Jackson et al. propose a role for B cell–intrinsic IFN-γ receptor signaling in spontaneous germinal center activation and autoantibody production.
Abstract
Dysregulated germinal center (GC) responses are implicated in the pathogenesis of human autoimmune diseases, including systemic lupus erythematosus (SLE). Although both type 1 and type 2 interferons (IFNs) are involved in lupus pathogenesis, their respective impacts on the establishment of autoimmune GCs has not been addressed. In this study, using a chimeric model of B cell-driven autoimmunity, we demonstrate that B cell type 1 IFN receptor signals accelerate, but are not required for, lupus development. In contrast, B cells functioning as antigen-presenting cells initiate CD4+ T cell activation and IFN-γ production, and strikingly, B cell–intrinsic deletion of the IFN-γ receptor (IFN-γR) abrogates autoimmune GCs, class-switched autoantibodies (auto-Abs), and systemic autoimmunity. Mechanistically, although IFN-γR signals increase B cell T-bet expression, B cell–intrinsic deletion of T-bet exerts an isolated impact on class-switch recombination to pathogenic auto-Ab subclasses without impacting GC development. Rather, in both mouse and human B cells, IFN-γ synergized with B cell receptor, toll-like receptor, and/or CD40 activation signals to promote cell-intrinsic expression of the GC master transcription factor, B cell lymphoma 6 protein. Our combined findings identify a novel B cell–intrinsic mechanism whereby IFN signals promote lupus pathogenesis, implicating this pathway as a potential therapeutic target in SLE.
Systemic lupus erythematosus (SLE) is a severe autoimmune disease characterized by class-switched autoantibodies (auto-Abs) targeting nuclear antigens. Despite an improved understanding of lupus pathogenesis, efficacious nontoxic therapies for this chronic disease are lacking. Although B cells have long been recognized as critical for lupus pathogenesis via production of pathogenic antinuclear Abs (ANAs), recent evidence has implicated dysregulated B cell signaling in the initiation of systemic autoimmunity (Shlomchik, 2009; Jackson et al., 2015). Thus, greater understanding of the specific B cell–intrinsic signals promoting breaks in germinal center (GC) B cell tolerance may inform the development of novel, targeted lupus therapies.
Although the site of initial activation of autoreactive B cells remains incompletely defined, several lines of evidence point to spontaneous autoimmune GCs as the likely source of auto-Ab–producing B cells. First, ANAs from lupus patients exhibit evidence of activation-induced cytidine deaminase (AID)–mediated somatic hypermutation (SHM) and class-switch recombination (CSR; Wellmann et al., 2005). Second, in mouse lupus models, a loss of auto-Abs after B cell–intrinsic MyD88 or TLR7 deletion is accompanied by a lack of spontaneous GCs (Becker-Herman et al., 2011; Teichmann et al., 2013; Hua et al., 2014; Jackson et al., 2014). Finally, ectopic GCs are frequently observed within inflamed target tissues, including kidneys from lupus nephritis patients (Aloisi and Pujol-Borrell, 2006; Vinuesa et al., 2009).
In this context, the Wiskott-Aldrich syndrome (WAS) chimera model of B cell–driven autoimmunity has provided important insights into the dysregulated B cell–intrinsic signals required for the generation of spontaneous autoimmune GCs (Becker-Herman et al., 2011; Jackson et al., 2014). In this model, B cells, but not other immune lineages, are deficient in the signaling adapter WAS protein. In the absence of WAS protein, B cells are modestly hyperresponsive to both B cell receptor (BCR) and TLR signals, resulting in spontaneous B cell–driven humoral autoimmunity characterized by spontaneous GCs, class-switched Abs, and immune complex glomerulonephritis. We recently used this model to show that B cell, and not myeloid, signals explain the opposing pathogenic and protective effects of TLR7 and TLR9 in systemic autoimmunity (Jackson et al., 2014), a finding that both confirmed the critical importance of dysregulated B cell signals in SLE and demonstrated the utility of this model in delineating B cell–intrinsic mechanisms in autoimmune pathogenesis.
IFNs are a family of inflammatory cytokines with important functions during pathogen infections. Both type 1 (IFN-α, -β, -ε, and -ω) and type 2 (IFN-γ) IFNs have been implicated in autoimmune pathogenesis in both human and animal studies (Baechler et al., 2003; Bennett et al., 2003; Kirou et al., 2005; Pollard et al., 2013). Although dysregulated type 1 IFN signals are clearly associated with SLE in humans, the relative importance of type 1 versus type 2 IFNs in driving B cell activation during spontaneous humoral autoimmunity has not been addressed. In this study, we dissect the B cell–intrinsic impacts of type 1 IFN and IFN-γ in lupus pathogenesis. Surprisingly, despite prominent effects of type 1 IFN on B cell activation in vitro, a lack of B cell type 1 IFN receptor (IFNAR) signals exerted minimal impacts on humoral autoimmunity in WAS chimeras. In contrast, WAS chimera autoimmunity was characterized by a marked expansion of IFN-γ–producing CD4+ T cells that was dependent on B cell antigen presentation in the context of MHC class II (MHCII). Strikingly, B cell–intrinsic deletion of the IFN-γ receptor (IFN-γR) abolished spontaneous autoimmune GCs and class-switched auto-Ab production. Although IFN-γ–mediated, B cell–intrinsic up-regulation of the T-box transcription factor T-bet was required for CSR to pathogenic Ig isotypes, T-bet deletion had no impact on spontaneous GC development. Instead, using in vitro studies with both mouse and human B cells, we demonstrate that IFN-γR signaling, in combination with integrated BCR, TLR, and/or CD40 signals, mediates high-level B cell lymphoma 6 protein (BCL-6) expression, thereby orchestrating a cell-intrinsic program required for B cell autoimmune GC formation.
RESULTS
B cell–intrinsic type 1 IFN receptor signals enhance, but are not required for, SLE development
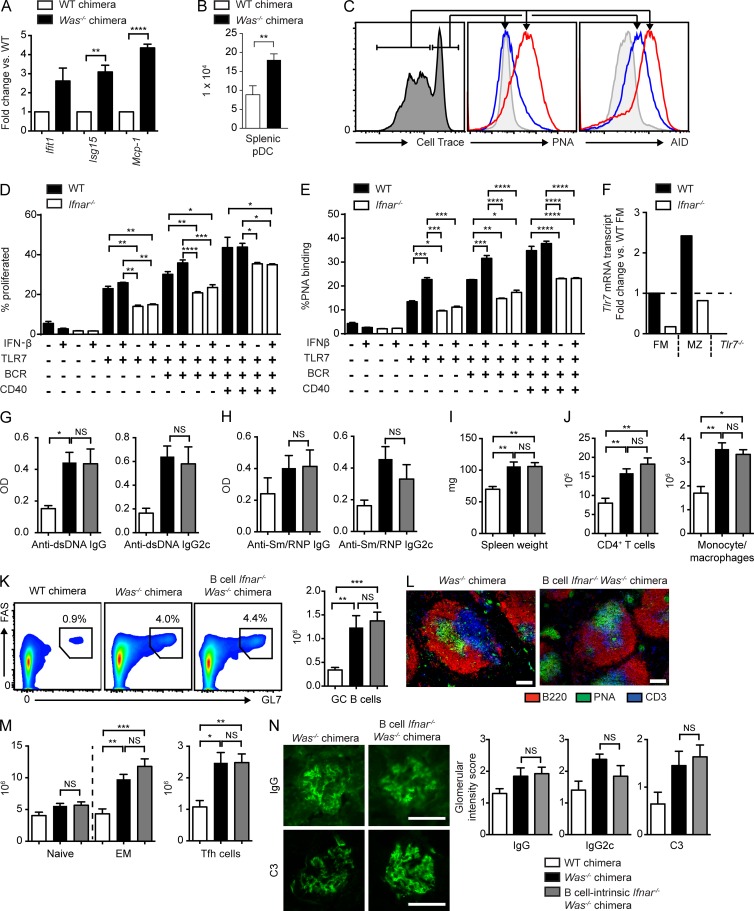
A prominent subset of lupus patients exhibits an IFN gene signature implicating type 1 IFN in lupus pathogenesis (Baechler et al., 2003; Bennett et al., 2003; Kirou et al., 2005). For this reason, we sought to determine whether diseased WAS chimera animals (Becker-Herman et al., 2011; Jackson et al., 2014) might manifest a similar transcriptional signature. Relative to WT chimera controls, splenocytes from WAS chimeras exhibited higher mRNA expression of several IFN-stimulated genes (Fig. 1 A). In addition, WAS chimeras developed a marked expansion of splenic plasmacytoid DCs, the major cellular source for type 1 IFN (Fig. 1 B). To model whether type 1 IFN likely exerts B cell–intrinsic impacts on spontaneous GCs in vivo, we stimulated B cells in vitro with anti-IgM, the TLR7 agonist R848, and anti-CD40 with or without exogenous IFN-β. Combined BCR-, TLR7-, and CD40-mediated signals synergized to promote B cell proliferation, and the subset of proliferating B cells bound peanut agglutinin (PNA) and expressed intranuclear AID, consistent with the adoption of an in vitro GC phenotype (Fig. 1 C). Exogenous IFN-β enhanced B cell proliferation and PNA binding (Fig. 1, D and E). In addition, type 1 IFN receptor–deficient (Ifnar−/−) B cells showed decreased activation even in the absence of exogenous IFN-β, which is likely explained by lower TLR7 expression in Ifnar−/− B cells (Fig. 1 F; Green et al., 2009).
Figure 1.
B cell–intrinsic IFNAR signals are not required for the development of humoral autoimmunity. (A) IFN-stimulated gene mRNA transcripts (fold change vs. WT chimera) in splenocytes from two or more independent chimeras. (B) Total splenic plasmacytoid DCs (pDCs) in WT (n = 5) and Was−/− (n = 9) mice from three independent chimeras. (C) Representative histograms. (Left) Gating of proliferated versus unproliferated subsets (assessed by Cell Trace dilution) in WT B cells stimulated with anti-IgM, R848, and IFN-β. (Middle) Relative surface PNA binding. (Right) AID expression in proliferated (red) versus unproliferated (blue) B cells. The gray histogram indicates unstimulated B cells. (D and E) Proliferation (D) and PNA binding (E) in splenic WT and Ifnar−/− B cells stimulated with the indicated combinations of anti-IgM, R848, anti-CD40, and IFN-β. (F) Tlr7 mRNA transcripts in sorted WT versus Ifnar−/− follicular mature (FM) and marginal zone (MZ) B cells (fold change vs. WT FM; Tlr7−/− is shown as a negative control). (G and H) Anti-dsDNA (G) and anti-Sm/RNP (H) IgG and IgG2c auto-Ab in WT (white), Was−/− (black), and B cell Ifnar−/− Was−/− (gray) chimeras at 12 (G) and 24 (H) wk after transplantation. (I) Spleen weights. (J) Splenic CD4+ T cells and CD11b+GR1lo monocytes/macrophages. (K) Representative FACS plots (left) and total number (right) of splenic PNA+FAS+ GC B cells. (L) Representative splenic sections stained with B220, PNA, and CD3. (M) Number of splenic naive (CD44LOCD62LHI) and EM (CD44HICD62LLO/HI) CD4+ T cells and PD1+CXCR5+ Tfh cells. (N, left) Representative glomerular IF staining. (Right) Intensity of glomerular IgG, IgG2c, and C3 staining scored from 0 to 3 by two independent blinded observers. (A, B, and G–N) Data are representative of four independent WT (n = 7), Was−/− (n = 18), and B cell Ifnar−/− Was−/− (n = 19) chimeras sacrificed at 24 wk after transplantation. (C–F) Data are representative of two or more independent experiments. Error bars indicate SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, by two-tailed Student’s t test (A and B), one-way ANOVA followed by Tukey’s multiple comparison test (D and E), or by the Kruskal-Wallis one-way ANOVA (G–N). Bars: (L) 100 µm; (N) 50 µm.
Together, these data suggested that B cell–intrinsic IFNAR signals likely promote humoral autoimmunity. To directly test this idea, we generated WAS B cell chimeras in which Ifnar deletion was limited to the B cell compartment by reconstituting lethally irradiated B cell–deficient (µMT) recipients with 20% WT, Was−/−, or Was−/−.Ifnar−/− BM together with 80% µMT BM. Surprisingly, B cell Ifnar deletion did not alter anti–double-stranded DNA (dsDNA) or Sm/RNP IgG auto-Abs (Fig. 1, G and H), nor titers of IgG2c subclass auto-Abs previously demonstrated to be critical for autoimmunity in this model (Jackson et al., 2014). This lack of impact on Sm/RNP Abs was particularly surprising because type 1 IFN promotes TLR7-dependent B cell activation (Fig. 1, D and E; Green et al., 2009) and B cell–intrinsic TLR7 signals are required for the generation of RNA-associated auto-Abs (Jackson et al., 2014). Consistent with auto-Ab titers, B cell IFNAR signals also exerted no impact on splenomegaly or CD4+ T cell/myeloid expansion (Fig. 1, I and J). Despite prominent in vitro effects of type 1 IFN, B cell–intrinsic Ifnar-deficient WAS chimeras developed robust splenic GCs, as reflected by FACS analysis and immunofluorescence (IF) staining (Fig. 1, K and L). Consistent with a lack of type 1 IFN impact on B cell activation, CD4+ effector/memory (EM) populations and follicular T helper cells (Tfh cells) were equally expanded with or without B cell Ifnar expression (Fig. 1 M). Finally, B cell–intrinsic ifnar deletion did not prevent immune complex glomerulonephritis (Fig. 1 N).
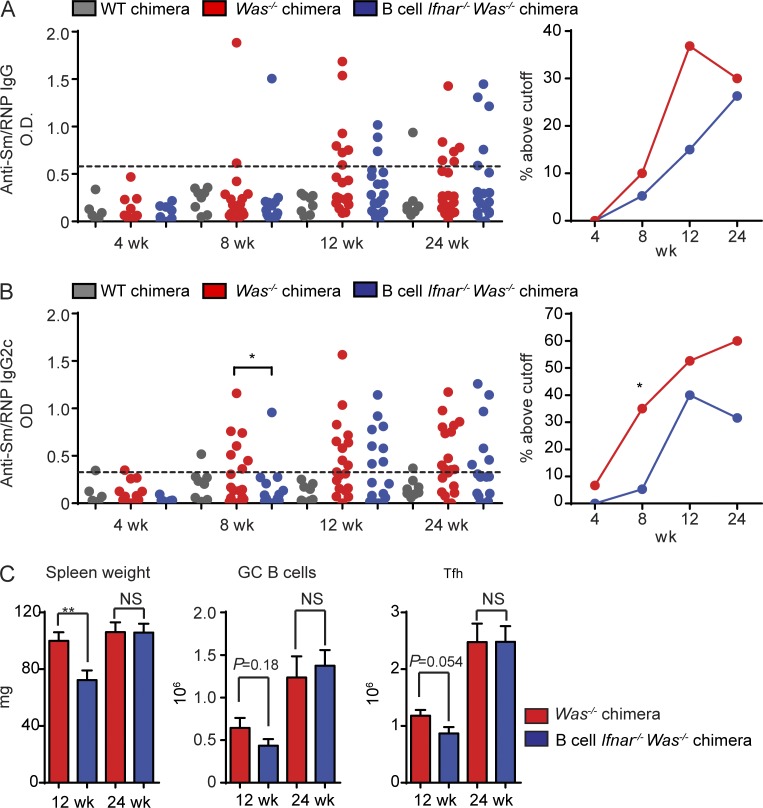
Lack of any impact of B cell IFNAR signals on the development of autoimmunity was surprising given human data implicating type 1 IFN gene signature in SLE pathogenesis (Baechler et al., 2003; Bennett et al., 2003; Kirou et al., 2005), the amelioration of autoimmunity by global Ifnar deletion in other mouse lupus models (Kiefer et al., 2012), and the prominent impacts of type 1 IFN on B cell activation in vitro. For this reason, we hypothesized that B cell–intrinsic IFNAR signals may accelerate the kinetics of auto-Ab development without being required for their generation. To test this hypothesis, we established independent cohorts of Was−/− and Was−/−.Ifnar−/− B cell chimeras and measured anti-Sm/RNP auto-Ab titers at 4, 8, 12, and 24 wk after transplantation. Because auto-Ab development in the WAS model is a stochastic process with an increasing proportion of chimeric mice developing high titer auto-Abs over time, we defined Sm/RNP positivity as an ELISA optical density greater than a predefined cutoff. Notably, B cell–intrinsic IFNAR signals accelerated the kinetics of Sm/RNP auto-Ab development, a finding that was reproducible in four independent Was−/− or Was−/−.Ifnar−/− cohorts (Fig. 2, A and B). However, the impact of B cell IFNAR signals on the rate of RNA-associated auto-Ab development was modest, with a significant increase in Sm/RNP IgG2c+ only at 8 wk after transplantation. We also sacrificed and evaluated separate cohorts of Was−/− and Was−/−.Ifnar−/− B cell chimeras at an earlier time point after transplantation (i.e., 12 instead of 24 wk). Similar to the impact on early auto-Ab titers, a lack of B cell IFNAR signaling delayed the development of splenomegaly, with a trend toward decreased Tfh cells and GC B cells at this early time point (Fig. 2 C).
Figure 2.
B cell–intrinsic IFNAR signals accelerate autoimmunity. (A and B) Serum anti-Sm/RNP IgG (A) and IgG2c (B) auto-Ab titers in WT (n ≥ 6), Was−/− (n ≥ 15) and B cell Ifnar−/− Was−/− (n ≥ 9) chimeras at the indicated times after transplantation. (Left) Auto-Ab optical density (OD). (Right) Percentage of mice with reactivity above cut-off (defined as more than three SDs above the mean optical density of WT C57/BL6 serum). (C) Spleen weight and total numbers of PNA+FAS+ GC B cells and PD1+CXCR5+ Tfh cells in Was−/− (n ≥ 10) and B cell Ifnar−/− Was−/− (n ≥ 9) chimeras sacrificed at 12 and 24 wk after transplantation. Error bars indicate SEM. *, P < 0.05; **, P < 0.01, by two-tailed Fischer’s exact test (A and B) or the Mann-Whitney U-test (C). Data are representative of four independent WT, Was−/−, and B cell Ifnar−/− Was−/− chimeras.
In summary, B cell–intrinsic deletion of the type 1 IFN receptor modestly decreased the rate of RNA-associated auto-Ab development, a finding that correlates with a slight delay in the development of splenic immune activation. However, despite clinical evidence implicating type 1 IFN in the pathogenesis of humoral autoimmunity, B cell–driven autoimmunity can develop in the absence of B cell IFNAR signals.
B cell antigen presentation promotes T cell activation in mouse lupus
Humoral autoimmunity in the absence of B cell–intrinsic IFNAR signals suggested that alternate cytokines might promote B cell activation in this model. Because CD4+ T cells are required for autoimmunity in the WAS chimera model (Becker-Herman et al., 2011), we hypothesized that CD4-derived cytokines might promote spontaneous autoimmune GCs. Although mouse lupus models driven by dysregulated B cell signals are frequently characterized by the expansion of activated CD4+ T cells (Becker-Herman et al., 2011; Dai et al., 2013; Lamagna et al., 2014), the mechanisms whereby B cells drive T cell activation during humoral autoimmunity have not been addressed. In addition to Ab formation, B cells present antigens to CD4+ T cells in the context of MHCII, implicating B cell APC function in directly initiating breaks in T cell tolerance (Shlomchik, 2009; Jackson et al., 2015).
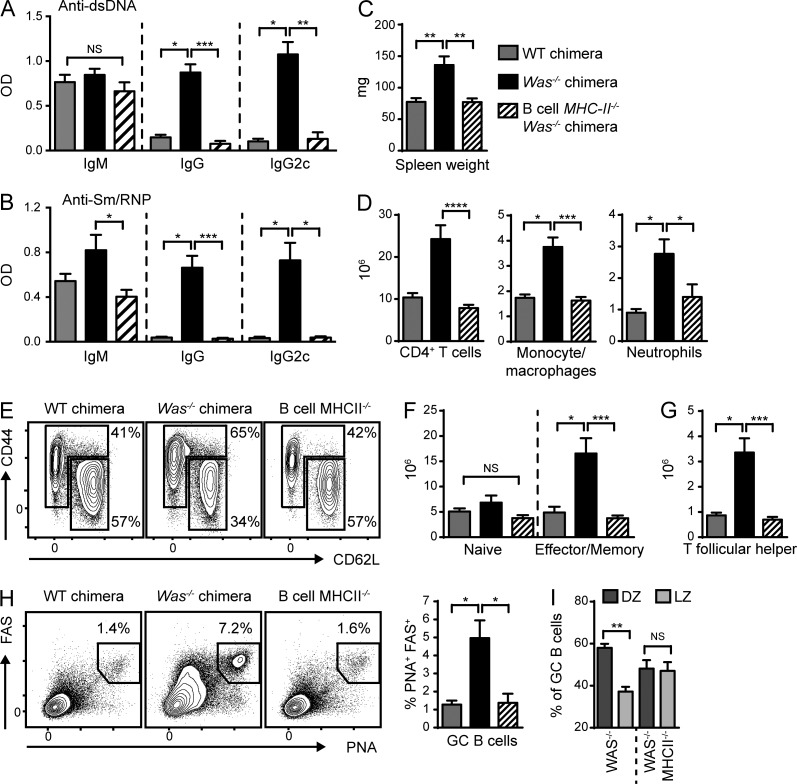
To test this idea, we generated WAS chimeras wherein all B cells lacked MHCII, whereas other myeloid APCs, including DCs, express MHCII. Strikingly, whereas IgM auto-Abs were only modestly affected, IgG and IgG2c subclass anti-dsDNA and Sm/RNP auto-Abs were abolished by B cell–intrinsic MCHII deletion (Fig. 3, A and B). Notably, this observation is consistent with a recent description of decreased serum auto-Abs in CD19cre MhcIlfl/fl mice on the lupus-prone MRL.Faslpr background (Giles et al., 2015). Although this previous study reported differing dependence of specific auto-Ab specificities on B cell MHCII expression, we observed a loss of both DNA- and RNA-associated IgG auto-Abs, a discrepancy possibly explained by incomplete Cre-mediated deletion of MHCII on B cells in the MRL.Faslpr model (Giles et al., 2015).
Figure 3.
B cell antigen presentation initiates systemic autoimmunity. (A and B) Anti-dsDNA (A) and anti-Sm/RNP (B) IgM, IgG, and IgG2c auto-Abs at 12 (A) and 24 (B) wk after transplantation. (C and D) Spleen weight (C) and the number of splenic CD4+ T cells, CD11b+GR1lo monocytes/macrophages, and CD11b+GR1+ neutrophils (D). (E) Representative FACS plots (gated on CD4+ T cells) showing naive (CD44LOCD62LHI) and EM (CD44HICD62LLO/HI) CD4+ T cells. Numbers indicate the percentages within the CD44LOCD62LHI and CD44HICD62LLO/HI gates. (F and G) Number of naive and EM CD4+ T cells (F) and PD1+CXCR5+ Tfh cells (G). (H) Representative FACS plots (gated on B220+) and percentage of splenic PNA+FAS+ GC B cells. (I) Proportion of PNA+FAS+ GC B cells within the DZ (CXCR4hiCD86lo) and light zone (LZ; CXCR4loCD86hi). Data are representative of two independent WT (n = 6), Was−/− (n = 8), and B cell MHCII−/−.Was−/− (n = 8) chimeras. Error bars indicate SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, by the Kruskal-Wallis one-way ANOVA.
In parallel with the loss of class-switched auto-Abs, the deletion of B cell MHCII was also sufficient to prevent splenomegaly and myeloid hyperplasia without affecting total B cell numbers (Fig. 3, C and D; and not depicted). In keeping with a role for B cell APC function in initiating T cell activation, CD4+ T cell expansion was markedly decreased in B cell MhcII−/− chimeras, a finding accounted for by the loss of activated EM T cells (Fig. 3, D–F). In addition, the generation of Tfh cells necessary for GC formation also required B cell MHCII (Fig. 3 G). Within the GCs, B cells proliferate and undergo SHM in the dark zone (DZ) and present captured antigens to cognate Tfh cells via MHCII in the light zone (Victora and Nussenzweig, 2012). In keeping with the importance of MHCII in this process, spontaneous GCs were markedly decreased in the absence of B cell MHCII expression (Fig. 3 H). Interestingly, a small subset of MhcII−/− B cells adopted a PNA+FAS+ GC phenotype. However, in the absence of B cell MHCII, the proportion of GC B cells within the DZ was diminished (Fig. 3 I), consistent with the demonstration that DZ B cell proliferation is directly proportional to the amount of MHCII-bound antigens presented to Tfh cells within the light zone (Gitlin et al., 2014).
Together, these data demonstrate the critical importance of B cell antigen presentation in both initiating T cell activation and sustaining spontaneous autoimmune GC responses in the WAS chimera model.
IFN-γ promotes autoimmunity in the WAS chimera model
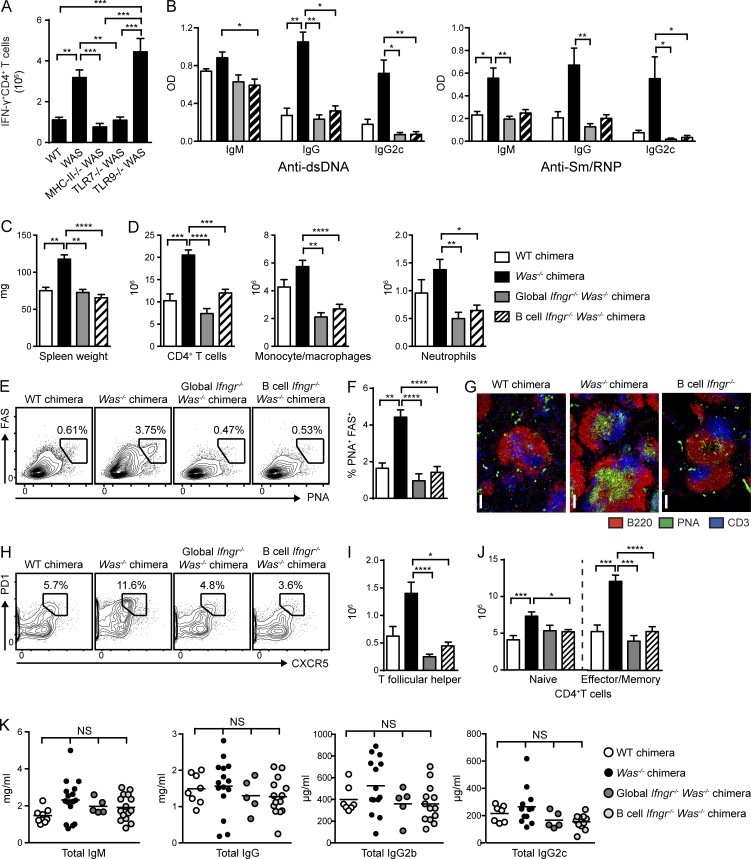
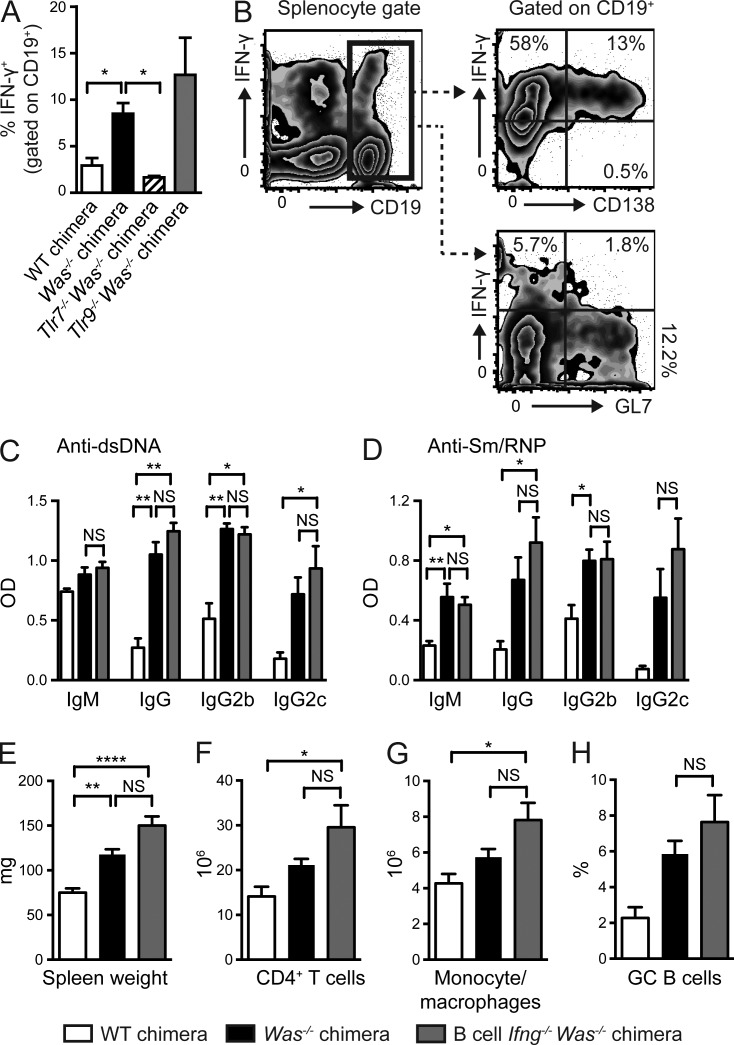
The requirement for B cell antigen presentation in promoting T cell activation in the WAS chimera model suggested that B cell–T cell interactions likely facilitated the production of a critical T cell–derived cytokine that enhanced spontaneous GC responses. For this reason, we quantified ex vivo cytokine production by CD4+ T cells from diseased WAS chimeras. We noted prominent expansion of IFN-γ+CD4+ T cells but no evidence for IL-4, IL-17A, or TNF production (Fig. 4 A and not depicted). Importantly, B cell MHCII deletion markedly decreased the number of IFN-γ+CD4+ T cells, confirming that B cell antigen presentation facilitates expansion of cytokine-producing T cells. B cell–intrinsic deletion of Tlr7 prevented, whereas deletion of B cell Tlr9 increased, the expansion of splenic IFN-γ+CD4+ T cells, a finding consistent with dual BCR/TLR signals in triggering this process and the opposing B cell–intrinsic impacts of TLR7 and TLR9 on disease outcome (Jackson et al., 2014).
Figure 4.
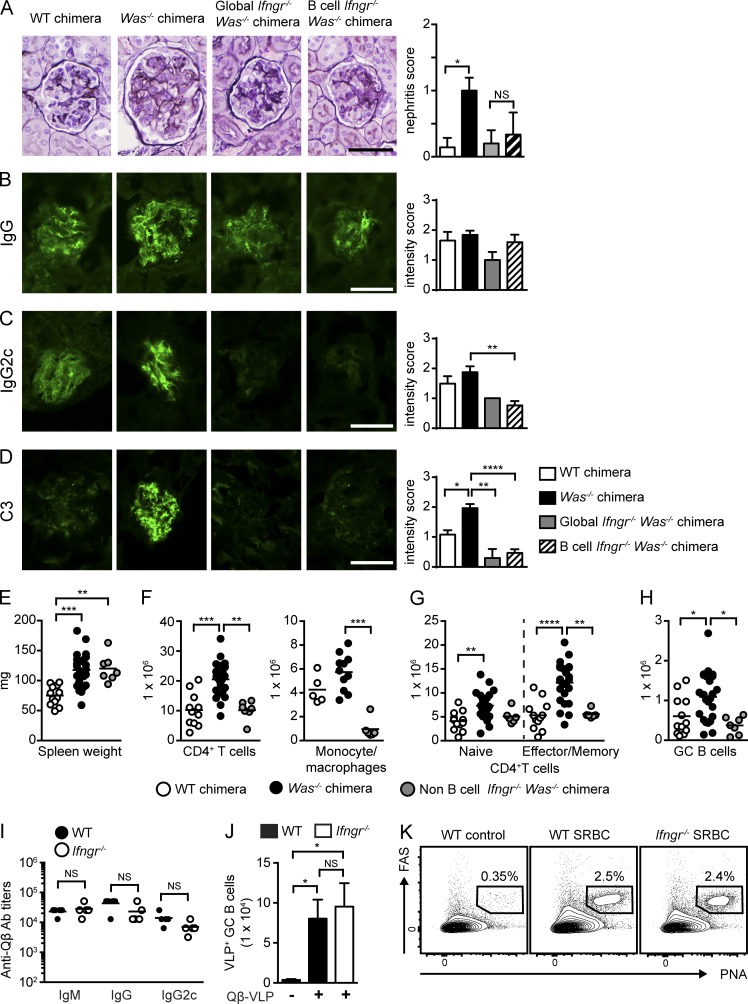
B cell–intrinsic IFN-γR signals promote spontaneous autoimmune GCs. (A) Number of IFN-γ+CD4+ T cells in WT, Was−/−, and B cell–intrinsic Tlr7−/−, Tlr9−/−, and MhcII−/− chimeras. (B) Anti-dsDNA and anti-Sm/RNP IgM, IgG, and IgG2c auto-Abs (12 wk after transplantation) in WT and Was−/− chimeras as well as Was−/− chimeras with global or B cell–intrinsic deletion of IFN-γR. (C) Spleen weight. (D) Number of splenic CD4+ T cells, CD11b+GR1lo monocyte/macrophages, and CD11b+GR1+ neutrophils. (E and F) Representative FACS plots (E; gated on CD19+) and percentage (F) of splenic PNA+FAS+ GC B cells. (G) Representative splenic sections stained with B220, PNA, and CD3. Bars, 100 µm. (H and I) Representative FACS plots (H; gated on CD4+) and number (I) of splenic PD1+CXCR5+ Tfh cells. (J) Number of naive (CD44LOCD62LHI) and EM (CD44HICD62LLO/HI) CD4+ T cells. (K) Total serum IgM, IgG, IgG2b, and IgG2c titers in the indicated chimeras. Data are representative of five WT (n = 10), five Was−/− (n = 18), two global Ifngr−/− Was−/− (n = 10), and four B cell–intrinsic Ifngr−/− Was−/− (n = 13) chimeras, as well as three Was−/−.Tlr7−/− (n = 14), three Was−/−.Tlr9−/− (n = 15), and two Was−/−.MhcII−/− (n = 8) chimeras sacrificed at 24 wk after transplantation. Error bars indicate SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, by the Kruskal-Wallis one-way ANOVA.
These findings suggested that T cell–derived IFN-γ may promote the activation of Was−/− B cells, resulting in humoral autoimmunity. To test this hypothesis, we first generated chimeras in which IFN-γR was absent on all cells, but WAS deficiency was restricted to the B cell compartment, by reconstituting irradiated Ifngr−/−.µMT recipients with 20% Was−/−.Ifngr−/− and 80% Ifngr−/−.µMT BM (henceforth called global Ifngr−/− WAS chimeras). Consistent with the role for IFN-γ in promoting IgG2c CSR (Snapper and Paul, 1987), global Ifngr deletion abrogated IgG2c auto-Ab–targeting dsDNA and Sm/RNP (Fig. 4 B). However, rather than a limited impact on the IgG2c subclass, total IgM and IgG auto-Abs were also markedly decreased in global Ifngr−/− WAS chimeras, suggesting that the lack of IFN-γ exerts a broader impact on the development of humoral autoimmunity.
In keeping with a proinflammatory role for IFN-γ in humoral autoimmunity, global IFN-γR deletion decreased splenomegaly by limiting the expansion of CD4+ T cells, monocyte/macrophages, and neutrophils (Fig. 4, C and D). IFN-γR deletion also abrogated the formation of spontaneous GCs, an observation consistent with a lack of class-switched IgG auto-Abs across IgG subclasses (Fig. 4, E–G). In parallel with decreased B cell activation, IFN-γR deletion also limited CD4+ T cell activation, as indicated by significantly decreased activated CD4+ EM subsets and a loss of Tfh cells (Fig. 4, H–J).
Together, these data demonstrate that IFN-γ exerts multiple impacts on the development of humoral autoimmunity in the WAS chimera model, including the production of class-switched auto-Abs, B cell activation and the development of spontaneous GCs, and expansion of activated CD4+ EM and Tfh cell subsets.
B cell–intrinsic IFN-γR deletion is sufficient to abrogate autoimmune GC formation
Decreased autoimmunity in Ifngr−/− WAS chimeras is consistent with previous studies demonstrating a role for IFN-γR in disease development in the MRLlpr, NZB/W, and Roquinsan/san mouse lupus models (Haas et al., 1997; Schwarting et al., 1998; Lee et al., 2012). However, with the exception of the Roquinsan/san model, where autoimmunity is promoted via IFN-γ–mediated expansion of Tfh cells (Lee et al., 2012), the cellular targets of IFN-γ-driven autoimmunity are unknown. For this reason, we examined the cell-intrinsic impacts of IFN-γ on humoral autoimmunity using independent mixed BM chimera models. First, we generated chimeras in which Was and Ifngr deficiency was limited to the B cell compartment (20% Was−/−.Ifngr−/− and 80% Ifngr+/+.µMT BM into irradiated Ifngr+/+.µMT recipients [B cell–intrinsic Ifngr−/− WAS chimeras]). Of note, despite the transfer of 20% Was−/−.Ifngr−/− BM, CD4+ T cells in these chimeras were overwhelmingly WT (>97%), consistent with the selective advantage of WT versus Was−/− T cells (Becker-Herman et al., 2011). In parallel, we generated chimeras with Was-deficient, but Ifngr-sufficient, B cells, whereas the majority of CD4+ T cells and myeloid cells were Was positive and Ifngr null (20% Was−/− and 80% Ifngr−/−.µMT BM into irradiated Ifngr−/−.µMT recipients [non–B cell Ifngr−/− WAS chimeras]).
Strikingly, B cell–intrinsic deletion of IFN-γR abrogated class-switched auto-Abs against the nuclear antigens dsDNA and Sm/RNP (Fig. 4 B). Although the reduction in IgG2c Abs was anticipated based on a previous study (Snapper and Paul, 1987), the complete absence of IgG auto-Abs mirrored the phenotype of global Ifngr−/− WAS chimeras. Importantly, these impacts of IFN-γ were specific to auto-Abs because the deletion of IFN-γR, whether globally or intrinsic to B cells, exerted minimal impacts on total serum IgM, IgG, or IgG2c levels (Fig. 4 K).
In parallel with a loss of class-switched auto-Abs, B cell–intrinsic IFN-γR deletion decreased splenomegaly and limited the expansion of CD4+ T cell and myeloid subsets to a similar extent as global Ifngr−/− WAS chimeras (Fig. 4, C and D). Most notably, deletion of IFN-γR abrogated spontaneous GCs as shown by absent splenic PNA+FAS+ B cells and a lack of PNA+ GCs by splenic IF staining (Fig. 4, E–G). In parallel with a loss of spontaneous GCs, splenic Tfh cells and activated CD4+ EM subsets were markedly decreased in B cell–intrinsic Ifngr−/− WAS chimeras (Fig. 4, H–J).
In parallel with a loss of auto-Abs, B cell–intrinsic Ifngr−/− WAS chimeras were protected from the development of lupus nephritis (Fig. 5 A). Consistent with prior work (Jackson et al., 2014), although glomerular IgG deposition was similar between experimental groups (Fig. 5 B), both global and B cell–intrinsic IFN-γR deletion limited the deposition of the pathogenic IgG2c subclass and decreased complement component C3 activation (Fig. 5, C and D).
Figure 5.
B cell–intrinsic IFN-γR deletion prevents immune complex glomerulonephritis; IFN-γ promotes autoimmunity but is not required for antiviral responses. (A, left) Representative renal histology. (Right) Glomerular inflammation score. Data are representative of two or more independent chimeras, WT (n = 6), Was−/− (n = 7), global Ifngr−/− Was−/− (n = 5), and B cell–intrinsic Ifngr−/− Was−/− (n = 6) chimeras. (B–D) IF staining for glomerular IgG (B), IgG2c (C), and C3 (D). (Left) Representative images. (Right) Intensity of glomerular IF staining. IF intensity scored by observers blinded to genotype. Data are representative of two or more independent chimeras, WT (n = 8), Was−/− (n = 16), global Ifngr−/− Was−/− (n = 5), and B cell–intrinsic Ifngr−/− Was−/− (n = 15) chimeras. (E–H) Spleen weight (E); number of splenic CD4+ T cells and CD11b+GR1lo monocyte/macrophages (F); number of naive and EM CD4+ T cells (G); and number of GC B cells (H). Data are representative of five WT (n = 11), five Was−/− (n = 18), and two non–B cell Ifngr−/− Was−/− (n = 7) chimeras. (I) Isotype-specific anti-Qβ Ab titers in WT (n = 4) and Ifngr−/− (n = 4) mice at day 7 after VLP immunization. (J) Total number of splenic Qβ-VLP+ GC B cells in immunized WT and Ifngr−/− mice. (K) Representative flow plots showing expansion of splenic PNA+FAS+ GC B cells in SRBC immunized WT and Ifngr−/− mice. Numbers indicate the percentages in the PNA+FAS+ gate. Lines indicate mean values. (I and J) Data are representative of two independent experiments. Bars, 50 µm. Error bars indicate SEM. Lines indicate mean values. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, by the Kruskal-Wallis one-way ANOVA (A–H and J) or the Mann-Whitney U-test (I).
Similar to the B cell–intrinsic role for IFN-γR signals in the generation of autoimmune GCs, deletion of IFN-γR on non–B cells also decreased systemic inflammation. Although spleen weights were not significantly altered, CD4+ T cell, monocyte/macrophage, and neutrophil expansion was limited in non–B cell Ifngr−/− WAS chimeras (Fig. 5, E and F). As predicted by the role for IFN-γ in driving T cell activation during humoral autoimmunity (Lee et al., 2012), the lack of IFN-γR on T cells prevented expansion of CD4+ EM subsets in WAS chimeras (Fig. 5 G). In parallel with absent T cell activation, the number of GC B cells was markedly decreased in non–B cell Ifngr−/− WAS chimeras despite intact B cell IFN-γR signaling in this model (Fig. 5 H).
Importantly, in contrast to the impact on autoimmune GC responses, T cell–dependent IgG and IgG2c Ab responses to Qβ virus-like particle (VLP) immunization (Hou et al., 2011) was not impacted by IFN-γR deletion (Fig. 5 I). Similarly, Ifngr-deficient mice developed robust GC responses after Qβ VLP or sheep RBC (SRBC) immunization (Fig. 5, J and K).
Thus, IFN-γ exerts a B cell–intrinsic impact on GC formation and on class-switched auto-Ab production that is context specific during the pathogenesis of humoral autoimmunity. Together, these data highlight a novel mechanism whereby IFN-γ promotes humoral autoimmunity via reciprocal regulation of B and T cell activation. Strikingly, in a mouse lupus model driven by dysregulated B cells, B cell–intrinsic IFN-γR deletion prevents systemic autoimmunity by abrogating the formation of spontaneous autoimmune GCs.
B cell IFN-γ production is not required for the development of spontaneous autoimmune GCs
In addition to auto-Ab production, B cells can impact autoimmune pathogenesis via the production of cytokines (Lund, 2008). Although B cell production of regulatory cytokines (including IL-10, IL-35, and TGF-β) has been shown to limit autoimmunity in several mouse models (Lund, 2008; Shen et al., 2014), to our knowledge, no prior studies have evaluated the role for B cell–derived effector cytokines in driving SLE. To test whether B cell–derived effector cytokines may impact autoimmunity, we first quantified B cell cytokine production by intracellular cytokine staining of splenic B cells from diseased WAS chimeras. Notably, whereas significant B cell IL-4, IL-10, and IL-17A production was not observed (not depicted), a prominent subset of Was−/− B cells produced IFN-γ (Fig. 6 A). In addition, IFN-γ+CD19+ B cells were markedly expanded by B cell–intrinsic TLR9 deletion but lost with B cell TLR7 deletion, correlating with opposing impacts of B cell TLR7 and TLR9 signals in autoimmunity (Jackson et al., 2014). Notably, the majority of IFN-γ–producing B cells were B220loCD138+ plasmablasts/plasma cells, not GL7+ GC B cells (Fig. 6 B), consistent with prior descriptions of plasma cells comprising the major cytokine-producing B cell subset (Dang et al., 2014).
Figure 6.
B cell–derived IFN-γ is not required for systemic autoimmunity. (A) Percentage IFN-γ+ B cells (gated on CD19+) in WT, Was−/−, and B cell–intrinsic Tlr7−/− and Tlr9−/− chimeras. (B) Representative flow plots demonstrating expression of the plasma cell marker, CD138, but not the GC B cell marker, GL7, on IFN-γ–producing CD19+ B cells. (C and D) Anti-dsDNA (C) and Sm/RNP (D) auto-Ab titers at 12 wk after transplantation. (E–H) Spleen weight (E) and the number of CD4+ T cells (F) and CD11b+GR1lo monocyte/macrophages (G), as well as the percentage of PNA+FAS+ GC B cells (H). Data are representative of two WT (n = 5), two Was−/− (n = 11), two B cell Ifng−/− Was−/− (n = 11), two Was−/−.Tlr7−/− (n = 14), and two Was−/−.Tlr9−/− (n = 15) chimeras sacrificed 24 wk after transplantation. (A and C–H) Error bars indicate SEM. (A) *, P < 0.05 for each experimental group relative to Was−/− chimera controls by the Mann-Whitney U-test. (C–H) *, P < 0.05; **, P < 0.01; ****, P < 0.0001, by the Kruskal-Wallis one-way ANOVA.
To test whether B cell–derived IFN-γ directly impacts humoral autoimmunity, we generated WAS chimeras in which B cells were unable to produce IFN-γ. Whereas deletion of B cell IFN-γR abrogated serum auto-Abs, class-switched auto-Abs against dsDNA and Sm/RNP developed in the absence of B cell–derived IFN-γ (Fig. 6, C and D). In addition, B cell IFN-γ–deficient WAS chimeras developed marked splenomegaly with splenic CD4+ T cells and myeloid expansion (Fig. 6, E–G). Furthermore, the lack of B cell IFN-γ did not prevent the development of spontaneous autoimmune GCs (Fig. 6 H). In summary, although we observed an expansion of IFN-γ–producing B cells in autoimmune WAS chimeras, B cell–derived IFN-γ was not required for auto-Ab production or development of spontaneous GCs, indicating that other cellular IFN-γ sources can compensate for the lack of B cell–derived IFN-γ.
T-bet promotes B cell–intrinsic IgG2c CSR but is not required for IFN-γ–driven spontaneous GCs
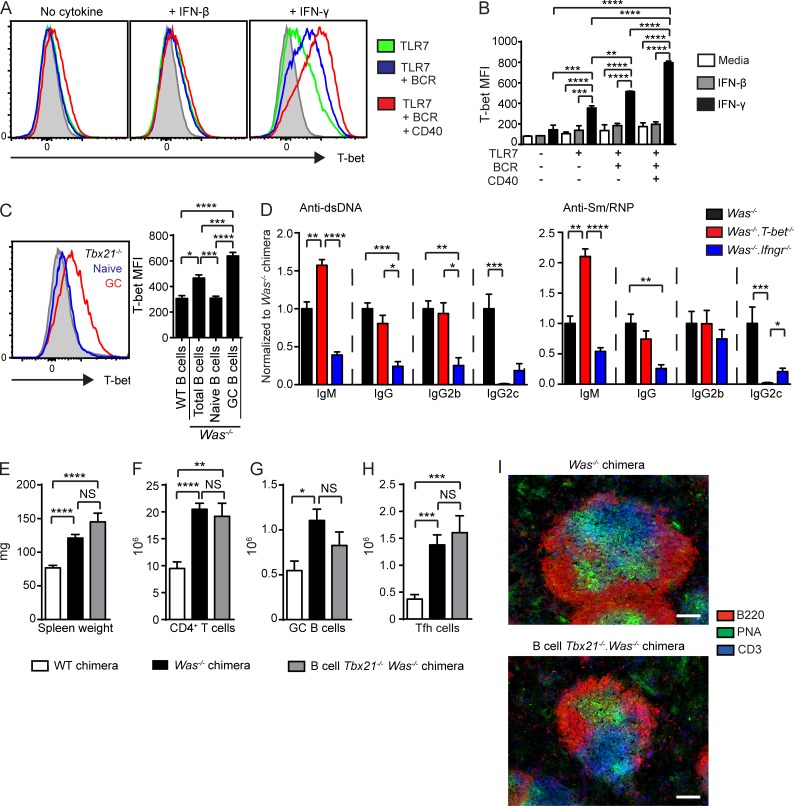
The Th1 cell–defining transcription factor T-bet (encoded by Tbx21) promotes B cell CSR to IgG2c and is required for the generation of IgG2c+ memory B cells (Peng et al., 2002; Gerth et al., 2003; Wang et al., 2012). Although B cell T-bet enhances viral clearance via the production of protective IgG2c Abs (Rubtsova et al., 2013), the importance of B cell–intrinsic T-bet in the pathogenesis of autoimmunity is unknown. Because both type 1 IFNs and IFN-γ have been demonstrated to induce B cell T-bet (de Goër de Herve et al., 2011; Rubtsova et al., 2013) but are differentially required for the development of spontaneous GCs in the WAS model, we compared the impact of IFN-β and IFN-γ on B cell T-bet expression at cytokine doses that equivalently enhanced B cell proliferation and PNA binding. Notably, although IFN-β up-regulated T-bet expression relative to media controls, IFN-γ induced a significantly greater increase in B cell T-bet expression (Fig. 7, A and B), a finding consistent with recently described synergistic roles for BCR, TLR7, and IFN-γ in B cell T-bet up-regulation (Rubtsova et al., 2013). Consistent with these in vitro data, we also observed increased in vivo T-bet expression in GC B cells from diseased WAS chimeras (Fig. 7 C). Together, these data demonstrate that IFN-γ promotes B cell–intrinsic up-regulation of T-bet and identify T-bet as a potential transcription factor promoting IFN-γ–driven autoimmune GCs.
Figure 7.
IFN-γ–mediated expression of T-bet promotes IgG2c CSR but is not required for spontaneous GC formation. (A) Representative histograms of intracellular T-bet staining of WT B cells stimulated with R848, anti-IgM, anti-CD40 with or without exogenous IFN-β or IFN-γ. The gray histogram indicates unstimulated WT B cells. (B) T-bet mean fluorescence intensity (MFI) in stimulated B cells. (A and B) Data are representative of two or more independent experiments. (C, left) Representative histogram of Was−/− chimera intranuclear T-bet expression in GC (PNA+GL7+) versus naive (non-GC; PNA−GL7−) WT B cells. The gray histogram indicates Tbx21−/− B cells. (Right) T-bet mean fluorescence intensity in total B cells from WT (n = 2) and Was−/− (n = 7) chimeras, as well as naive and GC B cells from Was−/− chimeras. (D) Anti-dsDNA (left) and Sm/RNP (right) isotype and subclass-specific auto-Abs (normalized to mean Was−/− chimera titer). (E–H) Spleen weight (E) and number of splenic CD4+ T cells (F), PNA+FAS+ GC B cells (G), and CXCR5+PD1+ Tfh cells (H). (I) Representative splenic sections stained with B220, PNA, and CD3 demonstrating PNA+ GCs in both Was−/− and B cell–intrinsic Tbx21−/−.Was−/− chimeras. Bars, 100 µm. (D–H) Data are representative of nine WT (n = 16), nine Was−/− (n = 34), and three B cell–intrinsic Was−/−.Tbx21−/− (n = 11) independent chimeras sacrificed at 24 wk. Error bars indicate SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, by one-way ANOVA followed by Tukey’s multiple comparison test (B and C) and by the Kruskal-Wallis one-way ANOVA (D–H).
To test this hypothesis, we generated WAS chimeras in which only B cells lacked T-bet. As predicted by prior studies (Peng et al., 2002; Gerth et al., 2003), B cell–intrinsic T-bet deletion abrogated IgG2c+ auto-Abs. However, in contrast with the lack of auto-Abs in the absence of B cell IFN-γR signals, similar titers of anti-dsDNA and Sm/RNP IgG and IgG2b Abs developed in B cell Tbx21−/− WAS versus WAS chimeras (Fig. 7 D), and, interestingly, IgM auto-Abs were increased in the setting of B cell T-bet deletion. Similarly, whereas B cell IFN-γR deletion prevented splenomegaly, the lack of B cell T-bet did not impact spleen size or CD4+ T cell and myeloid expansion in WAS chimeras (Fig. 7, E and F; and not depicted). T-bet–deficient chimeras also developed prominent spontaneous GCs with increased splenic PNA+FAS+ B cells and Tfh cells (Fig. 7, G and H) and prominent PNA+ GCs by splenic IF staining (Fig. 7 I). Therefore, B cell–intrinsic T-bet expression has no impact on the formation of autoimmune GCs but specifically impacts CSR to pathogenic auto-Ab subclasses.
IFN-γR synergizes with BCR, TLR, and CD40 signals to up-regulate BCL-6 in B cells
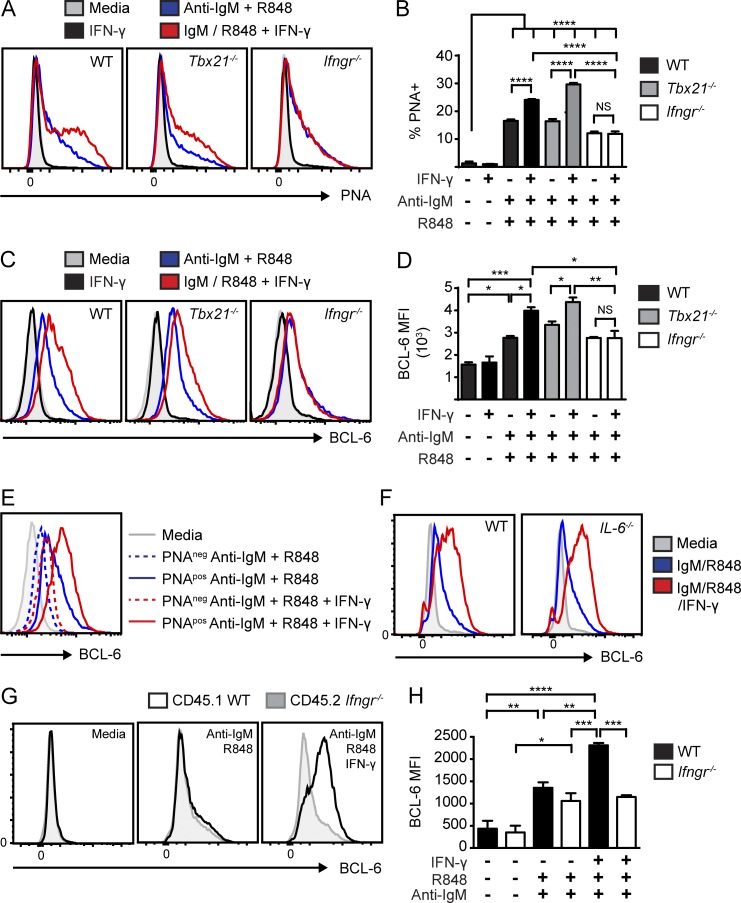
The aforementioned findings implied that IFN-γR signaling exerts a critical T-bet–independent impact on B cell activation and autoimmune GC formation. In an attempt to identify this program, we modeled initial B cell activation in vitro by stimulating WT, Tbx21−/−, and Ifngr−/− B cells with anti-IgM and R848 with and without IFN-γ. In vitro–stimulated mouse B cells adopted a GC phenotype as indicated by PNA binding as well as increased surface IL-21 receptor and AID expression (Fig. 8, A and B; and not depicted). Consistent with our in vivo data, IFN-γ increased PNA binding on BCR/TLR7-stimulated B cells in a manner that was dependent on IFN-γR, but not impacted by deletion of T-bet.
Figure 8.
IFN-γ promotes cell-intrinsic expression of BCL-6 in mouse B cells. (A–D) PNA binding (A), the percentages of PNA positivity (B), BCL-6 staining (C), and BCL-6 mean fluorescence intensity (MFI; D) in splenic WT, Tbx21−/−, and Ifngr−/− B cells stimulated with the indicated combinations of anti-IgM, R848, and IFN-γ. (E) BCL-6 in PNApos versus PNAneg B cells stimulated with anti-IgM/R848 or anti-IgM/R848 and IFN-γ. (F) BCL-6 in WT and Il-6−/− B cells stimulated with anti-IgM/R848 or anti-IgM/R848 and IFN-γ. In E and F, the gray histograms indicate unstimulated media controls. (G) Overlaid histograms of BCL-6 expression in co-cultured CD45.1 WT and CD45.2 Ifngr−/− B cells stimulated with media, anti-IgM/R848, and anti–IgM/R848/IFN-γ. (H) BCL-6 mean fluorescence intensity in co-cultured CD45.1 WT and CD45.2 Ifngr−/− B cells. Error bars indicate SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, by one-way ANOVA followed by Tukey’s multiple comparison test. Data are representative of two or more independent experiments.
The transcription factor BCL-6 is a master regulator of GC B cell and Tfh cell development (Bunting and Melnick, 2013). Thus, we hypothesized that IFN-γ might promote autoimmune GCs via B cell–intrinsic induction of BCL-6. Strikingly, although IFN-γ did not alter BCL-6 levels in resting cells, exogenous IFN-γ synergized with BCR and TLR7 signals to markedly increase BCL-6 in activated WT and Tbx21−/−, but not in Ifngr−/−, B cells (Fig. 8, C and D). Although BCL-6 levels were higher in stimulated PNApos versus PNAneg B cells, the increase in BCL-6 was not explained by IFN-γ–mediated expansion of GC-like B cells. Instead, exogenous IFN-γ specifically increased BCL-6 levels in PNApos B cells in response to anti-IgM and R848 stimulation (Fig. 8 E).
Activated B cells produce cytokines, including the proinflammatory cytokine IL-6 (Barr et al., 2012; Yehudai et al., 2012). Because IL-6 promotes Tfh cell development via induction of BCL-6 (Nurieva et al., 2009; Ma et al., 2012), we tested whether IFN-γ might promote BCL-6 expression via an indirect, IL-6-dependent, mechanism. Importantly, stimulated WT and IL-6 null exhibited equivalent BCL-6 up-regulation, indicating that IL-6 signals did not impact these in vitro events (Fig. 8 F). Furthermore, to assess whether other B cell–derived cytokines might indirectly influence BCL-6 expression, we established co-cultures of congenically marked WT and Ifngr−/− B cells and stimulated these cell mixtures with anti-IgM, R848, and IFN-γ. Notably, IFN-γ specifically increased BCL-6 expression only in stimulated WT B cells in these co-cultures (Fig. 8, G and H). Therefore, IFN-γR signaling synergizes with dual BCR and TLR7signals to promote BCL-6 expression via a direct cell-intrinsic mechanism in mouse B cells.
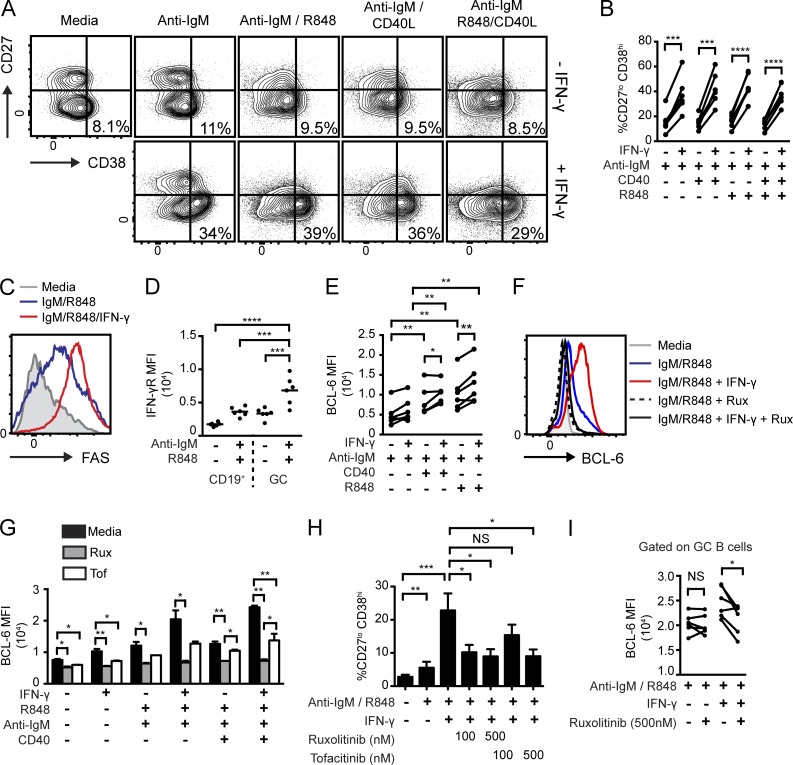
Importantly, similar to our finding in mouse cells, IFN-γ also promoted a GC phenotype in IgM, TLR7, and/or CD40L stimulated primary human B cells. After 72-h in vitro stimulation, IFN-γ treatment significantly increased the proportion of CD38+CD27− human B cells (Fig. 9, A and B), corresponding to an early GC phenotype (Jung et al., 2000). In addition, exogenous IFN-γ increased expression of the GC-marker FAS on BCR/TLR7-stimulated human B cells (Fig. 9 C). Importantly, whereas IFN-γR expression levels were low on unstimulated B cells, surface IFN-γR levels were increased on the CD38+CD27− GC subset after stimulation, implicating feed-forward mechanisms for IFN-γ in human GC responses (Fig. 9 D). Most notably, IFN-γ also synergized with BCR-, TLR7- and CD40-mediated signals to increase BCL-6 expression in activated primary human B cells (Fig. 9 E). Thus, IFN-γ synergizes with B cell activation signals to promote a GC phenotype and to increase BCL-6 expression in both mouse and human B cells.
Figure 9.
IFN-γ synergizes with BCR-, TLR7-, and CD40-dependent signals to promote a GC phenotype and BCL-6 expression in primary human B cells, and JAK1/2 inhibitors block these events. (A) Representative FACS plots showing IFN-γ–mediated expansion of the CD38+CD27− subset in stimulated primary human B cells. Numbers equal the percentages in the CD38+CD27− gate. (B) Percentage of CD38+CD27− B cells from independent donors stimulated with anti-IgM, R848, anti-CD40, and/or IFN-γ. (C) Overlaid histograms of surface FAS expression in human B cells stimulated with media, anti-IgM/R848, and anti–IgM/R848/IFN-γ. (D) Surface IFN-γR mean fluorescence intensity (MFI) in unstimulated versus anti-IgM/R848–stimulated human B cells gated on either total B cells (CD19+) or the CD38+CD27− subset (GC). (E) BCL-6 mean fluorescence intensity in stimulated human B cells. (F) Representative overlaid histogram of BCL-6 expression in mouse B cells stimulated as indicated and co-cultured with or without the JAK1/2 inhibitor, ruxolitinib (Rux; 500 nM). (G) BCL-6 mean fluorescence intensity in stimulated mouse B cells treated with or without the JAK inhibitors, Rux or tofacitinib (Tof; 500 nM). (H) Percentage of the CD38+CD27− GC phenotype of human B cells ± indicated concentrations of ruxolitinib or tofacitinib. (G and H) Error bars indicate SEM. (I) BCL-6 mean fluorescence intensity in human B cells stimulated ± 500 nM ruxolitinib (gated on CD38+CD27− GC B cells). (B, E, and I) Data are shown as a paired analysis of different stimulation conditions from individual human donors. (B, D, E, and G–I) *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, by paired two-tailed Student’s t test (B, E, and I) or one-way ANOVA followed by Tukey’s multiple comparison test (D, G, and H). Data are representative of two or more independent experiments.
The IFN-γR is composed of two subunits, IFNGR1 and IFNGR2, which associate with JAK1 and 2, respectively (Platanias, 2005). To test the requirement for JAK family activation in IFN-γ–mediated BCL-6 expression in B cells, we treated stimulated mouse and human B cells with ruxolitinib, a JAK1/JAK2 inhibitor approved for treatment of myelofibrosis (Verstovsek et al., 2012), or tofacitinib, a JAK1/JAK3 inhibitor approved in rheumatoid arthritis (Fig. 9, F–I; Lee et al., 2014). Notably, ruxolitinib blocked IFN-γ–mediated increases in BCL-6 expression and limited PNA binding in mouse B cells without impacting B cell survival (Fig. 9, F and G; and not depicted), whereas tofacitinib partially inhibited these effects, consistent with limiting JAK1, but not JAK2, signals. Similarly, ruxolitinib and tofacitinib completely or partially blocked the IFN-γ-mediated expansion of CD38+CD27− GC B cells in human B cell cultures, respectively (Fig. 9 H). Strikingly, consistent with findings in mouse B cells, IFN-γ–dependent BCL-6 up-regulation in human B cells was abolished by ruxolitinib (Fig. 9 I).
Together, these data highlight the novel observation that IFN-γR synergizes with BCR-, TLR-, and CD40-dependent signals to directly enhance BCL-6 expression in mouse and human B cells, a finding that provides a mechanistic explanation for loss of spontaneous autoimmune GCs in the absence of B cell–intrinsic IFN-γR expression.
DISCUSSION
IFN proteins exert pleiotropic effects during bacterial and viral infections. The IFN family comprises three major subtypes: type 1 (IFN-α, -β, -ε, and -ω), type 2 (IFN-γ), and type 3 (IFN-λ1, -λ2, and -λ3) IFN. In addition to physiological roles during infection, dysregulated IFN, in particular type 1 IFN, signals have been implicated in the development of autoimmunity. For example, a significant subset of SLE patients expresses a peripheral blood mononuclear cell type 1 IFN gene signature that correlates with disease severity (Baechler et al., 2003; Bennett et al., 2003; Kirou et al., 2005). Several human genetic polymorphisms within the type 1 IFN receptor signaling pathway, including variants within IRF5 and STAT4, are associated with increased lupus risk (Deng and Tsao, 2010). In addition, deletion of the type 1 IFN receptor, IFNAR, ameliorates disease in several mouse lupus models, although autoimmunity is notably exacerbated in Ifnar−/− MRL.Faslpr mice (Kiefer et al., 2012).
IFN-γ has also been implicated in SLE in both mouse and human studies. Elevated serum IFN-γ levels are observed in SLE patients (Pollard et al., 2013), and overexpression of IFN-γ is sufficient to promote positive ANAs and immune complex glomerulonephritis in transgenic mice (Seery et al., 1997). In addition, genetic or therapeutic targeting of IFN-γ abrogates autoimmunity in models of spontaneous or induced autoimmunity, including the NZB/NZW F1, MRL.Faslpr, and pristane mouse lupus models (Pollard et al., 2013). In this context, it is notable that significant overlap exists between genes regulated by type 1 and type 2 IFNs, suggesting a potentially important contribution of IFN-γ to the lupus IFN gene signature (Hertzog et al., 2011; Pollard et al., 2013). Consistent with this observation, clinical treatment with both IFN-α (type 1 IFN) and IFN-γ has been associated with the development of de novo SLE. These overlapping and, at times, contradictory roles for type 1 IFN and IFN-γ in lupus pathogenesis prompted us to dissect the cell-intrinsic impacts of these effector cytokines in humoral autoimmunity.
Mechanistically, type 1 IFNs are proposed to propagate an inflammatory feed-forward loop in which nucleic acid–containing immune complexes drive TLR7/TLR9-dependent type 1 IFN production by plasmacytoid DCs, resulting in enhanced autoantigen presentation and further activation of autoreactive B cells (Hall and Rosen, 2010). Because B cell TLR7 signals are required for spontaneous GCs in the WAS model (Jackson et al., 2014) and type 1 IFN enhanced TLR7-dependent B cell activation in vitro, we predicted that B cell–specific IFNAR deletion would similarly attenuate auto-Ab production and limit systemic autoimmunity in this model. Surprisingly, WAS chimeras developed prominent spontaneous GCs and high-titer class-switched auto-Abs, including IgG2c auto-Abs against RNA-associated autoantigens, that were unaffected by B cell–intrinsic Ifnar deletion, and a lack of B cell IFNAR signals only modestly delayed the kinetics of disease development. Although type 1 IFN may exert additional impacts on non–B cell lineages in this model, these findings suggested that alternative cytokine effectors are required to drive B cell activation in lupus.
Because B cell antigen presentation to CD4+ T cells in the context of MHCII initiates the expansion of IFN-γ–producing CD4+ T cells, we evaluated the impact of IFN-γ on the development of autoimmunity. Consistent with prior models (Haas et al., 1997; Schwarting et al., 1998; Lee et al., 2012), the development of systemic autoimmunity was abrogated in global Ifngr−/− WAS chimeras. A previous study using Roquinsan/san lupus-prone mice, in which IFN-γ promotes a T cell–intrinsic expansion of Tfh cells, suggested that CD4+ T cells are the major targets of IFN-γ action during autoimmune pathogenesis (Lee et al., 2012). Although complicated by variable IFN-γR chimerism in the myeloid compartment, our non–B cell Ifngr−/− WAS chimeras lend support to this model because the lack of IFN-γR on CD4+ T cells severely limited the development of spontaneous GCs.
Unexpectedly, B cell–intrinsic IFN-γR deletion abrogated the development of systemic autoimmunity. Because IFN-γ has previously been shown to promote IgG2c CSR, we initially predicted a limited impact of B cell–intrinsic IFN-γR deletion on the development of specific class-switched auto-Ab subclasses. In contrast, a lack of B cell IFN-γR signals markedly decreased all auto-Ab isotypes by eliminating spontaneous GCs. Although the role of B cell IFN-γR signals in human SLE has not yet been addressed, these data demonstrate that, in a mouse lupus model, IFN-γ drives autoimmune GCs via the reciprocal cell-intrinsic induction of Tfh cells and GC B cells. Surprisingly, although activated B cells from diseased WAS chimeras also produced IFN-γ, B cell–derived IFN-γ was redundant for the establishment of systemic autoimmunity. B cell cytokines have been shown to either promote or limit inflammatory responses after infectious challenges and during autoimmunity in mouse experimental models (Lund, 2008; Dang et al., 2014). Our findings demonstrate that the impact of B cell IFN-γ production is context specific and may be redundant in the setting of alternate cytokine-producing immune lineages.
Most notably, our data identify a novel, T-bet–independent mechanism underlying IFN-γ–driven spontaneous GCs. IFN-γR signals in B cells markedly up-regulate expression of the transcription factor T-bet and are required to promote CSR to the inflammatory IgG2c isotype after viral infection and during autoimmunity. Thus, these observations implicated T-bet transcriptional activity as potentially driving the IFN-γ–driven GC response. Surprisingly, although T-bet was essential for IgG2c auto-Ab CSR, the expansion of autoimmune GCs was unaffected by B cell–intrinsic T-bet deletion in WAS chimeras. In contrast, our in vitro studies demonstrate that IFN-γ integrates with BCR-, TLR-, and/or CD40-dependent signals to promote B cell–intrinsic expression of the critical transcription factor BCL-6 in both mouse and human primary B cells. Furthermore, using co-culture and/or genetic models, we show that IFN-γR signals directly orchestrate this transcriptional process. BCL-6 is required for the formation of both Tfh cells and GC B cells during a GC response. In B cells, BCL-6 acts as a transcriptional repressor, inhibiting terminal B cell differentiation by repressing IRF4 and BLIMP-1 and limiting the DNA damage response during SHM (Crotty et al., 2010). BCL-6 is first expressed shortly after antigen engagement and before the formation of established GCs (Kitano et al., 2011). Despite the critical importance of BCL-6 in GC responses, however, the B cell–intrinsic signals promoting or sustaining its expression are largely unknown (Basso and Dalla-Favera, 2012). Unexpectedly, our findings show that JAK1/2-mediated IFN-γR signals markedly enhance BCL-6 levels after initial B cell activation with BCR, TLR7, and/or CD40 signals and mediate this effect independently of T-bet, findings consistent with IFN-γ–dependent, T-bet–independent spontaneous GC formation in vivo.
Importantly, although B cell IFN-γR signals were required for autoimmune GCs, global IFN-γR deletion did not reduce T cell–dependent Ab responses, including anti-VLP Ab formation. Furthermore, previous work using human B and T cell co-cultures indicate that rather than driving GC responses, IFN-γR signals function to partially restrain Tfh cell–induced B cell Ab responses (Nündel et al., 2015). Thus, the B cell–intrinsic requirement for IFN-γR signals in auto-Ab responses appears to reflect the unique setting of self-antigen–driven dual BCR/TLR B cell activation and therefore may provide a unique context for therapeutic interventions that might spare protective, T cell–dependent Ab responses. Consistent with this idea, in vitro treatment with Food and Drug Administration–approved JAK inhibitors was sufficient to abolish the IFN-γ–mediated increase in BCL-6 expression in both mouse and human B cells. Given the importance of dysregulated B cell activation and spontaneous GCs in human lupus pathogenesis, our observations implicate this novel B cell pathway as a potential therapeutic target in SLE. Collectively, our findings suggest that IFN-γR blockade is likely to limit critical B cell–T cell interactions essential for the induction and/or expansion of autoimmune GC responses required for SLE and, possibly, in other disorders characterized by pathogenic auto-Abs.
MATERIALS AND METHODS
Mice
CD45.1 and CD45.2 C57BL/6, µMT, Was, Ifng−/−, Ifngr−/−, Ifnar−/−, Tbx21−/−, and MhcII−/− mice and the relevant mouse crosses were bred and maintained in the specific pathogen-free animal facility of Seattle Children’s Research Institute. All animal studies were conducted in accordance with the Seattle Children’s Research Institute Institutional Animal Care and Use Committee’s approved protocols.
BM transplantation
BM was harvested from the femora and tibiae of C57BL/6 (WT), Was−/−, Was−/−.Ifnar−/−, Was−/−.MhcII−/−, Was−/−.Ifngr−/−, Was−/−.Ifng−/−, Was−/−.Tbx21−/−, µMT, or Ifngr−/−.µMT mice. Single-cell suspensions were depleted for CD138+ cells (130–098-257; Miltenyi Biotec). CD138-depleted WT, Was−/−, Was−/−.Ifnar−/−, Was−/−.MhcII−/−, Was−/−.Ifngr−/−, Was−/−.Ifng−/−, or Was−/−.Tbx21−/− donor BM was mixed with µMT or Ifngr−/−.µMT BM at a 20:80 ratio, and 6 × 106 total BM was injected retroorbitally into lethally irradiated (450 cGy × 2 doses) µMT or Ifngr−/−.µMT recipients. Resulting BM chimeras were bled every 4–8 wk from the posttransplant date by retroorbital puncture and sacrificed 24–26 wk after transplantation. Data are representative of at least two independent experimental cohorts for each chimera.
Abs
Anti–mouse Abs used in this study include B220 (RA3-6B2), CD4 (RM4-5), Thy1.2 (53-2.1), CD138 (281-2), CXCR5 (2G8), CD86 (GL1), and CXCR4 (2B11/CXCR4) from BD; CD62L (MEL-14), CD11c (N418), Gr-1 (RB6-8C5), Ly5.1 (A20), Ly5.2 (104), CD11b (M1/70), GL7 (GL-7), PD-1 (J43), AID (mAID-2), T-bet (4B10), MHCII (M5/114.15.2), IFN-γ (XMG1.2), and BCL-6 (BCL-DWN) from eBioscience; goat anti–mouse IgM-, IgG-, IgG2b-, and IgG2c-HRP conjugated, unlabeled, or isotype from SouthernBiotech; CD19 (ID3), CD44 (IM7), CD4 (RM4-4), and Ly5.2 (104) from BioLegend; PNA (Fl-1071) from Vector Laboratories; and Fas (Jo2) from BD. Anti–human Abs used in this study include CD38 (HIT2), BCL-6 (K112-91), IgG (G18-145), and CD27 (L128) from BD, and Fas (DX2), CD19 (HIB19), IgM (MHM-88), and CD119 (GIR-208) from BioLegend.
Flow cytometry
Single-cell splenocyte suspensions were obtained as previously described (Becker-Herman et al., 2011) and incubated with fluorescence-labeled Abs for 20 min at 4°C. Intracellular cytokines were assessed by plating 106 cells/well in a 96-well plate and stimulating them for 5 h at 37°C with 5 µg/ml PMA (EMD Millipore), 1 µg/ml ionomycin (EMD Millipore), and GolgiStop (1:1,500 dilution; BD), after which intracellular staining was performed. Data were collected on a flow cytometer (LSR II; BD) and analyzed using FlowJo software (Tree Star). For human studies, cells were stained with a fixable viability dye for 10 min at room temperature followed by incubation with human TruStain FcX (BioLegend) for 10 min at 4°C. Surface Abs were stained for 20 min at 4°C followed by incubation with a transcription factor fixation/permeabilization solution (BD) for 40 min at 4°C. Intracellular Abs and transcription factor Abs were then stained for 40 min at 4°C in a permeabilization buffer (BD). Data collected with human samples were run on a cell sorter (FACSCanto II; BD).
Human subjects
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975 as revised in 2008, and were approved by the Benaroya Research Institute’s Institutional Review Board. PBMCs were derived from subjects participating in the Benaroya Research Institute control registry. Control participants were selected based on a lack of personal or family history of autoimmune disease. Written informed consent was obtained from all subjects before their participation.
In vitro stimulations
For mice, splenic B cells were purified from WT, Ifnar−/−, Tbx21−/−, and Ifngr−/− mice by CD43 microbead depletion (Miltenyi Biotec). Purified B cells were cultured in RPMI at 37°C for 48 h at 106 cells/well in a 96-well plate with or without 5 ng/ml R848, 1 µg/ml anti–mouse IgM F(ab')2 fragment (Jackson ImmunoResearch Laboratories, Inc.), 1 µg/ml anti–mouse CD40 (SouthernBiotech), recombinant mouse IFN-γ or IFN-β (200 and 300 U/ml, respectively; BioLegend), and 500 nM ruxolitinib or 500 nM tofacitinib. For co-culture experiments, congenically marked CD45.1 WT and CD45.2 Ifngr−/− B cells were stimulated together in 96-well plates (106 total cells/well). B cell surface marker and transcription factor expression was evaluated by flow cytometry. Cell proliferation was evaluated by Cell Trace violet (Invitrogen) dilution.
Total human B cells were purified by the Human B Cell Isolation kit II (Miltenyi Biotec). Total B cells were plated at 5 × 104 in a 96-well plate for 24 or 72 h with 10 µg/ml anti-IgM (Jackson ImmunoResearch Laboratories, Inc.), 5 µg/ml CD40L (PeproTech), or 3.75 µg/ml R848 (InvivoGen) with or without 10 µg/ml IFN-γ (R&D Systems). For JAK inhibitor experiments, ruxolitinib or tofacitinib was added at 100 nm or 500 nm at the initiation of culture. Cells were analyzed at 24- and 72-h time points.
Measurement of auto-Abs
For specific auto-Ab ELISAs, 96-well immuno plates (Thermo Fisher Scientific) were precoated overnight at 4°C with 100 µg/ml calf thymus dsDNA (D3664-5X2MG; Sigma-Aldrich) or 5 µg/ml Sm/RNP (ATR01-10; Arotec Diagnostic Limited). Plates were blocked for 1 h with 1% BSA in PBS before the addition of diluted serum for 2 h. Specific Abs were detected using goat anti–mouse IgM-, IgG-, or IgG2c-HRP (1:2,000 dilution; SouthernBiotech), and peroxidase reactions were developed using a OptEIA TMB substrate (BD). Absorbance at 450 nm was read using a plate reader (Victor 3; PerkinElmer), and data were analyzed using Prism software (GraphPad Software).
Quantitative PCR
RNA was isolated from total WT and wasp−/− chimeric mice 24 wk after transplantation using the RNeasy Micro kit (QIAGEN) and converted into cDNA by reverse transcription (Superscript II; Invitrogen). Real-time PCR was performed using an iCycler real-time PCR detection system with IQ SYBR green Supermix (Bio-Rad Laboratories), with mouse hypoxanthine-guanine phosphoribosyltransferase as a control. Primers used were as follows: Hprt, 5′-TTGCTGACCTGCTGGATTACA-3′ (forward) and 5′-CCCCGTTGACTGATCATTACA-3′ (reverse); Ifit, 5′-TGCTGAGATGGACTGTGAGG-3′ (forward) and 5′-CTCCACTTTCAGAGCCTTCG-3′ (reverse); Isg15, 5′-AAGCAGCCAGAAGCAGACTC-3′ (forward) and 5′-CACCAATCTTCTGGGCAATC-3′ (reverse); and Mcp-1, 5′-TAGTTTTTGTCACCAAGCTC-3′ (forward) and 5′-GATCTCATTTGGTTCCGATCC-3′ (reverse).
VLP and SRBC immunization
WT and Ifngr−/− mice were immunized intraperitoneally with 20 µg Qβ VLPs or SRBCs (15.2% by volume in sterile PBS). Spleens were harvested for flow analysis at day 7 after immunization. Anti-VLP Ab titers were determined as previously described (Hou et al., 2011).
Spleen and kidney IF staining
Mouse spleens and kidneys were embedded in an optimal cutting temperature compound and frozen over dry ice. 10-µm sections were cut on a cryostat, mounted on slides (Superfrost Excell; Thermo Fisher Scientific), and fixed in −20°C acetone for 20 min. After rehydration in a staining buffer (PBS, 1% goat serum, 1% BSA, and 0.1% Tween 20), slides were stained with B220-PE, CD3-APC, and PNA-FITC (spleens) or IgG-FITC, IgG2c-FITC, or C3-FITC (kidneys). Images were acquired using a microscope (DM6000B; Leica Biosystems), a camera (DFL300 FX; Leica Biosystems), and Application Suite Advanced Fluorescence software (Leica). For glomerular immune complex quantification, images were obtained using a constant exposure and scored from 0 to 3 by two independent observers blinded to the genotype.
Histopathology
Kidneys were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4 µm, and stained with Jones’ methenamine silver periodic acid–Schiff according to standard practices. Immunohistochemistry staining was performed using a Leica Biosystems Bond–automated immunostainer and HRP-conjugated secondary Abs. Histology images were acquired using a microscope (OptiPhot-2; Nikon) and a camera (EOS 5D Mark II; Canon). Glomerular inflammation was scored as follows: minimal mesangial expansion consistent with radiation injury (0+); focal glomerular changes with moderate mesangial expansion, GBM thickening/reduplication and glomerular hypercellularity (1+); or diffuse glomerular changes with severe mesangial expansion, GBM thickening/reduplication, and glomerular hypercellularity (2+). Pathology was scored by observers blinded to genotype.
Statistical evaluation
P-values were calculated using the two-tailed Student’s t test, the Mann-Whitney U-test, the two-tailed Fischer’s exact test, the one-way ANOVA followed by Tukey’s multiple comparison test, and the Kruskal-Wallis one-way ANOVA (GraphPad Software).
ACKNOWLEDGMENTS
The authors thank Jit Khim and Karen Sommer for assistance with mouse studies and laboratory management and Kelly Hudkins and Charles Alpers for renal histopathology staining and scoring.
This work was supported by the National Institutes of Health under the following grant numbers: R01HL075453 (to D.J. Rawlings), R01AI084457 (to D.J. Rawlings), R01AI071163 (to D.J. Rawlings), DP3DK097672 (to D.J. Rawlings), and K08AI112993 (to S.W. Jackson). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided by the Benaroya Family Gift Fund (to D.J. Rawlings), the American College of Rheumatology Research and Education Foundation Rheumatology Scientist Development Award (to S.W. Jackson), a Seattle Children’s Research Institute Pediatric Early Research Career award (to S.W. Jackson), and the Arnold Lee Smith Endowed Professorship for Research Faculty Development (to S.W. Jackson).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- Ab
- antibody
- AID
- activation-induced cytidine deaminase
- ANA
- antinuclear Ab
- BCL-6
- B cell lymphoma 6
- BCR
- B cell receptor
- CSR
- class-switch recombination
- dsDNA
- double-stranded DNA
- DZ
- dark zone
- EM
- effector/memory
- GC
- germinal center
- IF
- immunofluorescence
- IFN-γR
- IFN-γ receptor
- PNA
- peanut agglutinin
- SHM
- somatic hypermutation
- SLE
- systemic lupus erythematosus
- SRBC
- sheep RBC
- VLP
- virus-like particle
- WAS
- Wiskott-Aldrich syndrome
References
- Aloisi F., and Pujol-Borrell R.. 2006. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6:205–217. 10.1038/nri1786 [DOI] [PubMed] [Google Scholar]
- Baechler E.C., Batliwalla F.M., Karypis G., Gaffney P.M., Ortmann W.A., Espe K.J., Shark K.B., Grande W.J., Hughes K.M., Kapur V., et al. 2003. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA. 100:2610–2615. 10.1073/pnas.0337679100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr T.A., Shen P., Brown S., Lampropoulou V., Roch T., Lawrie S., Fan B., O’Connor R.A., Anderton S.M., Bar-Or A., et al. 2012. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6–producing B cells. J. Exp. Med. 209:1001–1010. 10.1084/jem.20111675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso K., and Dalla-Favera R.. 2012. Roles of BCL6 in normal and transformed germinal center B cells. Immunol. Rev. 247:172–183. 10.1111/j.1600-065X.2012.01112.x [DOI] [PubMed] [Google Scholar]
- Becker-Herman S., Meyer-Bahlburg A., Schwartz M.A., Jackson S.W., Hudkins K.L., Liu C., Sather B.D., Khim S., Liggitt D., Song W., et al. 2011. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J. Exp. Med. 208:2033–2042. 10.1084/jem.20110200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L., Palucka A.K., Arce E., Cantrell V., Borvak J., Banchereau J., and Pascual V.. 2003. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197:711–723. 10.1084/jem.20021553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting K.L., and Melnick A.M.. 2013. New effector functions and regulatory mechanisms of BCL6 in normal and malignant lymphocytes. Curr. Opin. Immunol. 25:339–346. 10.1016/j.coi.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S., Johnston R.J., and Schoenberger S.P.. 2010. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 11:114–120. 10.1038/ni.1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., James R.G., Habib T., Singh S., Jackson S., Khim S., Moon R.T., Liggitt D., Wolf-Yadlin A., Buckner J.H., and Rawlings D.J.. 2013. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J. Clin. Invest. 123:2024–2036. 10.1172/JCI66963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang V.D., Hilgenberg E., Ries S., Shen P., and Fillatreau S.. 2014. From the regulatory functions of B cells to the identification of cytokine-producing plasma cell subsets. Curr. Opin. Immunol. 28:77–83. 10.1016/j.coi.2014.02.009 [DOI] [PubMed] [Google Scholar]
- de Goër de Herve M.G., Durali D., Dembele B., Giuliani M., Tran T.A., Azzarone B., Eid P., Tardieu M., Delfraissy J.F., and Taoufik Y.. 2011. Interferon-alpha triggers B cell effector 1 (Be1) commitment. PLoS One. 6:e19366 10.1371/journal.pone.0019366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., and Tsao B.P.. 2010. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat. Rev. Rheumatol. 6:683–692. 10.1038/nrrheum.2010.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth A.J., Lin L., and Peng S.L.. 2003. T-bet regulates T-independent IgG2a class switching. Int. Immunol. 15:937–944. 10.1093/intimm/dxg093 [DOI] [PubMed] [Google Scholar]
- Giles J.R., Kashgarian M., Koni P.A., and Shlomchik M.J.. 2015. B cell–specific MHC class II deletion reveals multiple nonredundant roles for B cell antigen presentation in murine lupus. J. Immunol. 195:2571–2579. 10.4049/jimmunol.1500792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin A.D., Shulman Z., and Nussenzweig M.C.. 2014. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 509:637–640. 10.1038/nature13300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N.M., Laws A., Kiefer K., Busconi L., Kim Y.M., Brinkmann M.M., Trail E.H., Yasuda K., Christensen S.R., Shlomchik M.J., et al. 2009. Murine B cell response to TLR7 ligands depends on an IFN-β feedback loop. J. Immunol. 183:1569–1576. 10.4049/jimmunol.0803899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C., Ryffel B., and Le Hir M.. 1997. IFN-gamma is essential for the development of autoimmune glomerulonephritis in MRL/Ipr mice. J. Immunol. 158:5484–5491. [PubMed] [Google Scholar]
- Hall J.C., and Rosen A.. 2010. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat. Rev. Rheumatol. 6:40–49. 10.1038/nrrheum.2009.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog P., Forster S., and Samarajiwa S.. 2011. Systems biology of interferon responses. J. Interferon Cytokine Res. 31:5–11. 10.1089/jir.2010.0126 [DOI] [PubMed] [Google Scholar]
- Hou B., Saudan P., Ott G., Wheeler M.L., Ji M., Kuzmich L., Lee L.M., Coffman R.L., Bachmann M.F., and DeFranco A.L.. 2011. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity. 34:375–384. 10.1016/j.immuni.2011.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Z., Gross A.J., Lamagna C., Ramos-Hernández N., Scapini P., Ji M., Shao H., Lowell C.A., Hou B., and DeFranco A.L.. 2014. Requirement for MyD88 signaling in B cells and dendritic cells for germinal center anti-nuclear antibody production in Lyn-deficient mice. J. Immunol. 192:875–885. 10.4049/jimmunol.1300683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.W., Scharping N.E., Kolhatkar N.S., Khim S., Schwartz M.A., Li Q.Z., Hudkins K.L., Alpers C.E., Liggitt D., and Rawlings D.J.. 2014. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J. Immunol. 192:4525–4532. 10.4049/jimmunol.1400098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.W., Kolhatkar N.S., and Rawlings D.J.. 2015. B cells take the front seat: dysregulated B cell signals orchestrate loss of tolerance and autoantibody production. Curr. Opin. Immunol. 33:70–77. 10.1016/j.coi.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Choe J., Li L., and Choi Y.S.. 2000. Regulation of CD27 expression in the course of germinal center B cell differentiation: the pivotal role of IL-10. Eur. J. Immunol. 30:2437–2443. [DOI] [PubMed] [Google Scholar]
- Kiefer K., Oropallo M.A., Cancro M.P., and Marshak-Rothstein A.. 2012. Role of type I interferons in the activation of autoreactive B cells. Immunol. Cell Biol. 90:498–504. 10.1038/icb.2012.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirou K.A., Lee C., George S., Louca K., Peterson M.G., and Crow M.K.. 2005. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 52:1491–1503. 10.1002/art.21031 [DOI] [PubMed] [Google Scholar]
- Kitano M., Moriyama S., Ando Y., Hikida M., Mori Y., Kurosaki T., and Okada T.. 2011. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 34:961–972. 10.1016/j.immuni.2011.03.025 [DOI] [PubMed] [Google Scholar]
- Lamagna C., Hu Y., DeFranco A.L., and Lowell C.A.. 2014. B cell–specific loss of Lyn kinase leads to autoimmunity. J. Immunol. 192:919–928. 10.4049/jimmunol.1301979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.B., Fleischmann R., Hall S., Wilkinson B., Bradley J.D., Gruben D., Koncz T., Krishnaswami S., Wallenstein G.V., Zang C., et al. ORAL Start Investigators . 2014. Tofacitinib versus methotrexate in rheumatoid arthritis. N. Engl. J. Med. 370:2377–2386. 10.1056/NEJMoa1310476 [DOI] [PubMed] [Google Scholar]
- Lee S.K., Silva D.G., Martin J.L., Pratama A., Hu X., Chang P.P., Walters G., and Vinuesa C.G.. 2012. Interferon-γ excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 37:880–892. 10.1016/j.immuni.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Lund F.E. 2008. Cytokine-producing B lymphocytes-key regulators of immunity. Curr. Opin. Immunol. 20:332–338. 10.1016/j.coi.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Deenick E.K., Batten M., and Tangye S.G.. 2012. The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 209:1241–1253. 10.1084/jem.20120994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nündel K., Green N.M., Shaffer A.L., Moody K.L., Busto P., Eilat D., Miyake K., Oropallo M.A., Cancro M.P., and Marshak-Rothstein A.. 2015. Cell-intrinsic expression of TLR9 in autoreactive B cells constrains BCR/TLR7-dependent responses. J. Immunol. 194:2504–2512. 10.4049/jimmunol.1402425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Martinez G.J., Yang X.O., Tanaka S., Matskevitch T.D., Wang Y.H., and Dong C.. 2009. Bcl6 mediates the development of T follicular helper cells. Science. 325:1001–1005. 10.1126/science.1176676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S.L., Szabo S.J., and Glimcher L.H.. 2002. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl. Acad. Sci. USA. 99:5545–5550. 10.1073/pnas.082114899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias L.C. 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5:375–386. 10.1038/nri1604 [DOI] [PubMed] [Google Scholar]
- Pollard K.M., Cauvi D.M., Toomey C.B., Morris K.V., and Kono D.H.. 2013. Interferon-γ and systemic autoimmunity. Discov. Med. 16:123–131. [PMC free article] [PubMed] [Google Scholar]
- Rubtsova K., Rubtsov A.V., van Dyk L.F., Kappler J.W., and Marrack P.. 2013. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc. Natl. Acad. Sci. USA. 110:E3216–E3224. 10.1073/pnas.1312348110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting A., Wada T., Kinoshita K., Tesch G., and Kelley V.R.. 1998. IFN-γ receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Faslpr mice. J. Immunol. 161:494–503. [PubMed] [Google Scholar]
- Seery J.P., Carroll J.M., Cattell V., and Watt F.M.. 1997. Antinuclear autoantibodies and lupus nephritis in transgenic mice expressing interferon gamma in the epidermis. J. Exp. Med. 186:1451–1459. 10.1084/jem.186.9.1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P., Roch T., Lampropoulou V., O’Connor R.A., Stervbo U., Hilgenberg E., Ries S., Dang V.D., Jaimes Y., Daridon C., et al. 2014. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 507:366–370. 10.1038/nature12979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M.J. 2009. Activating systemic autoimmunity: B’s, T’s, and tolls. Curr. Opin. Immunol. 21:626–633. 10.1016/j.coi.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C.M., and Paul W.E.. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 236:944–947. 10.1126/science.3107127 [DOI] [PubMed] [Google Scholar]
- Teichmann L.L., Schenten D., Medzhitov R., Kashgarian M., and Shlomchik M.J.. 2013. Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity. 38:528–540. 10.1016/j.immuni.2012.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S., Mesa R.A., Gotlib J., Levy R.S., Gupta V., DiPersio J.F., Catalano J.V., Deininger M., Miller C., Silver R.T., et al. 2012. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 366:799–807. 10.1056/NEJMoa1110557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora G.D., and Nussenzweig M.C.. 2012. Germinal centers. Annu. Rev. Immunol. 30:429–457. 10.1146/annurev-immunol-020711-075032 [DOI] [PubMed] [Google Scholar]
- Vinuesa C.G., Sanz I., and Cook M.C.. 2009. Dysregulation of germinal centres in autoimmune disease. Nat. Rev. Immunol. 9:845–857. 10.1038/nri2637 [DOI] [PubMed] [Google Scholar]
- Wang N.S., McHeyzer-Williams L.J., Okitsu S.L., Burris T.P., Reiner S.L., and McHeyzer-Williams M.G.. 2012. Divergent transcriptional programming of class-specific B cell memory by T-bet and RORα. Nat. Immunol. 13:604–611. 10.1038/ni.2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann U., Letz M., Herrmann M., Angermüller S., Kalden J.R., and Winkler T.H.. 2005. The evolution of human anti-double-stranded DNA autoantibodies. Proc. Natl. Acad. Sci. USA. 102:9258–9263. 10.1073/pnas.0500132102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehudai D., Snir A., Peri R., Halasz K., Haj T., Odeh M., and Kessel A.. 2012. B cell-activating factor enhances interleukin-6 and interleukin-10 production by ODN-activated human B cells. Scand. J. Immunol. 76:371–377. 10.1111/j.1365-3083.2012.02752.x [DOI] [PubMed] [Google Scholar]