Abstract
Many of the most debilitating symptoms for psychiatric disorders such as schizophrenia remain poorly treated. As such, the development of novel treatments is urgently needed. Unfortunately, the costs associated with high failure rates for investigational compounds as they enter clinical trials has led to pharmaceutical companies downsizing or eliminating research programs needed to develop these drugs. One way of increasing the probability of success for investigational compounds is to incorporate alternative methods of identifying biological targets in order to more effectively screen new drugs. A promising method of accomplishing this goal for psychiatric drugs is to use functional magnetic resonance imaging (fMRI). fMRI investigates neural circuits, shedding light on the biology that generates symptoms such as hallucinations. Once identified, relevant neural circuits can be targeted with pharmacologic interventions and the response to these drugs measured with fMRI. This review describes the early use of fMRI in this context, and discusses the alpha7 nicotinic receptor agonist 3-(2,4-dimethoxybenzylidene) anabaseine (DMXB-A), as an example of the potential value of fMRI for psychiatric drug development.
Keywords: Biomarkers, functional magnetic resonance imaging, fMRI, nicotine, alpha7 nicotinic receptor, functional connectivity, SPEM
INTRODUCTION
Developing drugs to treat psychiatric conditions such as schizophrenia inherently involves risks and faces many challenges, but potentially could lead to new treatments that alleviate suffering of millions. Globally, 25 million people suffer from schizophrenia [1]. Since this disease is incurable, chronic, and presents clinically during early adulthood, it makes a large contribution to disability rates worldwide [2]. Furthermore, although current pharmacologic treatments for schizophrenia often successfully reduce psychotic symptoms, such as hallucinations and delusions, they minimally affect the emotional and cognitive symptoms that most greatly impact patients’ quality of life [3–5]. These symptoms lead to deficits in essential real-world skills such as vocational functioning, forming interpersonal relationships and participating in community activities. Additionally, current antipsychotic medications, based on full or partial dopamine antagonism, are associated with sedation, involuntary movements resembling Parkinson’s Disease (extrapyramidal side effects), or weight gain and lipid abnormalities that contribute to type 2 diabetes, heart attack, and stroke [5].
The suffering caused by this relatively common disease, in combination with the deficiencies in existing treatment options, suggests a need for developing new pharmacotherapies for schizophrenia. Difficulties inherent to psychiatric drug development, however, currently discourage research into this field [6–8]. These difficulties include long development times, high failure rates during late-stage clinical trials, weak validation, and poor predictive ability of current animal models, all of which contribute to an overall low probability of success for developing new psychologically active compounds [9–12]. Incorporating neuroimaging technologies, such as functional magnetic resonance imaging (fMRI), into the drug discovery process may represent a possible answer to many of these challenges [13,14]. Although the utility of this tool for drug development is currently being explored, the potential of this idea has generated increasing interest within many research communities. The cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia (CNTRICS) initiative centers on developing tools for future targeted drug development [15]. Additionally, the importance of private-public partnerships between psychiatric researchers and drug development companies, utilizing fMRI and other neuroimaging technologies, is increasingly recognized as indispensible for improving mental health treatment [16,17]. To explore the potential utility of this process, this brief review examines the early use of neuroimaging in the context of psychiatric drug development, and highlights a specific example of its use in the evaluation of a novel therapeutic compound, the nicotinic receptor agonist 3-(2,4-dimethoxybenzylidene) anabaseine (DMXB-A).
CHALLENGES IN PSYCHIATRIC DRUG DEVELOPMENT AND FMRI
Despite the need for new psychiatric drugs, many scientific challenges and barriers to success must be overcome before a pharmacologic compound can be developed into a novel therapeutic drug. One challenge is the low FDA approval rate of 8%, the rate lowest of all drug categories [9]. Drugs in this category spend the most time in clinical phases of testing, at 8 to 10 years on average [12]. While not specific to psychiatric drugs, the failure rate during clinical phases is particularly high, with less than 25% of all drugs succeeding in phase 2 clinical trials [11]. Phase 2 is the clinical ‘proof-of-concept’ stage, where the efficacy of the potential drug in treating a disease is directly tested in human subjects. Failure at this late stage of drug development is especially costly, both from the cost of phase 2 studies as well as the years of development necessary to reach this stage. Improving success rates at this stage has been proposed as the single most important factor needed to improve the drug development process [11].
Psychiatric drug development involves unique problems in phase 2 clinical trials. Ideally, drugs that reach this phase have known pharmacologic targets, related to known disease mechanisms, and will be tested at tolerated clinical doses that have been shown to affect a validated marker of biological response [11,18]. Since the pathology of psychiatric diseases is poorly understood, the selection of pharmacologic agents to target specific neuropathological processes is based upon limited evidence. Given that the brain is a relatively inaccessible organ, measuring drug concentrations within the brain and subsequently determining ideal drug dosing is difficult at best. As psychiatric diseases lack objective diagnostic tests, but instead are diagnosed based on structured clinical interview [19], validated markers of biological responses are currently unavailable [13]. fMRI may offer the potential to meet these challenges for psychiatric drug development by informing the selection of pharmacologic targets based on neural circuitry. Additionally, fMRI offers the opportunity to develop biological markers of drug response based on this circuitry, and provides information regarding drug concentrations needed to affect the circuitry [13,14,18].
NEUROIMAGING AND FMRI
Neuroimaging allows for investigation of the neural circuitry that underlies the effects of medications. fMRI measures fluctuations in the oxygen content of blood in the brain (see [13], or [14], for more detailed explanations). Other neuroimaging techniques measure electrical potentials on the scalp (electroencephalography, or EEG), magnetic fields outside of the skull (magnetoencephalography, or MEG), the breakdown of radioactive tracer molecules (positron emission tomography, or PET), or the absorption of infrared light (functional near-infrared spectroscopy, or fNIRS). fMRI holds particularly great potential to contribute to drug development due to its non-invasive nature, millimeter-level spatial resolution, and deep-brain coverage. Since fMRI measures activity without the use of radioactive tracers, it can be used for longitudinal studies involving repeated scans. The high spatial resolution of fMRI allows it to accurately identify even small changes in activity occurring in response to medications. Lastly, the ability to image subcortical structures, such as the thalamus, basal ganglia and hippocampus, allows for comparison of drug effects using the well-characterized neurochemistry and circuitry within these regions. In summary, the strengths of fMRI allow for examination of the neural circuitry underlying psychiatric pathology or for development of new biological markers of pharmacologic response, based on knowledge of existing neural circuitry and the chemistry of associated neurotransmitters.
TARGET IDENTIFICATION AND NEURAL CIRCUITS
Target identification, or the selection of a pharmacologic mechanism of action for drug development, is especially difficult in psychiatric disorders. Many neuropsychiatric disorders, such as schizophrenia, lack clearly identified pathologies or associated molecular targets. While existing antipsychotic drugs act by modifying synaptic communication via dopamine receptors, the side effect profiles of these drugs are problematic and often intolerable [20]. Furthermore, the prototypes of these medications were originally discovered through serendipity and careful observations in human subjects, rather than through a process of rational drug design [10,18]. Subsequently, their mechanisms of action were determined based on their receptor binding profiles. Animal assays probing these same mechanisms were then developed to investigate potential new medications. Given this process, these assays probe the same mechanisms of action as prototypical antipsychotic drugs, leading to discovery of new drugs with similar efficacy and side effect profiles as existing medications. These processes have not, therefore, led to new classes of antipsychotic medications or novel antipsychotic mechanisms of action [10]. In order to discover new, truly novel antipsychotic drugs that advance the treatment of schizophrenia, new pharmacologic targets need to be identified. The insight into the brain’s circuitry provided by fMRI, along with concurrent pharmacologic modification of this circuitry, may identify new pharmacologic targets.
A strength of fMRI is that it can relate behavior to the actions of drugs on functional brain regions, interconnected by white matter tracts to form neural circuits, in either animal models or human subjects. Pharmacologic targets based on neural circuits can be used to refine behavioral-based animal models, or to provide an objective marker of subjective responses reported by patients [13]. For example, pain is often measured using pain intensity scales, as reported by patients [21]. These subjective scales can be influenced by comorbid conditions, such as depression resulting from chronic pain [22]. Furthermore, they do not distinguish between types of pain such as hyperalgesia, an exaggerated pain response, and allodynia, a painful response to normally innocuous stimuli [23]. For these reasons, research into pain and analgesic compounds has focused on identifying objective measures of patients’ symptoms that are more clearly related to biology [22]. Using fMRI, Wise et al. [24] demonstrated that the analgesic remifentanil reduced pain-related activation in the insula and anterior cingulate cortex. Wager et al. [25] built upon those results, by combining fMRI and machine-learning techniques to identify a neurologic signature of pain as activation of the insula, anterior cingulate and somatosensory regions during heat-induced pain. Their resulting measure of activity within the brain is highly sensitive and specific to physical pain, and discriminates this type of pain from emotional pain resulting from social rejection. Lastly, this fMRI measure also is decreased by remifentanil [25]. In both of these cases, the fMRI response to medication confirmed subjective reports of symptoms, while providing additional objective information about the neural circuitry affected by the drug. Modification of this circuitry therefore represents a potential biological target that can be developed into biologic markers of a clinical response and used to screen new compounds [26].
BIOMARKERS OF NEURAL RESPONSES
Once identified, potential biological markers of pharmacologic response often need to be replicated to gain widespread acceptance as a precise tool for measuring the clinical effects of an intervention. The end result of this process is a validated biological marker, or biomarker. The National Institute of Health’s Biomarkers and Surrogate Endpoint Working Group defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal physiological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” [27]. Furthermore, this group systematically classified biomarkers according to their use and connection to the underlying biology. Type 0 biomarkers measure the natural history of the disease. Type I biomarkers measure intervention effects, as they relate to drug mechanisms. Type II biomarkers predict clinical benefits and can be used as surrogate endpoints. A surrogate endpoint is a biomarker that is able to predict clinical benefit based on epidemiological, therapeutic, or other evidence [27]. In drug development, identifying surrogate endpoints is especially important, since this type of biomarker can be used in place of clinical endpoints that are more difficult to measure, or that manifest late in the disease process [26].
Biomarkers based on neural circuitry have the potential to improve the prioritization of drug discovery resources by informing early decisions regarding whether or not to pursue development of a compound [26,27]. For example, consider choosing from among various pharmacologic compounds, any of which may potentially treat a psychiatric disorder. Using fMRI, type II biomarkers can be identified that measure patterns of pathologic activity in interconnected regions within the brain. Reduction in this activation pattern can be used as a “fingerprint” to screen for pharmacologic compounds with therapeutic potential [13]. This can be accomplished in healthy volunteers or, through translational fMRI, in laboratory animals as well. Compounds with this biomarker “fingerprint” can be prioritized for development. This demonstrated mechanism of action, directly related to psychopathology, increases the chances that the compound will succeed in phase 2 clinical trials in patients. Through connecting pharmacology with disease mechanisms at an earlier stage of drug development, fMRI could potentially cut costs, increase the probability of success, and improve the efficiency of psychiatric drug development [14].
In the manner described above, fMRI is increasingly used to help identify potential biomarkers for schizophrenia. For example, intrinsic hippocampal hyperactivity and default network hyperactivity are two promising, potential biomarkers for schizophrenia that have been identified in large part by fMRI [28]. The effectiveness of these biomarkers depends not only upon their ability to be associated with clinical outcomes, but also their ability to predict treatment response. As reviewed by Tregellas [28], both hippocampal hyperactivity and dysfunction within large-scale networks have demonstrated promise in fulfilling these criteria. These results, however, are in need of validation by larger studies, particularly in determining how effects of both established and investigational therapies may target these biomarkers to produce treatment effects. The widely replicated neural effects of nicotine in the disease may represent a particularly promising starting point for identifying biomarkers in schizophrenia.
NICOTINIC RECEPTORS AND SCHIZOPHRENIA
Nicotinic receptors have long been a target for psychiatric drug development due to high smoking rates in mental illness in general and schizophrenia in particular. Smoking rates in schizophrenia are as high as 80%, compared to less than 30% in the general population [29–31]. While the reasons for high smoking rates in schizophrenia are unknown, cigarette use has been suggested to be a form of self-medication in the disease [32–34]. Hypothesized reasons for this self-medication include improvement of negative symptoms, reduction of antipsychotic medication side-effects, and improvement of cognitive functioning [33]. Smoking also transiently improves multiple sensory processing problems in schizophrenia, including sensory gating deficits and smooth pursuit eye movement deficits (discussed below). Sensory gating deficits contribute to the inability to filter out irrelevant and potentially distressing environmental stimuli in the disease [32] and can be quantified with measures such as the P50 evoked potential and prepulse inhibition in human and animal subjects. These measures are abnormal in schizophrenia and are improved by nicotine or tobacco use [32,33,35]. As such, nicotinic receptors may be a useful target for pharmacologic intervention.
Many current pharmacologic therapies for schizophrenia target dopaminergic systems within the brain, a system partially under nicotinic control [36]. Different dopaminergic pathways in the brain may contribute to distinct symptoms of the illness. Pathways of the mesolimbic dopaminergic system, projecting to the nucleus accumbens, are believed to contribute to the positive symptoms of schizophrenia [36]. Current antipsychotic medications are full or partial dopamine antagonists, presumably improving hallucinations by decreasing dopamine release within the mesolimbic pathway [36]. Deficits within the mesocortical dopaminergic pathway, which projects to the prefrontal cortex, are believed to contribute to the negative symptoms of the disease. Increased dopamine release within this pathway may target the negative symptoms of the disease [36]. Agonists targeting the alpha7 nicotinic acetylcholine receptor (a7-nAChR) increase dopamine release in both pathways [37,38]. While increased dopamine release in the mesocortical pathway could potentially improve emotional blunting and cognitive symptoms, increase dopamine release within the mesolimbic pathway could potentially exacerbate hallucinations. Despite this, there is evidence suggesting that the a7-nAChR partial agonists have differential effects on these different pathways, and may even decrease dopamine release within the mesolimbic pathway in mouse models of schizophrenia [36,39].
Among nicotinic receptors, the alpha7 nicotinic acetylcholine receptor (a7-nAChR) is of particular interest in schizophrenia. Its involvement in the illness is supported by its pattern of expression within the brain, electrophysiological and genetic studies [32,35,40]. Decreased a7-nAChr expression in schizophrenia has been observed within many regions associated the pathology of the disease, such as the reticular thalamic nuclei [41], the cingulate cortex and frontal lobes [42,43], and the hippocampus [40]. Chromosome 15q13-14, the region which encompasses the CHRNA7 gene which codes for the a7-nAChR, is linked to schizophrenia [35]. Additionally, polymorphisms in the CHRNA7 upstream promoter region are linked to the development of schizophrenia, as well as to the sensory gating abnormalities associated with the disease. Lastly, the a7-nAChR partial agonist 3-(2,4-dimethoxy-benzylidene) anabaseine (DMXB-A) improves the blunted affect and emotional symptoms reported by patients with the disease [44]. This ability to improve emotional and cognitive deficits, symptoms not addressed by current treatment options [5], has created interest in developing new therapies based on alpha7 nicotinic agonists. How these agonists may improve cognitive symptoms and the neural circuitry involved, however, is largely unknown.
SMOOTH PURSUIT EYE MOVEMENT AS A POTENTIAL NICOTINIC BIOMARKER
The development of nicotinic-based therapies for schizophrenia could be improved by identifying a functional biomarker that measures clinically relevant response to the drug, along with the corresponding neural circuitry. One such possibility is the neuronal response associated with smooth pursuit eye movement (SPEM) deficits, an eye-tracking abnormality characteristic of the disease. In a SPEM task, subjects follow a dot as it steadily moves across a screen. Patients with schizophrenia show multiple impairments during the task, including increased anticipatory saccades as their eyes frequently dart in front of the moving dot. In comparison, a typical healthy subject’s eyes follow the dot more accurately, with minimal anticipatory saccades. This marker has been widely reproduced in a number of studies, is observed in both patients with schizophrenia and their relatives, and is not present in other psychiatric diseases [45–47].
The reproducibility and specificity of SPEM makes it of great interest in the study of schizophrenia, notably as a disease endophenotype under genetic control [47]. However, eye-tracking abnormalities were investigated before the increased interest in biomarkers and, by themselves, likely do not qualify as a biomarker under the rigorous definition of the term given above [26]. SPEM deficits do not seem to be linked to disease progression (type 0 biomarker), to a disease mechanism (type I) or to treatment response (type II). While SPEM deficits in schizophrenia are normalized by nicotine [48,49], the mechanism responsible for this effect is poorly understood. To increase the utility of SPEM as a potential biomarker, the technique must be more closely linked to the abnormal circuitry within the brain associated with schizophrenia.
Investigations using fMRI have built upon previous clinical studies by illuminating the neural circuitry involved in SPEM, how this circuitry responds to nicotine, and how it is disrupted in schizophrenia. Previously, SPEM deficits in schizophrenia had been linked to activity in frontal eye fields [50]. While this previous result marked an advance in understanding eye-tracking abnormalities, it was not linked to a core deficit of the disease. In contrast, using fMRI Tregellas et al. [51] demonstrated that eye-tracking abnormalities in schizophrenia are related to the hippocampus, a region linked to the pathology of schizophrenia [51]. The hippocampus is one of the most well-studied areas of the brain, with a well-described anatomy and physiology. Hippocampal dysfunction and abnormal circuitry are commonly reported in schizophrenia [28,52–55]. Furthermore, the medial temporal lobe in general and the hippocampus in particular show the most prominent grey matter deficits in schizophrenia [56,57]. Using fMRI to link SPEM to the hippocampus revealed a link between eye-tracking abnormalities and a hypothesized disease mechanism, thereby qualifying hippocampal hyperactivity during SPEM as a potential type I biomarker [40,51]. Furthermore, additional studies demonstrated that treatment with nicotine or the a7-nAChR partial agonist DMXB-A normalizes hippocampal hyperactivity during SPEM in schizophrenia, while potentially improving cognition in the disease [44,58–60]. This suggests that SPEM during fMRI may also represent a potential type II biomarker of treatment response.
CONNECTIVITY BIOMARKERS AND NICOTINIC RECEPTORS
In addition to SPEM, neuroimaging may potentially identify functional connectivity-related biomarkers of nicotinic therapeutic responses. While most often used to measure task-evoked activity within the brain, fMRI can be used to identify circuits of interconnected regions that form networks. These large-scale networks arise from intrinsic activity independent of psychological task performance, are present even during light anesthesia, are modifiable by nicotine and psychiatric medications, and, importantly, are conserved across species [61–64]. These characteristics make these potential biomarkers useful as a “language of translation” between psychiatric diseases and animal models of these diseases [13].
The Default Mode Network (DMN) is a well-characterized large-scale network that is active during mind-wandering, self-reflective thought and internally-focused mental states [65]. This network is primarily composed of the bilateral posterior cingulate cortex (PCC), precuneus, inferior parietal lobules (IPL) and medial prefrontal cortex (mPFC), but often includes the hippocampus and parahippocampal gyrus as well. Activity within DMN regions cluster together to form smaller subnetworks, with separate anterior and posterior subnetworks centered upon activity within the mPFC and PCC, respectively [65]. Homologues of the DMN have been identified in many species of primates and rodents [64,66,67]. In these species, midline cingulate, hippocampal, and lateral parietal regions are interconnected in a pattern remarkably similar to the DMN in humans. In schizophrenia, areas of the DMN are hyperactive and hyperconnected, and activity in these regions correlates with symptoms of the disease, such as hallucinations and delusions as well as emotional blunting [68]. These abnormalities extend to first-degree relatives of patients with schizophrenia. While the mPFC, PCC and parahippocampal gyrus are hyperactive both during psychological tasks and at rest in schizophrenia, parts of the precuneus show decreased activity [69]. The DMN hyperactivity observed in schizophrenia may indicate that the boundaries between external and internal mental activity are blurred, contributing to perceptual disturbances and hallucinations [70]. Connectivity between DMN subnetworks may change during the course of the disease. Patients experiencing their first episode of psychosis, who go on to be diagnosed with schizophrenia, show decreased connectivity between anterior and posterior DMN subnetworks [71,72]. This disconnectivity between DMN nodes does not appear to be present in subjects in later stages of the disease [68].
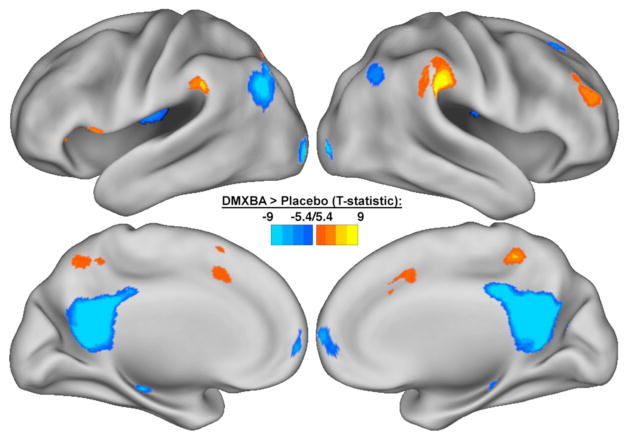
DMN activity and connectivity in schizophrenia are modulated by psychiatric medications [73–75] and nicotine [63]. The direction of the pharmacologic effect, however, depends on the region involved, the presence or absence of a cognitively demanding task, and the stage of the disease. In addition to normalizing hippocampal activity during SPEM, the nicotinic agonist DMXB-A also potentially improves connectivity within the DMN [73]. In subjects with schizophrenia, DMXB-A increases connectivity to the precuneus, while decreasing connectivity to the PCC, IPL and mPFC (Fig. 1), consistent with a normalization of connectivity deficits within the network [69]. Furthermore, polymorphisms in the regulatory sequence of the a7-nAChR subunit gene, CHRNA7, affect the response to DMXB-A [73]. Compared to the major allele, subjects carrying the minor allele of the 5′ regulatory sequence of CHRNA7 1831 base pairs upstream of the translation site had decreased response to DMXB-A in the PCC. This minor allele, which also has been linked to the development of schizophrenia [76], was associated with decreased improvement in cognitive measures in response to DMXB-A in patients [73].
Fig. 1.
Effects of 3-(2,4-dimethylbenzylidene)-anabaseine (DMXB-A) on default mode network (DMN) activity. Compared to placebo, DMXB-A decreased activity in DMN regions such as the posterior cingulate cortex, inferior parietal cortex, and medial frontal gyrus; in addition to increased activity in the precuneus and temporoparietal junction. Data are displayed as statistical map on the cortical surface, thresholded at p < 0.05 (FWE-corrected).
Antipsychotic drugs modulate connectivity between DMN subnetworks in schizophrenia, and this modulation may be related to the stage of the disease. During rest in healthy subjects, the antipsychotic and dopaminergic antagonist haloperidol decreases connectivity between DMN regions and posterior regions of the midbrain [75]. Similarly, the dopaminergic agonist levodopa increases connectivity between DMN regions and posterior midbrain regions in the same subjects. These midbrain regions minimally overlap with more anterior regions of the dopaminergic system, such as the substantia nigra or the ventral tegmental area.
During a working memory task, the atypical antipsychotic olanzapine increases DMN connectivity within the mPFC in patients [74]. This response to treatment may appear counterintuitive given the observed DMN hyperconnectivity in schizophrenia [68], but is consistent with evidence pointing to abnormal connectivity between DMN subnetworks early in the disease [71,72]. In the olanzapine study, most subjects were recovering from their first episode of psychosis and all were either drug naïve or drug free for at least two weeks [74]. The DMN identified in this study was predominantly located in posterior regions of the DMN, such as the PCC and IPL. Compared to healthy controls, patients recovering from first-episode psychosis demonstrate decreased connectivity between the mPFC and PCC [71,72]. Given these results, olanzapine treatment may normalize dysfunctional connectivity between the anterior and posterior nodes of the DMN present early in the course of the disease.
Antipsychotic dose, measured as chlorpromazine equivalents, may be related to DMN and sensorimotor network activity. During an auditory motor task, subjects with schizophrenia on higher drug doses demonstrate increased DMN activity and decreased motor network activity [77]. However, this association mirrors the differences between patients and healthy controls. In comparison with healthy controls, subjects with schizophrenia showed similarly increased DMN activity and decreased motor network activity [77]. Additionally, patients with more severe disease tend to be on higher doses of drugs, suggesting a potential confounding effect [78,79]. Since subjects on higher doses appear to have more severe abnormalities within these networks, the association between antipsychotic dose and network activity in this case may be an indication of disease severity and not an effect of medication.
NEURAL CIRCUITS AND BIOMARKERS IN ANIMAL MODELS
Drug effects on neural circuits, such as the effects of DMXB-A on DMN connectivity, are not limited to human subjects. Much of the preclinical work on DMXB-A and its pharmacologic target was carried out in animal models before the successful completion of phase 2 clinical trials [32]. Although the field of animal fMRI is still in its infancy, it has the potential to act as a unifying “language of translation” where the same technology measures the same neural circuitry in both patients with psychiatric disease as well as animal models of the disease [13]. Large-scale networks, including homologues of the human DMN, have been found in primates, rats, and mice [64]. Similar to the human DMN, the rodent DMN encompasses medial anterior regions, the cingulate gyrus, posterior parietal cortex and the hippocampal gyrus [66,80]. Connectivity biomarkers identified through fMRI may cross the species barrier between human subjects and animal models, thereby facilitating translational research and preclinical phases of drug discovery.
Current animal models of schizophrenia have substantial limitations and their ability to predict drug efficacy for human diseases has been poor [10]. These models are either based on genes with low penetrance, environmental insults that are not specific to the disease, or on non-specific behavioral tests that capture a single aspect of a complex diagnosis. fMRI can potentially improve the validation of current animal models by examining the neural circuitry that acts as a mediator between genotype or environmental insult and the disease phenotype [13]. Subsequently, fMRI can be used to investigate the effects of potential drugs on the relevant neural circuitry in these animal models, as well as in healthy human subjects. This process may improve the predictive validity of animal models by more closely linking genotype to phenotype, and by demonstrating that the potential drug affects the same mechanisms in humans. In this way, fMRI can act as a unifying technology that crosses between species, whereby results from human patients are used to refine an animal model, and results from the animal model are used to inform drug development involving human subjects.
CONCLUSION
Although there are many challenges involved in psychiatric drug development, they are not insurmountable. New technologies, such as fMRI, have the potential to improve efficiency at many phases in the drug development process. fMRI can contribute at preclinical phases in animal models, through identifying biological targets affected by pharmacological compounds, and by validating biomarkers based on neural circuitry. Incorporating fMRI in the early phases of this process allows potential drugs to be selected based upon their demonstrated effect on this pathologic circuitry, thereby improving the chances of demonstrating efficacy during a late-stage clinical trial. The example of DMXB-A, a compound developed to target the a7-nAChR, its beneficial effects on cognition in schizophrenia, and its success in a phase 2 trial [44] provide a demonstration of the possible benefits of fMRI involvement in the drug development process. Incorporating this new technology may result in more successful clinical trials and, potentially, the development of new drugs that will alleviate the widespread suffering caused by psychiatric diseases.
Acknowledgments
This work was supported by the Veterans Administration Biomedical Laboratory and Clinical Science Research and Development Service, the National Institutes of Health grants R01MH102224, R01DK103691, K01DK100445 and the Brain and Behavior Research Foundation.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
Send Orders for Reprints to reprints@benthamscience.ae
References
- 1.World Health Organization. Integrating mental health into primary care: a global perspective. Geneva: WHO Press; 2008. [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Bowie CR, Leung WW, Reichenberg A, et al. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63:505–11. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152–63. doi: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- 6.Abbott A. The drug deadlock. Nature. 2010;468:158–9. doi: 10.1038/468158a. [DOI] [PubMed] [Google Scholar]
- 7.Akil H, Brenner S, Kandel E, et al. The future of psychiatric research: genomes and neural circuits. Science. 2010;327:1580–1. doi: 10.1126/science.1188654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142. [DOI] [PubMed] [Google Scholar]
- 9.DiMasi JA, Feldman L, Seckler A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin Pharmacol Ther. 2010;87(3):272–7. doi: 10.1038/clpt.2009.295. [DOI] [PubMed] [Google Scholar]
- 10.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13(10):1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunnage ME. Getting pharmaceutical R&D back on target. Nat Chem Biol. 2011;7:335–9. doi: 10.1038/nchembio.581. [DOI] [PubMed] [Google Scholar]
- 12.Kaitin KI, DiMasi JA. Pharmaceutical innovation in the 21st century: new drug approvals in the first decade, 2000–2009. Clin Pharmacol Ther. 2011;89(2):183–8. doi: 10.1038/clpt.2010.286. [DOI] [PubMed] [Google Scholar]
- 13.Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov. 2006;5(5):411–24. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- 14.Wise RG, Preston C. What is the value of human FMRI in CNS drug development? Drug Discov Today. 2010;15(21–22):973–80. doi: 10.1016/j.drudis.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131–7. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarz AJ, Becerra L, Upadhyay J, Anderson J. A procedural framework for good imaging practice in pharmacological fMRI studies applied to drug development# 1: processes and requirements. Drug Discov Today. 2011;16(13/14):583–93. doi: 10.1016/j.drudis.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Insel TR, Voon V, Nye JS, Brown VJ. Innovative solutions to novel drug development in mental health. Neurosci Biobehav Rev. 2013;37:2438–44. doi: 10.1016/j.neubiorev.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pankevich DE, Altevogt BM, Dunlop J, Gage FH, Hyman SE. Improving and accelerating drug development for nervous system disorders. Neuron. 2014;84(3):546–53. doi: 10.1016/j.neuron.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (February 1996 Final), SCID-I/P. NY: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- 20.Lieberman JA, Stroup TS, McEvoy JP. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 21.Lasagna L. The psychophysics of clinical pain. The Lancet. 1962;280(7256):572–5. doi: 10.1016/s0140-6736(62)90445-2. [DOI] [PubMed] [Google Scholar]
- 22.Borsook D, Becerra L. Functional imaging of pain and analgesia--a valid diagnostic tool? Pain. 2005;117(3):247–50. doi: 10.1016/j.pain.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Schweinhardt P, Bountra C, Tracey I. Pharmacological FMRI in the development of new analgesic compounds. NMR Biomed. 2006;19(6):702–11. doi: 10.1002/nbm.1076. [DOI] [PubMed] [Google Scholar]
- 24.Wise RG, Rogers R, Painter D, et al. Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. NeuroImage. 2002;16(4):999–1014. doi: 10.1006/nimg.2002.1146. [DOI] [PubMed] [Google Scholar]
- 25.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368(15):1388–97. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank R, Hargreaves R. Clinical biomarkers in drug discovery and development. Nat Rev Drug Discov. 2003;2(7):566–80. doi: 10.1038/nrd1130. [DOI] [PubMed] [Google Scholar]
- 27.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 28.Tregellas JR. Neuroimaging biomarkers for early drug development in schizophrenia. Biol Psychiatry. 2014;76(2):111–9. doi: 10.1016/j.biopsych.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143(8):993–7. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 30.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284(20):2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 31.Winterer G. Why do patients with schizophrenia smoke? Curr Opin Psychiatry. 2010;23(2):112–9. doi: 10.1097/YCO.0b013e3283366643. [DOI] [PubMed] [Google Scholar]
- 32.Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24(2):189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- 33.Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29:1021–34. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Leonard S, Mexal S, Freedman R. Smoking, Genetics and schizophrenia: evidence for self medication. J Dual Diagn. 2007;3(3–4):43–59. doi: 10.1300/J374v03n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonard S, Freedman R. Genetics of chromosome 15q13-q14 in schizophrenia. Biol Psychiatry. 2006;60(2):115–22. doi: 10.1016/j.biopsych.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 36.Bencherif M, Stachowiak MK, Kucinski AJ. Alpha7 nicotinic cholinergic neuromodulation may reconcile multiple neurotransmitter hypotheses of schizophrenia. Med Hypotheses. 2012;78:594–600. doi: 10.1016/j.mehy.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 37.Huang M, Felix AR, Kwon S, et al. The alpha-7 nicotinic receptor partial agonist/5-HT3 antagonist RG3487 enhances cortical and hippocampal dopamine and acetylcholine release. Psychopharmacology. 2014;231(10):2199–210. doi: 10.1007/s00213-013-3373-5. [DOI] [PubMed] [Google Scholar]
- 38.Huang M, Felix AR, Flood DG, et al. The novel α7 nicotinic acetylcholine receptor agonist EVP-6124 enhances dopamine, acetylcholine, and glutamate efflux in rat cortex and nucleus accumbens. Psychopharmacology. 2014;231(23):4541–51. doi: 10.1007/s00213-014-3596-0. [DOI] [PubMed] [Google Scholar]
- 39.Kucinski A, Syposs C, Wersinger S, Bencherif M, Stachowiak MK, Stachowiak EK. α7 neuronal nicotinic receptor agonist (TC-7020) reverses increased striatal dopamine release during acoustic PPI testing in a transgenic mouse model of schizophrenia. Schizophr Res. 2012;136(1–3):82–7. doi: 10.1016/j.schres.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Freedman R, Adams CE, Leonard S. The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat. 2000;20(3–4):299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 41.Court J, Spurden D, Lloyd S, et al. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999;73(4):1590–7. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- 42.Marutle A, Zhang X, Court J, et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat. 2001;22(1–2):115–26. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- 43.Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10(8):1779–82. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- 44.Freedman R, Olincy A, Buchanan RW, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165(8):1040–7. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy DL, Holzman PS, Matthysse S, Mendell NR. Eye tracking dysfunction and schizophrenia: a critical perspective. Schizophr Bull. 1993;19(3):461–536. doi: 10.1093/schbul/19.3.461. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg DR, Sweeney JA, Squires-Wheeler E, Keshavan MS, Cornblatt BA, Erlenmeyer-Kimling L. Eye-tracking dysfunction in offspring from the New York High-Risk Project: diagnostic specificity and the role of attention. Psychiatry Res. 1997;66(2–3):121–30. doi: 10.1016/s0165-1781(96)02975-7. [DOI] [PubMed] [Google Scholar]
- 47.Ross RG, Olincy A, Mikulich SK, et al. Admixture analysis of smooth pursuit eye movements in probands with schizophrenia and their relatives suggests gain and leading saccades are potential endophenotypes. Psychophysiology. 2002;39(6):809–19. doi: 10.1111/1469-8986.3960809. [DOI] [PubMed] [Google Scholar]
- 48.Olincy A, Ross RG, Young DA, Roath M. Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacology. 1998;18(3):175–84. doi: 10.1016/S0893-133X(97)00095-X. [DOI] [PubMed] [Google Scholar]
- 49.Avila MT, Sherr JD, Hong E, Myers CS, Thaker GK. Effects of nicotine on leading saccades during smooth pursuit eye movements in smokers and nonsmokers with schizophrenia. Neuropsychopharmacology. 2003;28(12):2184–91. doi: 10.1038/sj.npp.1300265. [DOI] [PubMed] [Google Scholar]
- 50.Ross DE, Thaker GK, Holcomb HH, Cascella NG, Medoff DR, Tamminga CA. Abnormal smooth pursuit eye movements in schizophrenic patients are associated with cerebral glucose metabolism in oculomotor regions. Psychiatry Res. 1995;58(1):53–67. doi: 10.1016/0165-1781(95)02724-b. [DOI] [PubMed] [Google Scholar]
- 51.Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry. 2004;161(2):315–21. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- 52.Molina V, Sanz J, Sarramea F, Benito C, Palomo T. Prefrontal atrophy in first episodes of schizophrenia associated with limbic metabolic hyperactivity. J Psychiatr Res. 2005;39(2):117–27. doi: 10.1016/j.jpsychires.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Holt DJ, Weiss AP, Rauch SL, et al. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry. 2005;57(9):1011–9. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 54.Schobel SA, Chaudhury NH, Khan UA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167(10):1178–93. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 56.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–8. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 57.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2014;162(12):2233–45. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 58.Tregellas JR, Tanabe JL, Martin LF, Freedman R. FMRI of response to nicotine during a smooth pursuit eye movement task in schizophrenia. Am J Psychiatry. 2005;162(2):391–3. doi: 10.1176/appi.ajp.162.2.391. [DOI] [PubMed] [Google Scholar]
- 59.Tanabe J, Tregellas JR, Martin LF, Freedman R. Effects of nicotine on hippocampal and cingulate activity during smooth pursuit eye movement in schizophrenia. Biol Psychiatry. 2006;59(8):754–61. doi: 10.1016/j.biopsych.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Tregellas JR, Olincy A, Johnson L, Tanabe J. Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology. 2010;35(4):938–42. doi: 10.1038/npp.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greicius MD, Kiviniemi V, Tervonen O, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29(7):839–47. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci (Regul Ed) 2010;14(6):277–90. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Smucny J, Tregellas J. Nicotinic modulation of intrinsic brain networks in schizophrenia. Biochem Pharmacol. 2013;86(8):1163–72. doi: 10.1016/j.bcp.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smucny J, Wylie KP, Tregellas JR. Functional magnetic resonance imaging of intrinsic brain networks for translational drug discovery. Trends Pharmacol Sci. 2014;35(8):397–403. doi: 10.1016/j.tips.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 66.Upadhyay J, Baker SJ, Chandran P, et al. Default-mode-like network activation in awake rodents. PLoS One. 2011;6(11):e27839. doi: 10.1371/journal.pone.0027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci USA. 2009;106(14):5948–53. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106(4):1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garrity AG, Pearlson GD, McKiernan K. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164(3):450–7. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 70.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 71.Alonso-Solís A, Corripio I, de Castro-Manglano P, et al. Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophr Res. 2012;139(1–3):13–8. doi: 10.1016/j.schres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guerrero-Pedraza A, McKenna PJ, Gomar JJ, et al. First-episode psychosis is characterized by failure of deactivation but not by hypo- or hyperfrontality. Psychol Med. 2012;42(1):73–84. doi: 10.1017/S0033291711001073. [DOI] [PubMed] [Google Scholar]
- 73.Tregellas JR, Tanabe J, Rojas DC, et al. Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia. Biol Psychiatry. 2011;69(1):7–11. doi: 10.1016/j.biopsych.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sambataro F, Blasi G, Fazio L, et al. Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology. 2010;35(4):904–12. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cole DM, Oei NYL, Soeter RP, Both S, et al. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cerebr Cortex. 2013;23(7):1509–16. doi: 10.1093/cercor/bhs136. [DOI] [PubMed] [Google Scholar]
- 76.Stephens SH, Logel J, Barton A, et al. Association of the 5′-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophr Res. 2009;109(1–3):102–12. doi: 10.1016/j.schres.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abbott C, Juárez M, White T, et al. Antipsychotic dose and diminished neural modulation: a multi-site fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):473–82. doi: 10.1016/j.pnpbp.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nawata S, Yamauchi K, Ikegami N. Factors related to prescription dosage in Japanese psychiatric hospitals. Psychiatr Clin Neurosci. 2005;59(1):70–6. doi: 10.1111/j.1440-1819.2005.01334.x. [DOI] [PubMed] [Google Scholar]
- 79.Fujimaki K, Takahashi T, Morinobu S. Association of typical versus atypical antipsychotics with symptoms and quality of life in schizophrenia. PLoS One. 2012;7(5):e37087. doi: 10.1371/journal.pone.0037087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proc Natl Acad Sci USA. 2012;109(10):3979–84. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]