Abstract
2-Phenethyl isothiocyanate (PEITC), a natural product found as a conjugate in watercress and other cruciferous vegetables, is an inhibitor of the metabolic activation and lung carcinogenicity of the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in F344 rats and A/J mice. We carried out a clinical trial to determine whether PEITC also inhibits the metabolic activation of NNK in smokers. Cigarette smokers were recruited and asked to smoke cigarettes containing deuterium labelled [pyridine-D4]NNK for an acclimation period of at least one week. Then subjects were randomly assigned to one of two arms: PEITC followed by placebo, or placebo followed by PEITC. During the one-week treatment period, each subject took PEITC (10 mg in 1 ml of olive oil, 4 times per day). There was a one week washout period between the PEITC and placebo periods. The NNK metabolic activation ratio: [pyridine-D4]hydroxy acid/total [pyridine-D4]NNAL was measured in urine samples to test the hypothesis that PEITC treatment modified NNK metabolism. Eighty-two smokers completed the study and were included in the analysis. Overall, the NNK metabolic activation ratio was reduced by 7.7% with PEITC treatment (P = 0.023). The results of this trial, while modest in effect size, provide a basis for further investigation of PEITC as an inhibitor of lung carcinogenesis by NNK in smokers.
Keywords: 2-phenethyl isothiocyanate, PEITC, clinical trial, tobacco-specific lung carcinogen, chemoprevention
Introduction
Lung cancer, a devastating disease with a 5 year survival rate of less than 20%, killed 1,589,800 people in the world in 2012, about 4400 each day (1). Cigarette smoking accounts for 80% of this death toll in males and 50% in females (1). In the United States, cigarette smoking causes about 90% of all lung cancer deaths, which totaled 159,260 in 2014 (2). Eliminating or decreasing cigarette smoking are clearly the best approaches to save millions of lives; this has been partially successful in the United States, where the decrease in smoking prevalence is associated with a decrease in lung cancer (3). But this approach is limited because most cigarette smokers are addicted to nicotine and cannot quit their habit. About 70% of smokers try quitting each year, but less than 5% succeed (4). Even the best smoking cessation programs have six month success rates of only about 25% (5).
Chemoprevention is an alternate approach to decrease the risk for lung cancer in addicted smokers (6). In chemoprevention, one is treating the carcinogenic process rather than attempting to eradicate an established tumor. If carcinogenesis can be halted or even slowed, the chances of averting fatal lung cancer are increased. Tobacco smoke carcinogens are prime targets for chemoprevention of lung cancer; if their effects can be eliminated or decreased, the probability of developing lung cancer would also be decreased (6).
It has been 30 years since Chung et al first showed that phenethyl isothiocyanate (PEITC), which is found in substantial quantities as the conjugate gluconasturtiin in watercress and several other cruciferous vegetables, inhibited an obligatory step in the metabolic activation of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK, Figure S1) (7). NNK, which selectively induces lung tumors in rats, mice, and hamsters, is considered to be one of the most important carcinogens in tobacco smoke, and has been evaluated by the International Agency for Research on Cancer, along with the related carcinogen N′-nitrosonornicotine (NNN), as “carcinogenic to humans.” (8,9) Thus, decreasing the carcinogenicity of NNK by inhibiting its metabolic activation is one logical approach to chemoprevention of lung cancer in smokers.
Following the observation by Chung et al, multiple studies demonstrated that PEITC as well as its major metabolite N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine (PEITC-NAC) significantly inhibited lung tumorigenesis by NNK in mice and rats (10–17). Studies in the A/J mouse noted up to complete inhibition of lung tumor induction by NNK (for example, from 10.7 lung tumors per mouse to 0.3 lung tumors per mouse) when PEITC was given prior to NNK (11), but not when it was administered after NNK, indicating that its main effect was on metabolism or DNA binding of NNK. Similarly, three long term bioassays in F-344 rats, in which PEITC was added to the diet, clearly demonstrated significant inhibition of lung tumor induction by NNK (10,12,13). One study noted complete inhibition of lung tumor development (12).
Multiple studies have clearly demonstrated that the major mechanism by which PEITC inhibits carcinogenesis by NNK is inhibition of its metabolic activation by cytochrome P450 enzymes. This is the crucial step that produces highly reactive methylating and pyridyloxobutylating species that react with DNA to form a variety of mutagenic DNA adducts which are critical in lung carcinogenesis by NNK (Figure S1 (8,18). These studies, which have been carried out under a wide variety of conditions both in vitro and in vivo, with protocols varying from acute to chronic, and in some cases mimicking or coinciding with conditions used in the carcinogenicity studies, consistently demonstrate that PEITC decreases the formation of critical reactive metabolites of NNK, resulting in lower levels of DNA adducts in the lung, lower levels of hemoglobin adducts, and decreases in other endpoints, all reflecting inhibition of NNK metabolic activation (7,10–12,15,19–25). Nearly all of this can be traced to the inhibitory effects of PEITC on cytochrome P450 enzymes including human P450s 2A13, 2A6, 1A2, and 2B6, which are catalysts of NNK bioactivation (20,23,24,26–33). Consistent with these observations, we observed a significant effect of watercress consumption, as a source of PEITC, on NNK metabolism in smokers in a small clinical study (34). Excretion of the NNK metabolites NNAL plus NNAL-glucuronides increased upon watercress consumption, which would be expected upon inhibition of NNK metabolic activation (Figure S1). However, watercress consumption did not inhibit metabolism of coumarin or nicotine, both catalyzed by P450 2A6 (35,36).
Another aspect of chemoprevention by isothiocyanates involves the glutathione-S-transferase enzymes (GSTs) which catalyze the conjugation of isothiocyanates such as PEITC with glutathione, leading to the excretion in urine of PEITC-NAC. It has been hypothesized based on epidemiologic studies that individuals with GSTM1 or GSTT1 null-null genotypes would be protected from cancer more effectively by isothiocyanates than those with competent GST enzymes, because the isothiocyanates would be less effectively metabolized and excreted as their conjugates. Some support for this hypothesis has been obtained (37), but one study demonstrated no effect of GSTT1 or GSTM1 status on isothiocyanate excretion after consumption of watercress juice (38,39).
Thus, convincing data in the literature leave no doubt that PEITC can inhibit the metabolism and carcinogenicity of NNK in the rat and mouse lung. The primary aim of the randomized trial described here was to determine whether PEITC would also inhibit the metabolism of NNK in cigarette smokers. We applied our previously developed unique methodology based on the use of special cigarettes containing [pyridine-D4]NNK to allow us to quantify the extent of its metabolic activation to α-hydroxy metabolites which ultimately produce DNA adducts (Figure S1) (40). Formation of these metabolites was assessed by quantifying levels of [pyridine-D4]hydroxy acid, as opposed to the relatively large amounts of unlabeled urinary hydroxy acid produced as a minor urinary metabolite of nicotine in all smokers. To account for possible differences in the extent or topography of smoking of these cigarettes containing [pyridine-D4]NNK during the trial, we quantified the ratio [pyridine-D4]hydroxy acid:total [pyridine-D4]NNAL, where total [pyridine-D4]NNAL is the sum of [pyridine-D4]NNAL and its glucuronides (Figure S1). This ratio was hypothesized to decrease in subjects who took PEITC, due to the inhibition of cytochrome P450-catalyzed metabolic activation of [pyridine-D4]NNK and [pyridine-D4]NNAL (Figure S1). A secondary aim was to determine whether GSTM1 and GSTT1 genotype modified the impact of PEITC on NNK metabolism. This is the first clinical trial to assess the effects of pure PEITC on carcinogen metabolism in humans.
Materials and Methods
Drug Product
The use of PEITC and [pyridine-D4]NNK in this study were approved by the U.S. Food and Drug Administration under IND 74,037. Bulk PEITC was purchased from Sigma-Aldrich Corp, St. Louis, MO, and purified under GMP conditions at the Molecular and Cellular Therapeutics Facility, University of Minnesota. Purification was carried out on a Teledyne ISCO 1500 g flash column attached to a Teledyne ISCO Automated Chromatograpy System Combiflash Companion XL. PEITC was eluted by sequential washing with hexane and 80% hexane/20% ethanol. Fractions corresponding to PEITC standard, as determined by thin layer chromatography, were combined and the solvents were removed under reduced pressure. The purity of the PEITC was 99.2%, as determined by HPLC with UV detection at 254 nm, 1H-NMR, 13C-NMR, and GC-MS. Each dose used in this study consisted of 10 mg PEITC dissolved in 1 ml of olive oil N.F. (Gallipot, Inc). This solution was placed in a plastic syringe which each subject used to squirt the material into his or her mouth, 4 times per day, once every 4 h. Placebo was 1 ml of olive oil in a plastic syringe, with no added PEITC.
[pyridine-D4]4-Methylnitrosamino-1-(3-pyridyl)-1-butanone ([pyridine-D4]NNK)
[pyridine-D4]NNK was synthesized from [pyridine-D4]ethyl nicotinate (Toronto Research Chemicals) essentially as described for NNK (41). Its purity was >99% as determined by 1H-NMR, HPLC with UV detection, GC-MS, and direct inlet MS.
Cigarettes containing [pyridine-D4]NNK
Marlboro Virginia Blend (king size, hard pack) cigarettes, which contained 0.21 – 0.37 μg NNK/g wet weight tobacco, were purchased on the open market and modified by adding 0.300 μg [pyridine-D4]NNK to each cigarette. The addition of [pyridine-D4]NNK to the cigarettes was carried out with a specially designed microsyringe applicator system as previously described (40). The prepared cigarettes were conditioned at 25 °C and 60% relative humidity for 2 days, placed back in their original packs, 20 cigarettes per pack, and stored at 4 °C until being dispensed to study subjects. Twenty-five batches of these cigarettes were smoked under US Federal Trade Commission standard conditions; 10 batches were analyzed at the University of Minnesota, and contained 22.4 ± 2.1 ng/cigarette NNK and 21.5 ± 3.9 ng/cigarette [pyridine-D4]NNK in their mainstream smoke; and 15 batches were analyzed at the Centers for Disease Control and Prevention, Atlanta, GA and contained 18.8 ± 3.7 ng/cigarette NNK and 19.2 ± 2.4 ng/cigarette [pyridine-D4]NNK in their mainstream smoke. The use of these cigarettes was approved by the FDA Center for Tobacco Products, under Protocol Number P00006.
Study Design
This study was approved by the Institutional Review Boards of the University of Minnesota and the University of Pittsburgh and conducted at the University of Minnesota. The study was a phase II, randomized, placebo-controlled, double-blind, crossover clinical trial. The eligibility criteria for the study were: 1) current smokers of 10–45 cigarettes/day who were 21 years or older; 2) in apparent good physical health with normal liver function and no unstable medical conditions such as seizures or cancer; 3) in stable and good mental health, i.e., currently not experiencing unstable or untreated psychiatric diagnosis including substance abuse during the past 6 months; 4) currently not using any other tobacco or nicotine-containing products other than cigarettes; 5) not taking any drugs known to be substrates of cytochrome P450 1A2, 2B6 and 2A13 that may influence NNK metabolism; and 6) for female subjects, not pregnant or nursing. An overview of the study design is presented in Figure S2.
The duration of the study was approximately 5 weeks. After a telephone screening interview for subjects who responded to our local advertisement of the study, we invited those who were potentially eligible for the study to our research clinic for an orientation session. At the initial clinic visit, informed consent was obtained and subjects were asked to complete a questionnaire that asked for histories of tobacco use, medical conditions and medication use. A blood sample was collected for hemogram and liver function test. This blood sample also was used to determine both GSTM1 and GSTT1 genotypes for potential eligible subjects in the first two years of recruitment. After this visit (Week 1), subjects who were confirmed to be eligible were asked to smoke study cigarettes containing [pyridine-D4]NNK for at least one week (smoking adaptation) before the treatment began (Week 2). They were asked to smoke only these study cigarettes throughout the entire study period. Subjects were then randomly assigned to either the PEITC then placebo arm (PEITC-placebo group), or the placebo then PEITC arm (placebo-PEITC group) of the trial. During the treatment period, each subject was asked to take PEITC (10 mg in 1 ml olive oil, 4 times/day, once every 4h, for five days; Week 3 or 5) or the placebo agent (olive oil), on the same schedule (Week 3 or 5). There was a one-week washout period between the PEITC and placebo treatments (Week 4). Subjects were asked to avoid eating cruciferous vegetables, as instructed by a dietitian, throughout the study. Study participants were also asked to record any food and beverages consumed over 24h for 3 days (3rd, 4th and 5th) of each of the two treatment periods. The 24h food diaries were checked on a daily basis at the clinic visits during two treatment periods to confirm that the study participants did not consume any cruciferous vegetables during the past 24h.
The 24 h urine samples were collected at the following time points: end of the smoking adaptation period (week 2), three days (3rd, 4th, and 5th day) in each of the two treatment periods (weeks 3 and 5), and at the end of the washout period (week 4). Spot urine samples were collected on the 2nd day of each treatment period. Blood and buccal cell samples were collected at the following time points: baseline, end of the smoking adaptation period, each of the two treatment periods, and the washout period.
Analyses of Urinary Biomarkers
Total [pyridine-D4]NNAL and total NNAL were measured as described (40). Urine samples were treated with β-glucuronidase to release NNAL from its N- and O-glucuronides, and further purified and analyzed by LC-ESI-MS/MS. Free [pyridine-D4]NNAL and free NNAL were analyzed by the same method, but the urine samples were not treated with β-glucuronidase prior to their purification. Analysis of [pyridine-D4]hydroxy acid was performed by our recently developed methodology as described (42). Nicotine metabolites in urine including nicotine, cotinine and 3′-hydroxycotinine were analyzed as described (43). We used previously described methods, HPLC with UV-detection, for quantification of PEITC-NAC (44) and total isothiocyanates (45).
Endpoint Measures
The primary endpoint in this study was the ratio of urinary [pyridine-D4]hydroxy acid: total [pyridine-D4]NNAL. The rationale for use of this ratio is that it is not expected to be influenced by the number of cigarettes smoked per day by each subject or by smoking topography. As shown in Figure S1, [pyridine-D4]hydroxy acid is a urinary metabolite resulting from metabolic activation of [pyridine-D4]NNK. The other major urinary metabolite that results from this pathway is [pyridine-D4]keto acid. In the analysis of [pyridine-D4]hydroxy acid, the urine is treated with NaBH4, which converts all [pyridine-D4]keto acid to [pyridine-D4]hydroxy acid. Total [pyridine-D4]NNAL is the sum of the free metabolite and its glucuronides. As shown in Figure S1, total NNAL is produced by a pathway of NNK metabolism that is catalyzed mainly by carbonyl reductase enzymes, and total NNAL is considered to be an excellent biomarker of NNK exposure (46). These considerations lead to the conclusion that the ratio [pyridine-D4]hydroxy acid: total [pyridine-D4]NNAL is a biomarker of NNK/NNAL metabolic activation, which we hypothesized would be inhibited by PEITC. The deuterated compounds are necessary because hydroxy acid is a minor metabolite of nicotine, thus being present in smokers’ urine at more than 1000 times the amounts which could be generated in metabolism of NNK (47).
Secondary endpoints were urinary metabolites of NNK (total and free NNAL) and nicotine (total cotinine, 3′-hydroxycotinine, and their glucuronides). We calculated the total 3′-hydroxycotinine:total cotinine ratio, a measure of CYP2A6 activity, at the end of smoking adaptation period.
Statistical Analyses
All urinary biomarkers were expressed per mg of urinary creatinine to account for varying water content of individual urine samples. Given the markedly skewed distributions of the endpoint measures, formal statistical testing was performed on logarithmically (natural log) transformed values, and geometric means are presented.
The two-group t-test (for continuous variables) or chi-square statistics (for discrete or nominal variables) were used to compare the differences between the two treatment sequence assignments. The analysis of variance (ANOVA) method was used to examine the differences in urinary total ITC and PEITC-NAC among different GSTM1/GSTT1 genotypes. Spearman correlation was used to evaluate the correlations among urinary biomarkers measured before the PEITC treatment (at visit 2).
This study was a randomized crossover trial, a type of longitudinal study in which participants were randomly assigned to receive a sequence of treatment of PEITC or placebo. We used the linear mixed model with random effect (48) to simultaneously examine the effect of treatment (PEITC versus placebo), study period (period 1 versus period 2), treatment sequence (the carryover effect) and their interaction on the endpoint measurements in all subjects as well as in subgroup analyses stratified by subjects’ characteristics such as GSTM1 and/or GSTT1 genotypes, age, sex and smoking. A series of interaction terms between PEITC treatment and the baseline characteristics were included in the linear mixed models to evaluate the potential modifying effect of these factors on PEITC’s effect on the primary outcome measure – the [pyridine-D4]hydoxy acid:total [pyridine-D4]NNAL ratio. Because the statistical analyses for all the primary and secondary outcomes were done on the log-transformed variables, the difference of log-transformed means after back-transformation is presented as the percentage change, the equivalents of the ratio of the least-squared means on the original scale.
Statistical analyses were carried out using SAS software version 9.3 (SAS Institute, Cary, NC). All P-values reported are two-sided, and those that were less than 0.05 were considered to be statistically significant.
Results
Basic Observations
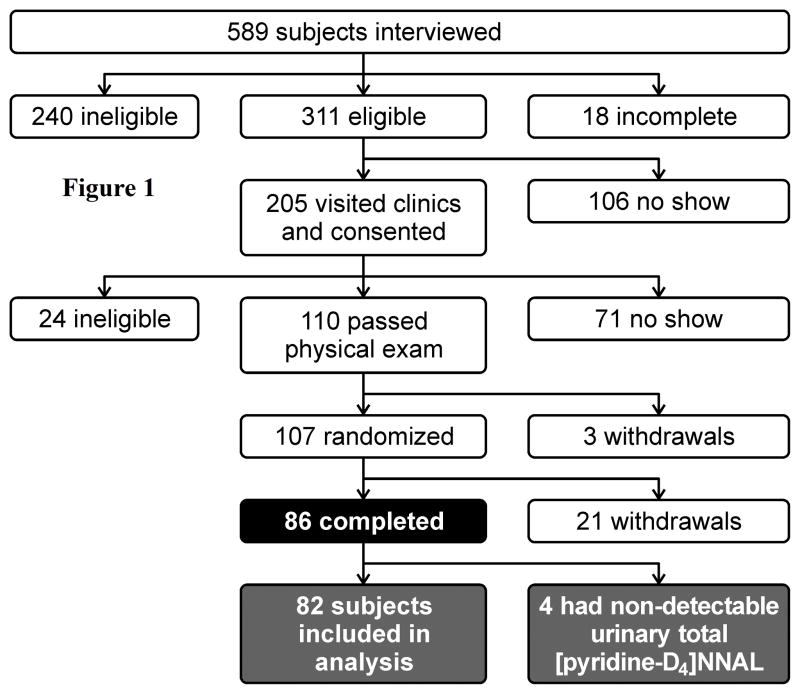
Figure 1 summarizes subject flow through the study. We interviewed 589 subjects, of whom 311 were potentially eligible for the study. Those subjects were invited to come to our research clinic for further evaluation of their eligibility. Of the 205 who visited our research clinic and signed consent forms, 110 passed the physical examination and enrolled in the study. Of those enrolled, 86 completed the entire course of the study and 24 withdrew. In addition, 4 subjects had undetectable urinary total [pyridine-D4]NNAL, indicating that they did not smoke the cigarettes containing [pyridine-D4]NNK, and thus were excluded from all statistical analysis.
Figure 1.
Profile of subject flow in the PEITC trial.
Among those who completed the study, the main adverse events during the PEITC treatment period were mild gastrointestinal (GI) disorders including dry mouth, taste alteration, stomach ache, belching, flatulence, and diarrhea; none of these were in the severe grade category.
Characteristics of the study subjects are summarized in Table 1. There were no significant differences in distributions of age, gender, race, level of education, smoking history (smoking intensity, duration, and age when beginning to smoke), and alcohol consumption between the PEITC-Placebo and Placebo-PEITC groups. Overall, the mean age (SD) was 41.0 (10.1) years. Among the 82 study participants, 46% were women, 67% were whites and 22% were African Americans. On average, study subjects smoked 19.1 (6.6) cigarettes per day and smoked cigarettes for 16.3 (9.8) years. Forty-seven percent had non-null and 12% null-null genotypes of both GSTM1 and GSTT1 genotypes.
Table 1.
Distributions of demographics and lifestyle factors and glutathione-S-transferase (GST) genotypes of study subjects by the treatment sequence assignment The PEITC Intervention Study 2008–2013
| Characteristics at baselinea | Treatment sequence assignment
|
Total | ||
|---|---|---|---|---|
| PEITC-Placebo | Placebo-PEITC | Pb | ||
| Number of subjects | 41 | 41 | 82 | |
| Age (years), mean (SD) | 40.9 (10.6) | 41.1 (9.6) | 0.939 | 41.0 (10.1) |
| Body mass index (kg/m2), mean (SD) | 28.0 (4.8) | 28.0 (6.3) | 0.957 | 28.0 (5.6) |
| Gender, n (%) | 0.376 | |||
| Male | 24 (59) | 20 (49) | 44 (54) | |
| Female | 17 (41) | 21 (51) | 38 (46) | |
| Race, n (%) | 0.839 | |||
| Africa American | 8 (20) | 10 (24) | 18 (22) | |
| Caucasian American | 28 (68) | 27 (66) | 55 (67) | |
| Others | 5 (12) | 4 (10) | 9 (11) | |
| Level of education, n (%) | 0.179 | |||
| High school or lower | 14 (34) | 20 (49) | 34 (41) | |
| College or higher | 27 (66) | 21 (51) | 48 (59) | |
| Cigarette smoking, mean (SD) | ||||
| Cigarettes/day | 19.3 (6.6) | 19.0 (6.7) | 0.843 | 19.1 (6.6) |
| Years of smoking | 14.7 (10.3) | 17.9 (9.4) | 0.157 | 16.3 (9.8) |
| Age at starting smoking (year) | 15.2 (4.9) | 15.1 (4.6) | 0.963 | 15.1 (4.7) |
| Age became regular smokers (year) | 18.6 (7.1) | 17.7 (5.2) | 0.514 | 18.1 (6.2) |
| Alcohol drinking, n (%)† | 0.784 | |||
| Never | 14 (35) | 17 (42) | 31 (39) | |
| Monthly or less | 14 (35) | 12 (30) | 26 (32) | |
| Greater than monthly | 12 (30) | 11 (28) | 23 (29) | |
| GSTM1 & GSTT1 genotypes, n (%) | 0.585 | |||
| Both present | 20 (49) | 18 (44) | 38 (46) | |
| Present/null | 3 (7) | 4 (10) | 7 (9) | |
| Null/present | 14 (34) | 11 (27) | 25 (30) | |
| Both null | 4 (10) | 8 (19) | 12 (15) | |
Abbreviations: SD, standard deviation.
2-sided P for the difference between the two treatment assignment groups based on the two-group t-test (for continuous variables) or χ2 test (for nominal or categorical variables).
Two subjects were excluded from this analysis due to missing data.
At the end of the smoking adaption (pre-intervention) period, study participants smoked an average of 20.9 (7.8) [pyridine-D4]NNK cigarettes per day. There was no statistical difference in mean [pyridine-D4]NNK cigarettes per day between the treatment sequence assignments at pre-intervention (Table 1) and during the intervention period (Table S1). The number of [pyridine-D4]NNK cigarettes smoked per day was comparable during study period 1 and period 2 (Table S1). At the pre-intervention stage, urinary levels of nicotine, NNK, and [pyridine-D4]NNK metabolites except urinary total nicotine were comparable between smokers assigned to the PEITC-Placebo and Placebo-PEITC groups (Table 2).
Table 2.
Distributions of urinary metabolites of nicotine, NNK and [pyridine-D4]NNK of study subjects by the treatment sequence assignment at the end of smoking adaptation (visit #2) The PEITC Intervention Study 2008–2013
| Urinary metabolites of nicotine and NNK | Treatment sequence assignment
|
Total | ||
|---|---|---|---|---|
| PEITC-Placebo | Placebo-PEITC | Pa | ||
| Number of subjects | 40b | 41 | 82 | |
| [pyridine-D4]NNK cigarettes/day, mean (SD)c | 21.6 (8.5) | 20.1 (7.1) | 0.419 | 20.9 (7.8) |
| Urinary metabolites, geometric mean (95% confidence interval) | ||||
| Total nicotine, ng/mg Cr | 2114 (1670,2676) | 3015 (2389,3806) | 0.039 | 2530 (2136, 2997) |
| Total cotinine, ng/mg Cr | 2839 (2368, 3402) | 3371 (2819, 4031) | 0.190 | 3096 (2725, 3519) |
| Total 3′-hydroxycotinine, ng/mg Cr | 6638 (5355, 8227) | 6993 (5657, 8646) | 0.736 | 6815 (5866, 7919) |
| Total nicotine equivalents, nmol/mg Cr | 45.1 (37.6, 54.0) | 58.1 (48.6, 69.5) | 0.054 | 51.2 (45.0, 58.3) |
| Total 3′-hydroxycotinne:total cotinine ratio | 2.34 (1.96, 2.79) | 2.07 (1.74, 2.47) | 0.348 | 2.20 (1.94, 2.49) |
| Total NNAL, pmol/mg Cr | 0.85 (0.65, 1.10) | 0.94 (0.73, 1.22) | 0.567 | 0.89 (0.75, 1.07) |
| Free NNAL, pmol/mg Cr | 0.24 (0.19, 0.30) | 0.29 (0.23, 0.36) | 0.276 | 0.26 (0.22, 0.31) |
| Total [pyridine-D4]NNAL, pmol/mg Cr | 0.24 (0.19, 0.31) | 0.26 (0.20, 0.33) | 0.697 | 0.25 (0.21, 0.30) |
| Free [pyridine-D4]NNAL, pmol/mg Cr | 0.08 (0.06, 0.10) | 0.09 (0.07, 0.11) | 0.546 | 0.08 (0.07, 0.10) |
| [Pyridine-D4]hydroxyl acid, pmol/mg Cr | 0.11 (0.09, 0.14) | 0.13 (0.11, 0.17) | 0.262 | 0.12 (0.11, 0.14) |
| [pyridine-D4]hydroxy acid:total [pyridine-D4]NNAL ratio | 0.47 (0.39, 0.55) | 0.52 (0.44, 0.62) | 0.398 | 0.49 (0.44, 0.56) |
2-sided P for the difference between the two treatment assignment groups based on the two-group t-test.
One subject did not provide the 24-hr urine sample at visit 2, thus, were excluded from the analysis.
Six subjects were excluded from analysis due to missing data (1 in PEITC-Placebo and 5 in Placebo-PEITC group); SD, standard deviation.
Correlations among various parameters measured in this study are shown in Table S2. The number of [pyridine-D4]NNK cigarettes per day, urinary levels of NNAL and [pyridine-D4]NNAL, both total and free, and all nicotine metabolites were highly correlated (P<0.001); [pyridine-D4]hydroxy acid was highly correlated with free and total [pyridine-D4]NNAL and with free and total NNAL.
Primary Outcome Analyses
Urinary levels of both PEITC-NAC and total isothiocyanates (ITC) were significantly elevated in the PEITC treatment periods compared to the placebo periods among all subjects by a magnitude of more than 150 times (Table S3). On average, approximately 29% of the daily PEITC intended dose was excreted as PEITC-NAC in the urine (Table S3).
Intake of PEITC reduced the urinary ratio of [pyridine-D4]hydroxy acid: total [pyridine-D4]NNAL by 7.7% (95% CI: 1.2% to 13.8%; P = 0.023) (Table 3). This reduction was primarily due to decreased [pyridine-D4]hydroxy acid, a biomarker of NNK metabolic activation, by PEITC. No statistically significant treatment sequence (i.e., carryover) effect was observed for urinary total [pyridine-D4]NNAL, [pyridine-D4]hydroxy acid or their ratio (Table S4). However, there was a statistically significant increase in urinary total [pyridine-D4]NNAL and a significant decrease in the ratio of [pyridine-D4]hydroxy acid: total [pyridine-D4]NNAL during study period 2 compared with study period 1 (Table S4).
Table 3.
Urinary levels of [pyridine-D4]NNK metabolites by PEITC treatment, The PEITC Intervention Study 2008–2013
| Urinary metabolites of [pyridine-D4]NNK | Geometric mean [pmol/mg creatinine]
|
|||
|---|---|---|---|---|
| Placebo (n= 82) | PEITC (n=82) | % difference (95% CI) | P for differencea | |
| Total [pyridine-D4]NNAL (pmol/mg creatinine) | 0.336 | 0.336 | −0.1 (−8.5, 9.1) | 0.980 |
| [pyridine-D4]Hydroxy acid (pmol/mg creatinine) | 0.150 | 0.140 | −6.7 (−13.8, 1.0) | 0.092 |
| [pyridine-D4]Hydroxy acid: [pyridine-D4] total NNAL ratio | 0.455 | 0.420 | −7.7 (−13.8, −1.2) | 0.023 |
2-sided P values were for PEITC treatment effect and derived from the mixed model.
The potential influence of GST genotype on PEITC metabolism was examined and the results are shown Table S5. Individuals possessing GSTM1 or both GSTM1 and GSTT1 genes had lower urinary concentrations of PEITC-NAC and total ITC than their counterparts lacking the genes, respectively.
The potential influence of GST genotype on PEITC’s effect on [pyridine-D4]NNK metabolism was examined and the results are shown Table 4. PEITC inhibited [pyridine-D4]NNK metabolism in subjects with the GSTT1 null genotype (an 11.1% decrease) but in GSTM1 non-nulls (an 8.8% decrease). The inhibitory effect of PEITC on [pyridine-D4]NNK metabolism was strongest in subjects lacking both the GSTM1 and GSTT1 genes (a 15.6% decrease, P=0.039), followed by those possessing both genes (a 9.4% decrease, P=0.045), but there was no effect in those possessing either the GSTM1 or the GSTT1 gene. However, the differences between these genotype groups were not statistically significant (all P values for interaction ≥ 0.436).
Table 4.
Urinary levels of [pyridine-D4]hydroxy acid:total [pyridine-D4]NNAL ratio by PEITC treatment stratified by GSTM1 and GSTT1 genotypes, The PEITC Intervention Study 2008–2013
| GST genotypes | No | Geometric means of [pyridine-D4]hydroxy acid:total [pyridine-D4]NNAL ratio
|
P for interactionb | |||
|---|---|---|---|---|---|---|
| Placebo | PEITC | % difference (95% CI) | P valuea | |||
| GSTM1 | 0.694 | |||||
|
| ||||||
| null | 37 | 0.423 | 0.397 | −6.2 (−16.5, 5.3) | 0.284 | |
| Present | 45 | 0.483 | 0.441 | −8.8 (−15.9, −1.1) | 0.031 | |
|
| ||||||
| GSTT1 | 0.574 | |||||
| null | 19 | 0.387 | 0.344 | −11.1 (−19.7, −1.6) | 0.037 | |
| Present | 63 | 0.478 | 0.446 | −6.7 (−14.2, 1.5) | 0.111 | |
|
| ||||||
| GSTM1 & GSTT1 | 0.436 | |||||
| Both null | 12 | 0.373 | 0.314 | −15.6 (−26.7, −2.9) | 0.039 | |
| Only one gene present | 32 | 0.445 | 0.431 | −3.1 (−14.6, 9.9) | 0.623 | |
| Both genes present | 38 | 0.496 | 0.450 | −9.4 (−17.4, −0.6) | 0.045 | |
2-sided P values were derived from the mixed models to test PEITC treatment effect within specific GST genotype group.
2-sided P values were derived from the mixed models to test the modifying effect of genotype on the PEITC’s effect on the [pyridine-D4]hydroxy acid:total [pyridine-D4]NNAL ratio.
We also examined the effect of PEITC on the urinary [pyridine-D4]hydroxy acid: total [pyridine-D4]NNAL ratio stratified by age (<40, ≥40 years), sex, years of smoking, total nicotine equivalents, and total 3′-hydroxycotinine:total cotinine ratio. Stronger effects of PEITC intake on the reduction of urinary [pyridine-D4]hydroxy acid: total [pyridine-D4]NNAL ratio were observed for subjects who were 40 years or older, were women, and had lower total nicotine equivalents or higher CYP2A6 activity (Table 5). However, the differences between these groups were not statistically significant (all P values for interaction ≥ 0.245).
Table 5.
Urinary levels of [pyridine-D4]hydroxy acid:total [pyridine-D4]NNAL ratio by PEITC treatment in stratified analysis, The PEITC Intervention Study 2008–2013
| Stratifying variable at baseline (visit 2) | No | Geometric means of [pyridine-D4]hydroxy acid:total [pyridine-D4]NNAL ratio
|
P for interactionb | |||
|---|---|---|---|---|---|---|
| Placebo | PEITC | % difference (95% CI) | P valuea | |||
| Age | 0.245 | |||||
| < 40 | 34 | 0.531 | 0.515 | −3.2 (−12.9, 7.7) | 0.559 | |
| ≥ 40 | 48 | 0.408 | 0.364 | −10.8 (−18.3, −2.5) | 0.015 | |
|
| ||||||
| Sex | 0.606 | |||||
| Men | 44 | 0.474 | 0.446 | −6.0 (−15.1, 4.0) | 0.238 | |
| Women | 38 | 0.434 | 0.394 | −9.3 (−17.2, −0.7) | 0.043 | |
|
| ||||||
| Years of smoking | 0.675 | |||||
| ≤10 | 22 | 0.511 | 0.465 | −9.0 (−21.8, 6.0) | 0.240 | |
| 11–20 | 30 | 0.441 | 0.423 | −4.2 (−13.0, 5.4) | 0.386 | |
| ≥ 21 | 30 | 0.436 | 0.385 | −11.6 (−22.1, 0.2) | 0.065 | |
|
| ||||||
| Total nicotine equivalentsc | ||||||
| < median | 40 | 0.507 | 0.453 | −10.7 (−18.8, −1.7) | 0.026 | 0.544 |
| ≥ median | 41 | 0.409 | 0.381 | −6.8 (−15.8, 3.3) | 0.186 | |
|
| ||||||
| Total 3′-hydroxycotinine: total cotinine ratioc | 0.345 | |||||
| < median | 40 | 0.463 | 0.438 | −5.6 (−14.0, 3.8) | 0.243 | |
| ≥ median | 41 | 0.446 | 0.395 | −11.5 (−19.6, −2.4) | 0.018 | |
2-sided P values were derived from the mixed models to test PEITC treatment effect within a subgroup by stratifying variable.
2-sided P values were derived from the mixed models to test PEITC treatment effect cross group of stratifying variable.
One subject did not provide the 24-hr urine sample at visit 2, thus, were excluded from the analysis.
Secondary Analyses
We analyzed the urine of some subjects for [pyridine-D4]4-hydroxy-1-(3-pyridyl)-1-butanone ([pyridine-D4] keto alcohol) and [pyridine-D4]4-hydroxy-1-(3-pyridyl)-1-butanol ([pyridine-D4] diol), which are primary metabolites of NNK and NNAL formed by their metabolic activation pathways (Figure S1), but neither was detectable.
Supplementation with PEITC significantly increased urinary total NNAL and total nicotine, and increased the ratio of 3′-hydroxycotinine glucuronides to free 3′-hydroxycotinine (Table S6). When data were analyzed by GSTM1 and GSTT1 genotypes, the effect of PEITC supplementation on the increased ratio of 3′-hydroxycotinine glucuronides to free 3′-hydroxycotinine was seen mainly in subjects who possessed either the GSTM1 (by 33.1%, 95% CI 6.8%–65.8%) or GSTT1 gene (by 31.3%, 95% CI 6.0% – 62.75), and was especially elevated among those who had both GSTM1 and GSTT1 genes (43.6%, 95% CI 15.5%–78.7%). There were no statistically significant effects of PEITC on urinary excretion of free NNAL, total cotinine, total 3′-hydroxycotinine, or total nicotine equivalents; and the total 3′-hydroxycotinine:total cotinine ratio, the glucuronides:free NNAL ratio, the glucuronides:free [pyridine-D4]NNAL ratio, or the glucuronides:free cotinine ratio (Table S6). GSTM1 and/or GSTT1 status did not modify the effects of PEITC on the urinary levels of these NNK and nicotine metabolites or their glucuronides/free ratios (data not shown).
Discussion
This is the first clinical trial of PEITC as an inhibitor of NNK metabolism in smokers. We used a unique design featuring cigarettes containing [pyridine-D4]NNK to allow specific evaluation of the potential effect of PEITC on NNK metabolism without confounding by nicotine metabolites. We report a significant but modest 7.7% inhibitory effect of PEITC on the metabolic activation of NNK in our smokers. Although modest, these findings were consistent with our hypothesis based on extensive studies of PEITC effects on NNK metabolism and carcinogenicity in laboratory animals. Stronger effects of PEITC on NNK metabolism were observed in subjects with the null genotype of both GSTM1 and GSTT1 (reduced by 15.6%), as well as in women, subjects 40 years or older, and those with higher total 3′-hydroxycotinine :total cotinine ratio, a phenotypic measure of CYP2A6.
We also observed a statistically significant effect of PEITC on glucuronidation of 3′-hydroxycotinine (a 22.9% increase) as in our previous study with watercress (35). The magnitude of the effect of PEITC on 3′-hydroxycotinine glucuronidation was greater in individuals with at least one copy of GSTM1 or GSTT1, especially in those possessing both GSTM1 and GSTT1 genes. These results suggest that PEITC may have a stronger effect on the induction of glucuronidation in individuals with the GSTM1 and GSTT1 genes, but as noted below, the effects of GSTM1 and GSTT1 status in this study were complex and are poorly understood.
Intake of PEITC borderline significantly increased urinary total nicotine (by 8.9%) and total nicotine equivalents (by 6.0%). This could be due to the bitter taste of PEITC delivered in olive oil by syringe. Subjects may have changed their smoking behavior to alleviate the unpleasant taste and smell of PEITC. However, we did not find that subjects consumed significantly more cigarettes in the PEITC treatment period than the placebo period. In our exploratory analysis (Table 5), a stronger inhibitory effect of PEITC on NNK metabolism was seen in smokers with lower total nicotine equivalents (a 10.7% decrease in [pyridine-D4]hydroxy acid:total [pyridine-D4]NNAL ratio) than higher total nicotine equivalents (a 6.8% decrease). However, the difference was not statistically significant (P = 0.544).
The daily dose of 40 mg PEITC was chosen based on the results of our study with watercress, in which subjects consumed a daily minimum amount of PEITC ranging from 19–38 mg for 3 days that effectively increased urinary total NNAL excretion (34). This dose did not show any toxic effects. In the Phase I study of PEITC, doses of up to 120 mg per day for 30 days were investigated. This highest dose caused some gastrointestinal distress and diarrhea, more than the 40 mg dose used here. While this 40 mg dose (0.5 mg/kg/day) is considerably less than the dose of PEITC used in our rat studies (20 mg/kg/day), so is the dose of NNK in our study subjects (0.000012 mg/kg/day), considerably less than that used in the rat studies (0.118 mg/kg/day). Thus, the PEITC:NNK ratio in our study subjects was about 42,000, which was about 250 times higher than the corresponding figure in the rat study (PEITC:NNK ratio = 170). PEITC is fat soluble. Thus, delivery of PEITC in olive oil avoids concerns about bioavailability; the phase 1 trial used the same form. The half-life of PEITC was 2.4h in the phase 1 study. Thus study subjects were asked to take PEITC (10 mg 4 times per day) to have a relatively stable internal dose of PEITC throughout the 24h per day.
Nevertheless, the dosing form of PEITC used in this study was not optimal and the dose may have been too low. The urinary excretion of PEITC equivalents (measured as PEITC-NAC) averaged 13.5 mg in 24 h in the study participants, which was approximately only one-third the amount of PEITC equivalents (36.6–37.7 mg) excreted as PEITC-NAC in the 24 h urine of 11 subjects who consumed six ounces of watercress per day (34). The lower excreted amount of PEITC-NAC in the present study suggests at least two possibilities – a low to moderate compliance among subjects who consumed a lower amount of PEITC than provided or retention of PEITC in the body that delays urinary excretion. Also, in the Phase I study with a daily dose of 120 mg PEITC, the maximum amount of PEITC-NAC in the urine was not reached until 2 weeks of daily dosing. This suggests that a longer dosing period and/or higher dose might have been more successful in our trial. Our study design was a compromise that was partially influenced by practical considerations such as the available dosage form.
Following the initial observation by London and co-workers that individuals with deletions in GSTM1 and GSTT1 who consumed isothiocyanates were at decreased risk for lung cancer (38), many studies have examined this relationship. A review of this literature suggests that cruciferous vegetable intake may be weakly and inversely associated with lung cancer risk with the strongest protective association among those with homozygous deletion for both GSTM1 and GSTT1, based on the hypothesis that these individuals would have more free isothiocyanate available (37). However, Dyba et al did not find any effect of GSTM1 and GSTT1 genotype on excretion of isothiocyanates after consumption of watercress juice, a rich source of PEITC (39). We also did not observe the predicted effect of glutathione S-transferase polymorphisms on PEITC metabolism. We saw a increases in urinary PEITC-NAC in the subjects who were null for GSTM1 and GSTT1 compared to those with the non-null genotypes. Similarly, subjects with GSTM1 null genotype had significantly more total isothiocyanates in their urine than those with the non-null genotype. These data suggest that GSTM1 and GSTT1 did not enhance the urinary excretion of ITC or PEITC. Based on our original hypothesis, we would have expected a stronger inhibitory effect of PEITC on NNK metabolic activation in those individuals with GST null genotypes. However, the present study did not show consistent results. PEITC had a stronger inhibitory effect in subjects with the null genotype of GSTT1 but not GSTM1. Furthermore, the inhibitory effect of PEITC on NNK metabolism was comparable in subjects who lacked both or possessed both genes. These data suggest a complex role of GSTM1 and GSTT1 in modifying the potential inhibitory effect of PEITC on NNK metabolism. It should also be noted that GSTP1, which has polymorphic forms but has not been shown to exist as a null, is also an excellent catalyst of PEITC metabolism (49), and that PEITC reacts easily with glutathione without catalysis. Further studies are required to elucidate the mechanisms by which GST null status affects isothiocyanate metabolism and lung cancer susceptibility.
The present study is larger and better controlled, both with respect to evaluation of NNK metabolism and specificity of the chemopreventive agent compared to our earlier study using watercress. Nevertheless, watercress consumption is an attractive way to deliver PEITC because of the relatively high concentrations of the PEITC precursor gluconasturtiin in this vegetable, which can be consumed in a more pleasing way than the dosing form used here, and without any significant concerns about toxicity. It will be important to build on the current trial results using watercress as the potentially protective agent.
The analysis of [pyridine-D4]hydroxy acid in urine is difficult because this highly polar metabolite is found in relatively low concentrations. The method, which involves two derivatization reactions, has been validated previously (42), but the analysis of more than 700 samples in the present trial was a challenge. The significant correlation of [pyridine-D4]hydroxy acid with total [pyridine-D4]NNAL (Spearman coefficient = 0.79, P<0.001, Table S2), comparing data obtained by different analysts who were blind to the origin of the samples, strongly supports the validity of the analytical chemistry methods used here.
While the metabolic ratio [pyridine-D4]hydroxy acid: total[pyridine-D4]NNAL is a good indicator of NNK/NNAL metabolism by α-hydroxylation, it is not the ideal biomarker. It would have been better to quantify the DNA adducts that result from α-hydroxylation of NNK and NNAL. While these have been well characterized and quantified in rats and mice treated with NNK (8,50), robust methods for their quantitation in available human specimens such as oral cells or blood are not available. The primary metabolites resulting from α-hydroxylation, [pyridine-D4]keto alcohol and [pyridine-D4]diol, might also have been better biomarkers of this process than [pyridine-D4]keto acid and [pyridine-D4]hydroxy acid that are further downstream, but we were unable to detect these metabolites in the urine of our subjects.
Although the reduction in the [pyridine-D4]hydroxy acid: total [pyridine-D4]NNAL ratio was modest in this study, we note that the extent of PEITC’s inhibition of tumor formation by NNK can be substantially greater than the inhibition of metabolism or adduct formation. In one study in rats, we observed that PEITC completely inhibited lung tumor formation by NNK, but the corresponding inhibition of NNK-hemoglobin adducts, a biomarker of its metabolic activation, was only about 40% (12). Similar observations have been reported in studies of inhibition of aflatoxin carcinogenesis in rats (51). Therefore, the ultimate effect of PEITC on NNK-induced lung cancer could be greater than predicted by a simple metabolic ratio.
In summary, we report promising results of the first clinical trial of PEITC as an inhibitor of carcinogen metabolism in smokers. We observed a modest, specific, and significant modulation of metabolism of the tobacco-specific lung carcinogen NNK in a direction that would predict efficacy in protection against cancer. Our results suggest some potentially important modifications in study design related to dose, dosage form, and length of the dosing period that could lead to greater efficacy in future trials.
Supplementary Material
Acknowledgments
Grant funding
This study was supported by the U.S. National Cancer Institute [R01 CA122244 to J-M. Yuan].
We thank Katrina Yershova for spiking [pyridine-D4]NNK in the study cigarettes, Meng Jing for performing assays for urinary hydroxyl acid, and Matthew Vang for performing assays for urinary NNAL and PEITC metabolites. We thank the subjects in this study for their conscientious participation, and Bob Carlson for editorial assistance.
Footnotes
Conflict of Interest Statement: We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Stewart BW, Wild CP. World Cancer Report 2014. Lyon, FR: IARC; 2014. [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.United States Department of Health and Human Services. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The Health Consequences of Smoking: 50 Years of Progress. [Google Scholar]
- 4.Gilpin EA, Pierce JP. Demographic differences in patterns in the incidence of smoking cessation: United States 1950–1990. Ann Epidemiol. 2002;12:141–50. doi: 10.1016/s1047-2797(01)00266-6. [DOI] [PubMed] [Google Scholar]
- 5.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- 6.Hecht SS, Kassie F, Hatsukami DK. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat Rev Cancer. 2009;9:476–88. doi: 10.1038/nrc2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung FL, Wang M, Hecht SS. Effects of dietary indoles and isothiocyanates on N-nitrosodimethylamine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone alpha-hydroxylation and DNA methylation in rat liver. Carcinogenesis. 1985;6:539–43. doi: 10.1093/carcin/6.4.539. [DOI] [PubMed] [Google Scholar]
- 8.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 89. Lyon, FR: IARC; 2007. Smokeless tobacco and tobacco-specific nitrosamines; pp. 41–583. [PMC free article] [PubMed] [Google Scholar]
- 10.Morse MA, Wang CX, Stoner GD, Mandal S, Conran PB, Amin SG, et al. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res. 1989;49:549–53. [PubMed] [Google Scholar]
- 11.Morse MA, Amin SG, Hecht SS, Chung FL. Effects of aromatic isothiocyanates on tumorigenicity, O6-methylguanine formation, and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Cancer Res. 1989;49:2894–97. [PubMed] [Google Scholar]
- 12.Hecht SS, Trushin N, Rigotty J, Carmella SG, Borukhova A, Akerkar SA, et al. Complete inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced rat lung tumorigenesis and favorable modification of biomarkers by phenethyl isothiocyanate. Cancer Epidemiol Biomarkers Prev. 1996;5:645–52. [PubMed] [Google Scholar]
- 13.Chung FL, Kelloff G, Steele V, Pittman B, Zang E, Jiao D, et al. Chemopreventive efficacy of arylalkyl isothiocyanates and N-acetylcysteine for lung tumorigenesis in Fischer rats. Cancer Res. 1996;56:772–78. [PubMed] [Google Scholar]
- 14.Jiao D, Smith TJ, Yang CS, Pittman B, Desai D, Amin S, et al. Chemopreventive activity of thiol conjugates of isothiocyanates for lung tumorigenesis. Carcinogenesis. 1997;18:2143–47. doi: 10.1093/carcin/18.11.2143. [DOI] [PubMed] [Google Scholar]
- 15.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metabol Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 16.Kassie F, Matise I, Negi M, Lahti D, Pan Y, Scherber R, et al. Combinations of N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine and myo-inositol inhibit tobacco smoke carcinogen-induced lung adenocarcinoma in A/J mice. Cancer Prev Res. 2008;1:285–97. doi: 10.1158/1940-6207.CAPR-08-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention. Vol. 9. Lyon, FR: IARC; 2004. Cruciferous Vegetables, Isothiocyanates, and Indoles; pp. 109–26. [Google Scholar]
- 18.Hecht SS. Progress and challenges in selected areas of tobacco carcinogenesis. Chem Res Toxicol. 2008;21:160–71. doi: 10.1021/tx7002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Z, Smith TJ, Thomas PE, Yang CS. Metabolic activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone as measured by DNA alkylation in vitro and its inhibition by isothiocyanates. Cancer Res. 1991;51:4798–803. [PubMed] [Google Scholar]
- 20.Conaway CC, Jiao D, Chung FL. Inhibition of rat liver cytochrome P450 isozymes by isothiocyanates and their conjugates: a structure-activity relationship study. Carcinogenesis. 1996;17:2423–27. doi: 10.1093/carcin/17.11.2423. [DOI] [PubMed] [Google Scholar]
- 21.Staretz ME, Koenig L, Hecht SS. Effects of long term phenethyl isothiocyanate treatment on microsomal metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in F344 rats. Carcinogenesis. 1997;18:1715–22. doi: 10.1093/carcin/18.9.1715. [DOI] [PubMed] [Google Scholar]
- 22.Sticha KRK, Staretz ME, Wang M, Liang H, Kenney PMJ, Hecht SS. Effects of benzyl isothiocyanate and phenethyl isothiocyanate on benzo[a]pyrene metabolism and DNA adduct formation in the A/J mouse. Carcinogenesis. 2000;21:1711–19. doi: 10.1093/carcin/21.9.1711. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima M, Yoshida R, Shimada N, Yamazaki H, Yokoi T. Inhibition and inactivation of human cytochrome P450 isoforms by phenethyl isothiocyanate. Drug Metab Dispos. 2001;29:1110–13. [PubMed] [Google Scholar]
- 24.von Weymarn LB, Chun JA, Hollenberg PF. Effects of benzyl and phenethyl isothiocyanate on P450s 2A6 and 2A13: potential for chemoprevention in smokers. Carcinogenesis. 2006;27:782–90. doi: 10.1093/carcin/bgi301. [DOI] [PubMed] [Google Scholar]
- 25.Morris ME, Dave RA. Pharmacokinetics and pharmacodynamics of phenethyl isothiocyanate: implications in breast cancer prevention. AAPS J. 2014;16:705–13. doi: 10.1208/s12248-014-9610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith TJ, Guo Z, Li C, Ning SM, Thomas PE, Yang CS. Mechanisms of inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone bioactivation in mouse by dietary phenethyl isothiocyanate. Cancer Res. 1993;53:3276–82. [PubMed] [Google Scholar]
- 27.Staretz ME, Hecht SS. Effects of phenethyl isothiocyanate on the tissue distribution of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and metabolites in F344 rats. Cancer Res. 1995;55:5580–88. [PubMed] [Google Scholar]
- 28.Konsue N, Ioannides C. Tissue differences in the modulation of rat cytochromes P450 and phase II conjugation systems by dietary doses of phenethyl isothiocyanate. Food Chem Toxicol. 2008;46:3677–83. doi: 10.1016/j.fct.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 29.Boysen G, Kenney PMJ, Upadhyaya P, Wang M, Hecht SS. Effects of benzyl isothiocyanate and 2-phenethyl isothiocyanate on benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolism in F-344 rats. Carcinogenesis. 2003;24:517–25. doi: 10.1093/carcin/24.3.517. [DOI] [PubMed] [Google Scholar]
- 30.Konsue N, Ioannides C. Modulation of carcinogen-metabolising cytochromes P450 in human liver by the chemopreventive phytochemical phenethyl isothiocyanate, a constituent of cruciferous vegetables. Toxicology. 2010;268:184–90. doi: 10.1016/j.tox.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Szaefer H, Krajka-Kuzniak V, Bartoszek A, Baer-Dubowska W. Modulation of carcinogen metabolizing cytochromes P450 in rat liver and kidney by cabbage and sauerkraut juices: comparison with the effects of indole-3-carbinol and phenethyl isothiocyanate. Phytother Res. 2012;26:1148–55. doi: 10.1002/ptr.3692. [DOI] [PubMed] [Google Scholar]
- 32.Stephens ES, Walsh AA, Scott EE. Evaluation of inhibition selectivity for human cytochrome P450 2A enzymes. Drug Metab Dispos. 2012;40:1797–802. doi: 10.1124/dmd.112.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshigae Y, Sridar C, Kent UM, Hollenberg PF. The inactivation of human CYP2E1 by phenethyl isothiocyanate, a naturally occurring chemopreventive agent, and its oxidative bioactivation. Drug Metab Dispos. 2013;41:858–69. doi: 10.1124/dmd.112.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hecht SS, Chung FL, Richie JP, Jr, Akerkar SA, Borukhova A, Skowronski L, et al. Effects of watercress consumption on metabolism of a tobacco-specific lung carcinogen in smokers. Cancer Epidemiol Biomarkers Prev. 1995;4:877–84. [PubMed] [Google Scholar]
- 35.Hecht SS, Carmella SG, Murphy SE. Effects of watercress consumption on urinary metabolites of nicotine in smokers. Cancer Epidemiol Biomarkers Prev. 1999;8:907–13. [PubMed] [Google Scholar]
- 36.Murphy SE, Johnson LM, Losey LM, Carmella GS, Hecht SS. Consumption of watercress fails to alter coumarin metabolism in humans. Drug Metab Dispos. 2001;29:786–88. [PubMed] [Google Scholar]
- 37.Lam TK, Gallicchio L, Lindsley K, Shiels M, Hammond E, Tao XG, et al. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18:184–95. doi: 10.1158/1055-9965.EPI-08-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.London SJ, Yuan JM, Chung F-L, Gao YT, CGA, Ross RK, et al. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet. 2000;356:724–29. doi: 10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- 39.Dyba M, Wang A, Noone AM, Goerlitz D, Shields P, Zheng YL, et al. Metabolism of isothiocyanates in individuals with positive and null GSTT1 and M1 genotypes after drinking watercress juice. Clin Nutr. 2010;29:813–18. doi: 10.1016/j.clnu.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stepanov I, Upadhyaya P, Feuer R, Jensen J, Hatsukami DK, Hecht SS. Extensive metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers. Cancer Epidemiol Biomarkers Prev. 2008;17:1764–73. doi: 10.1158/1055-9965.EPI-07-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hecht SS, Chen CB, Dong M, Ornaf RM, Hoffmann D, Tso TC. Studies on non-volatile nitrosamines in tobacco. Beiträge zur Tabakforschung. 1977;9:1–6. [Google Scholar]
- 42.Jing M, Wang Y, Upadhyaya P, Jain V, Yuan JM, Hatsukami DK, et al. Liquid chromatography-electrospray ionization-tandem mass spectrometry quantitation of urinary [pyridine-D4]4-hydroxy-4-(3-pyridyl)butanoic acid, a biomarker of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolic activation in smokers. Chem Res Toxicol. 2014;27:1547–55. doi: 10.1021/tx5001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy SE, Park S-SL, Thompson EF, Wilkens LR, Patel Y, Stram DO, et al. Nicotine N-glucurionidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35:2526–33. doi: 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung FL, Morse MA, Eklind KI, Lewis J. Quantitation of human uptake of the anticarcinogen phenethyl isothiocyanate after a watercress meal. Cancer Epidemiol Biomarkers Prev. 1992;1:383–88. [PubMed] [Google Scholar]
- 45.Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, et al. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: Relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998;7:775–81. [PubMed] [Google Scholar]
- 46.Joseph AM, Hecht SS, Murphy SE, Carmella SG, Le CT, Zhang Y, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14:2963–68. doi: 10.1158/1055-9965.EPI-04-0768. [DOI] [PubMed] [Google Scholar]
- 47.Hecht SS, Hatsukami DK, Bonilla LE, Hochalter JB. Quantitation of 4-oxo-4-(3-pyridyl)butanoic acid and enantiomers of 4-hydroxy-4-(3-pyridyl)butanoic acid in human urine: A substantial pathway of nicotine metabolism. Chem Res Toxicol. 1999;12:172–79. doi: 10.1021/tx980214i. [DOI] [PubMed] [Google Scholar]
- 48.Jones B, Kenward MG. Design and Analysis of Cross-Over Trials. 3. Boca Raton: Chapman and Hall; 2014. [Google Scholar]
- 49.Kolm RH, Danielson UH, Zhang Y, Talalay P, Mannervik B. Isothiocyanates as substrates for human glutathione transferases: structure-activity studies. Biochem J. 1995;311(Pt 2):453–59. doi: 10.1042/bj3110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balbo S, Johnson CS, Kovi RC, James-Yi SA, O’Sullivan MG, Wang M, et al. Carcinogenicity and DNA adduct formation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in F-344 rats. Carcinogenesis. 2014;35:2798–806. doi: 10.1093/carcin/bgu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson NM, Egner PA, Baxter VK, Sporn MB, Wible RS, Sutter TR, et al. Complete protection against aflatoxin B(1)-induced liver cancer with a triterpenoid: DNA adduct dosimetry, molecular signature, and genotoxicity threshold. Cancer Prev Res (Phila) 2014;7:658–65. doi: 10.1158/1940-6207.CAPR-13-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.