was protein dependent, being absent in boiled protein extracts (Fig. 3D). Based on these findings, we considered the fluorescence intensity increase to be representative of a bona fide ADPR cyclase activity. To determine the specific activity, we used commercial A. californica ADPR cyclase to generate a standard curve (Supplemental Fig. S1). This enabled us to estimate the specific activity in extracts of unstimulated Arabidopsis Col-0 to be around 0.01 to 0.015 units µg−1 total protein min−1 or units µg−1 protein min−1 (Fig. 3). An alternative assay based on the conversion of nicotinamide hypoxanthine dinucleotide (NHD) to cyclic inosine diphosphoribose (Graeff et al., 1996) resulted in a very similar estimate of Col-0 ADPR cyclase activity (Fig. 3E), while, as expected, 35S:Ac ADPR cyclase plants had significantly higher ADPR cyclase activity of 0.027 ± 0.0008 units µg−1 protein min−1 (P ≤ 0.001; Fig. 3E).
NO Is a Regulator of Arabidopsis ADPR Cyclase Activity
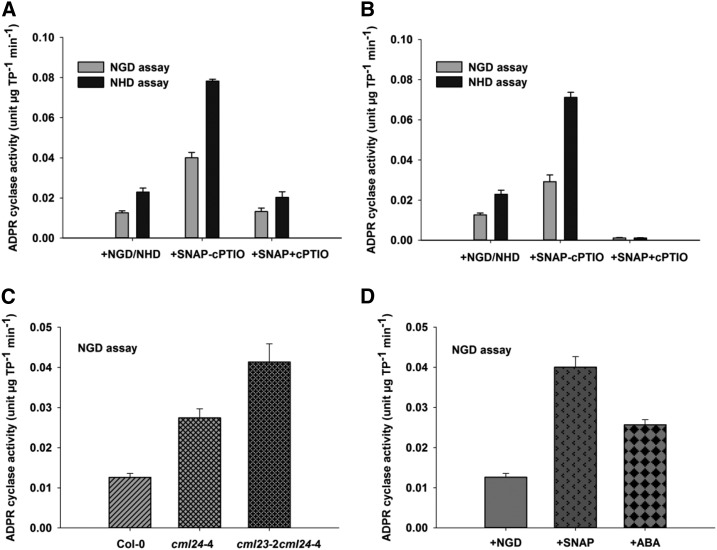
NO treatment of whole plants significantly increased the extractable ADPR cyclase activity using either NGD or NHD as substrate (P ≤ 0.001), and the NO scavenger cPTIO significantly reduced the effect of SNAP on extractable ADPR cyclase activity (Fig. 4, A and B). Similarly, adding SNAP to the extracted proteins also increased Arabidopsis ADPR cyclase activity, which was likewise reversed by cPTIO (Fig. 4B; P ≤ 0.001). This demonstrates that NO can regulate ADPR cyclase activity in a cell-free manner.
Figure 4.
ADPR cyclase activity in response to NO. A, ADPR cyclase activity in the soluble protein extract of SNAP (300 μm)-treated Col-0 plants. B, Effect of 300 μm SNAP on ADPR cyclase activity of the protein extracted from untreated Col-0 plants. C, ADPR cyclase activity in soluble protein extracts of cml24-4 and cml23-2 cml24-4 plants. D, ADPR cyclase activity in ABA (50 μm)-treated protein extracts of untreated Col-0 plants. Soluble protein extracts were prepared from 4- to 5-week-old plants, and equal amounts of protein (145 µg) were used to measure the ADPR cyclase activity by NGD or NHD assay. Data are presented as means of three biological replicates of three independent experiments, and error bars represent se. TP, Total protein.
To investigate whether physiologically relevant levels of NO can regulate ADPR cyclase activity in Arabidopsis, we measured it in lines carrying the calmodulin-like24-4 allele, which results in constitutively high NO (Tsai et al., 2007). Both cml24-4 and cml23-3 cml24-4 plants had significantly higher extractable ADPR cyclase activity compared with wild-type Col-0 plants (P ≤ 0.001; Fig. 4C).
ABA increases NO in guard cells (Neill et al., 2002); therefore, we tested the effect of this phytohormone on ADPR cyclase activity. Soluble protein extracts of Col-0 plants treated with 50 μm ABA had significantly higher ADPR cyclase activity of 0.026 ± 0.001 units µg−1 total protein min−1 (P ≤ 0.001; Fig. 4D) compared with untreated protein extracts of Col-0 plants (Fig. 4D). This activation appears to be physiologically relevant, because the activation by ABA was less than that due to the exogenous NO donor SNAP, which might be expected to cause very high levels of NO (P ≤ 0.001; Fig. 4D). NO-induced ADPR cyclase activity was inhibited by nicotinamide in a dose-dependent manner, with complete inhibition being achieved at 50 mm nicotinamide (Supplemental Fig. S2), consistent with the effect of nicotinamide on NO-induced increases in [Ca2+]cyt (Figs. 1D and 2, E and F).
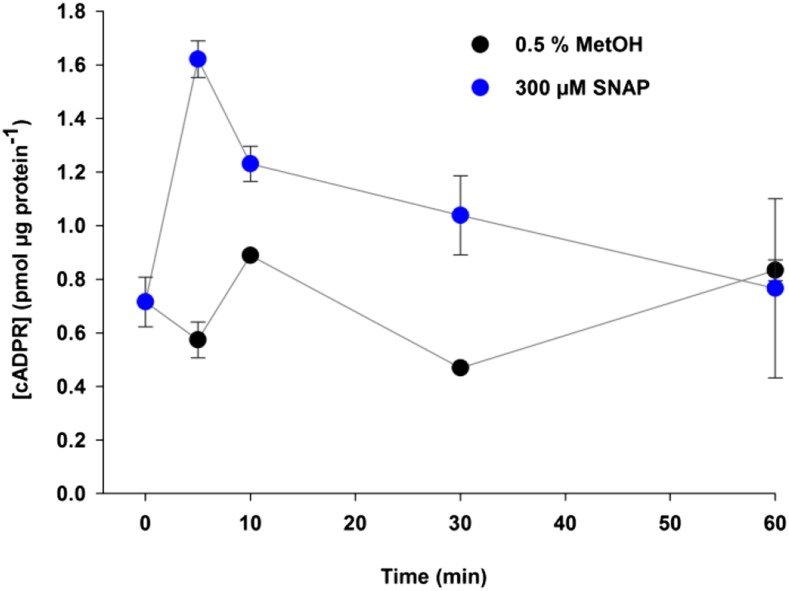
The activation of ADPR cyclase activity by NO was confirmed by the measurement of [cADPR] in Arabidopsis treated with 300 μm SNAP or 0.5% (v/v) methanol using a fluorescence-based coupled assay (Dodd et al., 2007). Before treatment, [cADPR] was 0.72 ± 0.09 pmol μg−1 protein (Fig. 5), and [cADPR] levels in the plants treated with the 0.5% (v/v) methanol control remained almost constant at all time points, varying from 0.47 ± 0.00 pmol μg−1 protein 30 min after the treatment to 0.89 ± 0.09 pmol μg−1 protein 60 min after the treatment (Fig. 5). The addition of 300 μm SNAP caused a fast increase of [cADPR] in the first 5 min, to 1.62 ± 0.34 pmol μg−1 protein, before slowly returning to resting levels at 60 min (0.77 pmol μg−1 protein; Fig. 5).
Figure 5.
SNAP triggers [cADPR] accumulation in Arabidopsis. Three-week-old Arabidopsis seedlings grown in 12 h of light/12 h of dark were treated with 300 μm SNAP or methanol control (0.5% [v/v] MetOH) by flooding the plates for 1 min. Each plate contained an average of 20 seedlings, and all of them were harvested in each time point. Three independent replicates were harvested at the beginning of the time course and 5, 10, 30, and 60 min after drug treatment. [cADPR] was estimated by a coupled assay, and each sample was measured at least twice. Error bars represent se.
DISCUSSION
NO Increases [Ca2+]cyt through a Pathway That Includes the Activation of ADPR Cyclase
We found that NO-mediated [Ca2+]cyt increases were abolished by incubation with the NADase inhibitor nicotinamide, that NO increases ADPR cyclase activity, and that NO stimulates the production cADPR, a Ca2+ agonist. These data and the insensitivity of NO-mediated increases in [Ca2+]cyt to GdCl3, an inhibitor of the plasma membrane-mediated influx of Ca2+, lead us to conclude that the primary pathway by which NO increases [Ca2+]cyt in Arabidopsis is through cADPR-mediated Ca2+ release from the ER and/or the vacuole, dependent on the activity of ADPR cyclase.
The conservation of the regulation of ADPR cyclase activity by NO between plants and animals could suggest a common ancestry for the pathway; alternatively, this might be an example of the convergent evolution of signaling in the plant and animal lineages. However, the lack of obvious orthologs for ADPR cyclase and ryanodine receptors in the Arabidopsis and other plant genomes makes it challenging to confirm either of these hypotheses. Our adaptation of ADPR cyclase activity assays for Arabidopsis, and the identification of both NO and cml24-4 mutants as activators of ADPR cyclase, provide a tool set that might aid in the isolation of the ADPR cyclase protein and the identification of the corresponding gene. This might provide information concerning potential evolutionary features of cADPR-dependent NO-induced increases in [Ca2+]cyt. Our discovery that cml24-4 plants have higher ADPR cyclase activity provides a potential genetic background to use in attempts to purify the enzyme. We have found that pharmacological tools can be used to activate and inhibit ADPR cyclase activity in a cell-free manner, which could be useful in confirming that a purified product represents a potential ADPR cyclase.
We found little evidence that cADPR signaling contributes to cold-, touch-, and H2O2-induced increases in [Ca2+]cyt. Cold-induced increases in [Ca2+]cyt are due to influx across the plasma membrane and efflux of Ca2+ from the vacuole (Knight et al., 1996), apparently through a cADPR-independent route. The analysis of H2O2-mediated [Ca2+]cyt signals revealed that the initial increase of [Ca2+]cyt was partially suppressed by 50 mm nicotinamide (Fig. 1B). However, this effect was much less than observed for NO, and we conclude that the bulk increase in [Ca2+]cyt in response to H2O2 is not ADPR cyclase dependent. [Ca2+]cyt elevations in response to H2O2 treatment arise primarily through the activation of hyperpolarization-activated Ca2+-permeable channels in the plasma membrane (Pei et al., 2000; Rentel and Knight, 2004). NaCl elevates [Ca2+]cyt within very short periods in plants (Knight et al., 1997; Kiegle et al., 2000; Knight, 2000; Moore et al., 2002). We also detected immediate rapid responses of [Ca2+]cyt to NaCl. Nicotinamide had some inhibitory effects but did not abolish NaCl-mediated [Ca2+]cyt increases (Fig. 1C). Based on this finding and studies with inhibitors of plasma membrane Ca2+ influx (Tracy et al., 2008), it appears that NaCl-induced increases involve both influx across the plasma membrane and cADPR-mediated Ca2+ release from the ER or vacuole. Release of Ca2+ from multiple stores through different pathways might permit the oscillatory [Ca2+]cyt signals induced by NaCl (Martí et al., 2013), which is a result of spatial heterogeneity (Tracy et al., 2008) and cell-specific dynamics (Martí et al., 2013).
NO Modulates Short-Term Ca2+ Responses in Arabidopsis
cADPR previously has been suggested to be involved in the NO signaling pathway in plants (Garcia-Mata et al., 2003; Lamotte et al., 2006; Zhang and Li, 2006), but measurements of NO regulation of ADPR cyclase activity and [cADPR] have not been reported. By measuring ADPR cyclase and cADPR levels, it has been possible to observe that the elevation of cADPR in response to NO is transitory (Fig. 5) and that [Ca2+]cyt returns rapidly to resting in the absence of cADPR synthesis (Fig. 2E). We conclude that cADPR-dependent NO-regulated [Ca2+]cyt signaling is most likely involved in shorter term responses that might occur in response to plant-pathogen interactions, symbiotic events, or hormones (Mur et al., 2013). If these rapid, short-term NO-mediated increases in [Ca2+]cyt are involved in longer term signaling, such as the photoperiodic regulation of flowering, they are likely to be very early in the signaling cascade.
It is not known how NO regulates ADPR cyclase activity, but we have shown this to occur in a cell-free extract; therefore, it is reasonable to suspect that the effect could be direct, through a mechanism such as nitrosylation. In mammals and sea urchins, NO increases the activity of ADPR cyclase through guanylate cyclase- and cGMP-dependent pathways (Galione, 1993, 1994). There are many possible sources of NO in plants, including enzymatic and nonenzymatic (Bethke et al., 2004; Crawford, 2006). One of the most established sources of NO in plants is nitrate reductase, which usually converts NO3− into NO2− but also may convert NO2− into NO in anaerobic conditions and when NO2− levels are high (Yamasaki et al., 1999; Rockel et al., 2002; Meyer et al., 2005; Crawford, 2006). Our data do not distinguish which of those are responsible for regulating ADPR cyclase, but we do demonstrate that high endogenous levels of NO, such as those achieved in cml24-4 mutants, are capable of increasing ADPR cyclase activity. Our identification of NO-regulated ADPR cyclase activity fills a gap in the NO signal transduction chain. There are likely to be additional regulators of ADPR cyclase activity, because the NO-induced cADPR increase was transient, contrasting with ABA-evoked cADPR increases, which were sustained for at least 1 h (Sánchez et al., 2004).
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Measurement of [Ca2+]cyt
Experiments were performed with Arabidopsis (Arabidopsis thaliana) ecotype Col-0, except where stated. Seeds were grown and [Ca2+]cyt was measured using aequorin in plants carrying CaMV35S:APOAEQUORIN as described by Martí et al. (2013). Seeds were sown in petri dishes and stratified in the dark at 4°C for 2 to 3 d. Petri dishes were then transferred to a growth cabinet (12 h of light/12 h of dark, 20°C, 50–60 μmol m−2 s−1 irradiance) for 7 to 12 d for [Ca2+]cyt measurement, 3 weeks for measuring [cADPR], and 4 to 5 weeks for ADPR cyclase activity measurement.
Measurement of ADPR Cyclase Activity in Protein Extracts
Preparation of Soluble Protein Extracts
Five to 10 g of whole rosette tissue, excluding roots, of 4- to 5-week-old Arabidopsis plants was homogenized using a pestle and mortar at 4°C in solution A (340 mm Glc [Fisher Scientific], 20 mm HEPES [Sigma], 1 mm MgCl2 [BDH Laboratory Supplies], 50 g mL−1 soybean trypsin inhibitor [Sigma], 10 µg mL−1 leupeptin [Sigma], and 10 µg mL−1 aprotinin [Sigma], pH 7.2, 3 mL g–1 fresh weight). The homogenate was filtered through two layers of Miracloth (Calbiochem), and the resulting filtrate was centrifuged at 2,000g for 5 min at 4°C to remove unbroken cell debris, tissues. etc. The supernatant was transferred into a 15-mL falcon tube and centrifuged at 12,000g for 15 min at 4°C (Beckman Coulter Avanti J-26XP centrifuge). After centrifugation, the supernatant was collected carefully and run through the PD-10 desalting column (GE Healthcare) according to the manufacturer’s protocols. Protein content was estimated with a protein assay kit (Bio-Rad Laboratories) using bovine serum albumin (New England Biolabs) as a standard.
NGD/NHD Assays of ADPR Cyclase Activity Using a Luminescence Spectrometer
A total of 145 μg of protein from Col-0 plants was taken in 1,200 μL of solution A (pH 7.2) in quartz cuvettes, and fluorescence intensity was measured for every minute up to 10 min at 21°C using a luminescence spectrometer (Perkin Elmer LS 55) set with the excitation wavelength at 300 nm and emission wavelength at 410 nm. After 10 min, 60 µL of 4 mm NGD (prepared in solution A, final concentration of 200 μm; Sigma) was added to reactions, and the resultant fluorescence intensity was measured for another 10 to 15 min. Additionally, fluorescence intensity was measured for every minute up to 10 to 15 min at 21°C for 1,200-μL reactions in solution A (pH 7.2) containing 145 μg of total protein, 145 μg of total protein + 200 μm NAD (Sigma), 145 μg of total protein + 200 μm NGD, and 145 μg of boiled protein (100°C; 10–15 min) + 200 μm NGD. For the NHD assay, 200 μm NHD (prepared in solution A, pH 7.2; Sigma) was used in place of NGD.
To test the effect of NO on ADPR cyclase activity in the protein extracts, 4- to 5-week-old Col-0, cml24-4, or cml23-2 cml24-4 plants were incubated in the presence or absence of 300 μm SNAP (Calbiochem) or 300 μm SNAP and 300 μm cPTIO (Sigma) for 40 to 50 min, separately. Alternatively, protein extracts of untreated Col-0 plants were incubated with 300 μm SNAP or 300 μm SNAP and 300 μm cPTIO for 40 to 50 min, separately. Total protein extract of Col-0 plants (4–5 weeks old) was incubated with 50 μm ABA for 1 h.
Reverse Cyclase Assay for [cADPR] Measurement
cADPR Isolation and cADPR Purification
Plants were dosed with 150 μm SNAP or 0.5% methanol by flooding for 1 min, after which all the liquid was taken out. Plants were harvested before dosing (0 min) and 5, 10, 30, and 60 min after. Only the aerial parts were harvested. Plants were pooled, frozen in liquid nitrogen, and stored at −80°C. Frozen samples were finely ground in liquid nitrogen. About 2 mg of frozen material was thawed and vortexed in 250 μL of ice-chilled HPLC-water (about 4°C; Fisher Scientific). Protein quantification was performed on 25 μL of the sample by Bradford assay. In order to precipitate proteins, 25 μL of 7 m perchloric acid (Sigma) was added to the samples and vortexed. One milliliter of ice-chilled 3:1 mix of 1,1,2-trichlorotrifluoroethane:tri-N-octylamine (Sigma) was added to separate cADPR from the rest of the plant extract. The mixture was vortexed and kept on ice until the precipitation of perchloric acid. Samples were centrifuged at 4°C for 10 min at 1,500g. After centrifugation, the samples had two phases separated by a white film. The aqueous phase (upper) was taken off, and 1 m NaPO4 buffer (pH 8) was added to a final concentration of 20 mm NaPO4. Contaminating nucleotides were removed from the isolated aqueous phase by enzymatic hydrolysis. Nucleotide pyrophosphatase (EC 3.6.1.9; Sigma) at 0.44 μg mL−1, 1.25 μg mL−1 alkaline phosphatase (EC 3.1.3.1; isolated from bovine intestinal mucosa; Sigma), and 0.06 μg mL−1 NADase (EC 3.2.2.5; isolated from porcine brain; Sigma) were added to the samples and incubated overnight at 37°C. After incubation, the enzymes were separated from the extract with 3,000 molecular weight cutoff filters (500 μL; Millipore) and spun at 4°C for 30 min at 13,000g. The final extract was diluted 1:1 with 200 mm phosphate buffer to a final concentration of 100 mm NaPO4.
Fluorescence-Based Cycling Assay
The cycling assay is based on a cycle of enzymatic conversions (Graeff and Lee, 2002). cADPR is first converted to NAD by ADPR cyclase (EC 3.2.2.5; from the marine sponge Axinella polypoides; a gift from E. Zocchi and Dr. S. Bruzzone, Universita di Genova) in the presence of high amounts of nicotinamide (Sigma). Next, alcohol dehydrogenase (EC 1.1.1.1; extracted from Saccharomyces cerevisiae; Sigma) converts ethanol and NAD into acetaldehyde and NADH. Finally, diaphorase (EC 1.8.1.4; extracted from Clostridium kluyveri; Sigma) converts NADH and resazurin (Sigma) into NAD and resorufin, a fluorescent substance that can be detected by a multifunctional microplate reader (FluoStar OPTIMA; BMG LabTech). To each well of black 96-well plates, 100 μL of sample was added. First, 50 μL of assay reagent (30 mm nicotinamide and 0.3 μg mL−1 ADPR cyclase in 100 mm phosphate buffer, pH 8) was added and left for 15 min, then 100 μL of cycling reagent (10 mm nicotinamide, 20 μm resazurin, 10 μm riboflavin 5-monophosphate [Sigma], 100 μg mL−1 alcohol dehydrogenase, 10 μg mL−1 diaphorase, and 2% ethanol in 100 mm NaPO4 buffer, pH 8) was added to each well, and fluorescence was measured at 30°C every 5 min for 8 h at 540 ± 5 nm excitation and 590 ± 5 nm emission. Diaphorase is frequently contaminated with nucleotides, so the enzyme was purified by incubation in 2% activated charcoal in 37°C for 30 min followed by centrifugation at 5,000g for 5 min. cADPR levels were estimated using 2, 5, 10, 50, 100, and 500 nm cADPR (Calbiochem) as standards. As the cADPR isolation and purification steps in the samples might lead to cADPR losses, those steps also were performed in the cADPR standards.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Preparation of the A. californica standard curve.
Supplemental Figure S2. ADPR cyclase activity in response to nicotinamide.
Supplementary Material
Acknowledgments
We thank Dr. María C. Martí and Matthew Stancombe for technical support.
Glossary
- [Ca2+]cyt
cytosolic free Ca2+ concentration
- ER
endoplasmic reticulum
- ABA
abscisic acid
- NO
nitric oxide
- H2O2
hydrogen peroxide
- SNAP
S-nitroso-N-acetylpenicillamine
- SNP
sodium nitroprusside
- cPTIO
2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- NGD
nicotinamide guanine dinucleotide
- Col-0
Columbia-0
- NHD
nicotinamide hypoxanthine dinucleotide
Footnotes
This work was supported by the Islamic Development Bank and the Cambridge Commonwealth Trust (to S.M.A.-A.), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (to C.T.H.), and the Biotechnology and Biological Sciences Research Council (grant no. BB/D017904/1 to A.N.D. and A.A.R.W.).
Articles can be viewed without a subscription.
References
- Allen GJ, Muir SR, Sanders D (1995) Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science 268: 735–737 [DOI] [PubMed] [Google Scholar]
- Bethke PC, Badger MR, Jones RL (2004) Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16: 332–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM. (2006) Mechanisms for nitric oxide synthesis in plants. J Exp Bot 57: 471–478 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Bowen HC, Maathuis FJM, Shabala SN, Tester MA, White PJ, Davies JM (2002) Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth. Plant J 32: 799–808 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Gardner MJ, Hotta CT, Hubbard KE, Dalchau N, Love J, Assie JM, Robertson FC, Jakobsen MK, Gonçalves J, et al. (2007) The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science 318: 1789–1792 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D (2010) The language of calcium signalling. Annu Rev Plant Biol 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Galione A. (1993) Cyclic ADP-ribose: a new way to control calcium. Science 259: 325–326 [DOI] [PubMed] [Google Scholar]
- Galione A. (1994) Cyclic ADP-ribose, the ADP-ribosyl cyclase pathway and calcium signalling. Mol Cell Endocrinol 98: 125–131 [DOI] [PubMed] [Google Scholar]
- Galione A, Lee HC, Busa WB (1991) Ca2+-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science 253: 1143–1146 [DOI] [PubMed] [Google Scholar]
- Galione A, White A, Willmott N, Turner M, Potter BV, Watson SP (1993) cGMP mobilizes intracellular Ca2+ in sea urchin eggs by stimulating cyclic ADP-ribose synthesis. Nature 365: 456–459 [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100: 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff R, Lee HC (2002) A novel cycling assay for cellular cADP-ribose with nanomolar sensitivity. Biochem J 361: 379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff RM, Walseth TF, Hill HK, Lee HC (1996) Fluorescent analogs of cyclic ADP-ribose: synthesis, spectral characterization, and use. Biochemistry 35: 379–386 [DOI] [PubMed] [Google Scholar]
- Guse AH, Lee HC (2008) NAADP: a universal Ca2+ trigger. Sci Signal 1: re10. [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Brownlee C (2004) The generation of Ca2+ signals in plants. Annu Rev Plant Biol 55: 401–427 [DOI] [PubMed] [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR (2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23: 267–278 [DOI] [PubMed] [Google Scholar]
- Knight H. (2000) Calcium signaling during abiotic stress in plants. Int Rev Cytol 195: 269–324 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J 12: 1067–1078 [DOI] [PubMed] [Google Scholar]
- Kraft R, Grimm C, Grosse K, Hoffmann A, Sauerbruch S, Kettenmann H, Schultz G, Harteneck C (2004) Hydrogen peroxide and ADP-ribose induce TRPM2-mediated calcium influx and cation currents in microglia. Am J Physiol Cell Physiol 286: C129–C137 [DOI] [PubMed] [Google Scholar]
- Lamotte O, Courtois C, Dobrowolska G, Besson A, Pugin A, Wendehenne D (2006) Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic Biol Med 40: 1369–1376 [DOI] [PubMed] [Google Scholar]
- Leckie CP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM (1998) Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc Natl Acad Sci USA 95: 15837–15842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC. (1993) Potentiation of calcium- and caffeine-induced calcium release by cyclic ADP-ribose. J Biol Chem 268: 293–299 [PubMed] [Google Scholar]
- Lee HC. (2001) Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol 41: 317–345 [DOI] [PubMed] [Google Scholar]
- Lee HC. (2005) Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling. J Biol Chem 280: 33693–33696 [DOI] [PubMed] [Google Scholar]
- Lee HC. (2006) Structure and enzymatic functions of human CD38. Mol Med 12: 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Aarhus R (1991) ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul 2: 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Aarhus R (1993) Wide distribution of an enzyme that catalyzes the hydrolysis of cyclic ADP-ribose. Biochim Biophys Acta 1164: 68–74 [DOI] [PubMed] [Google Scholar]
- Li PL, Tang WX, Valdivia HH, Zou AP, Campbell WB (2001) cADP-ribose activates reconstituted ryanodine receptors from coronary arterial smooth muscle. Am J Physiol Heart Circ Physiol 280: H208–H215 [DOI] [PubMed] [Google Scholar]
- Martí MC, Stancombe MA, Webb AAR (2013) Cell- and stimulus-type-specific cytosolic-free Ca2+ signals in Arabidopsis. Plant Physiol 163: 625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Lea US, Provan F, Kaiser WM, Lillo C (2005) Is nitrate reductase a major player in the plant NO (nitric oxide) game? Photosynth Res 83: 181–189 [DOI] [PubMed] [Google Scholar]
- Moore CA, Bowen HC, Scrase-Field S, Knight MR, White PJ (2002) The deposition of suberin lamellae determines the magnitude of cytosolic Ca2+ elevations in root endodermal cells subjected to cooling. Plant J 30: 457–465 [DOI] [PubMed] [Google Scholar]
- Muir SR, Sanders D (1997) Inositol 1,4,5-trisphosphate-sensitive Ca2+ release across nonvacuolar membranes in cauliflower. Plant Physiol 114: 1511–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LAJ, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV, Hall MA, Harren FJM, Hebelstrup KH, Gupta KJ (2013) Nitric oxide in plants: an assessment of the current state of knowledge. AoB Plants 5: pls052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Bewell MA, Siddiqua A, Dickinson GD, Galione A, Sanders D (2000) Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc Natl Acad Sci USA 97: 8693–8698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hancock JT (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128: 13–16 [PMC free article] [PubMed] [Google Scholar]
- Ozawa T. (2001) Ryanodine-sensitive Ca2+ release mechanism in non-excitable cells (Review). Int J Mol Med 7: 21–25 [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, et al. (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411: 595–599 [DOI] [PubMed] [Google Scholar]
- Rentel MC, Knight MR (2004) Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol 135: 1471–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico-Lemus M, Rodríguez-Garay B (2014) SNP as an effective donor of nitric oxide for in vitro plant cell and tissue culture. J Plant Biochem Physiol 2: 3 [Google Scholar]
- Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53: 103–110 [PubMed] [Google Scholar]
- Sánchez JP, Duque P, Chua NH (2004) ABA activates ADPR cyclase and cADPR induces a subset of ABA-responsive genes in Arabidopsis. Plant J 38: 381–395 [DOI] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K (2001) Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293: 1327–1330 [DOI] [PubMed] [Google Scholar]
- Thomas JM, Masgrau R, Churchill GC, Galione A (2001) Pharmacological characterization of the putative cADP-ribose receptor. Biochem J 359: 451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy FE, Gilliham M, Dodd AN, Webb AAR, Tester M (2008) NaCl-induced changes in cytosolic free Ca2+ in Arabidopsis thaliana are heterogeneous and modified by external ionic composition. Plant Cell Environ 31: 1063–1073 [DOI] [PubMed] [Google Scholar]
- Tsai YC, Delk NA, Chowdhury NI, Braam J (2007) Arabidopsis potential calcium sensors regulate nitric oxide levels and the transition to flowering. Plant Signal Behav 2: 446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmott N, Sethi JK, Walseth TF, Lee HC, White AM, Galione A (1996) Nitric oxide-induced mobilization of intracellular calcium via the cyclic ADP-ribose signaling pathway. J Biol Chem 271: 3699–3705 [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y, Takahashi S (1999) An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci 4: 128–129 [DOI] [PubMed] [Google Scholar]
- Yu JZ, Zhang DX, Zou AP, Campbell WB, Li PL (2000) Nitric oxide inhibits Ca2+ mobilization through cADP-ribose signaling in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 279: H873–H881 [DOI] [PubMed] [Google Scholar]
- Zhang AY, Li PL (2006) Vascular physiology of a Ca2+ mobilizing second messenger: cyclic ADP-ribose. J Cell Mol Med 10: 407–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.