Summary
Background
Several tyrosine kinase inhibitors (TKI) are available for treatment of patients with chronic myeloid leukemia in chronic phase (CML-CP). We analyzed long term response and compared outcomes of patients treated with 4 TKI modalities used as frontline therapy for CML-CP.
Methods
This is a retrospective cohort analysis of 482 patients with chronic phase CML treated in prospective clinical trials with frontline TKI modalities at a single institution. Patients were treated with imatinib 400 mg daily (n=68), imatinib 800 mg daily (n=200), dasatinib 50 mg twice daily or 100 mg daily (n=106) or nilotinib 400 mg twice a day (n=108). Primary end point of the study was to determine whether achieving complete cytogenetic response (CCyR) or major molecular response (MMR) has comparable prognostic implications regardless of the type of frontline TKI modality. Intention to treat analyses were performed for each TKI modality for response assessment and survival endpoints were analyzed using the Kaplan-Meier method and differences calculated by the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazard regression.
Findings
Overall, higher proportions of patients receiving imatinib 800 and 2nd generation TKI achieved complete cytogenetic response (CCyR), major molecular response (MMR) and ≥4.5 log reduction in BCR-ABL transcripts (MR4.5) at all time-points (3–60 months). Disease transformation occurred in 35/482 patients (7%), events occurred in 76/482 (16%) and 53/482 patients (11%) died. Overall, 5 year outcomes were event-free survival (EFS) 84%, failure-free survival (FFS) 70%, transformation-free survival (TFS) 92%, and overall survival (OS) 93%. Compared to other 3 treatment modalities, patients treated with imatinib 400 had significantly inferior EFS, FFS and TFS. Multivariate analysis demonstrated that therapy with imatinib 800, dasatinib or nilotinib predicted for EFS while FFS, TFS and OS were similar irrespective of the TKI used.
Interpretation
Treatment with imatinib 800, dasatinib or nilotinib demonstrates superior rates of responses, which are maintained even at longer follow up (5 years). Patient outcomes are improved after treatment with imatinib 800 and 2nd generation TKI’s as compared to imatinib 400. Results with imatinib 800 are similar to 2nd generation TKI with higher rate of discontinuation.
Keywords: Chronic myeloid leukemia in chronic phase, CML, CML-CP, Tyrosine kinase inhibitors (TKI), TKI, imatinib, nilotinib, dasatinib, cytogenetic response, molecular response
Introduction
Tyrosine kinase inhibitors (TKI) are the standard frontline treatment for patients with chronic myeloid leukemia (CML-CP) in chronic phase. Imatinib was the first of the TKI to be used in this setting. The eight-year follow up of the IRIS (International Randomized study of Interferon vs. STI571) multicenter clinical trial (the last available before the study was terminated) showed that therapy with imatinib resulted in a complete cytogenetic response (CCyR) rate of 83%, 45% discontinuation rate from imatinib and 8-year estimates for event-free survival (EFS) of 81%, progression-free survival (PFS) of 92%, and overall survival (OS) of 85% (93% when only CML related deaths were considered). Imatinib 800 mg has been used in an attempt to improve the outcome over what is achieved with standard-dose imatinib. Various studies have shown that treatment with second generation TKI such as dasatinib, nilotinib, and bosutinib result in deeper and faster responses with fewer transformations to accelerated and blast phase compared to imatinib. Based on these studies, dasatinib and nilotinib are now approved for initial therapy of CML-CP. Achieving early cytogenetic and molecular responses has been suggested to correlate with improved long-term clinical outcomes. The correlation of optimal cytogenetic and molecular responses at 3 months with improved long-term outcome is similar regardless of the TKI used.
However, none of the randomized studies has demonstrated an improved long-term outcome (i.e., EFS, OS) compared to imatinib, perhaps because of the short long-term follow-up. Furthermore, to our knowledge, no analysis is available to date including all available frontline TKI modalities. Therefore, we conducted this analysis including patients treated with any of 4 different TKI modalities at a single institution in consecutive or parallel trials with long-term follow-up data to determine the rates of cytogenetic and molecular responses achieved by each TKI up to 5 year follow-up and the prognostic impact of the responses achieved by each TKI at different time points on long term outcomes (EFS, FFS, TFS and OS).
Methods
All patients with CML-CP enrolled in consecutive or parallel clinical trials at MD Anderson Cancer Center using TKI as frontline therapy from July 31st 2000 to September 10th 2013 were included in this analysis. Patients were treated on protocols approved by the institutional review board and informed consent was obtained in accordance with the declaration of Helsinki. Inclusion and exclusion criteria were similar for all trials (Summarized in supplemental file, page 12). Follow up and response assessment were similar among all the trials: cytogenetic analysis every 3 months for the first year, then every 6 months for the next 2–3 years, then every 1–2 years. Real time polymerase chain reaction (RT-PCR) was generally assessed every 3 months for the first year, then every 6 months. Adherence to therapy was measured through a patient diary, pill count of returned medication, and patient interview during follow-up visits. Response criteria were as previously described. Cytogenetic response was assessed by conventional cytogenetic analysis done in bone marrow cells using the G-banding technique with at least 20 metaphases analyzed. Fluorescent in situ hybridization (FISH) on peripheral blood was used to evaluate response only when routine cytogenetic analysis was not successful (i.e., insufficient metaphases). Cytogenetic response categories included complete cytogenetic response (CCyR) (0% Ph-positive metaphases), partial cytogenetic response (PCyR) (1–35% Ph-positive metaphases), major cytogenetic response (MCyR) (≤35% Ph-positive metaphases) and minor cytogenetic response (>35% to 95% Ph-positive metaphases). Molecular response was assessed by RT-PCR and expressed as the BCR-ABL/ABL ratio (International Scale). A major molecular response (MMR) was defined as BCR-ABL/ABL transcript ratio ≤0.1%, and ≥4.5 log reduction in BCR-ABL transcripts (MR4.5) as a ratio of ≤0.0032%. Best response achieved at any time point and responses according to different time points were assessed. Only patients with typical BCR-ABL transcripts (b2a2 and/or b3a2) were included in the molecular analyses. Of note, patients treated with imatinib 400 initiated therapy between May 2001 and June 2001 when molecular analysis was not routinely done, therefore molecular response at 3 months is not available for imatinib 400. Undetectable molecular response prior to 2011 was done by confirming negative results through nested PCR. Therefore some of the older values may not be fully equivalent.
Statistical analysis
Event-free survival (EFS) was measured from the start of treatment to the date of any of the following events (as defined in the IRIS study) while on therapy: loss of complete hematologic remission (CHR, loss of major cytogenetic response (MCyR), progression to accelerated (defined as blasts ≥15%, blasts + promyelocytes ≥30%, basophils ≥20%, platelets <100×109/L, unrelated to therapy, or cytogenetic clonal evolution) or blast phase (defined as blasts ≥30%, or extramedullary disease), or death from any cause at any time while on study. Because of the limitations of this definition, we also measured the failure-free survival (FFS) that accounts for other events such as failure to achieve response at set times as defined by the European Leukemia Net (ELN), loss of CCyR, intolerance, or treatment discontinuation for any reason. Overall survival (OS) was measured from the time treatment was started to the date of death from any cause at any time or date of last follow-up. Transformation-free survival (TFS) was measured from the start of therapy to the date of transformation to accelerated or blast phase while on therapy or deaths on study (i.e. deaths on initial TKI).
Survival probabilities were estimated by the Kaplan-Meier method and compared by the log-rank test. Univariate and multivariate analyses were performed to identify whether the type of TKI modality can predict for patient outcomes. Variables with p-value ≤0.25 in the univariate analysis were entered into a multivariate model and analyzed using the Cox proportional hazard regression. A p-value of < 0.05 was considered significant. Survival endpoints were analyzed using the Kaplan-Meier method and differences calculated by the log-rank test. Statistical analyses were carried out using STATA/SE version 13.1 statistical software (Stata Corp. LP, College Station, Texas).
Role of the funding source
The study sponsor for all trials was MDACC. The supporters of the studies were Novartis (Imatinib and Nilotinib trials) and BMS (Dasatinib trial). The clinical trials were designed by JC and HK. The supporters reviewed provided drug and partial financial support for the conduct of the study. The supporters had no role in the collection, analysis or interpretation of the data. All authors had access to the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Patients
A total of 482 consecutive patients with newly diagnosed CML-CP treated with four TKI modalities were included in this analysis. The median age for the total cohort was 49 years (range, 18–86 years), the median follow-up was 90 months (interquartile range of 74 months). Most patients (70%) had low Sokal score. Patients were treated with imatinib 400 mg daily (n=68), imatinib 800 mg daily (n=200), dasatinib 50 mg twice daily or 100 mg daily (n=106), or nilotinib 400 mg twice daily (n=108). All TKI modalities were administered orally. Patient characteristics were comparable among patients treated with the different TKI modalities (Table 1). Imatinib was introduced in 2000 and therefore the follow-up is longer for patients treated with imatinib than with second generation TKIs. Disease transformation occurred in 35 patients (8%) including 7 patients with transformation to blast phase, 14 patients to accelerated phase and 14 on study deaths. A total of 53 (11%) patients died (including 14 deaths on study) and events occurred in 76 (16%) patients.
Table 1.
Patients and disease characteristics at the time of initial presentation on various TKI modalities.
| Imatinib 400 (n=68) | Imatinib 800 (n=200) | Dasatinib (n=106) | Nilotinib (n=108) | Overall (n=482) | |
|---|---|---|---|---|---|
| Median age, yrs (Range) | 49 [18–79] | 48 [19–85] | 48 [18–86] | 50 [18–86] | 49 [18–86] |
| Sokal Risk, % | |||||
| Low | 69 | 63 | 73 | 74 | 69 |
| Intermediate | 28 | 28 | 21 | 20 | 24 |
| High | 3 | 9 | 4 | 7 | 7 |
| WBC count (K/μL) Median (Range) | 22 (2 – 277) | 27 (2 – 283) | 26 (1 – 193) | 40 (1 – 342) | 28 (1 – 342) |
| Hemoglobin (g/dL) Median (Range) | 13 (8 – 16) | 12 (6 – 17) | 12 (7 – 16) | 12 (8 – 16) | 12 (6 – 17) |
| Peripheral blood blasts (%) Median (Range) | 0 (0 – 2) | 0 (0 – 12) | 0 (0 – 5) | 0 (0 – 7) | 0 (0 – 12) |
| Platelets (K/μL) (%) Median (Range) | 367 (103 – 1043) | 346 (15 – 1476) | 333 (86 – 1906) | 315 (73 – 2928) | 343 (15 – 2928) |
| Serum LDH (IU/L) Median (Range) | 367 (103 – 1043) | 346 (15 – 1476) | 333 (86 – 1906) | 315 (73 – 2928) | 343 (15 – 2928) |
| Splenomegaly, n(%) Spleen size >10 cm | 3 (5) | 17 (8) | 4 (4) | 4 (4) | 29 (6) |
| Median follow up in months (Interquartile Range) | 144 (60) | 116 (26) | 55 (49) | 49 (34) | 90 (74.1) |
Response and long term outcomes according to TKI modality
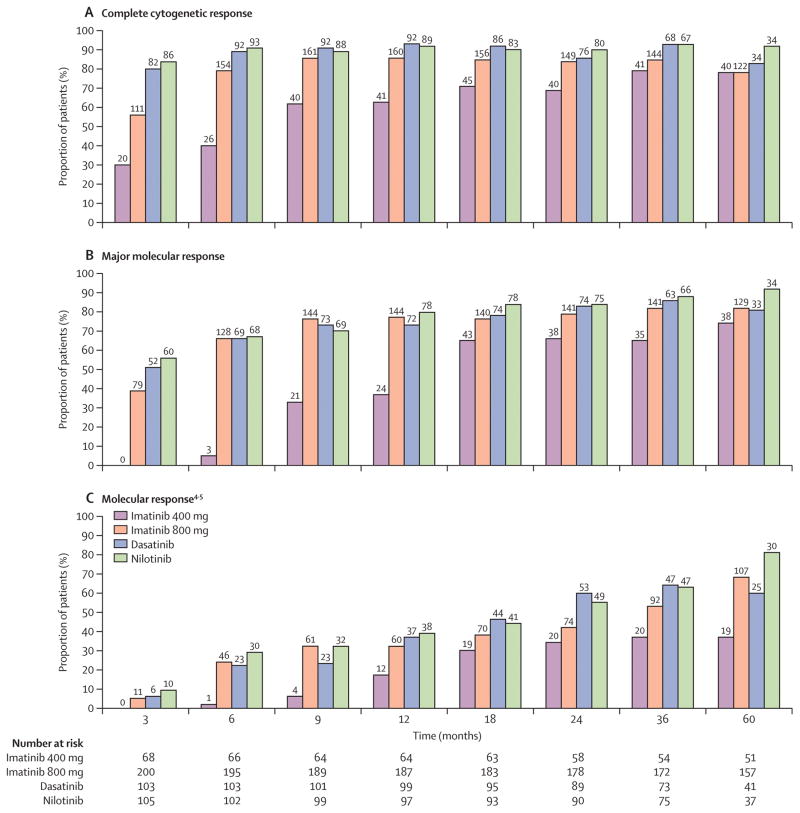
Overall, best cytogenetic responses for the total population were CCyR in 437/477 (92%) patients and 18/477 (4%) achieved PCyR. MMR was achieved in 412/477 (86%) patients and MR4.5 in 335/477 (70%) patients. According to specific TKI, the percentage of patients achieving CCyR was higher among patients treated with imatinib 800 (180/199; 90%), dasatinib (100/104; 96%) or nilotinib (99/107; 93%) as compared to those treated with imatinib 400 (58/67; 87%). Similarly, best MMR rates were higher with imatinib 800 (171/199; 86%), dasatinib (93/104; 90%) or nilotinib (97/107; 91%) than with imatinib 400 (51/67;76%). Fewer patients treated with imatinib 400 (38/67; 57%) achieved MR4.5 as compared to those treated with imatinib 800 (148/199; 74%), dasatinib (76/104; 73%) or nilotinib (76/107; 71%) (See supplemental Figure 1A–D). The median time to achieve MMR was 11 months for patients treated with imatinib 400 and 6 months each for those treated with imatinib 800, dasatinib and nilotinib.
We then compared outcomes according to the best cytogenetic responses. Patients who achieved CCyR had significantly better long-term outcome than those with PCyR or minor cytogenetic response (Supplemental Figure 2A–D). The 5-year estimates for EFS, FFS, TFS and OS among patients who achieved CCyR at any time were 89%, 77%, 94% and 95%, respectively (P<0.001 for each outcome). Similarly, the 5-year estimates for EFS, FFS, TFS and OS for patients that achieved MMR were 91%, 80%, 94% and 97%, respectively, (P<0.001 for each outcome) (Supplemental Figure 3A–D). We also compared EFS according to the type of response achieved at 12 months i.e MMR alone vs CCyR with no MMR vs no MMR, no CCyR. As expected, patients who achieved 12 months MMR or CCyR without MMR had better EFS as compared with patients who did not achieve MMR or CCyR (P<0.0001) (Supplemental Figure 4).
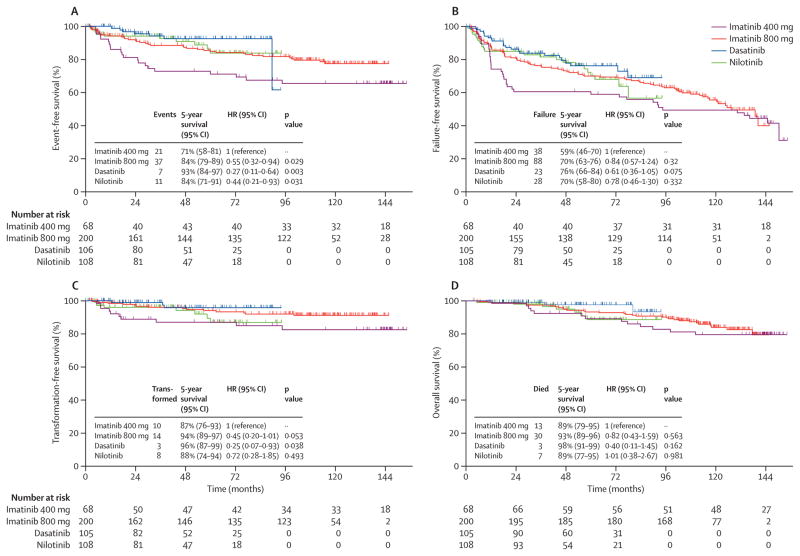
We then explored the difference in outcomes according to the type of TKI modality used (Figure 1A–D). Patients who received imatinib 400 exhibited significantly inferior EFS, as compared to other three TKI modalities (P = 0.009) while for FFS, TFS and OS it was (P = 0.353, 0.078 and P=0.381 respectively). Corresponding 95%CI and hazard ratios are mentioned in Figure 1(A–D) and in Table -1, supplemental tables 1–3 for EFS, FFS, TFS and OS respectively. Of note, patients who received imatinib 400 had higher frequencies of events, failure, transformations and deaths as compared to imatinib 800, dasatinib and nilotinib. Ten (2%) patients treated with imatinib 400 transformed to AP or BP (3 myeloid blast phase and 7 accelerated phase), compared to 5 (1%) on imatinib 800 (2 lymphoid blast phase, 3 accelerated phase), 2 on dasatinib (1%; 2 accelerated phase), and 4 on nilotinib (1%; 3 lymphoid blast phase, 1 accelerated phase).
Figure 1. Long term outcomes according to four TKI modalities (Imatinib 400, Imatinib 800, Dasatinib and Nilotinib).
A) Event free survival (EFS); median survival not reached in all 4 TKI modalities B) Failure free survival (FFS); median survival not reached for dasatinib and nilotinib and 94.4, 127.3 months in imatinib 400 and 800 respectively C) Transformation free survival (TFS); median survival not reached in all and D) Overall survival (OS); median survival not reached in all 4 TKI modalities. Corresponding Hazard ratios for each outcome according to TKI modality are shown in univariate analysis in Table-2, supplemental tables 1, 2 and 3 for EFS, FFS, TFS and OS respectively.
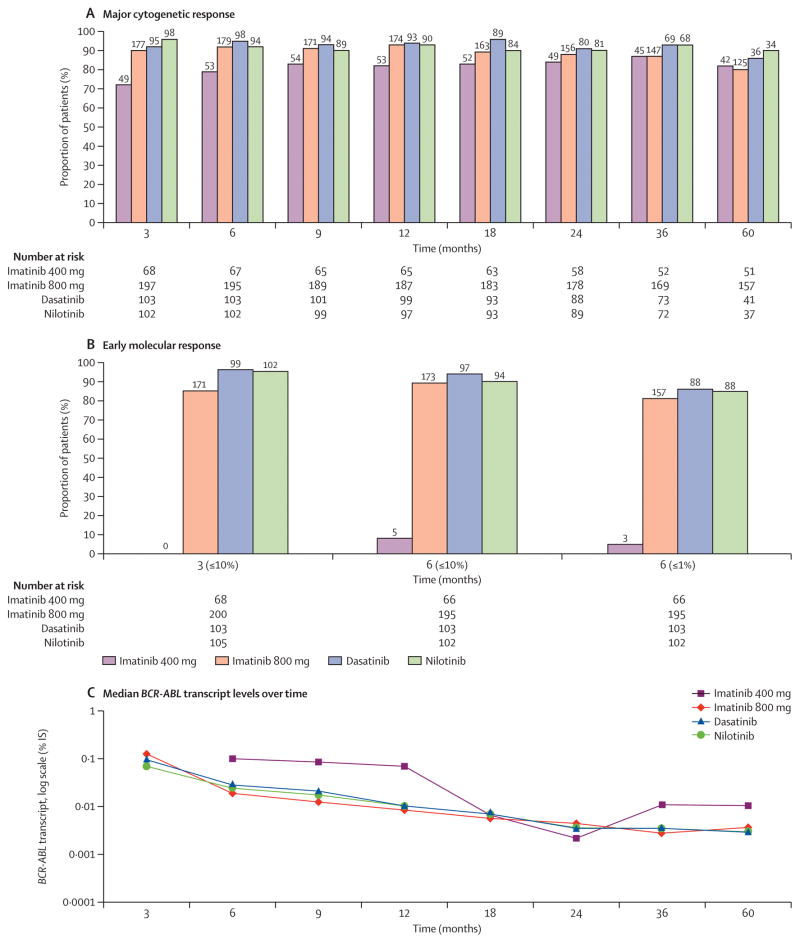
We then compared the response to therapy achieved by treatment modality at different time points (up to 60 months), on an intention to treat analysis. Only patients with values available at the time of assessment or who were under follow up were included in this analysis. Absolute numbers of evaluable patients according to TKI modality and at different time points are mentioned in figure 2 and 3. As shown in Figure 2A, patients taking imatinib 400 achieve CCyR at later time points and fewer of these patients achieve CCyR as compared to those treated with imatinib 800, dasatinib and nilotinib at all-time points. Only after approximately 36 months does the rate of CCyR for patients treated with imatinib 400 reaches similar levels to those achieved in the other cohorts. Similarly, Figure 2B–C shows that fewer patients taking imatinib 400 achieve MMR and MR4.5 at nearly all time points as compared to imatinib 800, dasatinib and nilotinib. MMR reaches a similar rate only after 60 months, while MR4.5 remains nearly half of that with other treatment modalities for at least 60 months. Patients receiving imatinib 800 had similar rates of CCyR, MMR and MR4.5 at all the time points to those receiving 2nd generation TKI. Supplemental table -1 shows 95%CI for all TKI modalities at different time points for CCyR and MMR. Similarly, fewer patients receiving imatinib 400 achieved major cytogenetic response (≤35% Ph+) and BCR-ABL/ABL (≤10%) at early time points or BCR-ABL/ABL (≤1%) at 6 months when compared with other TKI modalities (Figure 3A–B). Furthermore, prolonged (>12 months) reduction of median BCR-ABL was slowest with imatinib 400 compared to other TKI modalities over time (Figure 3C). Treatment with second generation TKI and imatinib 800 achieved earlier, deeper and sustained reduction in BCR-ABL transcripts.
Figure 2. Analysis of cytogenetic and molecular response at specific time points (3, 6, 9, 12, 18, 24, 36 and 60 months) by TKI modality.
A) Achievement of complete cytogenetic response (CCyR) (0% Ph-positive metaphases), percentages (top) and absolute numbers of evaluable patients (bottom) are shown for imatinib 400, imatinib 800, dasatinib and nilotinib respectively B) Achievement of major molecular response (MMR) (≤0.1% BCR-ABL-IS) percentages (top) and absolute numbers of evaluable patients (bottom) are shown for imatinib 400, imatinib 800, dasatinib and nilotinib respectively. Of note for imatinib 400 group, number of patients with available PCR values were (0 and 5 at 3 and 6 months respectively) C) Achievement of molecular response (MR4.5) (≤0.0032% BCR-ABL-IS).
Figure 3. Analysis of major cytogenetic and molecular responses (≤10% or ≤1%) BCR-ABL at specific time points by TKI modality.
– A) Achievement of major cytogenetic response (MCyR) (≤35% Ph-positive metaphases). B) Achievement of optimal molecular response (≤10% BCR-ABL-IS) at 3 and 6 months (≤1% BCR-ABL-IS). Absolute numbers of evaluable patients were (68, 66) for imatinib 400, (200, 195) for imatinib 800, (103, 103) for dasatinib and (105, 102) for nilotinib at 3 and 6 months respectively. Of note for imatinib 400 group, number of patients with available PCR values were (0 and 5 at 3 and 6 months respectively) C) Pattern of median BCR-ABL transcript levels over time (3, 6, 9, 12, 18, 24, 36 and 60 months) by TKI modality (log scale). Patients with imatinib 400 were not evaluable at 3 months due to lack of molecular data.
Overall, among the 53 patients who died, 16 were considered CML related deaths and 37 were non-CML related. Thirteen patients (19%) started on imatinib 400 died (7 transformed to blast phase, 4 second cancers (renal cell cancer, ovarian cancer, esophageal cancer and acute myeloid leukemia), 1 complications of stem cell transplant (SCT) and one unknown. For imatinib 800, 30 patients (15%) died including 2 that transformed to blast phase, 3 to accelerated phase and 25 due to other causes (6 cardiac diseases, 6 unknown cause, 3 other cancers, 3 neurological diseases, 2 car accidents, 2 complications of SCT, 1 pneumonia, 1 stroke with seizures and 1 suicide). Three patients (3%) died on dasatinib, (1 pancreatic cancer, 1 congestive heart failure and 1 infection). For nilotinib, 7 patients (6%) died (1 unknown, 1 stroke, 1 pulmonary embolism, 1 food poisoning, 1 sepsis, 1 complications of SCT and 1 died of post-operative complications for surgery done for another cancer).
Type of TKI and long term outcomes – Multivariate analysis
We then conducted univariate and multivariate analyses to find whether the TKI modality correlates with long term outcomes. Table-1 shows the results of these analyses for event free survival (EFS). Covariates with a p value <0.25 were included in the final multivariate model for EFS. Compared to imatinib 400, therapy with imatinib 800, dasatinib or nilotinib significantly predicted for longer EFS - imatinib 800 - HR=0.51, 95% CI 0.29–0.88 (p = 0.016), dasatinib HR=0.28, 95% CI 0.12–0.66 (p = 0.004), nilotinib - HR=0.42, 95% CI 0.20–0.89 (p = 0.024). In addition, the presence of splenomegaly at the time of initial presentation also predicted for inferior EFS- HR=2.14, 95% CI 1.03–4.48 (p = 0.043). In contrast, the type of TKI modality did not significantly predict for other long-term outcomes including failure free, transformation free and overall survival (Supplemental tables 2–4).
In a separate analysis we have assessed whether the correlation of the response achieved at different time points (at 3, 6, and 12 months) with long-term outcome was modulated by the TKI modality (Supplemental tables 5–8). Patients who achieved major cytogenetic response (MCyR) at 3 months, regardless of TKI modality, significantly predicted for longer EFS as compared to those patients who did not achieve such response at 3 months. MCyR at 3 months by either imatinib 400 - HR=0.31, 95% CI 0.13–0.74 (p = 0.009) or by imatinib 800, dasatinib or nilotinib (3TKI) - HR=0.18, 95% CI 0.09–0.37 (p <0.001) significantly predicted for longer EFS compared to those patients who did not achieve MCyR at 3 months. Similarly, achievement of MCyR at 3 months significantly predicted for FFS, TFS and OS irrespective of TKI modality. Furthermore, CCyR at 6, 12 and 18 months also significantly predicted for longer EFS, independently of the TKI modality. Furthermore, achievement of CCyR at 12 months was significantly predictive for FFS, TFS and OS irrespective of TKI modality.
Discontinuation rates of different TKI modalities
Overall, 163/482 (34%) patients have discontinued therapy. Percentages of patients who discontinued TKI therapy were 29/68 (43%) of those in the imatinib 400 cohort, 85/200 (43%) in the imatinib 800 cohort, 23/106 (21%) on dasatinib and 27/108 (25%) on nilotinib, respectively. To adjust for the different follow-up among different cohorts by treatment modality, we then compared the proportions of patients in each of the 4 TKI modality cohorts who discontinued therapy within 12 months, between 12 and 24 months and 24 to 36 months from start of therapy, respectively (see supplemental figure 5). Treatment discontinuation was more common among patients receiving imatinib compared to 2nd generation TKI’s.
Causes of discontinuation among the TKI modalities
Among the overall 163 patients who discontinued TKI therapy, the major reasons for treatment discontinuation were resistance (28%), toxicity (23%) or both (8%), patient choice (12%), deaths on study (8%), insurance/financial issues (6%), blast phase (5%), noncompliance (5%), other medical conditions (4%), and stem cell transplantation (1%).
Percentages of patients who discontinued therapy by TKI modality (imatinib 400, imatinib 800, dasatinib and nilotinib) were: resistance (19%, 12%, 6%, 3%), toxicity (7%, 8%, 7%, 7%) or both (1%, 3%, 3%, 2%), patient choice (6%, 6%, 3%, 1%), deaths on study (0%, 4%, 1%, 4%), insurance/financial issues (0%, 3%, 0%, 3%), blast phase (4%, 1%, 0%, 3%), noncompliance (3%, 1%, 2%, 3%), other medical conditions (3%, 2%, 0%, 0%), and stem cell transplantation (0%, 1%, 0%, 0%).
Of the 163 patients who discontinued therapy, 85 patients (18% of all treated patients; 53% of all those who discontinued therapy) discontinued therapy within 3 years from the start of treatment. The major reasons for treatment discontinuation among these 85 patients were resistance (24%), toxicity (24%) or both (15%), insurance/financial issues (9%), blast phase (9%), noncompliance (7%), deaths on study (5%), patient choice (3%), stem cell transplantation (2%) and other medical conditions (1%). Causes of discontinuation and the proportion of patients who discontinued therapy according to TKI modality and the duration of therapy are summarized in Table-2.
Table 2.
Analysis of factors predictive of event free survival (EFS) including the type of TKI modality.
| N | Events | Log-rank | HR | 95% CI HR | P-value | 5-year EFS | |
|---|---|---|---|---|---|---|---|
| *Univariate | |||||||
| TKI Type | |||||||
| Imatinib 400# | 68 | 21 | 0.009 | 72 | |||
| Imatinib 800 | 200 | 37 | 0.55 | (0.32–0.94) | 0.029 | 84 | |
| Dasatinib | 105 | 7 | 0.27 | (0.11–0.64) | 0.003 | 93 | |
| Nilotinib | 108 | 11 | 0.44 | (0.21–0.93) | 0.031 | 84 | |
| Sokal score | |||||||
| Low | 333 | 45 | 0.19 | 86 | |||
| Intermediate | 118 | 23 | 1.29 | (0.78–2.13) | 0.324 | 82 | |
| High | 30 | 8 | 1.91 | (0.90–4.06) | 0.092 | 76 | |
| Age, years | |||||||
| ≤55 | 322 | 50 | 0.91 | 84 | |||
| >55 | 159 | 26 | 1.03 | (0.64–1.65) | 0.913 | 84 | |
| Splenomegaly (≥10cm) | |||||||
| No | 445 | 64 | 0.003 | 86 | |||
| Yes | 36 | 12 | 2.44 | (1.32–4.53) | 0.005 | 70 | |
| Multivariate | |||||||
| TKI Type | |||||||
| Imatinib 400# | 68 | 21 | 72 | ||||
| Imatinib 800 | 200 | 37 | 0.51 | (0.29–0.88) | 0.016 | 84 | |
| Dasatinib | 105 | 7 | 0.28 | (0.12–0.66) | 0.004 | 92 | |
| Nilotinib | 108 | 11 | 0.42 | (0.20–0.89) | 0.024 | 84 | |
| Splenomegaly (≥10cm) | |||||||
| No | 445 | 64 | 86 | ||||
| Yes | 36 | 12 | 2.1 | (1.03–4.48) | 0.043 | 70 |
White blood cell (WBC) count, Hemoglobin, platelet count, peripheral blood blasts, serum lactate dehydrogenase (LDH) are not significant (p=NS; data not shown),
Imatinib 400 is the reference for comparison with other 3 TKI modalities
At the last follow-up, the median actual daily dose according to the TKI modality (imatinib 400, imatinib 800, dasatinib and nilotinib) is 400 mg, 600 mg, 80 mg and 800 mg, respectively, while the median daily dose after first 3 years of therapy was 400 mg, 800 mg, 100 mg, and 800 mg, respectively.
Subsequent therapies after discontinuation of initial TKI modality
Since overall survival can be influenced by successful salvage treatments which the patients may receive after failing or coming off of initial TKI, we then looked for subsequent therapies after excluding those patients who came off their initial TKI and subsequently lost to follow up. Overall 97 (20%) patients switched to other therapies after discontinuing initial TKI. Description of subsequent therapies is provided in supplemental table 9. Distribution according to TKI modality was imatinib 400, 17 (25%) patients; imatinib 800, 47 (24%) patients; dasatinib, 16 (15%) patients; and nilotinib, 17 (16%) patients. Of these 97 patients, 65 (67%) were in CCyR, 60 (62%) were in MMR and 77 (79%) were alive at the time of last follow up.
Discussion
In this study we have demonstrated that patients received imatinib 800 or dasatinib or nilotinib achieve early and deeper cytogenetic and molecular responses as compared to imatinib 400 and sustain this superiority over imatinib 400 even at 5 years follow up. Moreover, we have shown that the type of TKI modality can independently predict for event free but not overall survival. Development of 2nd generation TKI such as dasatinib and nilotinib has altered the selection of frontline TKI for the therapy of many patients with CML-CP. It is now well established that 2nd generation TKI’s can achieve earlier and deeper cytogenetic and molecular responses as compared to standard dose imatinib. Several studies have documented the positive impact of attaining early and deep cytogenetic and molecular responses on patient outcomes. Randomized studies such as ENESTnd and DASISION have shown that higher proportions of patients treated with the corresponding 2nd generation TKI (nilotinib and dasatinib, respectively) achieve deeper responses at earlier time points compared to standard dose imatinib. In other studies, high dose imatinib (800 mg) induced earlier and deeper molecular responses compared to standard dose imatinib. No direct comparisons through a randomized trial exist of higher dose-imatinib and 2nd generation TKI, or between the two approved 2nd generation TKI. Thus current treatment choices depend mostly on factors such as personal experience with each drug, patient comorbidities that may predispose to adverse events more frequently seen with one drug or another, personal preferences based on schedule, availability or cost.
In this analysis we reviewed the patterns of response and long term outcome achieved with 4 TKI modalities (imatinib 400, imatinib 800, dasatinib and nilotinib) in consecutive or parallel investigator-initiated clinical trials. This analysis suggests that results achieved with imatinib 400 mg daily (i.e., the standard dose of imatinib) are generally inferior to those achieved with any of the other three modalities. In contrast, there are no obvious differences in cytogenetic or molecular response rates between patients treated with imatinib 800 or any of the 2nd generation TKI. The same can be said about the long-term survival outcomes including EFS, FFS and TFS. In the absence of direct comparison of all 4 available modalities for treatment of CML through randomized trials, this analysis might be the best available indication to suggest that the response outcomes may be similar for higher-dose imatinib, dasatinib and nilotinib and that other criteria may be used for selecting the TKI best suited for each patient.
Importantly, no difference in overall survival can be identified between all four treatment modalities. This could be interpreted as somewhat surprising based on the known favorable impact that achieving early responses has on long term outcome. For example, achieving a deep response at 3 months from the start of therapy has been associated with an improved EFS (or progression free survival) and overall survival. Since routine testing for molecular response was not done in the early years of TKI therapy when nearly all patients treated with standard-dose imatinib in our series were treated, we focused on cytogenetic response, particularly major cytogenetic response at 3 months which is grossly equivalent to BCR-ABL/ABL ≤10%. We recently published the results in our series demonstrating that early responses at 3 months happened more frequently with imatinib 800 and 2nd generation TKI. Several analyses have demonstrated that such responses are associated with an improved PFS and OS. Still, neither the randomized trial of imatinib versus dasatinib or nilotinib, nor the present analysis including also imatinib 800, shows these early responses translating in an improvement in overall survival. It is possible that such benefit in survival may not become evident until many more years from the start of treatment, particularly when considering that the difference in 3-month response affects only a small subset of patients. However, it is also possible that effective intervention after failure is documented may negate the adverse prognostic significance of a slow response, at least in terms of survival. We have previously reported that when response to subsequent TKI therapy is accounted for, the outcome (i.e., “current EFS”) is significantly better than when the outcome is considered without accounting with the potential for salvage with sequential TKI. It is important to note that none of the patients included in this analysis who did not have an optimal response at 3 months received a different therapy at that time point. Patients who developed failure (resistance or intolerance) as per the ELN recommendations were offered therapy with other modalities, available to them at the time this occurred.
The kinetics of the response to imatinib 800 and 2nd generation TKI show that there is a higher rate of the deepest responses (e.g., MR4.5). Although the rates of MMR reach similar levels by 60 months of therapy, the rates of MR4.5 remain higher with imatinib 800 and 2nd generation TKI than with imatinib 400. Even at longer follow up, the maximum cumulative rate of MR4.5 in the cohort of patients treated with imatinib 400 is 37% at 5 years. These deeper responses do not seem to offer a benefit in the risk of transformation or death, but are important if one is to consider treatment discontinuation which is currently only considered for patients with sustained undetectable transcripts.
Furthermore, in multivariate analysis we have shown that therapy with imatinib 800, dasatinib and nilotinib can significantly predict for event free survival when compared to imatinib 400. However, type of TKI modality did not predict for failure free, transformation free and overall survival. These results may be explained by the fact that a large percentage of patients who fail on imatinib therapy can still achieve optimal response with 2nd generation TKI or imatinib 800 and this may nullify the negative impact on outcomes after failing imatinib 400.
In our series, the rates of treatment discontinuation are highest with imatinib compared to 2nd generation TKI. The reasons for treatment discontinuation are somewhat different between the two dose regimens of imatinib, with more patients discontinuing standard-dose imatinib therapy for resistance and higher-dose imatinib for intolerance. It should be noted that the rate of treatment discontinuation for toxicity is somewhat affected by availability of clinical trials intended to study the effect of change of therapy in patients with chronic low-grade adverse events. The rate of treatment discontinuation for 2nd generation TKI, after a median follow-up of over 4 years is ≤25% overall, and <20% by 3 years. These rates appear to be somewhat lower than those reported in the 3 year follow up of randomized trials ENESTnd (38% for imatinib arm and 28% for nilotinib) and DASISION (31% for imatinib arm and 29% for dasatinib). Also, the median dose at 3 years remains as intended for all treatment arms and drops slightly after 3 years for the higher-dose imatinib and dasatinib arms, suggesting that most patients can tolerate treatment as intended with adequate support in a dedicated CML clinic.
Our analysis should be considered with the caution warranted for comparisons across several studies over different (some sequential, other simultaneous) time periods. However, all of these studies were conducted at the same center, with the same eligibility criteria and follow-up. The rules used to manage these patients, in terms of monitoring, dose adjustments, and others were uniform, and the studies were all conducted prospectively. Thus, in the absence of a randomized trial comparing these treatment modalities, an analysis such as the one presented here might offer the best opportunity to define whether there might be differences in outcome between different treatment options. Furthermore, the median age of our patients was 49 years and 69% had low sokal risk score, characteristics that may suggest this population may not be fully representative of the global CML population. Also, molecular testing was not routinely done at 3 months in the early years of TKI therapy but instead was imitated when patients achieved complete cytogenetic response. Because of the nature of our trials, this affected mostly patients treated with imatinib 400 mg. Our findings that these patients have slower responses should be considered with caution because the lack of molecular testing at 3 months mostly affected this cohort. We tried to address this by using cytogenetic analysis that was routinely done, considering the established close correlation with cytogenetic analysis (i.e., BCR-ABL/ABL <10% is grossly equivalent to a major cytogenetic response). The impact that these differences may have in the generalizability of our findings and conclusions is unknown and should be taken in consideration.
Treatment selection for an individual patient is a complex decision that is affected by multiple factors, including availability, cost, familiarity with the drug, schedule of administration, risk factors for certain expected adverse events with a given agent, and others. Our results suggest that excellent results can be obtained with any of the options used particularly imatinib 800, dasatinib and nilotinib. The flexibility provided by the favorable results seen with all these options offers the physician the opportunity to select among several good approaches.
In conclusion, excellent results are obtained with all TKI modalities with a suggestion that patients treated with imatinib 800, dasatinib or nilotinib exhibited better long term response and outcomes as compared to imatinib 400, and higher proportions of patients who received imatinib discontinued treatment. With adequate management, transformation to accelerated and blast phase and death from CML is now rare with these treatment modalities.
Supplementary Material
Table 3.
Causes of discontinuation according to duration of therapy among different TKI modalities.
| Causes of discontinuation | % (Imatinib400/Imatinib800/Dasatinib/Nilotinib) | ||
|---|---|---|---|
| ≤ 12 months (%) | >12–24 months (%) | >24–36 months (%) | |
| Overall | (11,9,7,15) | (7,13,4,0) | (4,4,2,2) |
| Resistance | (3,1,0,1) | (3,2,1,0) | (3,1,2,1) |
| Toxicity | (4,2,2,5) | (1,2,2,0) | - |
| Resistance + Toxicity | (0,2,2,2) | (0,1,1,0) | (1,1,0,0) |
| Blast phase | (4,1,0,3) | (0,1,0,0) | - |
| Deaths | (0,1,0,1) | - | (0,1,0,0) |
| Patient Choice | (0,0,3,0) | - | - |
| Insurance/Financial | (0,1,0,2) | - | (0,1,0,1) |
| Non compliance | (0,1,1,2) | (3,0,0,0) | - |
| Stem cell transplant | (0,1,0,0) | - | - |
| Other medical condition | (0,1,0,0) | - | - |
Research in context.
Evidence before this study
We searched Medline and PubMed specifically for original research articles published on cytogenetic and molecular responses by different TKI modalities as frontline treatment for patients with CML in chronic phase. We identified relevant research articles (mentioned in reference section) which have demonstrated the superiority of dasatinib and nilotinib in randomized clinical trials in terms of response. We have also quoted studies which have documented deep impact on outcomes of early cytogenetic and molecular responses to TKI’s in frontline therapy of CML in chronic phase, which formed the basis for this study.
Added value of this study
This study provides insight into long term responses and outcomes of patients with chronic phase CML and provides a comparison of 4 commonly used TKI modalities. Since previous reports have compared only Imatinib 400 with either imatinib 800, dasatinib or nilotinib in randomized trials, our study provides a comparative analysis of 4 TKI modalities after a long follow up at a single center.
Implications of all the available evidence
We have identified that compared to imatinib 400, treatment with imatinib 800 or dasatinib or nilotinib is similar in terms of achieving durable and sustained cytogenetic and molecular responses and impacts patient outcomes to a similar degree.
Acknowledgments
Funding: Supported by the MD Anderson Cancer Center Support Grant CA016672 and Award Number P01 CA049639 from the National Cancer Institute.
Our funding was from the following sources - Supported in part by the MD Anderson Cancer Center Support Grant CA016672 (PI: Dr. Ronald DePinho) and Award Number P01 CA049639 (PI: Dr. Richard Champlin) from the National Cancer Institute. None of the authors are employed by NIH. JC is recipient of grant from NCI (PI of Project 1 of P01 CA049639).
Footnotes
Authorship Contributions
P.J., H.K. and J.C. designed the study.
G. N. G., P.J, H.K., and J.C. analyzed results.
P.J., G.N.G., M.L.A., K.S., C.G.R. and J.C. wrote the paper.
P.J., G.N.G., S.P., S.D., H.K., and J.C. did clinical correlation.
H.K., S.O.B., F.R., E.J., S.V., W.W., G.B. and J.C. contributed patient samples.
All authors reviewed and gave the final approval for the paper.
Conflicts of Interest Disclosures: J.C - Consultant: Pfizer, Ariad, Teva. Research support: Pfizer, Ariad, Chemgenex, Bristol Myers Squibb (BMS), Novartis, F.R. - research funding from BMS and honoraria from BMS, Novartis, and Pfizer. The remaining authors declare no competing financial interests.
References
- 1.Deininger M, O’Brien SG, Guilhot F, et al. International Randomized Study of Interferon Vs STI571 (IRIS) 8-Year Follow up: Sustained Survival and Low Risk for Progression or Events in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Treated with Imatinib. ASH Annual Meeting Abstracts. 2009;114(22):1126. [Google Scholar]
- 2.Baccarani M, Rosti G, Castagnetti F, et al. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet Study. Blood. 2009;113(19):4497–4504. doi: 10.1182/blood-2008-12-191254. [DOI] [PubMed] [Google Scholar]
- 3.Cortes JE, Baccarani M, Guilhot F, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol. 2010;28(3):424–430. doi: 10.1200/JCO.2009.25.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hehlmann R, Lauseker M, Jung-Munkwitz S, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29(12):1634–1642. doi: 10.1200/JCO.2010.32.0598. [DOI] [PubMed] [Google Scholar]
- 5.Deininger MW, Kopecky KJ, Radich JP, et al. Imatinib 800 mg daily induces deeper molecular responses than imatinib 400 mg daily: results of SWOG S0325, an intergroup randomized PHASE II trial in newly diagnosed chronic phase chronic myeloid leukaemia. Br J Haematol. 2014;164(2):223–232. doi: 10.1111/bjh.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hehlmann R, Muller MC, Lauseker M, et al. Deep Molecular Response Is Reached by the Majority of Patients Treated With Imatinib, Predicts Survival, and Is Achieved More Quickly by Optimized High-Dose Imatinib: Results From the Randomized CML-Study IV. J Clin Oncol. 2014;32(5):415–23. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 7.Cortes JE, Kantarjian HM, Goldberg SL, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. J Clin Oncol. 2009;27(28):4754–4759. doi: 10.1200/JCO.2008.20.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119(5):1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortes JE, Jones D, O’Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28(3):398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2302. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 12.Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28(3):392–397. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30(28):3486–3492. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merx K, Muller MC, Kreil S, et al. Early reduction of BCR-ABL mRNA transcript levels predicts cytogenetic response in chronic phase CML patients treated with imatinib after failure of interferon alpha. Leukemia. 2002;16(9):1579–1583. doi: 10.1038/sj.leu.2402680. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Pearson K, Ferguson JE, Clark RE. The early molecular response to imatinib predicts cytogenetic and clinical outcome in chronic myeloid leukaemia. Br J Haematol. 2003;120(6):990–999. doi: 10.1046/j.1365-2141.2003.04200.x. [DOI] [PubMed] [Google Scholar]
- 16.Branford S, Rudzki Z, Harper A, et al. Imatinib produces significantly superior molecular responses compared to interferon alfa plus cytarabine in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Leukemia. 2003;17(12):2401–2409. doi: 10.1038/sj.leu.2403158. [DOI] [PubMed] [Google Scholar]
- 17.Quintas-Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009;113(25):6315–6321. doi: 10.1182/blood-2008-07-166694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118(17):4541–4546. doi: 10.1182/blood-2011-04-348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116(19):3758–3765. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin D, Hedgley C, Clark RE, et al. Predictive value of early molecular response in patients with chronic myeloid leukemia treated with first-line dasatinib. Blood. 2012;120(2):291–294. doi: 10.1182/blood-2012-01-407486. [DOI] [PubMed] [Google Scholar]
- 21.Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30(3):232–238. doi: 10.1200/JCO.2011.38.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohm L, Arvidsson I, Barbany G, Hast R, Stenke L. Early landmark analysis of imatinib treatment in CML chronic phase: less than 10% BCR-ABL by FISH at 3 months associated with improved long-term clinical outcome. Am J Hematol. 2012;87(8):760–765. doi: 10.1002/ajh.23238. [DOI] [PubMed] [Google Scholar]
- 23.Hanfstein B, Muller MC, Hehlmann R, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML) Leukemia. 2012;26(9):2096–2102. doi: 10.1038/leu.2012.85. [DOI] [PubMed] [Google Scholar]
- 24.Jain P, Kantarjian H, Nazha A, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood. 2013;121(24):4867–4874. doi: 10.1182/blood-2013-03-490128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 27.Jabbour E, Kantarjian HM, O’Brien S, et al. Front-line therapy with second-generation tyrosine kinase inhibitors in patients with early chronic phase chronic myeloid leukemia: what is the optimal response? J Clin Oncol. 2011;29(32):4260–4265. doi: 10.1200/JCO.2011.36.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014;123(9):1353–60. doi: 10.1182/blood-2013-06-510396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123(4):494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortes J, Giles F, O’Brien S, et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-alpha. Blood. 2003;102(1):83–86. doi: 10.1182/blood-2003-01-0025. [DOI] [PubMed] [Google Scholar]
- 31.Al-Kali A, Kantarjian H, Shan J, et al. Current event-free survival after sequential tyrosine kinase inhibitor therapy for chronic myeloid leukemia. Cancer. 2011;117(2):327–335. doi: 10.1002/cncr.25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falchi L, Kantarjian HM, Wang X, et al. Significance of deeper molecular responses in patients with chronic myeloid leukemia in early chronic phase treated with tyrosine kinase inhibitors. Am J Hematol. 2013;88(12):1024–1029. doi: 10.1002/ajh.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.