Abstract
Objective
We examined the relation between aerobic fitness and inhibition in young children with and without ADHD-risk status.
Method
Participants (91 ADHD-risk, 107 typically-developing, Mage = 6.83, 53.5% male, and 68.2% Caucasian) completed an assessment of aerobic fitness and a flanker task requiring variable amounts of inhibitory control.
Results
Aerobic fitness was positively associated with inhibition. When inhibitory control demands were largest, the relation varied as a function of ADHD-risk status such that the link between aerobic fitness and inhibition was only significant for children with ADHD-risk. The relation between aerobic fitness, status, and inhibition was further moderated by age for interference control. Specifically, the positive relation between aerobic fitness and interference control was only significant for younger children with ADHD-risk.
Conclusion
A fitness–cognition link appears in young childhood that seems particularly salient for those in the earliest school years with ADHD-risk. The findings extend work on typically-developing children and suggest that exploring aerobic fitness interventions to address executive function impairments in children at risk for ADHD is warranted.
Keywords: fitness, ADHD, inhibition, executive function
Attention Deficit Hyperactivity Disorder (ADHD) is a highly prevalent neurobiological disorder, characterized by developmentally inappropriate presentations of inattention and/or hyperactivity/impulsivity (American Psychiatric Association, 2000, 2013). Individuals with ADHD typically experience significant impairment in multiple domains (American Psychiatric Association, 2000, 2013), including poor academic achievement (Frazier, Youngstrom, Glutting, & Watkins, 2007), grade retention (Loe & Feldman, 2007), and social maladjustment (Hoza, 2007). In addition to these impairments, a large proportion of children with ADHD exhibit one or more executive function deficits (Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005). Children possessing both ADHD and executive function deficits exhibit greater impairment and poorer outcomes than children with ADHD alone (Biederman et al., 2004). Therefore, understanding factors that may mitigate executive function deficits in children at-risk for ADHD is important. Research conducted with typically-developing children shows a positive association between aerobic fitness and executive function (Hillman, Buck, Themanson, Pontifex, & Castelli, 2009); however, there is a dearth of research investigating this association in children with elevated symptoms of ADHD.
Inhibition deficits are reliably observed in children with elevated ADHD symptoms (Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Inhibition is a component of executive function involving the capacity to suppress a dominant response (i.e., response suppression) and ignore competing information (i.e., interference control) in order to engage in an appropriate manner (Mullane, Corkum, Klein, & McLaughlin, 2009). When compared to typically-developing peers, children with ADHD consistently demonstrate poorer performance on computerized tasks of inhibition, including decreased reponse accuracy (Rubia, Smith, & Taylor, 2007; Wiersema, van der Meere, Roeyers, Van Coster, & Baeyens, 2006; Wodka et al., 2007) and poorer interference control (Mullane et. al, 2009). These impairments in inhibition are associated with increased hyperactive and inattentive behavior (Pliszka, Borcherding, Spratley, Leon, & Irick, 1997), poorer emotion regulation (Walcott & Landau, 2004), and academic difficulties (Biederman et al., 2004).
Researchers have observed a positive association between aerobic fitness and inhibitory aspects of executive functioning (Hillman, Erickson, & Kramer, 2008). Aerobic fitness is the ability to sustain aerobic physical activities and describes the physiological limit to the rate at which an individual can deliver and consume oxygen (Rowland, 1996). In typically developing 8 – 11 year old children, higher aerobic fitness is associated with better inhibition, including less variability in reaction time on an inhibitory control task (Wu et al., 2011). Moreover, in another typically developing sample (Mage = 9.4), higher fit children had superior allocation of attentional resources and better interference control in comparison to lower fit counterparts (Hillman et al., 2009). To date, little research has investigated the extent to which aerobic fitness may be beneficial for children with elevated ADHD symptoms.
Among the few studies examining this possible connection, Smith and colleagues (2013) implemented an eight-week, 26 minute/day moderate-to-vigorous physical activity intervention with young children (grades K-3) at-risk for ADHD and participants significantly improved on an inhibition task. Interestingly, other work has shown children ages 8 to 10 years with ADHD improve in inhibition performance even after a single bout of physical activity (Pontifex, Saliba, Raine, Picchietti, & Hillman, 2013). Because physical activity positively contributes to aerobic fitness levels (Rowlands, Eston, & Ingledew, 1999), these findings suggest that children with ADHD who are more aerobically fit may exhibit better inhibition than those who are less aerobically fit. Moreover, researchers have posited that children with executive function-related deficits may benefit more from executive function interventions than typically-developing peers (Diamond & Lee, 2011). Thus, the relation between aerobic fitness and inhibition may be stronger for children with elevated ADHD symptoms because they are more likely to experience executive function deficits as compared to their typically-developing peers. The present study examines this hypothesis.
Most work examining the relation between aerobic fitness and inhibition has targeted middle childhood and beyond (Hillman et al., 2009; Pontifex et al., 2013; Wu et al., 2011). However, it may be especially important to examine these relations during earlier phases of development when there is greater neuroplasticity (Mannuzza et al., 2008). Accordingly, the aim of this investigation was to examine the relation between aerobic fitness and inhibition in a sample of young children with and without risk of developing ADHD. Based on extant literature, we hypothesized that aerobic fitness would positively associate with inhibition performance and that this association would be stronger for children at-risk for ADHD than typically-developing children. We expected age to further moderate this association, such that aerobic fitness would be more beneficial at younger ages, especially when at-risk for ADHD.
Method
Participants
Data were taken from the baseline assessment of a larger physical activity intervention study on children with and without ADHD-risk (see Hoza et al., 2014). Young children were recruited from participating schools within two small suburban U.S. cities. Four participants in the larger study were missing data required for the present work, leaving 198 children (91 ADHD-risk, 107 typically-developing) for the present analyses who ranged in age from 4 to 8 years (Mage = 6.83, SDage = .97). The sample was 53.5% male, racially and ethnically diverse (68.2% Caucasian, 14.1% mixed race, 8.1% African American, 2.0% Asian, and 7.6% other races), and came from homes with at least one parent possessing some post-secondary education (74.0%).
Eligibility and status assignments were determined through a two-step screening. First, parents and teachers completed the ADHD-IV Rating Scale, a normed measure of DSM-IV ADHD symptoms (DuPaul, Power, Anastopoulos, & Reid, 1998). Second, parents completed the ADHD module of the National Institute of Mental Health Diagnostic Interview Schedule for Children, Version IV (DISC-IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000), an interviewer-administered structured clinical interview that assesses child psychiatric diagnoses. Additionally, parents and teachers completed the Impairment Rating Scale, a measure of child impairment across multiple domains (Fabiano et al., 2006).
ADHD-Risk Participants
Participants at or above the 90th percentile on the hyperactive/impulsive or total subscales of the ADHD-IV Rating Scales by parent or teacher report were eligible for secondary screening as a participant with ADHD-risk. To qualify as ADHD-risk, at the secondary screening participants had to exhibit the following attributes: (1a) Five or more symptoms of hyperactivity/impulsivity by parent report on the DISC-IV. (1b) If participants failed to meet five endorsed symptoms by parent report on the DISC-IV, similar to the methods used in the Multimodal Treatment Study of Children with ADHD (Hinshaw et al., 1997), up to two unique hyperactive/impulsive symptoms by teacher report on the ADHD-IV Rating Scale could be used to attain the required symptom count. (2) Impairment in two or more domains by parent and/or teacher report on the Impairment Rating Scale or by parent report on the DISC-IV. Our emphasis on hyperactivity/impulsivity and selection of a subdiagnostic threshold of five symptoms was based on our focus on young children and developmental considerations in diagnosing ADHD (see Barkley, 2003).
Typically-Developing Participants
Participants below the 90th percentile on the hyperactive/impulsive, inattentive, and total subscales of the ADHD-IV Rating Scales by parent and teacher reports were eligible for secondary screening as typically-developing (TD) participants. To qualify as TD, at the secondary screening participants had to exhibit four or fewer symptoms of hyperactivity/impulsivity and inattention by parent report on the DISC-IV.
Exclusion criteria were: (a) standardized scores less than 78 (i.e., 1.5 standard deviations below the mean) on non-verbal, verbal, and total IQ assessments from the Kaufman Brief Intelligence Test, Second Edition (Kaufman & Kaufman, 2004); (b) parent report of diagnosis of a pervasive developmental disorder, current seizure disorder, or intellectual disability; (c) taking medication for attentional or behavioral problems (which could impact cognitive assessment performance); (d) unable to participate in physical activity; (e) residing with current caretaker less than 6-months; and (f) no English-speaking caretaker with telephone access.
Considering all sources of symptom reporting, the mean numbers of unique symptoms endorsed were significantly higher (ps < .001) for the ADHD-risk group [hyperactivity/impulsivity symptoms: 8.5 (SD = 0.8); inattentive symptoms: 8.3 (SD = 1.3)] than those for the TD group [hyperactivity/impulsivity symptoms 2.4 (SD = 2.1); inattentive symptoms: 2.3 (SD = 2.4)].
Measures
Child Attentional Network Task (ANT)
The ANT (Rueda et al., 2004) is an individually administered computerized task that assesses inhibition. Participants respond to the direction of a centrally located target fish that is presented either individually or amid an array of distractor fish on each side. Participants indicate the direction of the target fish by pressing one of two buttons on a response pad corresponding to left or right. The task consists of congruent (target fish in the same direction of distractor fish), incongruent (target fish in the opposite direction of the distractor fish), and neutral trials (target fish presented individually). A 24 trial practice block was completed, followed by six blocks of 48 trials. Equal numbers of congruent, incongruent, and neutral trials appeared randomly within each block. The target and distractor fish were 3-cm-tall and were presented for 1700 ms followed by an interstimulus interval of 450 ms. Congruent and incongruent response accuracy as well as interference control (congruent response accuracy – incongruent response accuracy) were evaluated. The incongruent trials, relative to the congruent and neutral trials, result in perceptually-induced interference necessitating concurrent activation of both the correct response (elicited by the target fish) and the incorrect response (elicited by the distractor fish) before stimulus evaluation is complete. Thus, incongruent trials require greater amounts of response inhibition to execute the correct response (Spencer & Coles, 1999). The subtraction of incongruent response accuracy from congruent response accuracy isolates the interference control component of inhibition (Mullane et. al, 2009). Because individuals do not realistically benefit from increased task interference, negative interference scores (where incongruent response accuracy is greater than congruent response accuracy) were set to equal zero. Forns and colleagues (2014) support the validity and objectivity of the ANT with children.
Progressive Aerobic Cardiovascular Endurance Run (PACER)
The 15-meter version of the PACER (McClain, Welk, Ihmels, & Schaben, 2006) was administered in groups to assess aerobic capacity. Participants run back and forth at a specified pace (8.5 km/h, quickening by 0.5 km each minute) across a 15-meter area with lines at each end. A trial is completed when participants run from one end line to the other before the sound of a pace-keeping beep. When participants reach the line, they wait for the beep prior to running the next trial in the opposite direction. Each minute is designated by the sound of a triple beep to cue that the pace will quicken. When a participant fails to reach the end line by the beep, that trial is considered a miss. The trial number prior to the second miss (consecutive or nonconsecutive) is used as the final score. PACER scores demonstrate good reliability and validity in children and adolescents (Plowman & Meredith, 2013).
Procedures
Within participating schools, all families with children in grades K-2 were invited to participate. A letter and consent documents, along with the ADHD-IV rating scale, was sent home and returned by interested families. For consenting families, teachers completed the school version of the ADHD-IV about their child and a baseline screening session was scheduled if initial inclusion criteria were met. Families were compensated $50 for completing the secondary screening. During this session, which included collection of demographic information, the computerized ANT was administered individually to children by research assistants masked to ADHD-risk status. On a different day, in order to control for acute physical activity effects on inhibition, the PACER was administered in groups.
Data Analysis Strategy
Hierarchical multiple regression analyses were used to examine study hypotheses. Aerobic fitness, ADHD-risk status (i.e., ADHD-risk or TD), and participant age were entered in the first step of the model predicting inhibition. In the second step of the model, three two-way interactions were entered. Finally, to determine whether the relation between aerobic fitness, ADHD-risk status, and inhibition further varied as a function of age, a three-way interaction term (Fitness x Status x Age) was entered in the final step of the model. Following the method outlined by Aiken and West (1991), all continuous predictors in the model were mean-centered at entry into the model and ADHD-risk status was weight effect coded (ADHD-risk = −.54, TD = .46). When the hypothesized three-way interaction was not significant, a simplified model was examined including only the main effects and the hypothesized two-way Fitness x Status interaction. Significant interactions were decomposed following procedures outlined by Aiken and West (1991) and for the continuous moderator (i.e., participant age) simple slopes were computed at low (−1 SD) and high (+1 SD) levels. The Dawson and Richter (2006) online utility was used to plot simple slopes and conduct slope differences tests for three-way interactions.
Results
Preliminary Analyses
Means, standard deviations, and intercorrelations among study variables are presented in Table 1. As expected, both higher levels of aerobic fitness and being older were associated with better inhibition (i.e., higher levels of congruent and incongruent response accuracy and lower levels of interference control). Bivariate associations among ADHD-risk status and the response accuracy measures indicated TD participants were more accurate in their responses than ADHD-risk participants (congruent response accuracy: p = .01; incongruent response accuracy: p = .01). Unexpectedly, ADHD-risk status was not significantly correlated with interference control (p = .13).
Table 1.
Means, Standard Deviations, and Pearson’s Correlations among Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | M | SD | Range |
|---|---|---|---|---|---|---|---|---|---|
| 1. Aerobic fitness | -- | 12.12 | 4.39 | 2.00 – 32.00 | |||||
| 2. ADHD-risk status (ADHD-risk = 0; TD = 1) | .15* | -- | .54 | .50 | 0.00 – 1.00 | ||||
| 3. Participant age | .26*** | −.01 | -- | 6.83 | .97 | 4.44 – 8.90 | |||
| 4. Congruent response accuracy | .32*** | .18* | .39*** | -- | 87.59 | 12.40 | 35.42 – 100.00 | ||
| 5. Incongruent response accuracy | .31*** | .19** | .45*** | .77*** | -- | 78.06 | 19.06 | 14.58 – 100.00 | |
| 6. Interference control | −.17* | −.11 | −.34*** | −.23** | −.79*** | -- | 10.16 | 11.67 | 0.00 – 66.67 |
Notes. TD = typically developing;
p < .05,
p < .01,
p < .001.
Hierarchical Regression Analyses
Results for the initial models are presented in Table 2. For congruent response accuracy, the three-way interaction between aerobic fitness, ADHD-risk status, and age was not significant, p = .21. Moreover, the simplified model examining if the association between aerobic fitness and congruent response accuracy varied as a function of ADHD-risk status did not yield a significant two-way interaction, b = −.34, p = .37.
Table 2.
Initial Models Examining the Three-way Interaction of Aerobic Fitness, ADHD-risk Status, and Age Predicting Inhibition
| Outcome
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Congruent response accuracy | Incongruent response accuracy | Interference control | ||||||||
|
| ||||||||||
| Step | Variable | b | t | ΔR2 | b | t | ΔR2 | b | t | ΔR2 |
| 1 | Aerobic Fitness | .60 | 3.21** | .23 | .80 | 2.86** | .27 | −.19 | −1.04 | .14 |
| ADHD-risk Status | 3.84 | 2.43* | 6.23 | 2.63** | −2.35 | −1.49 | ||||
| Age | 4.35 | 5.19*** | 8.03 | 6.41*** | −3.93 | −4.70*** | ||||
| 2 | Fitness x Status | −.08 | −.21 | .02 | −1.15 | −1.93 | .03 | .98 | 2.46* | .03 |
| Fitness x Age | −.48 | −2.21* | −.48 | −1.46 | .02 | .07 | ||||
| Status x Age | −.47 | −.27 | −.70 | −.28 | −.05 | −.03 | ||||
| 3 | Fitness x Status x Age | −.55 | −1.26 | .01 | .51 | .77 | .00 | −.96 | −2.21* | .02 |
Notes. bs are unstandardized coefficients at the predictor’s entry into the equation;
p < .05,
p < .01,
p < .001.
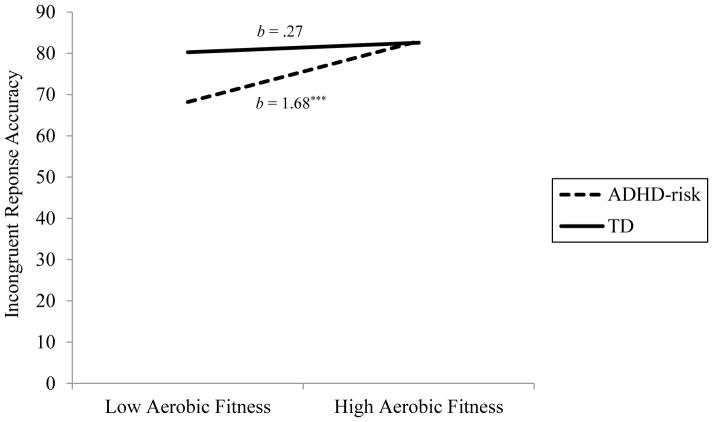
The three-way interaction predicting incongruent response accuracy was not significant, p = .44 (see Table 2). However, the simplified model showed the association of aerobic fitness with incongruent response accuracy to vary by ADHD-risk status, b = −1.41, p = .01, ΔR2 = .02. Simple slope analyses indicated that for ADHD-risk participants, better aerobic fitness associated with better inhibition (i.e., higher incongruent response accuracy scores p < .001, whereas this association was not significant for TD participants, p = .44 (see Figure 1).
Figure 1.
Two-way interaction of aerobic fitness and ADHD-risk status predicting incongruent response accuracy. Note. *** p < .001.
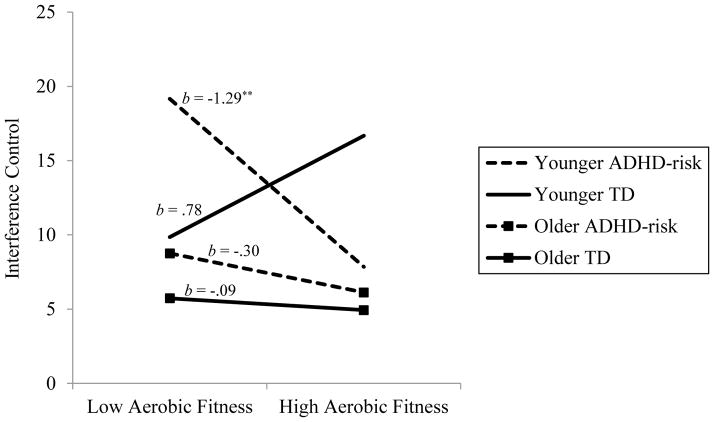
For interference control, the three-way interaction between aerobic fitness, ADHD-risk status, and age was significant (see Table 2). Simple slope analyses indicated that the association between aerobic fitness and interference control was only significant for younger, ADHD-risk status participants (p = .002), such that better aerobic fitness associated with better inhibition (i.e., lower scores on the interference control measure; see Figure 2). The magnitude of this association was significantly different from the same non-significant associations for younger, t(190) = 3.28, p = .001, and older, t(190) = 2.32, p = .02, TD participants, but not significantly different from the non-significant association for older, ADHD-risk participants, t(190) = 1.64, p = .10. In addition, the difference between the slopes for the younger and older TD participants was not significant, t(190) = −1.49, p = .14.
Figure 2.
Three-way interaction of aerobic fitness, ADHD-risk status, and age predicting interference control. Note. ** p < .01.
Discussion
We examined the association between aerobic fitness and inhibition in a sample of young children with and without risk for developing ADHD. As expected, bivariate correlations revealed that higher levels of aerobic fitness were associated with better overall performance across conditions of the flanker task. These results correspond with previous findings that higher-fit typically-developing children demonstrate superior performance than less-fit peers (Hillman et al., 2009; Wu et al., 2011). The present study enriches the knowledge base by extending this work to a sample of young children with and without ADHD-risk.
Our results suggest that the relation between aerobic fitness and inhibition (as measured by incongruent response accuracy) varies as a function of ADHD-risk status. Specifically, aerobic fitness and incongruent response accuracy were positively associated only for children identified as ADHD-risk. These findings support Diamond and Lee’s (2011) assertion that fitness may be more beneficial for individuals with known executive function deficits, such as children with ADHD-risk, than those without such deficits. Diamond and Lee further contend that engaging in activities that facilitate executive function development (e.g., improving aerobic fitness) during early childhood can reduce the gap between typically-developing children and those with deficient executive function. Our findings support this contention in that children of higher fitness had similar levels of incongruent response accuracy regardless of ADHD-risk status, whereas those with ADHD-risk and lower fitness appear to have an inhibition deficit.
Importantly, a significant three-way interaction between fitness, status, and age was observed for interference control. The association between aerobic fitness and interference control was only significant for younger children with ADHD-risk, for whom higher levels of aerobic fitness were associated with better interference control. For younger children with ADHD-risk, the magnitude of the association between fitness and interference control was significantly different than the association for both younger and older typically-developing children. However, the magnitude of this association for younger children with ADHD-risk was not statistically different from older children with ADHD-risk. Given findings that mechanisms promoting brain development (e.g., aerobic fitness) may have a greater impact in earlier stages of development when neuroplasticity is greater (Mundkur, 2005), it is possible that aerobic fitness is beneficial for interference control in both younger and older children with ADHD-risk; however, the effects in the current study were only apparent in younger children.
Interactive effects were not observed in the congruent task condition. The congruent task condition involves limited interference and thus places fewer demands on executive function and frontal lobe systems. As researchers have speculated that executive function deficits in children with ADHD result from frontal lobe abnormalities (Krain & Castellanos, 2006), it is possible that when little demand is placed upon the frontal lobe systems, children with ADHD-risk can perform similarly to typically-developing peers. In contrast, when greater demands are placed on the frontal lobe systems, children with ADHD-risk are likely to exhibit difficulty in accurate task completion, and thus benefit from the increased frontal lobe functioning associated with aerobic fitness (Voss et al., 2011). Therefore, the impact of aerobic fitness on inhibition is possibly most apparent on tasks making significant demands on the frontal lobe and executive function systems.
These promising findings must be interpreted in light of study limitations. First, our study was cross-sectional. Future research utilizing longitudinal designs to better understand how fitness may differentially influence inhibition development in children with ADHD or ADHD-risk relative to typically-developing children is necessary. Second, although our sample was fairly representative and diverse for the geographic regions involved (approximately 32% non-White), there were insufficient numbers of participants within non-White racial subgroups to pursue separate analyses by race/ethnicity. Third, our sample consisted of children at-risk for, but not necessarily diagnosed with, ADHD. Thus, it is difficult to know whether our results will generalize to children who meet full diagnostic criteria for ADHD or those with more severe symptomatology. Finally, our findings stem from a lab-based cognitive task. Replication employing ecologically valid assessments of inhibition is warranted.
Conclusion
Regardless of ADHD-risk status, children who were more aerobically fit exhibited better inhibition on a flanker task as measured by the congruent task condition. When greater demands on executive function were required, the relation between aerobic fitness and inhibition varied as a function of ADHD-risk status. Specifically, better aerobic fitness was associated with better inhibition for children with ADHD-risk, such that inhibition appeared normalized. This association was especially salient for inference control in the younger participants in the sample. Taken together, these findings highlight the potential for aerobic fitness intervention as a pathway for addressing executive functioning impairment in children in the earliest school years who possess ADHD-risk.
Acknowledgments
This research was supported primarily by grant number R01MH082893 from the National Institute of Mental Health to Betsy Hoza and John T. Green. This research was supported in part by the United States Department of Health and Human Services (USDHHS), Administration on Developmental Disabilities (ADD), grant award #90DD0645 to the Center on Disability and Community Inclusion, University of Vermont.
Footnotes
The views expressed in this paper are solely those of the authors, and do not necessarily reflect the views of the National Institute of Mental Health, the USDHHS, or the ADD and no official endorsement should be inferred.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Arlington, VA: American Psychiatric Publishing; 2000. text rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Barkley RA. Issues in the diagnosis of attention-deficit/hyperactivity disorder in children. Brain and Development. 2003;25:77–83. doi: 10.1016/S0387-7604(02)00152-3. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, Faraone SV. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. Journal of Consulting and Clinical Psychology. 2004;72:757–766. doi: 10.1037/0022-006X.72.5.757. [DOI] [PubMed] [Google Scholar]
- Dawson JF, Richter AW. Probing three-way interactions in moderated multiple regression: Development and application of a slope difference test. Journal of Applied Psychology. 2006;91:917–926. doi: 10.1037/0021-9010.91.4.917. [DOI] [PubMed] [Google Scholar]
- Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale IV: Checklists, Norms, and Clinical Interpretation. New York, NY: The Guilford Publications; 1998. [Google Scholar]
- Fabiano GA, Pelham WE, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, Burrows-MacLean L. A practical measure of impairment: Psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. Journal of Clinical Child and Adolescent Psychology. 2006;35:369–385. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Forns J, Esnaola M, López-Vicente M, Suades-González E, Alvarez-Pedrerol M, Julvez J, Sunyer J. The n-back test and the attentional network task as measures of child neuropsychological development in epidemiological studies. Neuropsychology. 2014;28:519–529. doi: 10.1037/neu0000085. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Glutting JJ, Watkins MW. ADHD and achievement meta-analysis of the child, adolescent, and adult literatures and a concomitant study with college students. Journal of Learning Disabilities. 2007;40:49–65. doi: 10.1177/00222194070400010401. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Buck SM, Themanson JR, Pontifex MB, Castelli DM. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Developmental Psychology. 2009;45:114–129. doi: 10.1037/a0014437. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, March JS, Abikoff H, Arnold LE, Cantwell DP, Conners CK, Hechtman LT. Comprehensive assessment of childhood attention-deficit hyperactivity disorder in the context of a multisite, multimodal clinical trial. Journal of Attention Disorders. 1997;1:217–234. doi: 10.1177/108705479700100403. [DOI] [Google Scholar]
- Hoza B. Peer functioning in children with ADHD. Journal of Pediatric Psychology. 2007;32(6):655–663. doi: 10.1093/jpepsy/jsm024. [DOI] [PubMed] [Google Scholar]
- Hoza B, Smith AL, Shoulberg EK, Linnea KS, Dorsch TE, Blazo JA, McCabe GP. A randomized trial examining the effects of aerobic physical activity on attention-deficit/hyperactivity disorder symptoms in young children. Journal of Abnormal Child Psychology. 2014 doi: 10.1007/s10802-014-9929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman brief intelligence test. 2. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clinical Psychology Review. 2006;26(4):433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. Journal of Pediatric Psychology. 2007;32(6):643–654. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein R, Truong N, Moulton J, Roizen E, Howell K, Castellanos F. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. American Journal of Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JJ, Welk GJ, Ihmels M, Schaben J. Comparison of two versions of the PACER aerobic fitness test. Journal of Physical Activity & Health. 2006;3(Suppl 2):S47–S57. [Google Scholar]
- Mullane JC, Corkum PV, Klein RM, McLaughlin E. Interference control in children with and without ADHD: a systematic review of Flanker and Simon task performance. Child Neuropsychology. 2009;15:321–342. doi: 10.1080/09297040802348028. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: Do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;517(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Mundkur N. Neuroplasticity in children. The Indian Journal of Pediatrics. 2005;72:855–857. doi: 10.1007/BF02731115. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Borcherding SH, Spratley K, Leon S, Irick S. Measuring inhibitory control in children. Journal of Developmental & Behavioral Pediatrics. 1997;18:254–259. [PubMed] [Google Scholar]
- Plowman SA, Meredith MD. Fitnessgram/Activitygram reference guide. 4. Dallas, TX: The Cooper Institute; 2013. [Google Scholar]
- Pontifex MB, Saliba BJ, Raine LB, Picchietti DL, Hillman CH. Exercise improves behavioral, neurocognitive, and scholastic performance in children with attention-deficit/hyperactivity disorder. The Journal of Pediatrics. 2013;162:543–551. doi: 10.1016/j.jpeds.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland TW. Developmental exercise physiology. Champaign, IL: Human Kinetics; 1996. [Google Scholar]
- Rowlands AV, Eston RG, Ingledew DK. Relationship between activity levels, aerobic fitness, and body fat in 8-to 10-yr-old children. Journal of Applied Physiology. 1999;86:1428–1435. doi: 10.1152/jappl.1999.86.4.1428. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A, Taylor E. Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychology. 2007;13:276–304. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, Posner MI. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Smith AL, Hoza B, Linnea K, McQuade JD, Tomb M, Vaughn AJ, Hook H. Pilot physical activity intervention reduces severity of ADHD symptoms in young children. Journal of Attention Disorders. 2013;17:70–82. doi: 10.1177/1087054711417395. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Coles MGH. The lateralized readiness potential: Relationship between human data and response activation in a connectionist model. Psychophysiology. 1999;36:364–370. doi: 10.1017/S0048577299970749. [DOI] [PubMed] [Google Scholar]
- Voss MW, Chaddock L, Kim JS, VanPatter M, Pontifex MB, Raine LB, Kramer AF. Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience. 2011;199:166–176. doi: 10.1016/j.neuroscience.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott CM, Landau S. The relation between disinhibition and emotion regulation in boys with attention deficit hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2004;33:772–782. doi: 10.1207/s15374424jccp3304_12. [DOI] [PubMed] [Google Scholar]
- Wiersema R, van der Meere J, Roeyers H, Van Coster R, Baeyens D. Event rate and event-related potentials in ADHD. Journal Child Psychology Psychiatry. 2006;47:560–567. doi: 10.1111/j.1469-7610.2005.01592.x. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, Larson JC, Fotedar S, Denckla MB, Mostofsky SH. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology. 2007;29:345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- Wu CT, Pontifex MB, Raine LB, Chaddock L, Voss MW, Kramer AF, Hillman CH. Aerobic fitness and response variability in preadolescent children performing a cognitive control task. Neuropsychology. 2011;25:333–341. doi: 10.1037/a0022167. [DOI] [PMC free article] [PubMed] [Google Scholar]