Abstract
The polarization of epithelial cells along an axis orthogonal to their apical-basal axis is increasingly recognized for roles in a variety of developmental events and physiological functions. While now studied in many model organisms, mechanistic understanding is rooted in intensive investigations of Planar Cell Polarity (PCP) in Drosophila. Consensus has emerged that two molecular modules, referred to here as the global and core modules, operate upstream of effector proteins to produce morphological PCP. Proteins of the core module develop subcellular asymmetry, accumulating in two groups on opposite sides of cells, consistent with proposed functions in producing cell polarity and in communicating that polarity between neighboring cells. Less clear are the molecular and cell biological mechanisms underlying core module function in the generation and communication of subcellular asymmetry, and the relationship between the global and core modules. In this review, we discuss these two unresolved questions, highlighting important studies and potentially enlightening avenues for further investigation. It is likely that results from Drosophila will continue to inform our views of the growing list of examples of PCP in vertebrate systems.
Keywords: Planar Cell Polarity, Subcellular asymmetry, Frizzled, Van Gogh, Flamingo, Core module, Global directional cue
Introduction
It is well appreciated that most cells assemble highly polarized structures that are essential for their specialized functions. In epithelial cells, the most obvious polarized feature is the universal apical-basal polarity that distinguishes the cell surface facing the external environment or lumen from that adjacent to the basal lamina. Extensive studies have revealed essential roles of apical-basal polarity in carrying out epithelial function and maintaining tissue homeostasis. At the same time, it has also been appreciated that epithelial cells can be polarized along the tissue surface, on an axis perpendicular to the apical-basal axis. This polarity, called planar cell polarity (PCP), is apparent in many epithelia in multi-cellular organisms. Understanding of the physiological significance of PCP, though often less apparent, has been steadily growing with the recent intensification of molecular genetic studies in various model organisms (Goodrich and Strutt, 2011). These efforts have shown that regulation of cellular function by PCP is important for processes including tissue morphogenesis (Keller, 2002), directional cell migration (Wada and Okamoto, 2009), and directional mechano-sensing (Kelly and Chen, 2007), and will be discussed by other reviews in this volume.
While control of planar cell polarity is largely distinct from that of apical-basal polarity, the core family of planar cell polarity proteins localize and appear to act apically at the adherens junctions (Goodrich and Strutt, 2011). As first discovered during acquisition of planar polarity in the Drosophila wing epithelium (Axelrod, 2001; Strutt, 2001), those proteins become asymmetrically localized in a highly stereotypical manner, such that a distal subset localizes at the distal cell cortex and interacts with a proximal subset in the neighboring cell, and vice versa, resulting in the polarized localization of both proximal and distal components within each cell (Vladar et al., 2009). In this review, we focus on our current understanding of the mechanisms that give rise to this asymmetric protein localization. We suggest that asymmetric protein localization is a characteristic and essential feature of planar polarized epithelia, based on a growing list of examples from both invertebrate and vertebrate systems. The majority of this review will discuss possible cell-autonomous and non-cell-autonomous mechanisms through which asymmetric protein localization arises. Our current understanding is based largely on experimental studies with Drosophila wing epithelium, in combination with mathematical simulations that examine the properties of proposed models. While studies with vertebrate models have to date yielded less mechanistic insight, numerous observations suggest substantial mechanistic conservation (Mitchell et al., 2009; Sienknecht et al., 2011).
We begin with a brief discussion of the three modules of planar polarity genes, and propose a hierarchical structure, a model first developed almost a decade ago (Tree et al., 2002a). Despite the elapsed time, the mechanisms underlying this organization have not been revealed. We believe that the model has proven to be a valid general framework for understanding planar cell polarity, despite some recent challenges, and that clarifying mechanisms will soon emerge. Given its controversial nature, the model deserves a quick revisit here.
Revisiting the three-tiered hierarchy of planar cell polarity
Original three-tiered hierarchy model
The existence of planar polarized features of many types of epithelial structures has enabled extensive genetic studies of the genes and molecular pathways that control planar cell polarity. On the basis of phenotype, as well as genetic interaction, cell biological and biochemical studies, these components can be classified as belonging to one of three distinct functional modules. We have argued previously that these three modules interact hierarchically (Tree et al., 2002a).
A highly conserved core module (including the proteins Frizzled (Fz), Dishevelled (Dsh), Van Gogh (Vang, aka Stramismus), Prickle (Pk), Flamingo (Fmi), and Diego (Dgo) produces molecular asymmetry within and between cells. Distinct proximal (Vang, Pk and Fmi) and distal (Fz, Dsh, Dgo and Fmi) complexes segregate to opposite sides of the cell, where they interact with the opposite complex in the neighboring cell at or near the adherens junctions. Feedback mechanisms between components of this key module ensure exclusive asymmetric protein localization during planar polarization by exclusion of oppositely oriented complexes from adjacent regions of the cell cortex, and by recruitment of the opposite complex in the neighboring cell (Goodrich and Strutt, 2011) The result is an amplification of localization asymmetry of these core PCP proteins, producing steep intracellular gradients from any initial biasing input. This polarity amplification is necessarily coupled with local alignment of polarity between neighboring cells (Amonlirdviman et al., 2005). The model predicts the observed interdependence of the asymmetric localization of each core PCP proteins (Bastock et al., 2003; Tree et al., 2002b), although depending on specific molecular mechanisms involved, allows for different degrees of residual function and polarization in the individual mutants (Axelrod, 2009). The majority of this review focuses on this core signal amplification module. As originally proposed,, a global module acts at the top of the hierarchy to provide directional information to orient polarization with respect to the tissue axes. In many vertebrate systems, PCP signaling relies on secreted Wnts, leading to the proposal that a global Wnt concentration gradient might directly provide such a directional cue (Goodrich and Strutt, 2011; Vladar et al., 2009; Wansleeben and Meijlink, 2011). The strength of the data for these assertions varies, but in at least some cases makes a reasonably strong argument. In contrast, in Drosophila, strong evidence argues against a direct contribution of Wnts to planar polarity (Casal et al., 2002; Chen et al., 2008; Lawrence et al., 2002). Instead, global directional cues are provided by a system involving oppositely oriented gradients of differential gene expression across the tissue axes. This module comprises the proteins Fat and Dachsous (Ft and Ds; both atyipcial cadherins) and Four-jointed (Fj; a golgi ectokinase) (Strutt, 2009). Ft and Ds form heterodimers that can orient in either direction at a given cell-cell interface. Fj acts on both Ft and Ds, making Ft a stronger ligand for Ds, and Ds a weaker ligand for Ft (Brittle et al., 2010; Simon et al., 2010). Ds and Fj are expressed in opposing gradients in each of the well-studied polarizing tissues in Drosophila (Casal et al., 2002; Ma et al., 2003; Yang et al., 2002), and are proposed to result in a biased orientation of Ft-Ds heterodimers at intercellular boundaries reflecting the direction of the Fj and Ds expression gradients (Simon, 2004; Strutt, 2009). This mechanism produces a subtle gradient of Ft activity within each cell, and epistasis studies in the eye suggest that Ft provides the critical output signal from this module (Yang et al., 2002). In other words, the mechanism converts tissue-wide expression gradients into subcellular gradients of Ft activity.
A distinguishing characteristic of phenotypes displayed by global module mutants is the presence of significant defects in planar polarity at the level of orientation, but with essentially all cells achieving full molecular and morphological asymmetry, thus distinguishing these mutants from mutants in the core and tissue-specific modules, in which molecular and morphological asymmetry are typically reduced or abolished. Perhaps the most illustrative example is the phenotype seen in large ft clones in the wing (Ma et al., 2003). Within these clones, the prehairs form complex swirling patterns, whereas wild-type hairs form parallel arrays. Furthermore, molecular polarization at the level of the core proteins remains intact, although mis-oriented, indicating that the mutant cells polarize, but no longer recognize the tissue axes (Ma et al., 2003). Additional evidence that the core module continues to polarize cells when the global module is disrupted comes from the observation that small ft mutant clones on Drosophila wings display normal polarity (Ma et al., 2003); the local alignment property of the core module aligns the mutant cells with each other (to form swirls), and with the polarity of the nearby wild-type cells outside the clone (to align with the tissue axis if the clone is small). Thus, the global module provides directionality to PCP, but is not required for cell polarization per se, provided the core module is intact.
It is important to point out that although the Ft/Ds/Fj global module as described contributes to PCP in all Drosophila tissues so far studied, several observations indicate that the current model is missing important pieces. When the Ds and Fj gradients are experimentally flattened, polarity of the ommatidia in the fly eye is severely disrupted as predicted (Simon, 2004). In contrast, planar polarity of the wing epithelium is more modestly affected, with much of the wing retaining a predominantly distal polarity. What accounts for the remaining distal directionality? While Ft is clearly important for global polarization, as evidenced by the disrupted polarity in loss-of-function ft clones, an extracellularly truncated fragment of Ft that cannot interact with Ds, and should therefore not be responsive to the Ds or Fj gradients, can rescue both the viability of ft mutant animals, and produce distal polarity in a substantial, predominantly distal, portion of the wing (Matakatsu and Blair, 2006; and our unpublished observations). From these observations, it is reasonable to conclude that there is an additional directional signal distinct from the gradients of Ds and Fj. Two more recent studies also provide support for the notion that additional polarizing signals are present, being organized by unknown cues at the dorsal-ventral boundary and perhaps also the anterior-posterior boundary (Brittle et al., 2012; Sagner et al., 2012). The nature of such a signal(s) is unknown. Resolution of these mysteries awaits further studies, as does determining whether any of these signals contribute to global PCP signaling in vertebrates, and if so, how their role relates to that of Wnt proteins.
Potential mechanisms by which the global module, as well as additional unidentified global cues, might transmit directional information to the core module remain poorly understood. One plausible postulate derives from the observation of a polarized apical microtubule network that is seen to have a slight excess of plus-ends on one side of the cell (Eaton et al., 1996; Shimada et al., 2006). Vesicles containing Fz have been observed to traverse the cell in a microtubule dependent fashion, moving in a plus-end biased direction (Shimada et al., 2006). Preliminary evidence suggests that the subcellular Ft gradient produced by the action of graded Ds and Fj expression (and recently visualized where the gradients are steep; Brittle et al., 2012) may produce the bias of this microtubule network. Perturbation of Ds, both by mutation and overexpression, produces alteration of microtubule orientation, providing at least some evidence that the global module orients the core module by organizing microtubules (Harumoto et al., 2010). Although appealing, this potential mechanism requires substantial additional validation. Whether additional unidentified global cues might work through regulating apical microtubules remains purely speculative.
At the bottom of the hierarchy reside the tissue specific effectors. Distinct sets of components are responsible for translating the molecular asymmetry of the core PCP proteins to the specific polarized outputs required in each tissue. These range from asymmetric cytoskeletal organization to asymmetric cell fate determination. Mutating these components therefore causes planar polarization defects of certain structures. Because these effectors are at the bottom of the hierarchy, their malfunction in general affects the polarized readouts without compromising the protein asymmetry, and thus the function, of the core PCP genes (Adler et al., 2004). Recently, some core PCP proteins have been suggested to be directly involved in polarizing cellular structures. For example, in vertebrate multi-ciliated cells, Dvl2 (a Dsh homolog) is associated with basal bodies of apical motile cilia, though direct evidence that it contributes to polarization of those cilia is thus far lacking (Park et al., 2008).
Reasons to reconsider?
A three-tiered hierarchy was initially proposed based on a variety of observations. The core module was proposed to regulate the tissue specific components in part based on their tissue specificity (Adler et al., 2000; Lee and Adler, 2002; Strutt et al., 2002) and on epistasis suggestive of this architecture (Lee and Adler, 2002; Yang et al., 2002). Upon its description, the global module was placed upstream of the core module by epistasis experiments in the eye (Yang et al., 2002), and by the simple yet powerful observation that core PCP proteins are incorrectly aligned within global mutant wing clones (Ma et al., 2003).
This three-tiered hierarchy model suggests a linear relationship between the global, core and tissue specific modules, in which the global module translates relatively shallow transcriptional gradients into subtle subcellular gradients, the core simultaneously amplifies subcellular asymmetry and locally aligns polarity, and the tissue specific modules read out polarity cues to produce morphological or cell fate asymmetry. Though the linear relationship of the modules can be inferred from the above observations, the nature of the molecular interactions between the three tiers remains largely unknown, and the lack of detailed mechanistic knowledge of the information flow between modules leaves open the possibility of other architectures for the relationship between modules.
The most direct challenges to a strictly linear three-tiered hierarchy model come from genetic studies of denticle polarity in the Drosophila adult abdomen and larval epidermis (Casal et al., 2006; Donoughe and DiNardo, 2011; Repiso et al., 2010). Two main observations have been proposed to be incompatible with the linear model. First, in both larval denticles and adult abdomen, double mutants constructed between components of the upstream global module and the core amplification module display stronger polarity defects than single mutants of each module alone (Casal et al., 2006; Donoughe and DiNardo, 2011; Repiso et al., 2010). Such enhancement of mutant phenotype has been argued to suggest that the upstream module and the core module can affect the downstream effectors (controlling denticle polarity) in parallel. Second, overexpression of upstream module components has been shown to alter denticle polarity in the abdomen even in the absence of an intact core signal amplification module (Casal et al., 2006). This was similarly interpreted to suggest the existence of a direct link from global directional cue to the tissue specific polarity readout. While it is plausible that the linear three-tiered model is indeed an incorrect universal description of planar polarity signaling, as these interpretations suggest, we argue that there is also an important interpretive flaw that renders the conclusions of both experiments ambiguous. A parallel relationship between the modules was inferred from the absence of an epistasis relationship between the global and core components; however, the mutants of the core module selected for these experiments might not fully abolish core module function, and therefore the observed results could be compatible with either a linear or non-linear (parallel) architecture between the modules. Given the available evidence, we think that the linear model remains most valid as a blueprint of the relationship between modules, but it is likely that only the emergence of molecular level understanding of signal transmission between modules will solidify (or eliminate) the linear three-tiered model. A more detailed discussion of this problem has been published elsewhere (Axelrod, 2009). A summary of the revised hierarchical model as described above can be found in Figure 1.
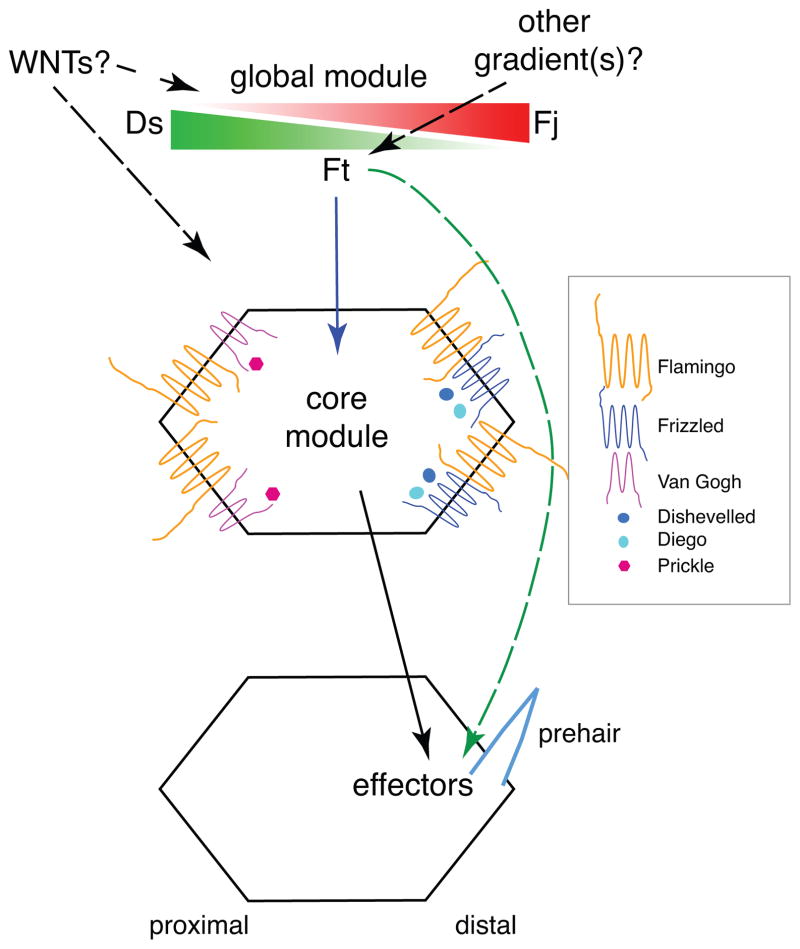
Figure 1.
Heirarchical model of the PCP signaling pathway. The pathway consists of three modules, the global, core, and tissue specific effector modules. According to the series model, the global module provides directional input to the core module (blue arrow) that establishes and amplifies subcellular asymmetry. This subcellular asymmetry is used to direct tissue specific effector module function within the cell. According to the parallel model, the global module communicates directly with the tissue specific effector module (green dashed arrow), without signaling to the core module. Directional information for the global module comes from tissue level expression gradients of Ds and Fj, but it is likely that other gradients are also important, at least in the wing (black dashed arrow). While Wnt proteins seem not to play a direct role in PCP signaling in Drosophila, they appear to do so in vertebrates (grey dashed arrows). Precisely how Wnt’s affect vertebrate PCP is unclear. Asymmetrically segregated core PCP proteins are shown. Various effector modules produce different tissue specific responses. Here, effectors establish the distal location for growth of a prehair, as on the wing and abdomen.
Asymmetric protein localization: a hallmark of planar cell polarity
Because regular planar polarized arrays of asymmetrically constructed cellular structures on the surface of epithelial cells have been appreciated for some time, the asymmetrically localized distribution of core PCP proteins suggested a striking feature of the planar cell polarity signaling mechanism that might underlie the molecular polarization of these cells. Indeed, we now believe that the segregation of these proteins to opposite sides of the cell is intimately linked to the mechanism that amplifies an initial input bias and locally aligns polarity between cells. Since its initial discovery in the Drosophila pupal wing epithelium (Axelrod, 2001; Strutt, 2001), similar asymmetrically localized distributions of core PCP proteins have been found in a substantial number of planar polarized epithelial types in both invertebrates and vertebrates.
In the developing fly eye disc, PCP proteins are found differentially enriched at the adherens junction between the two cells that will differentiate asymmetrically to become the R3 and R4 photoreceptor cells in each developing ommatidium (Das et al., 2002; Rawls and Wolff, 2003; Strutt et al., 2002). The enrichment of the Fz/Dsh complex on the prospective R3 cell side is thought to impose an initial bias that inhibits Notch signaling in this cell, biasing the asymmetric, lateral inhibition-mediated R3/R4 fate decision between the two equipotent precursors (Strutt et al., 2002). Asymmetric PCP protein localization was also found in the sensory organ precursor cells on the developing thorax (Bellaiche et al., 2004; Bellaiche et al., 2001). Vang is enriched on the anterior cortex while Fz is predominantly localized on the posterior side of the sensory organ precursor (SOP) cell. Their asymmetric localization predefines the anterior/posterior membrane domains, which subsequently determine the axis of SOP division and the asymmetric distribution of determinants of the daughter cell fates (Bellaiche et al., 2004; Bellaiche et al., 2001).
The generality of such polarized protein localization was supported by recent investigations on various vertebrate epithelia. In the ventral node of early mouse embryos, restricted localization of Vangl1, Vangl2, Pk2, Dvl2, and Dvl3 was observed prior to the posterior localization of the basal body (Antic et al., 2010; Hashimoto et al., 2010). Asymmetric localization of the basal body results in tilted growth of the motile primary cilium, thereby generating leftward directional fluid flow, which in turn gives rise to the left/right asymmetry of the body axis (Hashimoto et al., 2010; Song et al., 2010). Epidermal cells forming hair follicles on the mammalian skin also demonstrate planar polarized features: Celsr1, Vangl2 and Fz6 were found to localize asymmetrically. Through a mechanism not yet understood, the action of these asymmetrically localized proteins results in asymmetry within the developing follicle, producing a planar polarized tilt to hair growth (Devenport and Fuchs, 2008). In the auditory and vestibular epithelia in the inner ear of developing chicken and mouse embryos, asymmetric PCP protein crescents were observed both in the mechano-sensing hair cells and surrounding supporting cells, which is thought to be responsible for polarized positioning and orientation of the kinocilium and stereocilia required for correct mechanotransduction (Davies et al., 2005; Deans et al., 2007; Montcouquiol et al., 2006; Wang et al., 2006).
Similar protein asymmetry was also evident in several epithelial types with multiple, planar polarized, motile cilia. Ependymal cells on the ventricular lining of the brain, for example, demonstrate asymmetric Vangl2 localization, which is dependent on the Fmi orthologues Celsr2 and Celsr3 (Guirao et al., 2010; Tissir et al., 2010). Another well characterized example is the multi-cillated tracheal epithelial cells, where almost all known PCP proteins show asymmetric localization to opposite apical proximal and distal crescents, closely mimicking what we have learned from developing fly wing epithelium (Vladar et al., unpublished). It should be noted, however, that numerous other developmental events in vertebrates have been described to be under control of the PCP genes, or a set thereof, but for which robust asymmetric localization has not been observed (Vladar et al., 2009). These are typically non-epithelial, and how mechanistically similar they are to the epithelial PCP described here remains to be determined.
The ways and means to planar polarize a cell: mechanisms of achieving asymmetry
The process of segregating the proximal and distal PCP proteins to opposite regions of the adherens junction creates distinct domains at the cell cortex. Achieving this segregation requires an energy investment to overcome entropy. In this section, we discuss the active mechanisms through which such asymmetry is achieved and maintained.
Required components as revealed by genetics
As discovered in the Drosophila wing epithelium, a well-recognized and apparently conserved feature of the asymmetric protein localization mechanism is interdependence among the core PCP proteins for their asymmetric localizations (Goodrich and Strutt, 2011). The fully manifest asymmetric localization of each core PCP protein depends on the intact function of each of the other core proteins, suggesting a tight feedback-based mechanism.
Six proteins have been identified as important for establishing PCP, and whose localization satisfies these criteria. The atypical cadherin Flamingo (Fmi) was the first protein observed in a PCP-specific asymmetric localization, enriched on both the proximal and distal cortex of every cell during planar polarization of the wing (Shimada et al., 2001; Usui et al., 1999). Unipolar asymmetry was first independently seen for the seven-pass transmembrane protein Frizzled (Fz) (Strutt, 2001) and the cytosolic protein Dishevelled (Dsh) (Axelrod, 2001), both of which are enriched on the distal cortex of each wing epithelial cell. This distally localized PCP complex was later found to include Diego, another cytosolic protein with ankyrin repeats) (Das et al., 2004). On the opposite cell cortex, the four-pass transmembrane protein Vang (also known as Stbm) and the LIM-domain cytosolic protein Prickle (Pk) are enriched proximally (Bastock et al., 2003; Tree et al., 2002b). Importantly, correct apical localization of all six of these core PCP proteins at the adherens junction depends on the presence and function of the others. Protein localization asymmetry builds up slowly during fly wing development, beginning in the third instar, and showing the most prominent asymmetry during the hours just prior to the outgrowth of trichomes. After the planar polarized outgrowth of wing hairs, asymmetry is quickly lost.
Domineering nonautonomy: how to talk to your neighbor
Well prior to the characterization of PCP mutants in flies and the availability of modern genetic tools to make mosaic clones, transplantation experiments in larger insects showed that planar polarities in neighboring cells can influenced each other in a cell non-autonomous manner (Lawrence, 1975). Non-autonomy is now most readily observed using genetic mosaics to examine polarity in and around a clone of cells missing or over-expressing a PCP gene of interest. Of the six core proteins, loss-of-function clones of fz and vang strongly influence the polarity of neighboring non-mutant tissue, referred to as domineering non-autonomy (Adler et al., 2000; Casal et al., 2002; Taylor et al., 1998; Vinson and Adler, 1987). These observations clearly indicate that polarity disruption at a clone boundary can propagate between cells, and the influence of these effects can reach as far as tens of cells away from the clone boundary.
Associated with and underlying the non-autonomy seen in hair polarity patterns, Fz and Vang have been experimentally shown to recruit each other to adjacent sides of intercellular junctions in neighboring cells (Figure 2a)(Amonlirdviman et al., 2005; Bastock et al., 2003; Chen et al., 2008; Strutt and Strutt, 2009). In wild type polarized fly wing tissue, Fz crescents, on the distal edge of the cell, lie juxtaposed to the Vang enriched crescents on the proximal edge of the neighboring cells. This relationship between Fz and Vang holds true for each case of clonal manipulation examined in fly wings: Fz or Vang is recruited to the clonal boundary when its counterpart is over-expressed within the clone, and is absent on the clonal boundary if its counterpart is missing within the clone. A great deal of attention has been paid to this highly specific interaction between neighboring cells, which provides an elegant model to explain how a planar polarity signal propagates from cell to cell, serving to align polarity within a field and produce a coherent and error-free response (Amonlirdviman et al., 2005; Ma et al., 2003). Recent observations on both morphological and molecular polarity propagation in vertebrate systems suggest that this mechanism of domineering non-autonomy is conserved beyond the insect kingdom (Mitchell et al., 2009; Sienknecht et al., 2011).
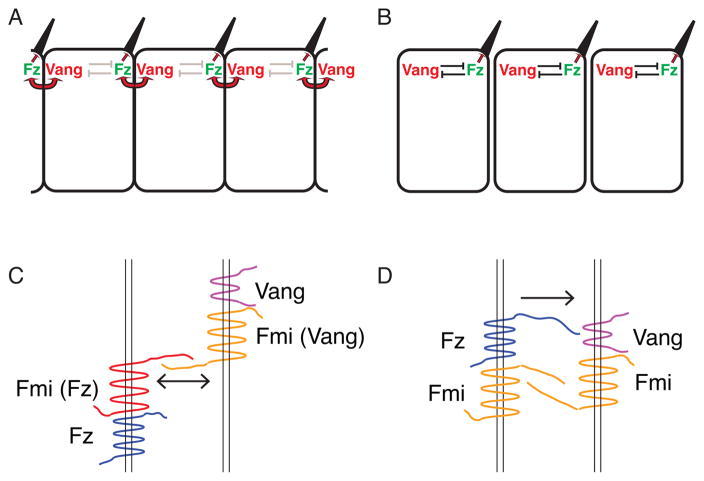
Figure 2.
Generation and amplification of asymmetry by the core module. (A) Intercellular signaling. Mutual intercellular recruitment between Fz in one cell and Vang in the neighboring cell (red arrows) is required for core module function. Initially, Fz and Vang are distributed uniformly around the adherens junctions, but become segregated to proximal and distal sides, as shown. (B) Intracellular signaling. Segregation of Fz and Vang requires mutual repulsion that requires activity of the cytoplasmic core proteins. The mechanism of this repulsion is not well defined. (C, D). Two models for the mutual intercellular recruitment between Fz and Vang. Chen et al., (2008) (C) propose that recruitment depends on asymmetric signaling through Flamingo homodimers in which each monomer adopt different functional states. The state associated with Vang (orange) is the basal state, while the state associated with Fz (red) is induced by interaction with Fz. No contact between Fz and Vang is required. In contrast, Wu et al., (2008) (D) propose unidirectional signaling that requires physical interaction between Fz and Vang, while symmetric Fmi homodimers scaffold the interaction.
Because of the central importance of this unique feature of PCP signaling, several groups independently investigated how specific interactions might mediate recruitment between the proximal and distal complexes. Through a variety of genetic experiments including manipulating PCP genes at clone boundaries, it was concluded that only three core PCP components are necessary for the mutual recruitment between Fz and Vang. Besides Fz and Vang themselves, the atypical cadherin Fmi is needed on both side of the adherens junction for this interaction to occur (Chen et al., 2008; Strutt and Strutt, 2007; Strutt, 2001; Strutt and Strutt, 2008). Since mutant forms of Fz and Vang lacking the majority of their extracellular domains can still recruit each other in the presence of Fmi, and since Fmi in a cell lacking either Fz or Vang recruits Fz from the neighboring cell, Chen et al., favored the proposal that the signal for the mutual recruitment between distal and proximal complexes is transmitted via Fmi dimers formed at the adherens junction, but not likely via direct interactions between Fz and Vang (Figure 2C) (Chen et al., 2008). This hypothesis raises the puzzling problem of how Fmi homodimers might transduce information asymmetrically such that a dimer associated with Fz on one side of the adherens junction selectively recruits Vang in the neighboring cell, and vice versa. Functional evidence for this asymmetry comes from the observation that the behavior of Fmi expressed in one cell to recruit Fz in the neighboring cell can be shifted by the presence of Fz to favor recruiting Vang in the neighboring cell (Chen et al., 2008). Though the physical basis for this asymmetry is not yet known, asymmetric conformations, post-translational modifications or unequal stoichiometries are possible explanations. While this asymmetric Fmi homodimer model remains our favored blueprint for further exploration of PCP signaling across the adherens junction, several groups have suggested alternative mechanisms. While it is universally agreed that the mutual recruitment between Fz and Vang complexes depends on the involvement of Fmi homodimers, there is disagreement regarding whether Fmi dimers asymmetrically transmit information between cells, or act solely act as a structural bridge to stabilize the Fz-Vang interaction, through which such information might flow. Wu et al., have presented evidence for a direct interaction between the extracellular CRD domain of Fz and Vang in vitro (Lawrence et al., 2004; Wu and Mlodzik, 2008). Physiological relevance of this interaction was argued based on the loss of PCP signaling observed when the CRD of Fz was replaced with that of the PCP-irrelevant Drosophila Fz2 gene. This negative result is discrepant with Chen et al.’s observation that a Fz derivative lacking the CRD can provide some PCP signaling activity. These dramatic differences in rescuing efficiency might reflect differences in protein expression or folding efficiency, and remain an important puzzle to be resolved.
Another clear distinction between the two models just described involves the directionality of polarity information transmission. According to the asymmetric Fmi homodimer model, polarity information flows in both directions such that cells on either side respond to the other (Figure 2C). The direct Fz-Vang interaction model as articulated by several groups argues for a unidirectional information flow in which Vang does not send information across the adherens junction but instead acts only as signaling receptor for the CRD domain of Fz (Figure 2D) (Lawrence et al., 2004; Wu and Mlodzik, 2008). This unidirectional model, however, does not readily explain how (at borders of fz - vang twin-clones) cells expressing Vang but not Fz cause repolarization of adjacent cells expressing Fz but not Vang (Strutt and Strutt, 2007).
A third, independent, investigation, by Strutt et al., contributed observations to this discussion that, while not providing resolution, must be accommodated by a correct model (Strutt and Strutt, 2008). Consistent with the asymmetric Fmi homodimer model, Strutt et al., presented evidence suggesting that Fmi exists in two alternative forms, either preferentially interacting with Fz, or with Vang, with the bias involving differential binding to the C-terminal cytoplasmic tail of Fmi. Furthermore, they found that Fmi preferentially binds Fz, rather than Vang, when present in excess. This differential affinity is in accordance with the observation that when produced in excess, Fmi molecules behave as though associated with Vang, and recruit Fmi-Fz complexes on the other side of the adherens junction (Chen et al., 2008). Furthermore, Strutt and Strutt observed that stable intercellular Fmi complexes form when neither cell expresses Vang and one cell expresses Fz, but not when neither cell expresses Fz and one expresses Vang. Because one side of the complex must have Fz, this result is consistent with the model of Chen et al. in which Fz induces a form of Fmi that selectively interacts with Fmi that is or is not associated with Vang.
The debate about how Fz and Vang recruit each other across intercellular boundaries is likely to continue as additional studies explore the interactions between Fz, Vang and Fmi at the adherens junctions. We expect that elucidating the biochemical characteristics and conformational nature of Fmi dimers will be a key milestone pushing our understanding of planar polarity propagation to a new mechanistic level.
Autonomous choices: focusing within a single cell
While much has been learned about the mutual recruitment between the distal Fz complexes and proximal Vang complexes (Figure 2A), it has yet to be rigorously determined whether these cross-junctional interactions can be solely responsible for achieving asymmetric protein localization in a self-organizing way. Mathematical modeling can simulate the acquisition of PCP protein asymmetry based on the mutual recruitment between Fz and Vang containing complexes in neighboring cells. To do so, the model must include repulsive interactions between the proximal and distal complexes within the same cell, and indeed, data implicating such interactions in part motivated the initial proposal of the feedback loop (Tree et al., 2002b). Additional genetic analyses have begun to define the component requirement and mechanism of this cell autonomous repulsion (Figure 2B). Three cytoplasmic core PCP genes, Dsh, Dgo and Pk, which are not required for the assembly of asymmetric Fz,Vang and Fmi containing intercellular complexes, are almost certainly playing essential roles in the cell autonomous repulsion between proximal and distal complexes required for amplifying asymmetry in a self-organizing fashion. When any of these three genes’ function is compromised, the mutant cell loses PCP protein asymmetry in a largely cell autonomous way.
These three cytoplasmic PCP genes also differ from Fz/Vang/Fmi in terms of the temporal requirement for their function. Fz/Vang/Fmi function are required from ~6h APF in fly pupual development for proper planar polarization of the wing tissue. Although they may act earlier, proper polarization can be achieved when Pk and Dsh function are present only after 16h APF.
Multiple lines of evidence indicate that the cytoplasmic core proteins stabilize or enhance accumulation of intercellular complexes, and because they act within the cell, the simplest explanation is that they act cell autonomously. The first comes from genetic experiments in which any one of these three PCP proteins is overexpressed within a clone of wing cells (Bastock et al., 2003; Das et al., 2004; Tree et al., 2002b). A substantial enhancement of protein localization at adherens junctions within the clone is observed, for most if not all other core PCP proteins. This suggests that these cytoplasmic factors can reinforce stable intercellular complex formation, either directly or indirectly. Among these, it is especially interesting to point out that overexpressing Dgo autonomously enhances clustering of Fmi molecules at the adherens junction (Jenny et al., 2005). Notably, accumulation of PCP complexes is similarly non-uniform at wild type junctions. These clusters likely resemble the recently reported discrete membrane domains undergoing unique protein turnover dynamics (Strutt et al., 2011).
These observations of enhancement of cortical PCP complex localization do not offer any direct hints toward how the cytoplasmic PCP proteins promote the amplification of asymmetry during polarization. One hint comes from the finding that at least some of these factors demonstrate mutually exclusive biochemical interactions. For example, both Pk and Dgo bind to Dsh in vitro. However, the Dsh-Pk interaction is strongly inhibited by the presence of Dgo, likely through competition for the same Dsh binding site (Jenny et al., 2005). We are just at the beginning of understanding what is likely a complex core PCP protein interaction network. Furthermore, it will be a considerable challenge to correlate the in vitro findings to the physiological roles of any specific interaction in vivo, where the network structure includes feedback mechanisms making predictions of pattern outcomes non-intuitive. We postulate that at least some of these protein interactions involving the cytoplasmic core PCP factors mediate the cell autonomous repulsive interactions between the Fz-containing distal complex and the Vang-containing proximal complex.
Additional cell autonomous mechanisms are likely required by each polarizing cell to interpret and transduce directional signals from the global module, or from other sources of directional information, to downstream modules. Some recent results suggest that a polarized apical microtubule network in fly wing cells may be oriented by signals from the global module, and in turn influence directional trafficking of vesicles containing core PCP proteins (Harumoto et al., 2010). Whether and how this aspect of cell autonomous regulation contributes to the establishment of core PCP protein asymmetry awaits more detailed exploration.
Building a unifying mechanism
Given the robust establishment of asymmetric core PCP protein localization in numerous polarized cell types in the animal kingdom, we postulate that the set of cell autonomous and non-autonomous mechanisms described in this review are conserved, at least in their essential mechanistic logic. During the decade since PCP protein localization asymmetry was initially discovered, the contribution of these process to the establishment of asymmetry has been intensively studied. Mechanistic molecular details of many of these processes have begun to emerge. Yet, despite the considerable progress, a relatively large amount is still to be learned about this fascinating process.
Genetic experiments, relying heavily on clonal analyses, have led to a model of the core PCP mechanism in which asymmetric complexes assembled between neighboring cells transmit polarity information between cells, serving both to amplify asymmetry once it is initiated, and to produce local polarity alignment between neighbors. Based on this basic biological principle, mathematical modeling, in various forms, has shown by mimicking the characteristic patterns of PCP protein localization in wild type as well as mutant genetic mosaic wings, that this kind of mechanism is a plausible description of how the core PCP module functions (Amonlirdviman et al., 2005; Burak and Shraiman, 2009; Le Garrec et al., 2006; Schamberg et al., 2010; Webb and Owen, 2004; Zhu, 2009). By continuing to combine modeling with biological experimentation, it should be possible to derive a much deeper understanding of the specific molecular interactions that underlie the feedback mechanism.
The nature of the global directional cue remains controversial. While we prefer a series model in which overall directional information comes from the Ft/Ds/Fj module and acts to bias the directionality of the core module, others argue for a parallel architecture in which each of these modules acts directly on the readout modules. In the series model, directionality comes from expression gradients, and while this could explain a variety of observations, it has shortcomings that must be addressed. In contrast, the parallel model requires that the core module acquire directionality via another, as yet undescribed, mechanism.
In the upcoming decade, we expect to see an even more integrative and interactive approach between experimental approaches including genetic, cell biological and biochemical methods, and increasingly sophisticated mathematical modeling techniques. Increasingly precise understanding will allow us to devise sophisticated genetic manipulations in vivo, that will aim to isolate and test a specific process that contributes to the polarization mechanism. More powerful experimental methods will begin to yield a much more detailed understanding of each molecular pathway and specific protein interaction, and ever more sophisticated modeling methods will contextualize their contributions to the PCP protein localization process and the eventual asymmetric outcome.
Acknowledgments
Work in the Axelrod lab is supported by grants from NIH/NIGMS. We thank Dr. Yi Guo for her artistic input and assistance preparing the figures.
References
- Adler PN, Taylor J, Charlton J. The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech Dev. 2000;96:197–207. doi: 10.1016/s0925-4773(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Adler PN, Zhu C, Stone D. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr Biol. 2004;14:2046–2051. doi: 10.1016/j.cub.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS One. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol. 2009;20:964–971. doi: 10.1016/j.semcdb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Bellaiche Y, Beaudoin-Massiani O, Stuttem I, Schweisguth F. The planar cell polarity protein Strabismus promotes Pins anterior localization during asymmetric division of sensory organ precursor cells in Drosophila. Development. 2004;131:469–478. doi: 10.1242/dev.00928. [DOI] [PubMed] [Google Scholar]
- Bellaiche Y, Gho M, Kaltschmidt JA, Brand AH, Schweisguth F. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat Cell Biol. 2001;3:50–57. doi: 10.1038/35050558. [DOI] [PubMed] [Google Scholar]
- Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr Biol. 2010;20:803–810. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle A, Thomas C, Strutt D. Planar polarity specification through asymmetric subcellular localization of Fat and Dachsous. Curr Biol. 2012;22:907–914. doi: 10.1016/j.cub.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burak Y, Shraiman BI. Order and stochastic dynamics in Drosophila planar cell polarity. PLoS Comput Biol. 2009;5:e1000628. doi: 10.1371/journal.pcbi.1000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J, Struhl G, Lawrence PA. Developmental compartments and planar polarity in Drosophila. Curr Biol. 2002;12:1189–1198. doi: 10.1016/s0960-9822(02)00974-0. [DOI] [PubMed] [Google Scholar]
- Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, Nusse R, Axelrod JD. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–1105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- Das G, Reynolds-Kenneally J, Mlodzik M. The atypical cadherin Flamingo links Frizzled and Notch signaling in planar polarity establishment in the Drosophila eye. Dev Cell. 2002;2:655–666. doi: 10.1016/s1534-5807(02)00147-8. [DOI] [PubMed] [Google Scholar]
- Davies A, Formstone C, Mason I, Lewis J. Planar polarity of hair cells in the chick inner ear is correlated with polarized distribution of c-flamingo-1 protein. Dev Dyn. 2005;233:998–1005. doi: 10.1002/dvdy.20376. [DOI] [PubMed] [Google Scholar]
- Deans MR, Antic D, Suyama K, Scott MP, Axelrod JD, Goodrich LV. Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear. J Neurosci. 2007;27:3139–3147. doi: 10.1523/JNEUROSCI.5151-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoughe S, DiNardo S. dachsous and frizzled contribute separately to planar polarity in the Drosophila ventral epidermis. Development. 2011;138:2751–2759. doi: 10.1242/dev.063024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton S, Wepf R, Simons K. Roles for Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila. J Cell Biol. 1996;135:1277–1289. doi: 10.1083/jcb.135.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, Mirzadeh Z, Cremer H, Montcouquiol M, Sawamoto K, Spassky N. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, Uemura T. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19:389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, Hamada H. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol. 2010;12:170–176. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Kelly M, Chen P. Shaping the mammalian auditory sensory organ by the planar cell polarity pathway. Int J Dev Biol. 2007;51:535–547. doi: 10.1387/ijdb.072344mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA. The structure and properties of a compartment border: the intersegmental boundary in Oncopeltus. Ciba Found Symp. 1975;0:3–23. doi: 10.1002/9780470720110.ch2. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Casal J, Struhl G. Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development. 2002;129:2749–2760. doi: 10.1242/dev.129.11.2749. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131:4651–4664. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]
- Le Garrec JF, Lopez P, Kerszberg M. Establishment and maintenance of planar epithelial cell polarity by asymmetric cadherin bridges: a computer model. Dev Dyn. 2006;235:235–246. doi: 10.1002/dvdy.20617. [DOI] [PubMed] [Google Scholar]
- Lee H, Adler PN. The function of the frizzled pathway in the Drosophila wing is dependent on inturned and fuzzy. Genetics. 2002;160:1535–1547. doi: 10.1093/genetics/160.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 2009;19:924–929. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, Murdoch J, Warchol ME, Wenthold RJ, Kelley MW. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls AS, Wolff T. Strabismus requires Flamingo and Prickle function to regulate tissue polarity in the Drosophila eye. Development. 2003;130:1877–1887. doi: 10.1242/dev.00411. [DOI] [PubMed] [Google Scholar]
- Repiso A, Saavedra P, Casal J, Lawrence PA. Planar cell polarity: the orientation of larval denticles in Drosophila appears to depend on gradients of Dachsous and Fat. Development. 2010;137:3411–3415. doi: 10.1242/dev.047126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagner A, Merkel M, Aigouy B, Gaebel J, Brankatschk M, Julicher F, Eaton S. Establishment of Global Patterns of Planar Polarity during Growth of the Drosophila Wing Epithelium. Curr Biol. 2012;22:1296–1301. doi: 10.1016/j.cub.2012.04.066. [DOI] [PubMed] [Google Scholar]
- Schamberg S, Houston P, Monk NA, Owen MR. Modelling and analysis of planar cell polarity. Bull Math Biol. 2010;72:645–680. doi: 10.1007/s11538-009-9464-0. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr Biol. 2001;11:859–863. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Sienknecht UJ, Anderson BK, Parodi RM, Fantetti KN, Fekete DM. Non-cell-autonomous planar cell polarity propagation in the auditory sensory epithelium of vertebrates. Dev Biol. 2011;352:27–39. doi: 10.1016/j.ydbio.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of fat:dachsous binding by the cadherin domain kinase four-jointed. Curr Biol. 2010;20:811–817. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D. Gradients and the specification of planar polarity in the insect cuticle. Cold Spring Harb Perspect Biol. 2009;1:a000489. doi: 10.1101/cshperspect.a000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D, Johnson R, Cooper K, Bray S. Asymmetric localization of frizzled and the determination of notch-dependent cell fate in the Drosophila eye. Curr Biol. 2002;12:813–824. doi: 10.1016/s0960-9822(02)00841-2. [DOI] [PubMed] [Google Scholar]
- Strutt D, Strutt H. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev Biol. 2007;302:181–194. doi: 10.1016/j.ydbio.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt DI. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001;7:367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–1564. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Asymmetric localisation of planar polarity proteins: Mechanisms and consequences. Semin Cell Dev Biol. 2009;20:957–963. doi: 10.1016/j.semcdb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Strutt H, Warrington SJ, Strutt D. Dynamics of core planar polarity protein turnover and stable assembly into discrete membrane subdomains. Dev Cell. 2011;20:511–525. doi: 10.1016/j.devcel.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Qu Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, Fujimori T, Labeau J, Tyteca D, Courtoy P, Poumay Y, Uemura T, Goffinet AM. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Semin Cell Dev Biol. 2002a;13:217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002b;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: the developing cell’s compass. Cold Spring Harb Perspect Biol. 2009;1:a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Okamoto H. Roles of noncanonical Wnt/PCP pathway genes in neuronal migration and neurulation in zebrafish. Zebrafish. 2009;6:3–8. doi: 10.1089/zeb.2008.0557. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansleeben C, Meijlink F. The planar cell polarity pathway in vertebrate development. Dev Dyn. 2011;240:616–626. doi: 10.1002/dvdy.22564. [DOI] [PubMed] [Google Scholar]
- Webb SD, Owen MR. Intra-membrane ligand diffusion and cell shape modulate juxtacrine patterning. J Theor Biol. 2004;230:99–117. doi: 10.1016/j.jtbi.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–469. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Zhu H. Is anisotropic propagation of polarized molecular distribution the common mechanism of swirling patterns of planar cell polarization? J Theor Biol. 2009;256:315–325. doi: 10.1016/j.jtbi.2008.08.029. [DOI] [PubMed] [Google Scholar]