Synopsis
Driven by major scientific advances in analytical methods, biomonitoring, computational tools, and a newly articulated vision for a greater impact in public health, the field of exposure science is undergoing a rapid transition from a field of observation to a field of prediction. Deployment of an organizational and predictive framework for exposure science analogous to the “systems approaches” used in the biological sciences is a necessary step in this evolution. Here we propose the Aggregate Exposure Pathway (AEP) concept as the natural and complementary companion in the exposure sciences to the Adverse Outcome Pathway (AOP) concept in the toxicological sciences. Aggregate exposure pathways offer an intuitive framework to organize exposure data within individual units of prediction common to the field, setting the stage for exposure forecasting. Looking farther ahead, we envision direct linkages between aggregate exposure pathways and adverse outcome pathways, completing the source to outcome continuum for more efficient integration of exposure assessment and hazard identification. Together, the two pathways form and inform a decision-making framework with the flexibility for risk-based, hazard-based, or exposure-based decision making.
WHY ENVIRONMENTAL HEALTH NEEDS AN ORGANIZATIONAL FRAMEWORK FOR EXPOSURE SCIENCE
Exposure science is a field of study that seeks to understand the nature of contact between physical, chemical or biologic stressors and humans or other ecosystem elements for the purpose of protecting ecologic and public health1. Historically, exposure assessment has played a complimentary role with the fields of epidemiology and toxicology, helping identify and mitigate health impacts of environmental exposures, of which lead and radon serve as good examples1.
Recognizing the historical value of exposure science and recent demands to meet the growing need to conduct more comprehensive exposure assessment (thousands of stressors), more quickly and more accurately, a committee of the National Academy of Sciences (NAS) recently called for an extensive expansion of human and ecological exposure assessment1. Ideally, an expanded technological base and infrastructure would support the characterization of exposure to all endogenous and exogenous chemicals and other stressors across the life-time of an organism or community of interest, commonly referred to as the exposome2. Looking beyond exposure characterization, the committee envisioned a transformed field of science enabled by a predictive framework with the ability to forecast exposures with improved accuracy. To realize this vision, exposure science would need to “adopt a systems-based approach that, to the extent possible, considers exposures from source to dose and dose to source and considers multiple levels of integration…1.” It is clear that data and information emerging from an invigorated and expanding field of exposure science should be organized in a framework that not only promotes forecasting of exposures, but provides the necessary linkages between source and internal exposure. Informed by data comprising the full pathway from source to internal exposure, environmental health decisions could be made based on either the effects initiated by an exposure, control of contributing sources of chemical exposures, or both. But more than four years after the committee report, an organizational framework to enable a “systems” based approach has yet to emerge. In this context, the framework would be a layered structure that describes the elements of exposure pathways, the relationship between those elements, and how data describing the elements is stored and utilized for selected outputs, such as exposure assessment, exposure prediction or public health decision making.
THE AOP FRAMEWORK AS A FOUNDATION
Fortuitously, most of the elements of an organizing framework that meet the needs of the exposure science community with the power to drive richer integration with the fields of toxicology and epidemiology are similar to the elements of the increasingly successful and maturing Adverse Outcome Pathway (AOP) framework. An AOP is a conceptual framework that organizes existing knowledge concerning biologically plausible and empirically supported links between molecular level perturbation of a biological system and an adverse outcome at a level of biological organization of regulatory relevance3.
The concept of an AOP was first articulated by Ankley and colleagues in response to rapidly expanding regulatory demands to assess the ecological risks of chemical exposures for a more expansive set of biological outcomes3. The AOP framework met critical needs to organize rapidly emerging toxicity data streams and formalize relationships between biological elements (e.g., binding to receptor, gene expression, cellular response, tissue response, adverse outcome), promoting the use of mechanistic information and development of computational models of pathways. The value of the AOP framework is evidenced by the rapid progress in moving from concept to application. In 2012, the Organization for Economic Co-operation and Development (OECD) launched an AOP development program, and subsequently an international AOP knowledgebase project, which together have produced guidance documents 4, 5, and an online resource (https://aopwiki.org) that now has more than 100 AOPs at various stages of development contributed by governmental, academic, and industrial stakeholders from around the world.
Exposure science has the same set of needs for organizing rapidly emerging information, across many levels of environmental and biological organization. In addition, there is a need for better integration of exposure and toxicity data. We believe the AOP framework and the existing supporting infrastructure can be seen as ready for modification, adoption and eventual implementation as the guiding framework for exposure science.
THE AGGREGATE EXPOSURE PATHWAY CONCEPT
Here we propose the Aggregate Exposure Pathway (AEP) concept as the natural and complementary companion in the exposure sciences to the AOP concept in the toxicological sciences. The AEP framework is an extension of earlier calls for better integration of exposure data with the existing AOP framework, starting with Ankley and colleagues’ inclusion of “concepts of dosimetry” in the AOP definition3 and expanded by Groh and coworkers, the first to recommend “initiating a systematic collection of the information on exposure, chemical properties, and toxicokinetics” within the AOP framework6.
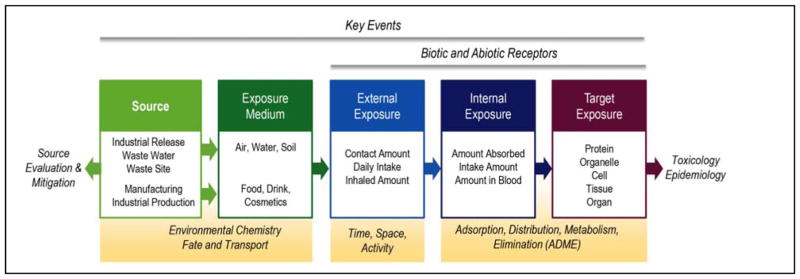
An AEP is the assemblage of existing knowledge concerning biologically, chemically and physically plausible, empirically supported links between introduction of a chemical or other stressor into the environment and its concentration at a site of action, i.e. target site exposure as defined by the NAS1 (Figure 1). It may be relevant to exposure assessment, risk assessment, epidemiology, or all three. The target site exposure (the terminal outcome of the AEP), along with the molecular initiating event from the AOP, represent the point of integration between an AEP and an AOP. We envision AEPs that comprise a sequence of key events describing the introduction of a chemical (or other stressor) into the environment from sources, fate and transport through one or more environmental media, external exposure sources, patterns of exposure, and the biokinetic processes that together produce the target site exposure (Figure 1). This proposal intentionally considers biokinetic processes leading to target site exposures as part of exposure science and the AEP, consistent with a vision of exposure science spanning all levels of physical and biological organization necessary to capture the movement of chemicals from source to the site of action1.
Figure 1.
The principle components of an Aggregate Exposure Pathway (AEP) cover all necessary levels of ecological, biological and physical organization from sources to target tissue. Each box represents a key event which is a measurable change in a chemical state and concentration that is essential, but not necessarily sufficient, for the movement of a chemical from a source to the target site exposure. Each arrow represents a key event relationship which links a pair of key events. AEP’s can be used to accumulate information for source mitigation, or use in epidemiology and toxicology.
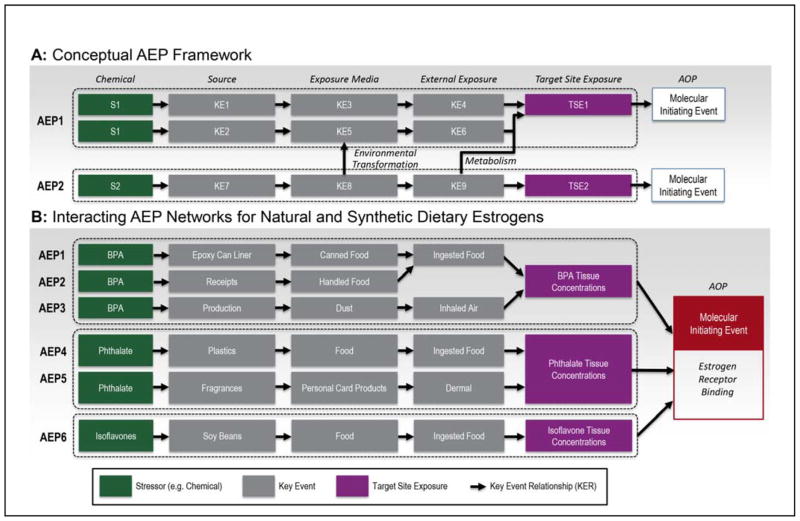
The AEP definition intentionally builds on the conceptual AOP framework (Figure 2A),3,7, 8 and where possible, adheres to the standards, style, structure and definitions created for the AOP. Embracing the AOP framework and retaining its structure and terminology is one of several steps we take to provide a foundation for the longer term goal of full integration of the AEP and AOP frameworks. The basic building blocks of an AEP retain the naming conventions used for AOPs, with revisions to describe specialized elements unique to AEPs: Key Events (KEs) describe the obligate steps through the AEP (Table 1, Figure 2A); and Key Event Relationships (KERs) describe the linkages between KEs, establishing the order of events. The target site exposure is a specialized KE8 describing the concentration or amount of a stressor (and timing/duration) at the site of action that corresponds to a molecular initiating event for an AOP. Aggregation of chemical-specific AEPs produces an interacting network of exposure pathways sharing KEs, converging on common target site exposures, and in the example provided, common molecular initiating events of the AOP framework, such as estrogen receptor binding (Figure 2B).
Figure 2.
A) Aggregate Exposure Pathway building blocks adapted from the Adverse Outcome Pathway conventions. Chemical transformations and metabolism can produce chemicals that connect AEPs. B) Aggregation of chemical-specific AEPs for a group of natural and synthetics estrogens showing the emergent interacting network of AEPS through sharing KEs, converging on common target site exposures for each estrogen and a common molecular initiating event for receptor dependent AOPs.
Table 1.
Primary Components of an AEP (AEP)a.
| Key Event (KE) |
|
| Key Event Relationship (KER) |
|
| Target Site Exposure (TSE) |
|
The structure of the table, and description of the building blocks of the AEP are intentionally drawn from the corresponding table in Villeneuve et al. 8 and concepts in Ankley et al. 3 with the minimal necessary modifications to assure the parallelism and complimentary nature of the AEP and AOP are conveyed.
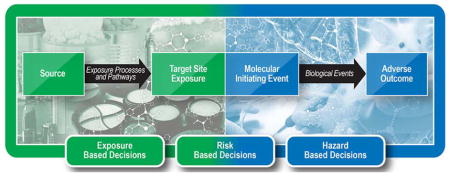
Termination of the AEP at the target site exposure is a unique aspect of the AEP. By describing the pathway from source to the site of action, where the molecular initiating event triggering an AOP occurs, the AEP-AOP linkage provides an intuitive, natural linkage from source and outcome (Figure 3). In an ideal case with a fully defined AEP and AOP, the comparison of the target site exposure concentration of a chemical with the concentration predicted to sufficiently perturb the molecular initiating event and activate the AOP will give a margin of safety estimate needed for risk assessment.
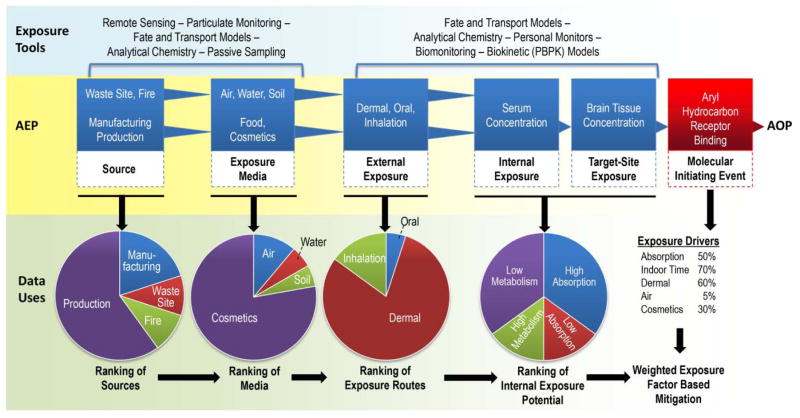
Figure 3.
Conventional and emerging exposure science tools, from exposomics, biomonitoring and computational exposure construction will be used to characterize key events and construct AEPs. Aggregation of AEPs can be used to weigh the influence of assembled key events on aggregate exposure for purposes of prioritizing source mitigation efforts.
The AOP framework, with its natural dependency on exposure and pharmacokinetic information for any risk-based application, may often drive AEP development. On the other hand, as momentum in the acquisition of exposure data for a greater fraction of the ~80,000 chemicals in commerce grows, AEP development is also expected to drive AOP development.
Flexibility would be a key component of the AEP and AOP frameworks. Full AEP development may not always be warranted. For example, for screening-level assessments, truncated AEPs detailing only elements of the pathway from external exposure to internal exposure may be sufficient. AEPs may conclude at any level of ecological organization appropriate for the needs of the assessment it supports, for example, from source to deposition on a plant or element of the built environment. However, developers of AEPs should strive for completeness. Doing so opens opportunities to contribute to the evolution and expansion of exposure science through the acquisition and organization of exposure data.
The AEP framework offers an intuitive approach for organization of exposure data. The framework promotes identification of data gaps, as well as identification and ranking of common or critical exposure pathways. Prediction of chemical concentrations and transformations, within the physical or biological elements of an exposure pathway or across the elements of a pathway, is empowered. Looking farther ahead, we envision direct linkages between AEPs and AOPs (Figures 2 and 3), completing the source to outcome continuum and setting the stage for more efficient integration of exposure and toxicity data for decision making. Together these frameworks form and inform a decision making framework with the flexibility for risk-based, hazard-based or exposure-based decision making.
DEFINING THE TERM “EXPOSURE” FOR AGGREGATE EXPOSURE PATHWAY APPLICATIONS
In some application areas, the terms dose and exposure are each used, sometimes interchangeably and without convention, to describe exposures at different levels of biological organization (e.g., external site of contact or internal site of action). The NAS report on exposure science for the 21st century defined the field of exposure science as the study of the contact between stressors and receptors1. Thus the field, and the term exposure, encompasses the full breadth of processes and conditions from source to any abiotic or biotic receptor. Here we embrace this definition of exposure and formally adopt exposure as the only necessary term for the field. Exposure refers to the amount of a stressor reaching buildings, soil microbes, humans, tissues, or cells, without arbitrary determinations regarding when the exposure becomes a dose1. The identity, amount, location, and duration/time(ing) of a stressor coming in contact with a receptor comprise the four necessary parameters for characterizing an exposure of interest. Establishment of an exposure science ontology, proposed recently by Mattingly and colleagues 9, is a necessary step in the evolution towards a productive AEP framework.
PRINCIPLES GUIDING AGGREGATE EXPOSURE PATHWAY DEVELOPMENT
While over-specification of the process for developing AEPs here might limit evolution of the AEP concept best accomplished in workshops, the thoughtful approaches articulated by Groh, Villeneuve, 7, 8 and others, as well as the OECD, 4, 5 serve as a positive starting point. Five “core” principles were proposed for AOPs: AOPs are 1) not chemical specific, 2) modular, 3) a pragmatic unit of development and evaluation, 4) functional units of prediction and 5) constantly evolving “living” documents7, 8. These principles, with two exceptions, would also provide sufficient flexibility and structure to assure consistency, utility and continued evolution of the AEP framework. Principle one would be modified to reflect the chemical-specific nature of exposure pathways (Figure 2), although some elements of an AEP, for example KER’s related to a metabolic process affecting the fate of multiple compounds, may be chemical agnostic and reusable. While the AEP concept maintains the spirit of the third principle, the pragmatic unit in an exposure pathway would be the contributions from all sources to a single target site exposure. For example, a single chemical could move through several environmental media (air, water, soil) and enter a target species through more than one pathway (dermal, inhalation, oral) leading to a single target site exposure (Figure 2B). In comparison, the unit of development for AOPs is a restricted pathway connecting a single molecular initiating event with a single adverse outcome.
Where the AOP framework was fit to a single purpose of understanding the pathway from molecular initiating event to adverse outcome, we see that the AEP framework may serve several purposes, and thus may need greater flexibility than the AOP framework. AEPs may be constructed for purposes of understanding source contributions, the role of exposure pathways, or may be driven by the need to supply the human exposure to target site exposure information for an AOP. Additional modification and extension of these principles to reflect aspects unique to AEPs would be expected, but might best emerge from multi-stakeholder workshops similar to those convened to establish the AOP framework. We expect that these issues will be widely discussed with guidance emerging from contributions from multiple stakeholders.
AGGREGATE EXPOSURE PATHWAY APPLICATIONS IN ENVIRONMENTAL HEALTH
We envision a broad range of application areas emerging from implementation of the AEP framework within publically accessible, web-based tools like those in development for AOPs (https://aopkb.org/). The development of AEPs will enable general activities such as data acquisition, organization, access and mining, and those that enabled by the availability of these data, such as aggregation of exposure pathways by source, chemical classes, exposure routes, common AOPs. Another example would be identification of new target site exposures of concern and, by extension, AOPs in need of development. Application areas already common to the field of exposure science would also be enhanced by the AEP framework, for example, exposure modeling, weighted source assessment and mitigation, and cumulative risk assessment. Some specific examples follow.
Exposure Modeling
Modular descriptions of exposure pathways would allow use of individual modules as units of prediction (e.g., CalTox, IMPACT, USEtox), and the collection of modules for prediction of a full exposure pathway. Several research programs involve development of exposure modules that cover various elements of the AEP, such as near-field exposures during the use of consumer products10, 11. In addition, construction of aggregate exposure models has been an active area of research outside of the agency and within the ecological/environmental fate and transport field, for pesticides and consumer products12–27. Development of these models can be viewed as an initial step toward construction of modular, predictive AEPs able to estimate exposures that, in turn, can be directly linked to hazard data and AOPs.
Weighted Source Assessment and Mitigation
Querying a growing database of AEPs would produce new insights into the relative importance of key sources, biological or environmental processes contributing to either total external exposure, or perhaps more importantly, their concentration at one or more specific target site exposure linked to AOPs of regulatory concern (Figure 3). By extension, the AEP and AOP frameworks would be used together to identify key sources, exposure or biokinetic processes controlling exposure for classes of compounds operating through single AOPs, allowing joint weighting by exposure level, source, or biological potency (Figure 3). This way, source mitigation priorities, or research priorities for reducing uncertainty related to missing essential data could be identified properly through weighting of both hazard and exposure. For example, Tolls and colleagues estimated emissions of adhesives and sealants through aggregation of multiple use and manufacturing scenarios for purposes of environmental risk assessment28.
Cumulative risk assessment
Groups of compounds acting through a common AOP would be conducted rapidly and efficiently through mining of AEP data. For example, existing extensive information on concentrations of synthetic and natural plant-derived estrogens in food supply, consumer products, cash register receipts could be utilized to assemble networks of AEPs for all estrogenic compounds, e.g. the estrome, for purposes of weighing sources of exposure and developing mitigation strategies or calculating the probability of effects from one or more estrogen receptor initiated AOPs (Figure 2B).
AEP-AOP INTEGRATION FOR ENVIRONMENTAL HEALTH
Creation and adoption of an effective organizational structure for exposure science and emergence of a supporting computational infrastructure are initial steps towards the more transformational goal of a formal linkage between the organizational frameworks for exposure science and toxicology. AEPs, with their complimentary network of exposure pathways, are not invoked here as a stand alone concept parallel to the AOP. Where Perkins saw a system of interactive AOPs with common key events as a AOP network (cited in 29), we envision a similar AEP network capturing the cumulative exposure from multiple chemicals across several exposure pathways, with direct linkage to AOP networks (Figure 2B). AEPs would produce target site exposures for comparison with concentrations expected to trigger the molecular initiating event for the corresponding AOP (Figure 2B), providing interacting organizational frameworks from which exposure, hazard, or risk-based decisions could be made.
The recent emergence and rapid growth of integrating high throughput exposure predictions and high throughput toxicity testing data30–34 for high throughput prioritization exemplifies the value and impact of AEP-AOP integration. In our view, these efforts reflect the use of AEPs embedded in computational models to provide external exposures to large numbers of compounds, and to translate these exposures into equivalent serum or tissue concentrations (i.e. target site exposure). Comparison of estimated target site exposures to concentrations found in high throughput test systems that are sufficient for triggering selected molecular initiating events, as surrogates for adverse outcomes, can be made. These emerging high throughput approaches have been successfully combined to make risk-based ranking of almost 200 compounds34. We also envision databases of accumulated information on interacting AEP-AOPs would transform the assessment of hazards/risks arising from the cumulative effects of multiples stressors acting on a single AOP (cumulative risk assessment), and the aggregate effects of exposure to all chemicals producing exposure to each chemical at a site of action (aggregate risk assessment, Figure 2B). In the extreme, AEPs would help identify cases where the AOP is not relevant for a specific chemical because it cannot reach the site of action, for example, formaldehyde is proposed to cause leukemia through an AOP involving DNA damage to cells of the lymphohemopoetic system35, but it has recently been shown not to reach systemic tissues including the bone marrow36.
FUTURE DIRECTIONS AND CONCLUSIONS
The improvements in public health through better integration of exposure science and toxicological science envisioned by the NAS1, 37 will occur more quickly, more completely and have more immediate impact if there is an organizing framework and infrastructure that allows archiving and efficient use of these data for prediction and decision making. Our proposal to utilize the AEP concept as the organizational framework for exposure science, builds on the long history of aggregate exposure assessments as a key feature of the field and recent technological advances in computational exposure modeling and informatics (e.g. AOP Wiki, Effectopedia). By articulating the basic elements, uses and impact of the AEP framework here, we intend to initiate a broader effort, to include workshops and additional manuscripts, that will produce more complete guidance, address the use of exposure tools for developing AEP’s, and the challenges and uncertainties in accumulating the necessary exposure data.
Completing the source to outcome continuum by joining the AEP and AOP networks sets the stage for more efficient integration of toxicity testing information and exposure information, creating opportunities for development and deployment of novel computational tools that enable more comprehensive, more rapid exposure-based, hazard-based, and risk-based decision making38.
The incentive for establishing the AEP or other organizational framework for exposure science will continue to grow in proportion to the rapid growth of exposure science, invigorated by a new vision for the field and fueled by new investments by the National Institutes of Health, Environmental Protection Agency, and other federal agencies. Development of the AEP framework will undoubtedly benefit substantially from the existing organizational support and physical/computational infrastructure now supporting the AOP framework (e.g. the wiki). Ideally, supporting infrastructure, similar in structure and function to those developed for the AOP program, would be developed for a complimentary AEP program.
Acknowledgments
This work (JGT) was supported by P30 ES000210 (Oregon State University-PNNL) and P42 ES016465 by the National Institute of Environmental Health Sciences and the Laboratory Directed Research and Development program at the Pacific Northwest National Laboratory (PNNL) and is a contribution of the Global Forensic Chemical Exposure Assessment for the Environmental Exposome project. PNNL is a multi-program national laboratory operated by Battelle for the DOE under Contract DE-AC05-76RLO 1830.
Footnotes
DISCLAIMER
The U.S. Environmental Protection Agency has provided administrative review and has approved for publication. The views expressed in this paper are those of the authors and do not necessarily reflect the views of the U.S. Environmental Protection Agency.
References
- 1.NRC. Exposure Science in the 21st Century: a Vision and a Strategy. National Academies Press; Washington, DC: 2012. [PubMed] [Google Scholar]
- 2.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–50. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 3.Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–41. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 4.(OECD), O. f. E. C.-o. a. D; Development, O. f. E. C.-o. a, editor. Guidance Document On Developing and Assessing Adverse Outcome Pathways. Organization for E; Paris: 2013. p. 45. [Google Scholar]
- 5.(OECD), O. f. E. C.-o. a. D; Development, O. f. E. C.-o. a, editor. Users Handbook Supplement to the Guidance Document On Developing and Assessing AOPs. Organization for E; Paris: 2013. p. 53. [Google Scholar]
- 6.Groh KJ, Carvalho RN, Chipman JK, Denslow ND, Halder M, Murphy CA, Roelofs D, Rolaki A, Schirmer K, Watanabe KH. Development and application of the adverse outcome pathway framework for understanding and predicting chronic toxicity: II. A focus on growth impairment in fish. Chemosphere. 2015;120:778–92. doi: 10.1016/j.chemosphere.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. Adverse outcome pathway development II: best practices. Toxicol Sci. 2014;142(2):321–30. doi: 10.1093/toxsci/kfu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol Sci. 2014;142(2):312–20. doi: 10.1093/toxsci/kfu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattingly CJ, McKone TE, Callahan MA, Blake JA, Hubal EA. Providing the missing link: the exposure science ontology ExO. Environ Sci Technol. 2012;46(6):3046–53. doi: 10.1021/es2033857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wambaugh JF, Wang A, Dionisio KL, Frame A, Egeghy P, Judson R, Setzer RW. High throughput heuristics for prioritizing human exposure to environmental chemicals. Environ Sci Technol. 2014;48(21):12760–7. doi: 10.1021/es503583j. [DOI] [PubMed] [Google Scholar]
- 11.Isaacs KK, Glen WG, Egeghy P, Goldsmith MR, Smith L, Vallero D, Brooks R, Grulke CM, Ozkaynak H. SHEDS-HT: an integrated probabilistic exposure model for prioritizing exposures to chemicals with near-field and dietary sources. Environ Sci Technol. 2014;48(21):12750–9. doi: 10.1021/es502513w. [DOI] [PubMed] [Google Scholar]
- 12.Tozer SA, Kelly S, O’Mahony C, Daly EJ, Nash JF. Aggregate exposure modelling of zinc pyrithione in rinse-off personal cleansing products using a person-orientated approach with market share refinement. Food Chem Toxicol. 2015;83:103–10. doi: 10.1016/j.fct.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Safford B, Api AM, Barratt C, Comiskey D, Daly EJ, Ellis G, McNamara C, O’Mahony C, Robison S, Smith B, Thomas R, Tozer S. Use of an aggregate exposure model to estimate consumer exposure to fragrance ingredients in personal care and cosmetic products. Regul Toxicol Pharmacol. 2015;72(3):673–82. doi: 10.1016/j.yrtph.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy MC, Glass CR, Fustinoni S, Moretto A, Mandic-Rajcevic S, Riso P, Turrini A, van der Voet H, Hetmanski MT, Fussell RJ, van Klaveren JD. Testing a cumulative and aggregate exposure model using biomonitoring studies and dietary records for Italian vineyard spray operators. Food Chem Toxicol. 2015;79:45–53. doi: 10.1016/j.fct.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy MC, Glass CR, Bokkers B, Hart AD, Hamey PY, Kruisselbrink JW, de Boer WJ, van der Voet H, Garthwaite DG, van Klaveren JD. A European model and case studies for aggregate exposure assessment of pesticides. Food Chem Toxicol. 2015;79:32–44. doi: 10.1016/j.fct.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Delmaar C, Bokkers B, ter Burg W, Schuur G. Validation of an aggregate exposure model for substances in consumer products: a case study of diethyl phthalate in personal care products. J Expo Sci Environ Epidemiol. 2015;25(3):317–23. doi: 10.1038/jes.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosens I, Delmaar CJ, Ter Burg W, de Heer C, Schuur AG. Aggregate exposure approaches for parabens in personal care products: a case assessment for children between 0 and 3 years old. J Expo Sci Environ Epidemiol. 2014;24(2):208–14. doi: 10.1038/jes.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tornero-Velez R, Davis J, Scollon EJ, Starr JM, Setzer RW, Goldsmith MR, Chang DT, Xue J, Zartarian V, DeVito MJ, Hughes MF. A pharmacokinetic model of cis- and trans-permethrin disposition in rats and humans with aggregate exposure application. Toxicol Sci. 2012;130(1):33–47. doi: 10.1093/toxsci/kfs236. [DOI] [PubMed] [Google Scholar]
- 19.Cowan-Ellsberry CE, Robison SH. Refining aggregate exposure: example using parabens. Regul Toxicol Pharmacol. 2009;55(3):321–9. doi: 10.1016/j.yrtph.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Andrew Clayton C, Pellizzari ED, Whitmore RW, Quackenboss JJ, Adgate J, Sefton K. Distributions, associations, and partial aggregate exposure of pesticides and polynuclear aromatic hydrocarbons in the Minnesota Children’s Pesticide Exposure Study (MNCPES) J Expo Anal Environ Epidemiol. 2003;13(2):100–11. doi: 10.1038/sj.jea.7500261. [DOI] [PubMed] [Google Scholar]
- 21.Pang Y, MacIntosh DL, Camann DE, Ryan PB. Analysis of aggregate exposure to chlorpyrifos in the NHEXAS-Maryland investigation. Environ Health Perspect. 2002;110(3):235–40. doi: 10.1289/ehp.02110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellweg S, Demou E, Bruzzi R, Meijer A, Rosenbaum RK, Huijbregts MAJ, McKone TE. Integrating Human Indoor Air Pollutant Exposure within Life Cycle Impact Assessment. Environmental Science & Technology. 2009;43(6):1670–1679. doi: 10.1021/es8018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jolliet O, Ernstoff AS, Csiszar SA, Fantke P. Defining Product Intake Fraction to Quantify and Compare Exposure to Consumer Products. Environ Sci Technol. 2015;49(15):8924–31. doi: 10.1021/acs.est.5b01083. [DOI] [PubMed] [Google Scholar]
- 24.Kephalopoulos S, Bruinen de Bruin Y, Arvanitis A, Hakkinen P, Jantunen M. Issues in consumer exposure modeling: towards harmonization on a global scale. J Expo Sci Environ Epidemiol. 2007;17(Suppl 1):S90–100. doi: 10.1038/sj.jes.7500605. [DOI] [PubMed] [Google Scholar]
- 25.McKone TE. Control, D. o. T. S, editor. CalTOX, A Multimedia Total Exposure Model For Hazardous-Waste Sites. Sacramento, California: 1993. [Google Scholar]
- 26.Rosenbaum RK, Huijbregts MA, Henderson AD, Margni M, McKone TE, van de Meent D, Hauschild MZ, Shaked S, Li DS, Gold LS. USEtox human exposure and toxicity factors for comparative assessment of toxic emissions in life cycle analysis: sensitivity to key chemical properties. The International Journal of Life Cycle Assessment. 2011;16(8):710–727. [Google Scholar]
- 27.Wormuth M, Demou E, Scheringer M, Hungerbühler K. Assessments of Direct Human Exposure—The Approach of EU Risk Assessments Compared to Scenario-Based Risk Assessment. Risk Anal. 2007;27(4):979–990. doi: 10.1111/j.1539-6924.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 28.Tolls J, Gomez D, Guhl W, Funk T, Seger E, Wind T. Estimating emissions from adhesives and sealants uses and manufacturing for environmental risk assessments. Integrated environmental assessment and management. 2015 doi: 10.1002/ieam.1662. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Reyero N. Are adverse outcome pathways here to stay? Environ Sci Technol. 2015;49(1):3–9. doi: 10.1021/es504976d. [DOI] [PubMed] [Google Scholar]
- 30.Wambaugh JF, Wetmore BA, Pearce R, Strope C, Goldsmith R, Sluka JP, Sedykh A, Tropsha A, Bosgra S, Shah I, Judson R, Thomas RS, Woodrow Setzer R. Toxicokinetic Triage for Environmental Chemicals. Toxicol Sci. 2015;147(1):55–67. doi: 10.1093/toxsci/kfv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wetmore BA, Allen B, Clewell HJ, 3rd, Parker T, Wambaugh JF, Almond LM, Sochaski MA, Thomas RS. Incorporating population variability and susceptible subpopulations into dosimetry for high-throughput toxicity testing. Toxicol Sci. 2014;142(1):210–24. doi: 10.1093/toxsci/kfu169. [DOI] [PubMed] [Google Scholar]
- 32.Wetmore BA, Wambaugh JF, Allen B, Ferguson SS, Sochaski MA, Setzer RW, Houck KA, Strope CL, Cantwell K, Judson RS, LeCluyse E, Clewell HJ, 3rd, Thomas RS, Andersen ME. Incorporating High-Throughput Exposure Predictions with Dosimetry-Adjusted In Vitro Bioactivity to Inform Chemical Toxicity Testing. Toxicol Sci. 2015 doi: 10.1093/toxsci/kfv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wetmore BA, Wambaugh JF, Ferguson SS, Li L, Clewell HJ, 3rd, Judson RS, Freeman K, Bao W, Sochaski MA, Chu TM, Black MB, Healy E, Allen B, Andersen ME, Wolfinger RD, Thomas RS. Relative impact of incorporating pharmacokinetics on predicting in vivo hazard and mode of action from high-throughput in vitro toxicity assays. Toxicol Sci. 2013;132(2):327–46. doi: 10.1093/toxsci/kft012. [DOI] [PubMed] [Google Scholar]
- 34.Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ, 3rd, Dix DJ, Andersen ME, Houck KA, Allen B, Judson RS, Singh R, Kavlock RJ, Richard AM, Thomas RS. Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol Sci. 2012;125(1):157–74. doi: 10.1093/toxsci/kfr254. [DOI] [PubMed] [Google Scholar]
- 35.Checkoway H, Dell LD, Boffetta P, Gallagher AE, Crawford L, Lees PS, Mundt KA. Formaldehyde Exposure and Mortality Risks From Acute Myeloid Leukemia and Other Lymphohematopoietic Malignancies in the US National Cancer Institute Cohort Study of Workers in Formaldehyde Industries. J Occup Environ Med. 2015;57(7):785–94. doi: 10.1097/JOM.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu R, Lai Y, Hartwell HJ, Moeller BC, Doyle-Eisele M, Kracko D, Bodnar WM, Starr TB, Swenberg JA. Formation, Accumulation, and Hydrolysis of Endogenous and Exogenous Formaldehyde-Induced DNA Damage. Toxicol Sci. 2015;146(1):170–82. doi: 10.1093/toxsci/kfv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NRC. Toxicity Testing in the 21st Century: A Vision and a Strategy. National Academies of Sciences; Washington, DC: 2007. [Google Scholar]
- 38.Cohen Hubal EA, Richard AM, Shah I, Gallagher J, Kavlock R, Blancato J, Edwards SW. Exposure science and the U.S. EPA National Center for Computational Toxicology. J Expo Sci Environ Epidemiol. 2010;20(3):231–6. doi: 10.1038/jes.2008.70. [DOI] [PubMed] [Google Scholar]