Abstract
Wingless-related integration site (Wnt) signaling has proven to be a fundamental mechanism in cardiovascular development as well as disease. Understanding its particular role in heart formation has helped to develop pluripotent stem cell differentiation protocols that produce relatively pure cardiomyocyte populations. The resultant cardiomyocytes have been used to generate heart tissue for pharmaceutical testing, and to study physiological and disease states. Such protocols in combination with induced pluripotent stem cell technology have yielded patient-derived cardiomyocytes that exhibit some of the hallmarks of cardiovascular disease and are therefore being used to model disease states. While FDA approval of new treatments typically requires animal experiments, the burgeoning field of tissue engineering could act as a replacement. This would necessitate the generation of reproducible three-dimensional cardiac tissues in a well-controlled environment, which exhibit native heart properties, such as cellular density, composition, extracellular matrix composition, and structure-function. Such tissues could also enable the further study of Wnt signaling. Furthermore, as Wnt signaling has been found to have a mechanistic role in cardiac pathophysiology, e.g. heart attack, hypertrophy, atherosclerosis, and aortic stenosis, its strategic manipulation could provide a means of generating reproducible and specific, physiological and pathological cardiac models.
Keywords: Pluripotent Stem Cells, Stem Cell Differentiation, Wnt Signaling, Cardiac Tissue Engineering, Engineered Cardiac Tissue, Cardiovascular Disease
INTRODUCTION
Through decades of diligent work, researchers have come to understand the enormous effect a cell’s environment has on cellular processing and responses. Embryogenesis is a prime example of how precisely timed exposure to specific doses of intracellular and extracellular factors can induce cell differentiation and axis patterning [1,2]. In the case of the heart, this precise molecular signaling begins in the embryo and continues in the fetus [3] and is heavily dependent upon the wingless-related integration site (Wnt) family of molecules [4]. This article will provide a brief overview of the role of the canonical (β-catenin-dependent) Wnt signaling pathway in heart development, cardiac repair, cardiovascular disease and its current and potential utility in cardiac tissue engineering.
OVERVIEW OF CANONICAL WNT SIGNALING
Wnt genes and proteins play a critical role in cell fate decisions, axis patterning, cell proliferation, and cellular migration [5–8]. The Wnt family of proteins are secreted signaling molecules that initiate a variety of intracellular signaling pathways [9]. In this review, we will restrict our discussion to the canonical Wnt/β-catenin signaling pathway in cardiogenesis, cardiac repair and cardiac disease development. A more detailed description of Wnt signaling can be found in the following reviews of the topic [9–12].
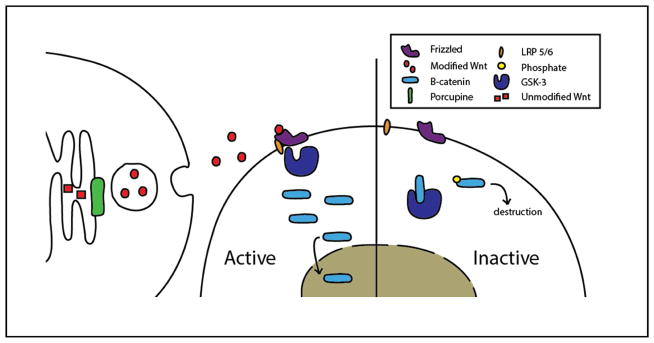
Wnt signaling begins with the secretion of Wnt ligands into the extracellular space, which requires the post-translational modification of the Wnt proteins by the resident ER acyltransferase Porcupine [13] (Figure 1). In the canonical pathway, Wnt binds to the receptor Frizzled (Frz) [14] and the co-receptor, low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6), to form a trimeric complex. Formation of this complex inhibits the glycogen synthase kinase 3 (GSK-3) destruction complex, which prevents the degradation and promotes the stabilization of the cytosolic β-catenin pool [15]. Accumulation of β-catenin in the cytoplasm results in its translocation to the nucleus where it binds to T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors that turn on Wnt-responsive genes.
Figure 1. Schematic of Wnt signaling.
Wnt molecules are modified and marked for export from cells by Porcupine. Wnt molecules bind to the extracellular receptor Frizzled, which causes an association with the co-receptor LRP 5/6. Formation of this trimeric complex inhibits GSK-3 from phosphorylating and marking β-catenin for degradation. Free β-catenin accumulates in the cytosol and enters nucleus where it regulates gene transcription. In the absence of Wnt, GSK-3 phosphorylates the cytosolic pool of β-catenin to induce rapid turnover and maintain a very low free β-catenin pool.
WNT SIGNALING IN CARDIOGENESIS
During gastrulation, the embryo becomes organized into three main germ layers—the ectoderm, mesoderm and endoderm, which establish the nascent shape of vital organs. The mammalian heart derives from the mesoderm, which originates from an earlier transient structure, the primitive streak. While the precise regulation of primitive streak formation and germ layer induction is not yet understood in its entirety, bone morphogenic protein 4 (BMP4), Nodal, and Wnt signaling pathways are all involved [3,10,16]. The coordinated regulation of these three pathways by means of the variable expression levels of agonists and the restricted expression of inhibitors to defined regions, has been demonstrated to create signaling domains that guide primitive streak and germ layer development [17].
MIMICKING HEART DEVELOPMENT IN VITRO
While cardiogenesis is a tightly regulated and complex process, it has been demonstrated that it can be mimicked in vitro starting with human pluripotent stem cells (hPSCs). Spontaneously beating cardiac-like cells can be obtained by culturing hPSCs as embryoid bodies (EBs) with serum rich media, wherein the pluripotent cells follow their innate cardiogenic pathway [18]. While the beating cells obtained through this method were found to possess cardiac-specific ion channels and intracellular contractile proteins, there was no specificity of cardiac cell subtype, i.e. a mixed population of cells with ventricle, atrial, and pace-maker action potential profiles were obtained [19], and this protocol yielded <1% cardiomyocytes (CMs). Early attempts at directed differentiation used timed administration of activin A to initiate Nodal-like signaling in monolayers of hPSCs to induce mesoderm and endoderm. This step was followed by administration of BMP4 to further specify cardiac fate. This protocol improved the yield of CMs to >30% [20]. However, greater success has been obtained by manipulating the Wnt/β-catenin signaling pathway in directed differentiation cultures. The role of Wnt/β-catenin signaling in cardiogenesis is biphasic: activation of Wnt/β-catenin signaling during EB formation (before specification of three germ layers) was found to promote mouse embryonic stem cell differentiation into CMs, whereas activation in the late phase after EB formation (during gastrulation) inhibited CM formation [21,22]. By including the Wnt inhibitor dickkopf-related protein 1 (DKK1)—a Wnt inhibitor that binds LRP6 [23,24]—during the stage of cardiac mesoderm induction and specification, the CM yield was increased to >50% [25]. Furthermore, Lian and colleagues robustly generated directed differentiation cultures with yields of up to 98% CMs by sequential treatment with a small molecule Wnt agonist, CHIR99021, followed by a small molecule Wnt antagonist, Wnt inhibitor of Wnt production 4 (IWP4) [26,27]. More recently, differentiation strategies further manipulating cellular signaling have shown control over individual cardiac cell types such as atrial, ventricular and nodal cells [28–30].
Although briefly reviewed here, cardiogenesis is a precise multi-step process. Despite this, researchers have harnessed our as yet incomplete understanding of heart development to generate robust protocols for the directed differentiation of hPSCs into cardiomyocytes. Such protocols have revolutionized the field of cardiac tissue engineering by providing a ready and reliable source of cardiomyocytes from which cardiac tissues can be generated for regenerative therapy applications or as in vitro models.
WNT SIGNALING IN CARDIAC REPAIR AND DISEASE
With a thorough understanding of the implications of Wnt signaling in cardiac development, disease, and repair on a cellular basis, it stands to reason that we may one day be able to exploit these processes in the development of engineered heart tissues. To this end, we will provide an overview of the various physiological and pathological processes in which there is evidence of Wnt/β-catenin signaling.
Wnt signaling abnormalities have been identified as risk factors for various cardiovascular diseases and can be used clinically to predict future cardiovascular complications. For example, mutations in the LRP6 protein have been linked to early coronary artery disease and hypertension [31], and decreased Wnt1 protein levels have been associated with premature myocardial infarction (MI) in patients [32]. In addition, high serum levels of the Wnt antagonists secreted frizzled related protein-3 (sFRP-3), dickkopf-1 (DKK-1), and Wnt inhibitory factor-1 (WIF-1) were identified in patients with symptomatic aortic stenosis in contrast to healthy controls, and the circulating levels of WIF-1 and DKK-1 were found to be predictive of mortality [33]. The differentiation of porcine valve interstitial cells into osteoblast-like cells, a process involved in the calcification of the aortic valve, was found to significantly increase the expression of GSK-3β and β-catenin [34].
Wnt signaling has also been implicated in a variety of cardiac repair processes that at times appear contradictory, indicating a highly sophisticated and complex regulatory mechanism. Activation of Wnt signaling through Wnt3a has been shown to interfere with cardiac progenitor self-renewal, whereas inhibition of Wnt signaling through sFRP-based antagonism or myocyte-specific loss of β-catenin has been demonstrated to be cardioprotective [35–38]. In addition, activation of the Wnt/β-catenin signaling pathway can promote fibrosis in cardiac repair, whereas antagonism of Wnt3a or global overexpression of the sFRP family of Wnt antagonists inhibits fibrosis and inflammation [39,40], [41,42]. Although able to produce distinct effects, both activation and inhibition of Wnt signaling plays a complex and necessary role in both the protection and repair of cardiac tissue post MI. The beneficial outcomes of attenuating fibrosis through inhibiting Wnt signaling have been demonstrated by a reduction in infarct size (18% of left ventricle circumference vs. 30% in controls) 30 days post MI in mice overexpressing sFRP-1 (also known as FrzA) [35]; and a reduction in infarct expansion and improved cardiac function 5 weeks post MI in mice treated with a Wnt3a/Wnt5a inhibitor [43]. Additionally, the co-expression of Frz1 and Wnt3a has been shown to promote the differentiation of fibroblasts to myofibroblasts, specialized cells that function in cardiac repair inflammatory response [44]. However, inhibition of Frz receptors have also been demonstrated to increase myofibroblast numbers and improve cardiac function [45]. Equally contradictory, activation of Fzr1 and Frz2 by Wnt3a was found to attenuate fibroblast migration [44], while Frz2 was found to be upregulated in migrating myofibroblasts [46]. It is evident that inhibition of Wnt/β-catenin signaling leads to positive cardioprotective and anti-fibrotic effects, but its role in myofibroblast function is unclear. It should be noted that though scar formation and inflammation are fundamental components of the repair process post injury, when excessive or chronic in nature, they can contribute to pathological cardiac remodeling resulting in cardiac dilation and impaired function [47–49].
Wnt/β-catenin signaling processes are not-only involved in regulating fibrosis and inflammation in cardiac repair; recent findings have also linked Wnt signaling to endothelialization and angiogenesis in injured heart tissue, including the endothelial-to-mesenchymal transition (EndMT). Activation of Wnt signaling was found to mark cells undergoing the EndMT seven days post MI in a murine model [50]. Similar to fibrosis and inflammation, in the case of angiogenesis both the activation and inhibition of Wnt signaling have been implicated. The Wnt antagonist sFRP-1/FrzA was found to be expressed during neovessel formation but not in fully mature vessels; to promote migration and tube formation; and when overexpressed, to promote an increased density of vessels characterized by a comparatively larger, longer, more mature phenotypes relative to controls [46]. However, Wnt activation through Wnt1 has also been demonstrated to have proangiogenic effects in human endothelial progenitor cells and to promote increased blood flow and capillary density when injected into murine ischemic hind limbs [47]. There is a need for further investigation as it is possible that the choice of pathway might yield independent downstream outcomes that are not apparent in these experiments.
Cardiac hypertrophy is a common response by cardiomyocytes to mechanical and neurohormonal stimuli. However, post MI hypertrophy results from the inundation of these biomechanical stresses on the myocardium. In adults, hypertrophy is typically associated with pathological cardiac remodeling, impaired cardiac function and ultimately heart failure [51,52]. Expression of β-catenin is indicative of canonical Wnt signaling in a cell, and has been implicated in the development of hypertrophy in cardiomyocytes. Cytosolic β-catenin was found to be elevated and sustained for a longer period in neonatal rat ventricular cardiomyocytes treated with hypertrophic stimuli and in rat hearts subjected to pressure overload [36]. Similarly, heterozygous deletion of β-catenin in a pressure overload mouse model induced a significant upregulation of the fetal gene program—β-myosin heavy chain, atrial and brain natriuretic peptides (ANP and BNP, respectively)—compared to wild-type controls; however no differences in functional improvements were observed between the groups [53]. Promotion of cardiac hypertrophy has been linked to overexpression of the Wnt signaling agonist Dishevelled-1 (Dvl-1), characterized by an increased heart-to-body weight ratio, increased cardiomyocyte size, a 12-fold increase in ANP expression, increased left ventricular dilation and reduced ejection fraction relative to the control at 3 months, with premature death before 6 months, in a rat pressure overload model [51]. Furthermore, depletion of the Dvl-1 protein has been shown to induce the reverse effect of reducing cardiac hypertrophy by maintaining left ventricular wall thickness and decreasing ANP and BNP expression, compared to wild-type controls in a murine pressure overload model after 7 days [38]. Attenuation of hypertrophy has also been attributed to increased activity of the Wnt inhibitor GSK-3β [15,38,54] and to overexpression of the Wnt inhibitor DKK-3 [55]; whereas exacerbation of cardiac hypertrophy has been attributed to the Wnt agonist Dapper-1 [56] and deletion of DKK-3. Hence, with few exceptions it can be said that activation of Wnt/β-catenin signaling promotes cardiac hypertrophy and inhibition of Wnt/β-catenin signaling has the opposite effect.
Wnt signaling has also been linked to the development of atherosclerotic plaques. Macrophage-induced inflammation in blood vessels walls has been implicated in the development of atherosclerosis [57]. While Wnt5a proteins have been found in macrophages stimulated by bacterial pathogens [58,59], they have also been found in the macrophage-rich regions in both murine and human atherosclerotic lesions [59], implying their importance in both pathological an physiological development of atherosclerosis. Genetic phenotype assessment of atherosclerotic tissue further implicates Wnt5a as an inflammatory mediator in this disease [60]. Recent findings have shown that various LRP co-receptors are highly expressed in atherosclerotic lesions, and can control the rate of lipid accumulation in aortic vessels through macrophages involvement [61,62]. LRP5 knockout mice fed a hypercholesterolemic diet developed larger atherosclerotic lesions, as well as increased serum cholesterol levels and increased total lipid coverage along vessel walls, compared with wild-type mice [62]. The activation of Wnt/β-catenin signaling is therefore postulated to be protective against atherosclerotic progression. This is also evidenced by the report that the Wnt inhibitor DKK-1 was increased in both the serum and lesions of ApoE knockout mice and patients with clinical atherosclerosis [63].
Table 1 summarizes the number and diversity of processes that have been linked to Wnt/β-catenin signaling in cardiac repair and cardiovascular disease. This complex network of molecular communication is astonishing and illustrates the absolutely critical function of this pathway in the cardiac system. With each additional investigation, our understanding of the relationship between Wnt signaling and the heart grows, as does the potential utility of Wnt signaling molecules and regulators as a source of therapeutic solutions for cardiac disease and as a source of targets that can be manipulated for in vitro and in vivo disease and cardiac repair model development. However, it is obvious that the Wnt signaling pathway is finely-tuned and will require quite a high degree of accuracy in the choice of regulator, concentration and timing used, to be able to control the outcome with precision.
Table 1.
Summary of processes linked to Wnt/β-catenin signaling.
| Disease | Wnt Molecule | Study Focus | Experimental Model | Physiological Effect | Ref. |
|---|---|---|---|---|---|
| Cardiac Injury | SFRP2, Wnt1 Wnt3a, Wnt5a, Fz2 | Infarcted heart tissue |
|
|
[44,46,50] |
| SFRP2 | Scar formation |
|
|
[42] | |
| FrzA, Wnt1 | Angiogenesis |
|
|
[64] | |
| sFRP-1/FrzA | Infarcted heart tissue |
|
|
[35] | |
| β-catenin | Infarcted heart tissue |
|
|
[37,53,65] | |
| Hypertrophy | Dvl-1 | Infarcted heart tissue |
|
|
[38,51] |
| DKK3 | Circulation |
|
|
[55] | |
| Dapper-1 | Infarcted heart tissue |
|
|
[56,66] | |
| Atherosclerosis | Wnt5a | Macrophage rich region of atherosclerotic lesions | Mouse atherosclerosis model | Inflammatory mediator in atheroscleorosis and macrophage recruitment | [59,60] |
| LRP family | Atherosclerotic lesions |
|
|
[61] | |
| LRP5 | Circulation |
|
|
[62] | |
| Aortic Stenosis | Wnt antagonists (sFRP-3, DKK-1, WIF-1) | Circulation, calcified valves |
|
|
[33] |
| β-catenin | Valve interstitial cells |
|
|
[34] |
CARDIAC TISSUE ENGINEERING
As our understanding of the outcomes of activating and inhibiting Wnt pathway at specific times in the development, repair and pathological processes develops, it can be envisioned that we will be able to generate a large variety of specialized cardiac tissue models. Current models are capable of recreating diseased 3D tissue in a well-controlled micro-environment to closely mimic pathophysiological condition in cellular density, composition and extracellular matrix proteins [67]. Specific diseases such as cardiac hypertrophy, atherosclerosis and aortic stenosis can be mimicked in a 3D tissue engineered environment [68–71]. Although these studies do not directly investigate the role of Wnt related signaling, its function is essential to obtain contractile cardiac cells from PSCs. Similar models could prove useful in delineating more of the specific roles Wnt signaling plays in each disease. We will be able to model cardiac repair in vitro and follow the process towards a physiological or pathological outcome. We will then be able to use these in vitro tissue models or in vivo animal models for drug discovery and regenerative therapy investigation which could act to reduce, refine and potentially replace the usage of animal models [72].
Wnt/β-catenin signaling has been demonstrated to be a vital component of the heart in development, cardiac repair and in cardiac disease pathogenesis. It has also been demonstrated that by manipulating the Wnt/β-catenin signaling cascade in vitro hPSCs can be differentiated to provide a large robust supply of cardiac cells for tissue engineering and regenerative therapy applications, a much needed resource. However, the potential of the Wnt/β-catenin pathway in tissue engineering and modeling applications needs to be further explored. Given the central role of the Wnt/β-catenin signaling pathway in both health and disease, it is obvious that any manipulation will need to be very strategic and precise in order for specified outcomes to be achieved, but the success of the directed differentiation protocols shows that it is possible and probable to achieve this goal.
Supplementary Material
Acknowledgments
This work is funded by the Canadian Institutes of Health Research (CIHR) Operating Grant (MOP-126027), NSERC Discovery Grant (RGPIN 326982-10), and National Institutes of Health grant (2R01 HL076485). M.R. is supported by Canada Research Chair (Tier 2) and Steacie Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dequéant ML, Pourquié O. Segmental patterning of the vertebrate embryonic axis. Nat Rev Genet. 2008;9:370–382. doi: 10.1038/nrg2320. [DOI] [PubMed] [Google Scholar]
- 2.Perrimon N, Pitsouli C, Shilo BZ. Signaling Mechanisms Controlling Cell Fate and Embryonic Patterning. Cold Spring Harb Perspect Biol. 2012;4:a005975. doi: 10.1101/cshperspect.a005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murry CE, Keller G. Differentiation of Embryonic Stem Cells to Clinically Relevant Populations: Lessons from Embryonic Development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 5.Mentink RA, Middelkoop TC, Rella L, Ji N, Tang CY, Betist MC, et al. Cell Intrinsic Modulation of Wnt Signaling Controls Neuroblast Migration in C. elegans. Dev Cell. 2014;31:188–201. doi: 10.1016/j.devcel.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Bakre MM, Hoi A, Mong JCY, Koh YY, Wong KY, Stanton LW. Generation of Multipotential Mesendodermal Progenitors from Mouse Embryonic Stem Cells via Sustained Wnt Pathway Activation. J Biol Chem. 2007;282:31703–31712. doi: 10.1074/jbc.M704287200. [DOI] [PubMed] [Google Scholar]
- 7.Vasiev B, Balter A, Chaplain M, Glazier JA, Weijer CJ. Modeling Gastrulation in the Chick Embryo: Formation of the Primitive Streak. PLoS ONE. 2010;5:e10571. doi: 10.1371/journal.pone.0010571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaldis P, Pagano M. Wnt Signaling in Mitosis. Dev Cell. 2009;17:749–750. doi: 10.1016/j.devcel.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 9.De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin. 2011;43:745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 10.Gessert S, Kühl M. The Multiple Phases and Faces of Wnt Signaling During Cardiac Differentiation and Development. Circ Res. 2010;107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 11.Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci. 2002;115:3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Yang J, Evans PM, Liu C. Wnt signaling: the good and the bad. Acta Biochim Biophys Sin. 2008;40:577–594. doi: 10.1111/j.1745-7270.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, et al. Small molecule–mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blankesteijn WM, van de Schans VAM, ter Horst P, Smits JFM. The Wnt/frizzled/GSK-3 beta pathway: a novel therapeutic target for cardiac hypertrophy. Trends Pharmacol Sci. 2008;29:175–180. doi: 10.1016/j.tips.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty MP, Kamerzell TJ, Dawn B. Chapter 7 - Wnt Signaling and Cardiac Differentiation. In: Tang Y, editor. Prog Mol Biol Transl Sci. Academic Press; 2012. [accessed June 25, 2015]. pp. 153–174. http://www.sciencedirect.com/science/article/pii/B9780123984593000071. [DOI] [PubMed] [Google Scholar]
- 17.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 18.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 19.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flügel L, et al. Patient-Specific Induced Pluripotent Stem-Cell Models for Long-QT Syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 20.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 21.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, et al. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, et al. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 24.Semënov MV, Tamai K, Brott BK, Kühl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol CB. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 26.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E1848–1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Huang C, Wu P, Yang J, Song T, Chen Y, et al. Cardiac stem cells differentiate into sinus node-like cells. Tohoku J Exp Med. 2010;222:113–120. doi: 10.1620/tjem.222.113. [DOI] [PubMed] [Google Scholar]
- 29.Christoffels VM, Smits GJ, Kispert A, Moorman AFM. Development of the Pacemaker Tissues of the Heart. Circ Res. 2010;106:240–254. doi: 10.1161/CIRCRESAHA.109.205419. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–587. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goliasch G, Wiesbauer F, Kastl SP, Katsaros KM, Blessberger H, Maurer G, et al. Premature myocardial infarction is associated with low serum levels of Wnt-1. Atherosclerosis. 2012;222:251–256. doi: 10.1016/j.atherosclerosis.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Askevold ET, Gullestad L, Aakhus S, Ranheim T, Tønnessen T, Solberg OG, et al. Secreted Wnt modulators in symptomatic aortic stenosis. J Am Heart Assoc. 2012;1:e002261. doi: 10.1161/JAHA.112.002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu G, Chen T, Zhou H, Sun K, Li J. Role of Wnt/β-catenin signaling pathway in the mechanism of calcification of aortic valve. J Huazhong Univ Sci Technol Med Sci Hua Zhong Ke Ji Xue Xue Bao Yi Xue Ying Wen Ban Huazhong Keji Daxue Xuebao Yixue Yingdewen Ban. 2014;34:33–36. doi: 10.1007/s11596-014-1228-x. [DOI] [PubMed] [Google Scholar]
- 35.Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, et al. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003;108:2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 36.Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, et al. Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci U S A. 2003;100:4610–4615. doi: 10.1073/pnas.0835895100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelarayán LC, Noack C, Sekkali B, Kmecova J, Gehrke C, Renger A, et al. Beta-Catenin downregulation attenuates ischemic cardiac remodeling through enhanced resident precursor cell differentiation. Proc Natl Acad Sci U S A. 2008;105:19762–19767. doi: 10.1073/pnas.0808393105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Schans VAM, van den Borne SWM, Strzelecka AE, Janssen BJA, van der Velden JLJ, Langen RCJ, et al. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension. 2007;49:473–480. doi: 10.1161/01.HYP.0000255946.55091.24. [DOI] [PubMed] [Google Scholar]
- 39.Deb A. Cell-cell interaction in the heart via Wnt/β-catenin pathway after cardiac injury. Cardiovasc Res. 2014;102:214–223. doi: 10.1093/cvr/cvu054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daskalopoulos EP, Hermans KCM, Janssen BJA, Matthijs Blankesteijn W. Targeting the Wnt/frizzled signaling pathway after myocardial infarction: a new tool in the therapeutic toolbox? Trends Cardiovasc Med. 2013;23:121–127. doi: 10.1016/j.tcm.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, et al. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci U S A. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laeremans H, Hackeng TM, van Zandvoort MAMJ, Thijssen VLJL, Janssen BJA, Ottenheijm HCJ, et al. Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation. 2011;124:1626–1635. doi: 10.1161/CIRCULATIONAHA.110.976969. [DOI] [PubMed] [Google Scholar]
- 44.Laeremans H, Rensen SS, Ottenheijm HCJ, Smits JFM, Blankesteijn WM. Wnt/frizzled signalling modulates the migration and differentiation of immortalized cardiac fibroblasts. Cardiovasc Res. 2010;87:514–523. doi: 10.1093/cvr/cvq067. [DOI] [PubMed] [Google Scholar]
- 45.Turner NA, Porter KE. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis Tissue Repair. 2013;6:5. doi: 10.1186/1755-1536-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blankesteijn WM, Essers-Janssen YPG, Verluyten MJA, Daemen MJAP, Smits JFM. A homologue of Drosophila tissue polarity gene frizzled is expressed in migrating myofibroblasts in the infarcted rat heart. Nat Med. 1997;3:541–544. doi: 10.1038/nm0597-541. [DOI] [PubMed] [Google Scholar]
- 47.Blankesteijn WM, Creemers E, Lutgens E, Cleutjens JP, Daemen MJ, Smits JF. Dynamics of cardiac wound healing following myocardial infarction: observations in genetically altered mice. Acta Physiol Scand. 2001;173:75–82. doi: 10.1046/j.1365-201X.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 48.van den Borne SWM, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 49.Sun Y, Kiani MF, Postlethwaite AE, Weber KT. Infarct scar as living tissue. Basic Res Cardiol. 2002;97:343–347. doi: 10.1007/s00395-002-0365-8. [DOI] [PubMed] [Google Scholar]
- 50.Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech. 2011;4:469–483. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malekar P, Hagenmueller M, Anyanwu A, Buss S, Streit MR, Weiss CS, et al. Wnt signaling is critical for maladaptive cardiac hypertrophy and accelerates myocardial remodeling. Hypertension. 2010;55:939–945. doi: 10.1161/HYPERTENSIONAHA.109.141127. [DOI] [PubMed] [Google Scholar]
- 52.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 53.Qu J, Zhou J, Yi XP, Dong B, Zheng H, Miller LM, et al. Cardiac-specific haploinsufficiency of beta-catenin attenuates cardiac hypertrophy but enhances fetal gene expression in response to aortic constriction. J Mol Cell Cardiol. 2007;43:319–326. doi: 10.1016/j.yjmcc.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90:1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Liu Y, Zhu XH, Zhang XD, Jiang DS, Bian ZY, et al. Dickkopf-3 attenuates pressure overload-induced cardiac remodelling. Cardiovasc Res. 2014;102:35–45. doi: 10.1093/cvr/cvu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hagenmueller M, Riffel JH, Bernhold E, Fan J, Zhang M, Ochs M, et al. Dapper-1 induces myocardial remodeling through activation of canonical Wnt signaling in cardiomyocytes. Hypertension. 2013;61:1177–1183. doi: 10.1161/HYPERTENSIONAHA.111.00391. [DOI] [PubMed] [Google Scholar]
- 57.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 58.Pereira CP, Bachli EB, Schoedon G. The wnt pathway: a macrophage effector molecule that triggers inflammation. Curr Atheroscler Rep. 2009;11:236–242. doi: 10.1007/s11883-009-0036-4. [DOI] [PubMed] [Google Scholar]
- 59.Christman MA, Goetz DJ, Dickerson E, McCall KD, Lewis CJ, Benencia F, et al. Wnt5a is expressed in murine and human atherosclerotic lesions. Am J Physiol Heart Circ Physiol. 2008;294:H2864–2870. doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- 60.Kim J, Kim J, Kim DW, Ha Y, Ihm MH, Kim H, et al. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J Immunol Baltim Md 1950. 2010;185:1274–1282. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 61.Borrell-Pagès M, Romero JC, Juan-Babot O, Badimon L. Wnt pathway activation, cell migration, and lipid uptake is regulated by low-density lipoprotein receptor-related protein 5 in human macrophages. Eur Heart J. 2011;32:2841–2850. doi: 10.1093/eurheartj/ehr062. [DOI] [PubMed] [Google Scholar]
- 62.Borrell-Pages M, Romero JC, Badimon L. Cholesterol modulates LRP5 expression in the vessel wall. Atherosclerosis. 2014;235:363–370. doi: 10.1016/j.atherosclerosis.2014.05.922. [DOI] [PubMed] [Google Scholar]
- 63.Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 64.Dufourcq P. FrzA, a Secreted Frizzled Related Protein, Induced Angiogenic Response. Circulation. 2002;106:3097–3103. doi: 10.1161/01.CIR.0000039342.85015.5C. [DOI] [PubMed] [Google Scholar]
- 65.Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, et al. Beta-catenin downregulation is required for adaptive cardiac remodeling. Circ Res. 2007;100:1353–1362. doi: 10.1161/01.RES.0000266605.63681.5a. [DOI] [PubMed] [Google Scholar]
- 66.Hagenmueller M, Riffel JH, Bernhold E, Fan J, Katus HA, Hardt SE. Dapper-1 is essential for Wnt5a induced cardiomyocyte hypertrophy by regulating the Wnt/PCP pathway. FEBS Lett. 2014;588:2230–2237. doi: 10.1016/j.febslet.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 67.Yoshida Y, Yamanaka S. Recent stem cell advances: induced pluripotent stem cells for disease modeling and stem cell-based regeneration. Circulation. 2010;122:80–87. doi: 10.1161/CIRCULATIONAHA.109.881433. [DOI] [PubMed] [Google Scholar]
- 68.Hirt MN, Sörensen NA, Bartholdt LM, Boeddinghaus J, Schaaf S, Eder A, et al. Increased afterload induces pathological cardiac hypertrophy: a new in vitro model. Basic Res Cardiol. 2012;107:307. doi: 10.1007/s00395-012-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robert J, Weber B, Frese L, Emmert MY, Schmidt D, von Eckardstein A, et al. A Three-Dimensional Engineered Artery Model for In Vitro Atherosclerosis Research. PLoS ONE. 2013;8:e79821. doi: 10.1371/journal.pone.0079821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ge X, Ren Y, Bartulos O, Lee MY, Yue Z, Kim KY, et al. Modeling Supravalvular Aortic Stenosis Syndrome With Human Induced Pluripotent Stem Cells. Circulation. 2012;126:1695–1704. doi: 10.1161/CIRCULATIONAHA.112.116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maleki H, Shahriari S, Labrosse M, Pibarot P, Kadem L. An In Vitro Model of Aortic Stenosis for the Assessment of Transcatheter Aortic Valve Implantation. J Biomech Eng. 2014;136:054501–054501. doi: 10.1115/1.4026576. [DOI] [PubMed] [Google Scholar]
- 72.Flecknell P. Replacement, reduction and refinement. ALTEX. 2002;19:73–78. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.