Diterpene resin acids are major conifer defense compounds and important precursors for industrial bioproducts and are formed through a modular system of diterpene synthases and cytochrome P450s.
Abstract
Cytochrome P450 enzymes of the CYP720B subfamily play a central role in the biosynthesis of diterpene resin acids (DRAs), which are a major component of the conifer oleoresin defense system. CYP720Bs exist in families of up to a dozen different members in conifer genomes and fall into four different clades (I–IV). Only two CYP720B members, loblolly pine (Pinus taeda) PtCYP720B1 and Sitka spruce (Picea sitchensis) PsCYP720B4, have been characterized previously. Both are multisubstrate and multifunctional clade III enzymes, which catalyze consecutive three-step oxidations in the conversion of diterpene olefins to DRAs. These reactions resemble the sequential diterpene oxidations affording ent-kaurenoic acid from ent-kaurene in gibberellin biosynthesis. Here, we functionally characterized the CYP720B clade I enzymes CYP720B2 and CYP720B12 in three different conifer species, Sitka spruce, lodgepole pine (Pinus contorta), and jack pine (Pinus banksiana), and compared their activities with those of the clade III enzymes CYP720B1 and CYP720B4 of the same species. Unlike the clade III enzymes, clade I enzymes were ultimately found not to be active with diterpene olefins but converted the recently discovered, unstable diterpene synthase product 13-hydroxy-8(14)-abietene. Through alternative routes, CYP720B enzymes of both clades produce some of the same profiles of conifer oleoresin DRAs (abietic acid, neoabietic acid, levopimaric acid, and palustric acid), while clade III enzymes also function in the formation of pimaric acid, isopimaric acid, and sandaracopimaric acid. These results highlight the modularity of the specialized (i.e. secondary) diterpene metabolism, which produces conifer defense metabolites through variable combinations of different diterpene synthase and CYP720B enzymes.
Oleoresin is a major chemical defense of conifers, consisting of complex and variable mixtures of over 100 different monoterpenes, sesquiterpenes, and diterpenes (Phillips and Croteau, 1999; Keeling and Bohlmann, 2006a, 2006b; Zulak and Bohlmann, 2010; Bohlmann, 2012). In species of spruce (Picea spp.) and pine (Pinus spp.), oleoresin accumulates in large quantities in resin ducts in all major organs and their different tissues. When herbivores or pathogens damage these tissues, constitutive and induced oleoresin exudes from severed resin ducts as a chemical and physical defense. In the case of forest insect pests, the extent of herbivory on an individual pine or spruce tree can range from isolated feeding or oviposition by a few insects to mass attack by hundreds or thousands of insects, as, for example in the epidemic phase of a bark beetle outbreak. Depending on the severity of an insect attack, the oleoresin defense of a conifer may fully protect a tree by deterring, repelling, or killing the herbivore or, in case of an overwhelming mass attack, may become ineffective as a defense (Boone et al., 2011; Raffa, 2014).
Diterpenes and monoterpenes are the two major constituents of the oleoresin defense of spruces and pines, where diterpenes accumulate predominantly in the oxidized form of diterpene resin acids (DRAs; Supplemental Fig. S1; Martin et al., 2002; Zulak et al., 2009; Hamberger et al., 2011; Hall et al., 2013a, 2013b; Keefover-Ring et al., 2016). In addition to their natural role in conifer defense, humans have used DRAs for centuries as versatile biochemicals. For example, DRAs were used in large quantities in the traditional naval stores industry to protect wooden ships and boats against fouling (Langenheim, 2003). In modern industrial applications, DRAs are converted into an array of biochemicals and bioproducts such as coatings, polymers, inks, flavors, and fragrances (Bohlmann and Keeling, 2008; Hillwig et al., 2011; Zerbe and Bohlmann, 2015a). Conifer DRAs are of considerable structural diversity, which results from the combined activities of multienzyme families of diterpene synthases (diTPSs) and cytochrome P450 monooxygenases (P450s) in a modular biosynthetic system (Hamberger et al., 2011; Zerbe and Bohlmann, 2015a, 2015b).
In the first part of DRA biosynthesis, diTPSs catalyze the multistep cyclization and rearrangement of geranylgeranyl diphosphate (GGPP; Supplemental Fig. S1) to produce various bicyclic or, more commonly, tricyclic diterpene structures (Peters, 2010; Zerbe and Bohlmann, 2015a). Families of diTPSs, including the isopimaradiene synthases (ISO) and levopimaradiene/abietadiene synthases (LAS), have been characterized in several spruce and pine species (Martin et al., 2004; Ro and Bohlmann, 2006; Keeling et al., 2011; Zerbe et al., 2012; Hall et al., 2013b). A general picture of conifer diTPSs has emerged, with the majority representing bifunctional class I/II enzymes with two active sites (Zhou et al., 2012; Zerbe and Bohlmann, 2015b). These diTPSs catalyze the initial bicyclization of GGPP into (+)-copalyl diphosphate (CPP) at the class II active site. CPP is then released and subsequently bound by the class I active site (Peters et al., 2001), where cleavage of the diphosphate group of CPP and additional cyclization and rearrangements via intermediate carbocations occur. Known ISO enzymes complete the formation of the diterpene olefins isopimaradiene and sandaracopimaradiene by deprotonation of a postulated sandaracopimarenyl cation intermediate (Keeling et al., 2008; Hall et al., 2013b). In contrast, LAS enzymes produce the unstable 13-hydroxy-8(14)-abietene, which subsequently dehydrates to form abietadiene, levopimaradiene, palustradiene, and neoabietadiene (Keeling et al., 2011). In addition, monofunctional class I diTPSs (PIM and ISO) were recently characterized in lodgepole pine (Pinus contorta) and jack pine (Pinus banksiana), producing pimaradiene and isopimaradiene (Hall et al., 2013b). In brief, this set of conifer diTPSs accounts for the biosynthesis of the diterpene olefins isopimaradiene, pimaradiene, abietadiene, levopimaradiene, palustradiene, and neoabietadiene plus the ephemeral 13-hydroxy-8(14)-abietene (Supplemental Fig. S1).
In the second part of DRA biosynthesis, the diterpene olefins produced by diTPSs are oxidized at C18 (Supplemental Fig. S1). Two cytochrome P450 enzymes of the CYP720B subfamily, loblolly pine (Pinus taeda) CYP720B1 (Ro et al., 2005) and Sitka spruce (Picea sitchensis) CYP720B4 (Hamberger et al., 2011), have been described to catalyze the three-step oxidation of several diterpene olefins to the corresponding DRAs via intermediate diterpene alcohols and aldehydes. CYP720B genes have been found in different gymnosperms, but they appear to be missing in angiosperms. Twelve different CYP720B members were annotated in Sitka spruce, which fall into four clades, I to IV (Hamberger et al., 2011). The only two functionally characterized CYP720B enzymes, PtCYP720B1 and PsCYP720B4, belong to clade III, while the functions of members of the other clades are unknown (Hamberger et al., 2011).
Here, we characterized previously unknown and unexpected functions of two members of CYP720B clade I, CYP720B2 and CYP720B12, and compared these with clade III enzymes in three different Pinaceae species, Sitka spruce, lodgepole pine, and jack pine. While all characterized enzymes contribute to the biosynthesis of DRAs present in the oleoresin, we found that clade I and III enzymes use different substrates. Specifically, we found that, unlike clade III enzymes, clade I enzymes did not convert diterpene olefins but were active with the unstable 13-hydroxy-8(14)-abietene product of LAS. This work substantially expands our knowledge of the biochemical functions of CYP720B enzymes in different conifer species and establishes 13-hydroxy-8(14)-abietene as a relevant biosynthetic intermediate and P450 substrate in the DRA chemical defense system.
RESULTS
Discovery, Annotation, and Phylogeny of CYP720Bs in Six Different Conifer Species
A previous analysis of the Sitka spruce transcriptome and full-length (FL) complementary DNA (cDNA) sequences revealed 12 different CYP720B family members (Hamberger et al., 2011). We used the Sitka spruce sequences to explore genome and transcriptome assemblies of other conifer species, specifically white spruce (Picea glauca), Norway spruce (Picea abies), loblolly pine, lodgepole pine, and jack pine (Birol et al., 2013; Hall et al., 2013a; Nystedt et al., 2013; Neale et al., 2014; Wegrzyn et al., 2014; Warren et al., 2015). Including CYP720Bs of a white spruce hybrid (P. glauca × Picea engelmannii; Ralph et al., 2008) and intraspecific minor variants, which may represent allelic differences, we obtained a set of 62 different CYP720B sequences. Among these, we identified and annotated nine distinct FL CYP720B members in white spruce, seven in loblolly pine, and five each in Norway spruce, lodgepole pine, and jack pine (Table I; Fig. 1). Given the different levels of gene space coverage of the various transcriptome data and the highly fragmented nature of conifer genome assemblies (De La Torre et al., 2014; Warren et al., 2015), the CYP720Bs found in the six different species almost certainly represent only subsets of the complete CYP720B subfamily in each of these species. Nonetheless, they provide a multispecies topography of this apparently gymnosperm-specific (or perhaps conifer-specific) group of P450s (Fig. 1).
Table I. CYP720B members identified in transcriptomes and genomes of different spruce and pine species.
Using Sitka spruce (Ps) CYP720B cDNA reference sequences (Hamberger et al., 2011), CYP720B members were identified as present (+) in white spruce (Pg), Norway spruce (Pa), loblolly pine (Pt), lodgepole pine (Pc), and jack pine (Pb) transcriptome (T) or genome (G) sequences. Not included are intraspecific minor variants, which may represent allelic differences, as shown in Figure 1.
| Clade | CYP720B | Ps |
Pg |
Pa |
Pt |
Pc |
Pb |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| cDNA | T | G | T | G | T | G | T | T | ||
| I | CYP720B2 | + | + | + | + | + | + | + | + | + |
| CYP720B12 | + | + | + | + | + | + | + | + | + | |
| CYP720B11 | + | + | + | + | ||||||
| CYP720B10 | + | + | + | + | + | |||||
| II | CYP720B13 | + | ||||||||
| CYP720B14 | + | |||||||||
| CYP720B15 | + | + | + | |||||||
| CYP720B16 | + | |||||||||
| CYP720B17 | + | + | + | + | ||||||
| III | CYP720B1 | + | + | + | + | |||||
| CYP720B4 | + | + | + | + | ||||||
| CYP720B5 | + | + | + | |||||||
| CYP720B7 | + | + | + | |||||||
| IV | CYP720B8 | + | + | + | + | + | ||||
| CYP720B9 | + | + | + | |||||||
Figure 1.
Phylogeny of the gymnosperm-specific CYP720B family with representatives from seven different spruce and pine species based on genome and transcriptome analyses and FL cDNA cloning. Shown is a maximum likelihood tree of 62 CYP720B protein sequences from Sitka spruce (Ps), white spruce (Pg), Norway spruce (Pa), hybrid white spruce (Pge), loblolly pine (Pt), jack pine (Pb), and lodgepole pine (Pc). The phylogeny includes sequences described by Hamberger et al. (2011) and sequences summarized in Table I plus variants thereof. The CYP720B sequences cluster into four clades, I to IV. The only two previously functionally characterized CYP720B enzymes, PtCYP720B1 and PsCYP720B4 of clade III, are shown in boldface black letters. CYP720B enzymes tested for functions in this study are depicted in red. PtCYP720B1v3, labeled with an asterisk, was annotated previously as PtCYP720B6 (Hamberger et al., 2011) and reannotated based on our phylogeny, which identified a larger cluster of CYP720B1 members. The scale bar represents 0.1 amino acid substitutions per site. Bootstrap confidence values over 60% are given. The tree is rooted using Arabidopsis (Arabidopsis thaliana) AtCYP720A1 as an outgroup. Putative allelic variants (v) are indicated.
A phylogeny of the deduced CYP720B protein sequences (Fig. 1) confirmed the pattern of the previously established four clades, I to IV (Hamberger et al., 2011), which appear to have arisen by repeated gene duplications. Within a clade, the pairwise sequence identity is at least 63% (for clades I and III, see Supplemental Table S1). Several CYP720B members, specifically CYP720B2, CYP720B8, CYP720B10, and CYP720B12, are represented with orthologs in spruce and pine species, suggesting that some of the proposed gene duplications that gave rise to the multiple CYP720Bs in each species occurred prior to the separation of spruce and pine lineages more than 100 million years ago (Savard et al., 1994; Wang and Ran, 2014). In contrast, CYP720B11 sequences were only found in the three pine species but lacked apparent orthologs in the transcriptome and genome sequences of the three spruce species.
Clade I and III CYP720Bs Functions in DRA Biosynthesis
Only two CYP720B members, PtCYP720B1 and PsCYP720B4 (both of which belong to clade III), were shown previously to function in DRA biosynthesis (Ro et al., 2005; Hamberger et al., 2011). Therefore, it was not possible to predict a priori if members of other clades also function in DRA biosynthesis and if they would act on a similar broad spectrum of diterpene substrates and catalyze similar three-step oxidations as the two characterized clade III enzymes (Hamberger et al., 2011). Since clade I sequences were well represented across the transcriptomes of the six pine and spruce species investigated in this study, supporting an important role in the Pinaceae, we selected members from across this clade for comparative functional characterization with clade III members. Clade I members selected for biochemical characterization included Sitka spruce PsCYP720B2 and PsCYP720B12; lodgepole pine PcCYP720B2, PcCYP720B10v1, PcCYP720B10v2, PcCYP720B11, and PcCYP720B12; and jack pine PbCYP720B2, PbCYP720B10, PbCYP720B11, and PbCYP720B12. For comparison, we characterized the clade III members lodgepole pine PcCYP720B1 and jack pine PbCYP720B1, with Sitka spruce PsCYP720B4 as a reference (Hamberger et al., 2011; Fig. 1).
To assess the activities of these CYP720Bs in DRA biosynthesis, we initially used the previously established yeast (Saccharomyces cerevisiae) in vivo assay system that allows coexpression of CYP720B cDNAs with conifer diTPSs and yeast geranylgeranyl diphosphate synthase (GGPPS; Ro et al., 2005; Hamberger et al., 2011). We used two different yeast platform strains, one expressing a LAS diTPS and the other expressing an ISO diTPS. The BY4741 yeast strains used in this study also carried a chromosomally integrated NADPH-dependent cytochrome P450 reductase (CPR) from lodgepole pine (PcCPR) for stable CPR expression. The BY4741 GGPPS/LAS/CPR platform strain produced abietadiene as the principal diterpene olefin (Fig. 2, peak 1; Supplemental Fig. S2). Growing this strain in a pH 7 buffered environment led to an additional accumulation of lower amounts of levopimaradiene, neoabietadiene, and palustradiene (Supplemental Fig. S2). The BY4741 GGPPS/ISO/CPR platform strain produced isopimaradiene as the principal diterpene olefin (Fig. 3, peak 6). In both platform strains, candidate CYP720Bs were expressed using the pYeD60 plasmid (Hamann and Møller, 2007).
Figure 2.
CYP720B-dependent in vivo formation of DRAs in recombinant yeast in vivo assays. Total ion gas chromatography-mass spectrometry (GC-MS) profiles are from extracts from engineered yeast strains expressing GGPPS, LAS, and CPR in the absence (top trace) or presence of different clade I or III CYP720B enzymes from Sitka spruce (Ps), jack pine (Pb), or lodgepole pine (Pc). Yeast expressing GGPPS, LAS, and CPR without CYP720B accumulate the diterpene olefin abietadiene (peak 1; top trace). Unlabeled peaks represent yeast metabolites, some of which are masking the additional LAS products palustradiene, levopimaradiene, and neoabietadiene (Supplemental Fig. S2). IS represents the internal standard dichlorodehydroabietic acid. In the presence of clade III CYP720B4 and CYP720B1 and clade I CYP720B2 and CYP720B12, the DRAs palustric acid (peak 2), levopimaric acid (peak 3), abietic acid (peak 4), and neoabietic acid (peak 5) accumulate. Clade I CYP720B10 and CYP720B11 did not show DRA formation in this assay. Retention times and mass spectra of metabolites identified as abietadiene (peak 1), palustric acid (peak 2), levopimaric acid (peak 3), abietic acid (peak 4), and neoabietic acid (peak 5) match those of authentic standards. The mass spectra of these metabolites produced in the engineered yeast cells and the mass spectra of the authentic standards are shown in Supplemental Figure S3. The corresponding olefin profiles and substrate depletion of the yeast in vivo assays are shown in Supplemental Figure S2, revealing the olefins palustradiene, levopimaradiene, and neoabietadiene in addition to abietadiene (peak 1), as produced in yeast expressing GGPPS, LAS, and CPR.
Figure 3.
CYP720B-dependent in vivo formation of isopimaric acid in engineered yeast. GC-MS total ion profiles are from extracts from engineered yeast strains expressing GGPPS, ISO, and CPR in the absence (top trace) or presence of different clade I or III CYP720B enzymes. Yeast expressing GGPPS, ISO, and CPR without CYP720B accumulate the diterpene olefin isopimaradiene (peak 6; top trace). Isopimaradiene is converted to isopimaric acid (peak 7) in the presence of the clade III enzymes PsCYP720B4, PbCYP720B1, and PcCYP720B1. Retention times and mass spectra of metabolites identified as isopimaradiene (peak 6) and isopimaric acid (peak 7) match those of authentic standards (Supplemental Fig. S3). Unlabeled peaks represent yeast metabolites in the extracts. IS represents the internal standard dichlorodehydroabietic acid.
Expression of the clade III CYP720B cDNAs, PsCYP720B4, PbCYP720B1, and PcCYP720B1, in the GGPPS/LAS/CPR strain yielded four DRAs, palustric acid (peak 2), levopimaric acid (peak 3), abietic acid (peak 4), and neoabietic acid (peak 5; Fig. 2). In the case of the CYP720B1 activities, DRA formation also was reflected in reduced diterpene olefin levels (Fig. 2; Supplemental Fig. S2). Very similar results of DRA formation and olefin depletion were obtained for expression of the clade I proteins PsCYP720B2, PsCYP720B12, PcCYP720B2, PcCYP720B12, PbCYP720B2, and PbCYP720B12 in the GGPPS/LAS/CPR strain. This contrasted with a lack of diterpene olefin conversion in strains expressing clade I lodgepole pine and jack pine PcCYP720B10, PcCYP720B11, PbCYP720B10, or PbCYP720B11.
Expression of the clade III CYP720B cDNAs, PsCYP720B4, PbCYP720B1, and PcCYP720B1, in the GGPPS/ISO/CPR strain resulted in the formation of isopimaric acid (Fig. 3, peak 7; Supplemental Fig. S3) and depletion of isopimaradiene. In contrast to clade III members, none of the clade I members formed detectable levels of isopimaric acid or other oxidation products of isopimaradiene when expressed in the GGPPS/ISO/CPR strain.
In summary, this in vivo screen of clade I and III CYPD720B candidates in combination with the LAS and ISO diTPSs revealed some commonalities and some unique features in the activities of different clade I and III members toward diterpene olefins, which were consistent across the species Sitka spruce, lodgepole pine, and jack pine. While the clade I members CYP720B10 and CYP720B11 did not show any DRA biosynthetic activity in this screen, the clade I members CYP720B2 and CYP72B12 appeared to have a role in DRA metabolism, with the formation of abietic acid, levopimaric acid, neoabietic acid, and palustric acid. The clade III enzymes CYP720B4 and CYP720B1 also formed abietic acid, levopimaric acid, neoabietic acid, and palustric acid and, in addition, produced isopimaric acid. None of the alcohol or aldehyde intermediates in the formation of palustric acid, levopimaric acid, abietic acid, neoabietic acid, and isopimaric acid were detected in this screen, suggesting that all of the active CYP720Bs catalyze the efficient three-step oxidation at C18 without substantial accumulation of the intermediates with lower oxidation states.
In Vitro Assays Reveal Differences in Substrate Use of Clade I and III CYP720Bs with 24 Different DRA Precursors
To comprehensively assess the activities of clade I and III members with relevant DRA pathway substrates (in particular the different diterpene olefins, alcohols, and aldehydes), we prepared microsome fractions of the recombinant P450s for in vitro assays. Microsome preparations contained individual CYP720Bs and PcCPR. We confirmed the successful expression of all CYP720B P450 proteins with CO difference spectra (Supplemental Fig. S4). As substrates, we used a previously described panel of 24 DRA precursors (Hamberger et al., 2011), which included the eight olefins abietadiene, palustradiene, levopimaradiene, neoabietadiene, pimaradiene, isopimaradiene, sandaracopimaradiene, and dehydroabietadiene and the corresponding eight different C18 alcohols and eight C18 aldehydes (Supplemental Fig. S1). The NADPH- and CPR-dependent activities of the 14 CYP720Bs were qualitatively assessed in triplicate assays by the presence or absence of detectable DRA product formation using liquid chromatography-mass spectrometry (LC-MS) analysis (Table II). Microsomes containing PsCYP720B4 protein were used as a positive control (Hamberger et al., 2011), confirming activity with all 24 tested substrates (Table II). The newly identified clade III enzymes, jack pine PbCYP720B1 and lodgepole pine PcCYP720B1, also were active with all 24 substrates. Consistent with the lack of activity observed in in vivo assays, clade I lodgepole pine and jack pine PcCYP720B10, PcCYP720B11, PbCYP720B10, and PbCYP720B11 did not show detectable activity in vitro using representative diterpenol substrates.
Table II. In vitro activity of clade I and clade III CYP720Bs with 24 different substrates, representing DRA precursor diterpene olefins, alcohols, and aldehydes.
The formation (blue) or lack of detectable formation (yellow) of DRAs observed with three independent microsomal preparations for each CYP720B candidate and substrate is summarized.
 |
When testing microsomes that contained the individual clade I enzymes PsCYPP720B2, PsCYP720B12, PbCYPP720B2, PbCYP720B12, PcCYPP720B2, or PcCYP720B12 with all 24 substrates, none of these assays yielded detectable DRA formation (Table II). Furthermore, we could not detect any formation of the C18 alcohols or aldehydes in these assays. These results were surprising, because they appeared to contradict some of the results of the yeast in vivo assays (Figs. 2 and 3). Based on the yeast in vivo assays, it was reasonable to expect that the CYP720B2 and CYP720B12 enzymes were not active with isopimaradiene and perhaps the corresponding isopimaradienol and isopimaradienal. However, it was unexpected that the CYP720B2 and CYP720B12 proteins showed no activity in in vitro assays with abietadiene, palustradiene, levopimaradiene, and neoabietadiene as substrates.
The discrepancy between the in vitro and in vivo results observed with CYP720B2 and CYP720B12 of all three spruce and pine species led us to the hypothesis that these clade I enzymes do not use abietadiene, palustradiene, levopimaradiene, and neoabietadiene as substrates, which are the ultimate products of the diTPS LAS, but may be active on the initial, ephemeral LAS product 13-hydroxy-8(14)-abietene (Keeling et al., 2011). This hypothesis was tested as described below.
CYP720Bs of Clade I, But Not Clade III, Use the Ephemeral LAS Product 13-Hydroxy-8(14)-Abietene as a Substrate to Produce DRAs
The model conifer diTPS LAS produces an unstable initial product, 13-hydroxy-8(14)-abietene (Supplemental Fig. S1), which dehydrates spontaneously to form abietadiene, levopimaradiene, palustradiene, and neoabietadiene (Keeling et al., 2011). The unstable 13-hydroxy-8(14)-abietene is not commercially available, and also could not be chemically synthesized, as a substrate for enzyme assays. Attempts by us and others to reproduce chemical synthesis using a previously described method (Zhang et al., 2005) were unsuccessful. Hence, we designed a sequential in vitro assay system, which allowed us to test if the clade I enzymes CYP720B2 and CYP720B12 act on 13-hydroxy-8(14)-abietene as a substrate in DRA biosynthesis. In this assay system, we used a heterologously expressed and purified LAS from lodgepole pine (Hall et al., 2013b) to convert GGPP into 13-hydroxy-8(14)-abietene (Fig. 4, peak 8). After an initial assay incubation to allow for 13-hydroxy-8(14)-abietene accumulation, which was detected by LC-MS with APCI interface (Keeling et al., 2011), microsome preparations containing the individual CYP720B enzymes and PcCPR were added to the assay system. The addition of microsomes containing clade III PsCYP720B4 did not result in the detectable conversion of 13-hydroxy-8(14)-abietene [i.e. we did not observe the formation of oxidized derivatives of 13-hydroxy-8(14)-abietene, nor did we detect the depletion of this compound; Fig. 4]. In contrast, the addition of microsomes containing the clade I PsCYP720B12 caused complete depletion of the 13-hydroxy-8(14)-abietene peak (Fig. 4). LC-MS monitoring of m/z 303 for DRAs detected the de novo occurrence of two products in these assays (Fig. 4). Retention time and mass spectra of the first product (peak 2-5) were consistent with the overlapping retention times and the mass spectra of the four DRAs, palustric acid (peak 2), levopimaric acid (peak 3), abietic acid (peak 4), and neoabietic acid (peak 5). The second product (peak 9) also showed the dominant m/z 303 mass fragment characteristic for DRAs, but the longer retention time was indicative of a more polar compound.
Figure 4.
Clade I members CYP720B2 and CYP720B12 converting the LAS product 13-hydroxy-8(14)-abietene in a sesquential diTPS and P450 assay system. A, Normal-phase atmospheric pressure chemical ionization (APCI) LC-MS scans of in vitro assays with recombinant LAS and sequential incubation with Sitka spruce PsCYP720B4 or PsCYP720B12. Total ion chromatograms (TIC) and extracted ion chromatograms at mass-to-charge ratio (m/z) 303 (EIC303) are shown. Retention times and mass spectra of DRAs (convoluted peak 2-5) match those of authentic standards. The retention time and mass spectrum of 13-hydroxy-8(14)-abietene (peak 8) are in agreement with previously published results (Keeling et al., 2011). Peaks 9 and 10 represent epimers of 13-hydroxy-8(14)-abietic acid. The peak labeled with an asterisk represents yeast microsome sterols. The top two traces are ion chromatograms of control assays using microsomes transformed with empty vectors in the absence or presence of the LAS substrate GGPP. B, GC-MS scans of extracts from a sequential incubation of GGPP with recombinant LAS and microsomes containing recombinant Sitka spruce PsCYP720B12 expressed in the yeast strain BY4741:PcCPR. Total ion chromatograms and the extracted ion chromatograms at m/z 374 (EIC 374) and m/z 421 (EIC 421) are shown; m/z 374 is the characteristic ion for trimethylsilyl derivatives of palustric acid (peak 2), levopimaric acid (peak 3), abietic acid (peak 4), and neoabietic acid (peak 5), and m/z 421 is the characteristic ion for 13-hydroxy-8(14)-abietic acid (peaks 9 and 10). C, Extracted ion GC-MS scans at m/z 421 of sequential assays testing different clade I and III recombinant CYP720B enzymes of Sitka spruce (Ps) and lodgepole pine (Pc) with 13-hydroxy-8(14)-abietene. The formation of 13-hydroxy-8(14)-abietic acid (peaks 9 and 10) was detected in the presence of clade I enzymes CYP720B2 and CYP720B12 but not with the clade III enzymes PsCYP720B4 and PcCYP720B1.
To gain further insights into the nature of peak 2-5 and peak 9, we derivatized the product of the sequential LAS/PsCYP720B12 assay system with N,O-bis(trimethylsilyl)trifluoroacetamide followed by GC-MS analysis (Fig. 4). This allowed for the separation and detection of the four DRAs, palustric acid (peak 2), levopimaric acid (peak 3), abietic acid (peak 4), and neoabietic acid (peak 5), in addition to product peak 9 and a weaker peak 10. The latter two peaks had the same mass fragmentation pattern with a characteristic ion at m/z 421 (Fig. 4; Supplemental Fig. S5). Using chemical ionization, we detected a molecular ion at m/z 464 ([M+H]+ at m/z 465) for peaks 9 and 10 (Supplemental Fig. S5). Taking into account both the LC-MS and GC-MS data, we propose that peaks 9 and 10 are epimers of 13-hydroxy-8(14)-abietic acid. The characteristic ion at m/z 421 after electron impact ionization is likely the result of the loss of a propyl fragment (m/z = 43). Epimers of 13-hydroxy-8(14)-abietene were described previously by Keeling et al. (2011).
These results obtained from sequential LAS/PsCYP720B12 assays suggested that clade I PsCYP720B12 converts the epimers of 13-hydroxy-8(14)-abietene (peak 8) to 13-hydroxy-8(14)-abietic acid (peaks 9 and 10), which subsequently dehydrate to palustric acid (peak 2), levopimaric acid (peak 3), abietic acid (peak 4), and neoabietic acid (peak 5; Fig. 4). The clade I enzymes PsCYP720B2, PbCYP720B2, PbCYP720B12, PcCYP720B2, and PcCYP720B12 showed the same activity toward 13-hydroxy-8(14)-abietene as PsCYP720B12. The formation of 13-hydroxy-8(14)-abietic acid was shown by detection of the characteristic fragment at m/z 421 of the derivatized assay product (Fig. 4). In contrast, when 13-hydroxy-8(14)-abietene produced by LAS in sequential assays was incubated with microsomes that contained the clade III enzymes PsCYP720B4, PbCYP720B1, and PcCYP720B1, no conversion of the initial LAS product and no accumulation of 13-hydroxy-8(14)-abietic acid were observed (Fig. 4).
DISCUSSION
Using a sequential LAS/CYP720B assay system (Fig. 4) allowed us, to our knowledge for the first time, to assess the recently discovered, ephemeral LAS product 13-hydroxy-8(14)-abietene as a substrate for downstream P450 reactions in DRA biosynthesis. This assay system resolved the above-noted apparent discrepancy of clade I enzyme activities observed in the yeast in vivo activity screen (Fig. 2) and the in vitro assays with individual isolated substrates (Table II). The formation of DRAs with the clade I CYP720B2 and CYP720B12 enzymes in yeast in vivo assays can be explained by the P450 enzymes accessing the short-lived 13-hydroxy-8(14)-abietene product of the LAS when coexpressed in yeast cells. The immediate products of these yeast in vivo activities of CYP720B2 and CYP720B12 would be the unstable 13-hydroxy-8(14)-abietic acid, which spontaneously dehydrates, leading to the detected accumulation of the DRAs palustric acid, levopimaric acid, abietic acid, and neoabietic acid (Fig. 2). This observation is in agreement with the results of the sequential LAS/CYP720B in vitro assays. It is also consistent with results obtained from in vitro assays with the panel of 24 individual substrates, in which CYP720B2 and CYP720B12 clade I enzymes did not convert the diterpene olefins palustradiene, levopimaradiene, abietadiene, and neoabietadiene and their corresponding C18 alcohols and aldehydes.
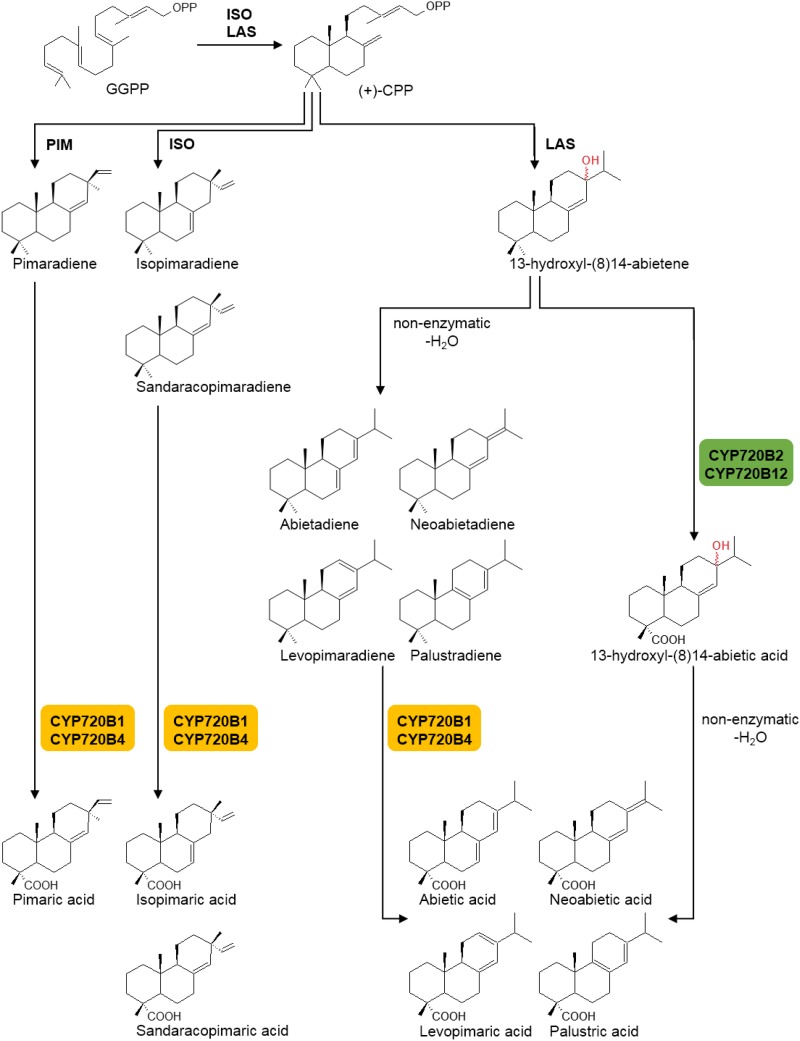
Taken together, employing complementary assay systems revealed unique features of the clade I and III CYP720Bs with regard to the specific diterpene substrates that these P450s can utilize in DRA biosynthesis (Fig. 5). The clade I enzymes use the short-lived 13-hydroxy-8(14)-abietene, and the clade III enzymes use the dehydration compounds abietadiene, levopimaradiene, palustradiene, and neoabietadiene. Taking into account the spontaneous dehydration of 13-hydroxy-8(14)-abietic acid formed by the clade I P450s, the clade I and III enzymes ultimately yield the same four DRAs, palustric acid, levopimaric acid, abietic acid, and neoabietic acid, all of which are found in the oleoresin of spruce and pine, albeit through alternative routes (Fig. 5). In addition, the clade III enzymes also can account for the formation of isopimaric acid, pimaric acid, and sandaracopimaric acid, but clade I enzymes do not appear to have a role in forming these three DRAs. Moreover, the clade III enzyme PsCYP720B4 appeared to be highly active in the biosynthesis of dehydroabietic acid (Hamberger et al., 2011).
Figure 5.
Schematic of a modular metabolic system of DRA biosynthesis. The diTPS modules ISO, LAS, and PIM convert GGPP via the intermediate CPP into a suite of diterpene olefins. In the case of LAS, the initial and unstable diTPS product is 13-hydroxy-8(14)-abietene. Here, we provide evidence that the clade I enzymes CYP720B2 and CYP720B12 (green) convert 13-hydroxy-8(14)-abietene to the corresponding 13-hydroxy-8(14)-abietic acid, which then undergoes dehydration to form the DRAs abietic acid, levopimaric acid, neoabietic acid, and palustric acid. In an alternative route, 13-hydroxy-8(14)-abietene undergoes spontaneous dehydration to form the diterpene olefins abietadiene, levopimaradiene, neoabietadiene, and palustradiene, which are then converted into the corresponding DRAs by the clade III enzymes CYP720B1 and CYP720B4 (orange). The same clade III enzymes also convert the olefins pimaradiene, isopimaradiene, and sandaracopimaradiene to the corresponding DRAs.
The CYP720B clade I product 13-hydroxy-8(14)-abietic acid is unstable under in vitro conditions, and its identification in our assays followed similar lines of evidence as established previously for the identification of 13-hydroxy-8(14)-abietadiene produced by LAS (Keeling et al., 2011). DRAs with a hydroxyl group at C13 have not been found in the oleoresin of spruce, lodgepole pine, or jack pine (Miller et al., 2005; Zulak et al., 2009; Hall et al., 2013b). However, diterpenoids with a hydroxyl or ether group at the C13 position exist in the oleoresin of Pinus massoniana (Yang et al., 2010). The lack of detection of 13-hydroxy-8(14)-abietic acid in spruce, lodgepole pine, and jack pine could be due to dehydration in planta. Conversely, if substantial amounts of 13-hydroxy-8(14)-abietic acid and its precursor 13-hydroxy-8(14)-abietadiene are stable in planta, and dehydration is an effect of extraction and analysis conditions, the diversity of DRAs in planta might be larger than is currently known. Under this scenario, the CYP720B clade III enzymes might be more relevant in the formation of pimaric acid, isopimaric acid, and sandaracopimaric acid (Fig. 5).
The results of our comparative characterization highlight alternative, but convergent, routes of DRA biosynthesis involving clade I and III CYP720Bs (Fig. 5). Accounting for known spruce and pine diTPSs (Keeling and Bohlmann, 2006b; Keeling et al., 2011; Hall et al., 2013b) and P450s of DRA biosynthesis (Ro and Bohlmann, 2006; Hamberger et al., 2011; this study), we propose an extended DRA pathway system that incorporates elements of modularity, potential redundancy, and anastomosis (Jennewein and Croteau, 2001). Sets of multiple diTPS and P450 modules are the result of gene duplications and functional diversification during the evolution of the TPS-d and CYP720B gene families of gymnosperm specialized diterpene metabolism. These gene families are related in origin and/or function to similar diTPSs and P450s of general (i.e. primary) GA biosynthesis, although only the genes of specialized metabolism appear to exist with multimember, functionally diverse gene families, contrasting with low-copy-number or single-copy genes in general metabolism (Keeling et al., 2010; Warren et al., 2015). While some diTPS and CYP720B functions appear to be redundant within the respective gene families, closer inspection revealed variations in substrate utilization and products (e.g. clade I and III CYP720Bs, LAS, and ISO) as well as cases of subfunctionalization between bifunctional and monofunctional diTPSs (Hall et al., 2013b) and neofunctionalization (e.g. LAS and ISO; Keeling et al., 2008). The different roles of clade I and III CYP720Bs also may represent a case of pathway anastomosis: clade I CYP720Bs efficiently convert the initial LAS product 13-hydroxy-8(14)-abietene (but not its default dehydration products) into DRAs. Such default dehydration products, abietadiene, palustradiene, levopimaradiene, and neoabietadiene, arising from 13-hydroxy-8(14)-abietene would be utilized by clade III CYP720Bs and reconnected to DRA biosynthesis. Pathway anastomosis, or biosynthetic convergence, in diterpene specialized (i.e. secondary) metabolism may be biologically relevant in the context of metabolite damage control, as discussed in a recent review by Hanson et al. (2016). In addition, clade III P450s appear to have an extended substrate range that also includes isopimaradiene. The observation that clade I and III CYP720Bs can use the presence of a C13 hydroxyl group, distal to the target C18 to distinguish between their substrates, is of particular interest for future work on comparative structure-function analysis of these P450s. The broad spectrum of diterpene substrates covered by clade I and III P450s, combined with their efficiency at catalyzing the three-step C18 oxidation, explains the predominance of the DRAs (as opposed to lesser oxidized diterpenes) in conifer oleoresin. In addition, the diTPS modules of this system create the fundamental diterpene scaffold diversity of this pathway system.
Recent transcript analysis of CYP720B members in Sitka spruce showed that clade I CYP720B2 and CYP720B12 and clade III CYP720B4 transcripts were expressed with similar tissue-specific patterns predominantly in the stem bark and roots (Hamberger et al., 2011), which are two major sites of resin accumulation in spruce. These three CYP720Bs also showed similar selective high abundance of transcript levels in laser microdissected cortical resin ducts (Hamberger et al., 2011). This supports a partly redundant or anastomotic role of P450s of these two clades in synergistically producing major DRAs. Noteworthy, the clade I CYP720B10, for which we were not able to identify a biochemical function, was only detected at very low levels in Sitka spruce across different organs and tissues (Hamberger et al., 2011), suggesting a less important role in DRA or diterpene metabolism. CYP720B11 was not found in spruce.
In future work, it would be of great interest to selectively remove the activities of individual diTPS and CYP720B modules from this metabolic system of DRA biosynthesis (Fig. 5) to further test for the degree and biological relevance of functional specialization or redundancy as well as anastomosis in planta. However, despite some recent success of RNA interference down-regulation in this system (Hamberger et al., 2011), altering or editing of gene expression remains a challenge in conifers.
MATERIALS AND METHODS
Materials
The Saccharomyces cerevisiae strain BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) was used for the expression of cytochrome P450 enzymes. Escherichia coli DH5 α-Select chemically competent cells (Bioline) were used for routine cloning, and E. coli BL21DE3-C41 cells were used for the expression of recombinant diTPS. Diterpene resin acid standards were purchased from Orchid Celmark. The corresponding diterpene olefins, alcohols, and aldehydes were synthesized from the acids at Best West Laboratories as described previously (Ro et al., 2005).
Yeast Expression Strain with Chromosomal Integration of a Conifer CPR
To streamline the functional characterization of P450 proteins in yeast, an S. cerevisiae strain was generated that contains the lodgepole pine (Pinus contorta) PcCPR stably integrated into the BY4741 genome. PcCPR was identified in the lodgepole pine transcriptome (Hall et al., 2013a). FL cDNA was amplified using gene-specific primers (Supplemental Table S2) and cloned into pYeDP60u (Hamann and Møller, 2007). Stable integration of PcCPR into the BY4741 genome was achieved following the method described by Jensen et al. (2011). In brief, the expression cassette, including the FL cDNA of PcCPR, GAL10-cyc1 promoter, and GAL10-cyc1 terminator, was PCR amplified from the pYeDP60u-PcCPR plasmid using primers that introduced AvrII and FseI restriction sites in the 5′ and 3′ ends, respectively (Supplemental Table S2). The expression cassette was then cloned into pUC19-MGA1 using AvrII and FseI sites to produce pUC19-PcCPRexpress. Digestion of pUC19-PcCPRexpress with SbfI led to a linearized fragment containing the PcCPR expression cassette and the KANMX selection gene flanked by two intergenic yeast sequences that allow homologous recombination and stable integration into the yeast genome. The linearized fragment was transformed into BY4741 using the LiCl method (Gietz and Schiestl, 2007). Correct insertion of the PcCPR expression cassette into the BY4741 genome was verified by PCR analysis. The CPR activity of select recombinant BY4741 colonies carrying the PcCPR expression cassette was assayed using the Cytochrome C Reductase (NADPH) Assay Kit (Sigma). All transformed colonies showed substantial cytochrome c reduction activities compared with the BY4741 strain, and strain BY4741:PcCPR1 was chosen for further analysis.
CYP720B Discovery
To expand the known portfolio of CYP720Bs in different conifer species, we screened the published transcriptome and genome assemblies described for white spruce (Picea glauca) and hybrid white spruce (P. glauca × Picea engelmannii; Ralph et al., 2008; Birol et al., 2013; Warren et al., 2015), Norway spruce (Picea abies; Nystedt et al., 2013), loblolly pine (Pinus taeda; Neale et al., 2014; Wegrzyn et al., 2014), jack pine (Pinus banksiana), and lodgepole pine (Hall et al., 2013a). The transcriptome and genome assemblies were searched using the TBLASTN tool with all of the previously identified Sitka spruce (Picea sitchensis) CYP720B cDNA sequences as query (Hamberger et al., 2011) with an E-value threshold of 1e–50 and a minimum translated read length of 100 amino acids. Candidate sequences were manually reassembled and validated to obtain a final set of CYP720B candidates. Maximum likelihood phylogenetic analyses were performed on aligned amino acid sequences using the software MEGA version 6 (Tamura et al., 2011) with default parameters and 500 bootstrap repetitions. Phylogenetic trees were visualized using TreeView 1.6.6 (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html; Page, 1996).
Cloning of CYP720B FL cDNAs and Yeast Transformation
For cloning of FL cDNAs of CYP720B candidates, RNA was extracted from bark and xylem of a 5-year-old Sitka spruce, a 2-year-old jack pine, and a 2-year-old lodgepole pine and converted to cDNA using SuperScript III Reverse Transcriptase (Invitrogen) as described previously (Kolosova et al., 2004). FL cDNAs were amplified from cDNAs by PCR using Phusion Hot Start II DNA Polymerase (Thermo Scientific) and gene-specific primers (Supplemental Table S2). Primers were designed based on the sequence discovery described above. PCR products were cloned into pYeDP60u by either In-Fusion Cloning (Clontech) or the USER cloning strategy (Hamann and Møller, 2007), and inserts were sequence verified. PsCYP720B4 was cloned into pYeD60u from the previously described pESC-Leu::PsCYP720B4 plasmid (Hamberger et al., 2011). Plasmid transformation of yeast strain BY4741:PcCPR was conducted using the LiCl method (Gietz and Schiestl, 2007). Transformed yeast strains were selected on plates with appropriate synthetic complete dropout selection medium and grown at 30°C for 48 h. Details on yeast medium and culture conditions were described previously (Pompon et al., 1996; Ro et al., 2005).
Yeast in Vivo Assays for CYP720B Activity
To assess CYP720B activity in the in vivo yeast system, BY4741:PcCPR cells were cotransformed with plasmids harboring GGPPS, a diTPS, and CYP720B FL cDNAs. The plasmids containing GGPPS and diTPSs, pESC-His::PaLAS/ScGGPPS and pESC-His::PaISO/ScGGPPS, were described previously (Ro et al., 2005; Hamberger et al., 2011). These plasmids allow expression of the yeast GGPPS and Norway spruce LAS or ISO enzymes in yeast cells to produce DRA precursors as substrates for candidate P450s (Ro et al., 2005; Hamberger et al., 2011). Transformed yeast cells were grown to an optical density at 600 nm (OD 600) of 0.6 before transfer to buffered (0.25 mm HEPES, pH 7) minimal selection medium containing 2% Gal (w/v) to induce protein expression. After growth for 16 h, yeast cells were pelleted by centrifugation at 3,200g for 10 min. Cell pellets were disrupted using glass beads (425–600 μm; Sigma), and diterpenes were extracted twice with 5 mL of pentane:diethyl ether (2:1, v/v) with 2 mm dichlorodehydroabietic acid as an internal standard. Organic solvent extracts were pooled, and anhydrous sodium sulfate was added to remove residual water. The organic phase was transferred to a gas chromatography vial and concentrated to 0.2 mL under N2 gas. Extracts were stored at −20°C or immediately derivatized and analyzed by GC-MS. Prior to gas chromatography analysis, extracts were derivatized by adding 5 μL of N,O-bis(trimethylsilyl)trifluoroacetamide (with 10% trimethylsilyl; Sigma) to 40 μL of the extract. The reactions were kept at room temperature for at least 1 h before GC-MS analysis.
Microsome Preparation
BY4741:PcCPR cells transformed with plasmids containing candidate CYP720Bs were grown and microsomes were prepared as described previously (Pompon et al., 1996). In brief, 50 mL of selective dropout media was inoculated 1:10 with an overnight culture starting at an OD 600 of 0.2 and grown at 30°C and 170 rpm for 24 h. A volume of 200 mL of YPDE medium (1% yeast extract (w/v), 2% bacto-peptone (w/v), 5% ethanol (v/v), and 2% dextrose (w/v)) was inoculated with the 50 mL culture and incubated for another 24 h at 30°C and 170 rpm. Expression of CYP720Bs was induced by changing cultures to 200 mL of YPG medium (1% yeast extract (w/v), 2% bacto-peptone (w/v), and 2% Gal (w/v)). Cells were grown for another 12 to 16 h at 30°C and 170 rpm. Yeast cells were pelleted by centrifugation at 2,000g for 10 min, washed once with 5 mL of TEK (50 mm Tris-HCl, pH 7.5, 1 mm EDTA, and 100 mm KCl), and resuspended in TES2 buffer (50 mm Tris-HCl, pH 7.5, 1 mm EDTA, 600 mm sorbitol, 5 mm dithiothreitol, and 0.25 mm phenylmethylsulfonyl fluoride). All subsequent steps were performed at 4°C. Yeast cell were disrupted mechanically using acid-washed glass beads (425–600 μm; Sigma) and manual shaking for 3 × 30 s. The cell homogenate was centrifuged at 10,000g for 15 min followed by ultracentrifugation of the supernatant at 100,000g for 1 h. Microsomes were suspended and homogenized using a Potter-Elvehjem homogenizer in a buffer containing 50 mm Tris-HCl buffer, pH 7.5, 1 mm EDTA, and 30% (v/v) glycerol. Microsome preparations were used directly for enzyme assays or stored at −80°C. CO difference spectra of microsomes carrying recombinant CYP720Bs and PcCPR were measured according to Guengerich et al. (2009), and the concentrations of CYP720B proteins were calculated based on an extinction coefficient of 91,000 m−1 cm−1 (Supplemental Fig. S4).
In Vitro P450 Assays
Microsome preparations containing candidate CYP720Bs and PcCPR were assayed for activity with diterpene substrates as described previously by Hamberger et al. (2011). In brief, assays contained 50 mm potassium phosphate, pH 7.5, 20 μm substrate, and 50 μL of the microsome preparation in a total volume of 300 μL. Substrates were added, dissolved in acetonitrile (alcohols), ethyl acetate (aldehydes), or diethyl ether (olefins), depending on the substrate polarity. Enzyme reactions were initiated by adding 0.8 mm NADPH, incubated at 30°C for 1 h, and stopped by adding twice 500 μL of ethylacetate. Organic layers were combined and transferred to new gas chromatography vials, evaporated under N2 gas, and resuspended in 200 μL of acetonitrile. LC-MS analysis was used to measure the formation of DRAs.
Sequential in Vitro LAS/P450 Assays
The diterpenol 13-hydroxy-8(14)-abietene is not available as a stable substrate. Therefore, we developed a sequential in vitro assay system in which a purified PcLAS enzyme was used to produce this compound as a substrate for assays with CYP720B candidates. An N-terminal truncated version of lodgepole pine PcLAS was expressed in E. coli BL21DE3-C41 cells and purified by Ni2+ affinity chromatography as described previously (Keeling et al., 2008; Hall et al., 2013b). Purified PcLAS was stored in 25 mm HEPES (pH 7.4), 100 mm KCl, and 10% glycerol (v/v). Protein concentrations were determined using the Pierce BCA (bicinchoninic acid) protein assay kit (Thermo Scientific). For the sequential assay, single-vial PcLAS enzyme assays with 40 μm GGPP (Sigma) as substrate were completed in 50 mm HEPES (pH 7.2), 10 μm MgCl2, 5% glycerol (v/v), and 5 mm dithiothreitol using 50 μg of purified PcLAS in a total volume of 500 μL. After 1 h at 30°C, 50 μL of the assay was removed to test for the formation of 13-hydroxy-8(14)-abietene. The second step of the sequential assay was initiated by adding 45 μL of microsome preparations, expressing PcCPR and candidate CYP720Bs, and 5 μL (10 mm) of NADPH to the remaining assay volume. The assay was incubated for another 30 min at 30°C before stopping the reaction by adding 500 μL of pentane:diethyl ether (2:1, v/v) with 2 mm dichlorodehydroabietic acid as an internal standard and vortexing. The organic phase was transferred to a new gas chromatography vial. Derivatization of DRAs was performed as described above.
GC-MS and LC-MS Analyses
Derivatized extracts from in vivo and in vitro assays were analyzed on an Agilent 6890A Series gas chromatography system with an Agilent 7683 autosampler coupled to an Agilent 5975 Inert XL mass spectrometric detector operating in electron ionization mode at 70 eV (Agilent Technologies). An Agilent HP-5ms (5% phenylmethyl siloxane, 30 m, 250-μm i.d., 0.25-μm film) column was used to separate the compounds at a flow rate of 1 mL min−1 helium. The injector was operated in pulsed splitless mode, and 0.5 to 2 μL of the derivatized extracts was injected. The temperatures of the mass spectrometry transfer line, source, and quadrupole were 230°C, 250°C, and 150°C, respectively. The oven program for the HP5 column was as follows: 40°C for 1 min; ramp of 40°C min−1 to 300°C; and 300°C for 3 min. The same temperature program was used when the HP-5 column was used on an Agilent gas chromatography system in chemical ionization mode with CH4 as reagent gas.
Underivatized extracts from in vitro assays were analyzed by LC-MS on an LC-MSD-Trap-XCD Plus 1100 Series device (Agilent) on a Zorbax SBC18 rapid-resolution HT column (4.6 mm i.d. × 50 mm, 1.8-μm pore size; Agilent) in positive electrospray ionization mode (dry temperature, 350°C). Samples of 10 μL were injected with a column flow of 1 mL min−1 in isocratic mode (acetonitrile:water, 85%:15% + 0.2% formic acid, (v/v)) with the diode array detector scanning from 190 to 400 nm for a run time of 10 min.
To test for 13-hydroxy-8(14)-abietene and its oxidized derivatives, LC-MS analyses were conducted on an Agilent 1100 LC-MSD-Trap-XCT Plus mass spectrometer with an APCI interface. Chromatography was completed on a Zorbax Rx-SIL silica column (4.6 mm i.d. × 150 mm, 5-μm pore size; Agilent) with isocratic elution of pentane:ether (80:20, v/v) at 30°C at 1.4 mL min−1. Postcolumn, 0.1 mL min−1 0.2% formic acid in pentane:ether (80:20, all v/v) was added via a T-fitting by syringe pump to assist ionization. APCI-mass spectrometry conditions were as follows: APCI temperature, 350°C; dry temperature, 325°C; nebulizer, 60 p.s.i.; dry gas flow, 7 L min−1; high-voltage capillary, 3 kV; and positive mode, 40 to 350 atomic mass unit scan range.
Sequence data from this article can be found in the National Center for Biotechnology Information GenBank under the following accession numbers: PbCYP720B1v1 (KJ845665), PbCYP720B1v2 (KJ845666), PbCYP720B2 (KJ845667), PbCYP720B10 (KJ845668), PbCYP720PB11 (KJ845669), PbCYP720B12 (KJ845670), PcCYP720B1 (KJ845671), PcCYP720B2 (KJ845672), PcCYP720B10v1 (KJ845673), PcCYP720B10v2 (KJ845674), PcCYP720B11 (KJ845675), PcCYP720B12 (KJ845676), and PcCPR (KJ914574).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Diterpene structures.
Supplemental Figure S2. Formation of diterpene olefins in yeast in vivo assays.
Supplemental Figure S3. Mass spectra of diterpenoids.
Supplemental Figure S4. Reduced CO difference P450 spectra of isolated microsomes containing CYP720 proteins.
Supplemental Figure S5. Chemical ionization.
Supplemental Table S1. Amino acid sequence identity of clade I and III CYP720Bs from Sitka spruce, white spruce, jack pine, and lodgepole pine.
Supplemental Table S2. Primers for the amplification of FL cDNAs
Supplementary Material
Acknowledgments
We thank Dr. David Nelson (University of Tennessee, Memphis) for naming of P450 genes, Dr. Philipp Zerbe (University of California, Davis) and Dr. Bjoern Hamberger (Michigan State University) for critically reading the article prior to submission, and Karen Reid (University of British Columbia) for outstanding laboratory management.
Glossary
- DRA
diterpene resin acid
- diTPS
diterpene synthase
- P450
cytochrome P450 monooxygenase
- GGPP
geranylgeranyl diphosphate
- ISO
isopimaradiene synthase
- LAS
levopimaradiene/abietadiene synthase
- CPP
(+)-copalyl diphosphate
- cDNA
complementary DNA
- FL
full-length
- GGPPS
geranylgeranyl diphosphate synthase
- CPR
cytochrome P450 reductase
- LC-MS
liquid chromatography-mass spectrometry
- APCI
atmospheric pressure chemical ionization
- m/z
mass-to-charge ratio
- GC-MS
gas chromatography-mass spectrometry
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council of Canada Discovery Grant Program, Genome Canada, and Genome British Columbia to J.B. in support of the LSARP SMarTForests Project, the Michael Smith Laboratories, and the University of British Columbia Distinguished Scholar Program.
Articles can be viewed without a subscription.
References
- Birol I, Raymond A, Jackman SD, Pleasance S, Coope R, Taylor GA, Yuen MM, Keeling CI, Brand D, Vandervalk BP, et al. (2013) Assembling the 20 Gb white spruce (Picea glauca) genome from whole-genome shotgun sequencing data. Bioinformatics 29: 1492–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann J. (2012) Pine terpenoid defences in the mountain pine beetle epidemic and in other conifer pest interactions: specialized enemies are eating holes into a diverse, dynamic and durable defence system. Tree Physiol 32: 943–945 [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Keeling CI (2008) Terpenoid biomaterials. Plant J 54: 656–669 [DOI] [PubMed] [Google Scholar]
- Boone CK, Aukema BH, Bohlmann J, Carroll AL, Raffa KF (2011) Efficacy of tree defense physiology varies with bark beetle population density: a basis for positive feedback in eruptive species. Can J For Res 41: 1174–1188 [Google Scholar]
- De La Torre AR, Birol I, Bousquet J, Ingvarsson PK, Jansson S, Jones SJN, Keeling CI, MacKay J, Nilsson O, Ritland K, et al. (2014) Insights into conifer giga-genomes. Plant Physiol 166: 1724–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH (2007) Frozen competent yeast cells that can be transformed with high efficiency using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2: 1–4 [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Martin MV, Sohl CD, Cheng Q (2009) Measurement of cytochrome P450 and NADPH-cytochrome P450 reductase. Nat Protoc 4: 1245–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DE, Yuen MMS, Jancsik S, Quesada AL, Dullat HK, Li M, Henderson H, Arango-Velez A, Liao NY, Docking RT, et al. (2013a) Transcriptome resources and functional characterization of monoterpene synthases for two host species of the mountain pine beetle, lodgepole pine (Pinus contorta) and jack pine (Pinus banksiana). BMC Plant Biol 13: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DE, Zerbe P, Jancsik S, Quesada AL, Dullat H, Madilao LL, Yuen M, Bohlmann J (2013b) Evolution of conifer diterpene synthases: diterpene resin acid biosynthesis in lodgepole pine and jack pine involves monofunctional and bifunctional diterpene synthases. Plant Physiol 161: 600–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Møller BL (2007) Improved cloning and expression of cytochrome P450s and cytochrome P450 reductase in yeast. Protein Expr Purif 56: 121–127 [DOI] [PubMed] [Google Scholar]
- Hamberger B, Ohnishi T, Hamberger B, Séguin A, Bohlmann J (2011) Evolution of diterpene metabolism: Sitka spruce CYP720B4 catalyzes multiple oxidations in resin acid biosynthesis of conifer defense against insects. Plant Physiol 157: 1677–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson AD, Henry CS, Fiehn O, de Crecy-Lagard V (2016) Metabolic damage and metabolite damage control in plants. Annu Rev Plant Biol 67: (in press) 10.1146/annurev-arplant-043015-111648 [DOI] [PubMed] [Google Scholar]
- Hillwig ML, Mann FM, Peters RJ (2011) Diterpenoid biopolymers: new directions for renewable materials engineering. Biopolymers 95: 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennewein S, Croteau R (2001) Taxol: biosynthesis, molecular genetics, and biotechnological applications. Appl Microbiol Biotechnol 57: 13–19 [DOI] [PubMed] [Google Scholar]
- Jensen NB, Zagrobelny M, Hjernø K, Olsen CE, Houghton-Larsen J, Borch J, Møller BL, Bak S (2011) Convergent evolution in biosynthesis of cyanogenic defence compounds in plants and insects. Nat Commun 2: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefover-Ring K, Trowbridge A, Mason CJ, Raffa KF (2016) Rapid induction of multiple terpenoid groups by Ponderosa pine in response to bark beetle-associated fungi. J Chem Ecol 42: 1–12 [DOI] [PubMed] [Google Scholar]
- Keeling CI, Bohlmann J (2006a) Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol 170: 657–675 [DOI] [PubMed] [Google Scholar]
- Keeling CI, Bohlmann J (2006b) Diterpene resin acids in conifers. Phytochemistry 67: 2415–2423 [DOI] [PubMed] [Google Scholar]
- Keeling CI, Dullat HK, Yuen M, Ralph SG, Jancsik S, Bohlmann J (2010) Identification and functional characterization of monofunctional ent-copalyl diphosphate and ent-kaurene synthases in white spruce reveal different patterns for diterpene synthase evolution for primary and secondary metabolism in gymnosperms. Plant Physiol 152: 1197–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling CI, Madilao LL, Zerbe P, Dullat HK, Bohlmann J (2011) The primary diterpene synthase products of Picea abies levopimaradiene/abietadiene synthase (PaLAS) are epimers of a thermally unstable diterpenol. J Biol Chem 286: 21145–21153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling CI, Weisshaar S, Lin RPC, Bohlmann J (2008) Functional plasticity of paralogous diterpene synthases involved in conifer defense. Proc Natl Acad Sci USA 105: 1085–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova N, Miller B, Ralph S, Ellis BE, Douglas C, Ritland K, Bohlmann J (2004) Isolation of high-quality RNA from gymnosperm and angiosperm trees. Biotechniques 36: 821–824 [DOI] [PubMed] [Google Scholar]
- Langenheim JH. (2003) Plant Resins: Chemistry, Evolution, Ecology and Ethnobotany. Timber Press, Portland, OR [Google Scholar]
- Martin D, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129: 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Fäldt J, Bohlmann J (2004) Functional characterization of nine Norway spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant Physiol 135: 1908–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Madilao LL, Ralph S, Bohlmann J (2005) Insect-induced conifer defense: white pine weevil and methyl jasmonate induce traumatic resinosis, de novo formed volatile emissions, and accumulation of terpenoid synthase and putative octadecanoid pathway transcripts in Sitka spruce. Plant Physiol 137: 369–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale DB, Wegrzyn JL, Stevens KA, Zimin AV, Puiu D, Crepeau MW, Cardeno C, Koriabine M, Holtz-Morris AE, Liechty JD, et al. (2014) Decoding the massive genome of loblolly pine using haploid DNA and novel assembly strategies. Genome Biol 15: R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystedt B, Street NR, Wetterbom A, Zuccolo A, Lin YC, Scofield DG, Vezzi F, Delhomme N, Giacomello S, Alexeyenko A, et al. (2013) The Norway spruce genome sequence and conifer genome evolution. Nature 497: 579–584 [DOI] [PubMed] [Google Scholar]
- Page RD. (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12: 357–358 [DOI] [PubMed] [Google Scholar]
- Peters RJ. (2010) Two rings in them all: the labdane-related diterpenoids. Nat Prod Rep 27: 1521–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RJ, Ravn MM, Coates RM, Croteau RB (2001) Bifunctional abietadiene synthase: free diffusive transfer of the (+)-copalyl diphosphate intermediate between two distinct active sites. J Am Chem Soc 123: 8974–8978 [DOI] [PubMed] [Google Scholar]
- Phillips MA, Croteau RB (1999) Resin-based defenses in conifers. Trends Plant Sci 4: 184–190 [DOI] [PubMed] [Google Scholar]
- Pompon D, Louerat B, Bronine A, Urban P (1996) Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol 272: 51–64 [DOI] [PubMed] [Google Scholar]
- Raffa KF. (2014) Terpenes tell different tales at different scales: glimpses into the chemical ecology of conifer-bark beetle-microbial interactions. J Chem Ecol 40: 1–20 [DOI] [PubMed] [Google Scholar]
- Ralph SG, Chun HJE, Kolosova N, Cooper D, Oddy C, Ritland CE, Kirkpatrick R, Moore R, Barber S, Holt RA, et al. (2008) A conifer genomics resource of 200,000 spruce (Picea spp.) ESTs and 6,464 high-quality, sequence-finished full-length cDNAs for Sitka spruce (Picea sitchensis). BMC Genomics 9: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro DK, Arimura G, Lau SYW, Piers E, Bohlmann J (2005) Loblolly pine abietadienol/abietadienal oxidase PtAO (CYP720B1) is a multifunctional, multisubstrate cytochrome P450 monooxygenase. Proc Natl Acad Sci USA 102: 8060–8065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro DK, Bohlmann J (2006) Diterpene resin acid biosynthesis in loblolly pine (Pinus taeda): functional characterization of abietadiene/levopimaradiene synthase (PtTPS-LAS) cDNA and subcellular targeting of PtTPS-LAS and abietadienol/abietadienal oxidase (PtAO, CYP720B1). Phytochemistry 67: 1572–1578 [DOI] [PubMed] [Google Scholar]
- Savard L, Li P, Strauss SH, Chase MW, Michaud M, Bousquet J (1994) Chloroplast and nuclear gene sequences indicate late Pennsylvanian time for the last common ancestor of extant seed plants. Proc Natl Acad Sci USA 91: 5163–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Ran JH (2014) Evolution and biogeography of gymnosperms. Mol Phylogenet Evol 75: 24–40 [DOI] [PubMed] [Google Scholar]
- Warren RL, Keeling CI, Yuen MMS, Raymond A, Taylor GA, Vandervalk BP, Mohamadi H, Paulino D, Chiu R, Jackman SD, et al. (2015) Improved white spruce (Picea glauca) genome assemblies and annotation of large gene families of conifer terpenoid and phenolic defense metabolism. Plant J 83: 189–212 [DOI] [PubMed] [Google Scholar]
- Wegrzyn JL, Liechty JD, Stevens KA, Wu LS, Loopstra CA, Vasquez-Gross HA, Dougherty WM, Lin BY, Zieve JJ, Martínez-García PJ, et al. (2014) Unique features of the loblolly pine (Pinus taeda L.) megagenome revealed through sequence annotation. Genetics 196: 891–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang NY, Liu L, Tao WW, Duan JA, Tian LJ (2010) Diterpenoids from Pinus massoniana resin and their cytotoxicity against A431 and A549 cells. Phytochemistry 71: 1528–1533 [DOI] [PubMed] [Google Scholar]
- Zerbe P, Bohlmann J (2015a) Enzymes for synthetic biology of ambroxide-related diterpenoid fragrance compounds. Adv Biochem Eng Biotechnol 148: 427–447 [DOI] [PubMed] [Google Scholar]
- Zerbe P, Bohlmann J (2015b) Plant diterpene synthases: exploring modularity and metabolic diversity for bioengineering. Trends Biotechnol 33: 419–428 [DOI] [PubMed] [Google Scholar]
- Zerbe P, Chiang A, Yuen M, Hamberger B, Hamberger B, Draper JA, Britton R, Bohlmann J (2012) Bifunctional cis-abienol synthase from Abies balsamea discovered by transcriptome sequencing and its implications for diterpenoid fragrance production. J Biol Chem 287: 12121–12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Ma YG, Sun CL, Qiu YH, Lu C (2005) First total synthesis of (±)-13-hydroxy-8(14)-abietene. Chin Chem Lett 16: 727–728 [Google Scholar]
- Zhou K, Gao Y, Hoy JA, Mann FM, Honzatko RB, Peters RJ (2012) Insights into diterpene cyclization from structure of bifunctional abietadiene synthase from Abies grandis. J Biol Chem 287: 6840–6850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulak KG, Bohlmann J (2010) Terpenoid biosynthesis and specialized vascular cells of conifer defense. J Integr Plant Biol 52: 86–97 [DOI] [PubMed] [Google Scholar]
- Zulak KG, Lippert DN, Kuzyk MA, Domanski D, Chou T, Borchers CH, Bohlmann J (2009) Targeted proteomics using selected reaction monitoring reveals the induction of specific terpene synthases in a multi-level study of methyl jasmonate-treated Norway spruce (Picea abies). Plant J 60: 1015–1030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.