Abstract
Implantation of mechanical circulatory support (MCS) devices – ventricular assist devices and the total artificial heart – has emerged as a vital therapy for advanced and end-stage heart failure. Unfortunately, MCS patients face the requirement of life-long antiplatelet and anticoagulant therapy to combat thrombotic complications resulting from the dynamic and supraphysiologic shear stress conditions associated with such devices, whose effect on platelet activation is poorly understood. We developed a syringe-capillary viscometer – the “platelet hammer” – that repeatedly exposed platelets to average shear stresses up to 1000 dyne/cm2 for as short as 25 ms. Platelet activation state was measured using a modified prothrombinase assay, with morphological changes analyzed using scanning electron microscopy. We observed an increase in platelet activation state and post-high shear platelet activation rate, or sensitization, with an increase in stress accumulation (SA), the product of shear stress and exposure time. A significant increase in platelet activation state was observed beyond an SA of 1500 dyne-s/cm2, with a marked increase in pseudopod length visible beyond an SA of 1000 dyne-s/cm2. Utility of the platelet hammer extends to studies of other shear-dependent pathologies, and may assist development of approaches to enhance the safety and effectiveness of MCS devices and objective antithrombotic pharmacotherapy management.
Keywords: platelet activation, mechanical circulatory support, shear stress, thrombosis, antithrombotic agents
INTRODUCTION
Mechanical circulatory support (MCS) has emerged as an effective lifesaving therapy for patients with advanced and end-stage heart failure (1). The most recent INTERMACS database report reflects the growing use, efficacy and acceptability of MCS systems, with over 11,000 implants between 2006 and the last quarter of 2014 (2, 3). Attendant with the use of MCS systems is the clinical requirement for long-term antiplatelet and anticoagulant therapy to prevent device-related thrombosis. Despite concomitant antithrombotic drug use, some patients have potentially catastrophic thrombotic complications, including thrombus-induced pump failure and neurovascular events at a rate of 1 to 6% per patient year (4). These events are known to be initiated by platelet activation resulting from the dynamic and pathological fluid shear stress conditions in these devices (5). While several studies have attempted to determine what hemodynamic conditions are required to initiate and sustain platelet activation, examination of this behavior while mimicking the repeated short bursts of pathological shear stresses found in MCS devices is lacking.
Traditionally, closed-loop systems have been utilized, consisting of minimally reactive polymeric tubing with affixed MCS devices through which blood or platelet preparations are recirculated, to evaluate shear-mediated platelet activation and hemolysis (6-10). Over the years, a variety of in vitro devices have been developed or adapted to study platelet activation due to pathological shear stresses and exposure times, serving as proxies for in vitro flow loops containing devices or in vivo studies, to minimize blood or animal study requirements. Researchers initially used cone-and-plate viscometers (CPV), syringe pumps or compressed gas piston systems driving platelet-rich plasma (PRP) through capillary tubes, or coaxial Couette viscometers to measure serotonin release, platelet factor 3 availability, beta thromboglobulin release, shape change or LDH liberation (11-14). Microchannels and microcapillaries have been used for analysis of aggregation, adhesion, and thrombus formation at shear stresses approaching 1400 dyne/cm2 (15-19). Our group developed the Hemodynamic Shearing Device (HSD), a computer-controlled modified cone-plate-Couette viscometer, to emulate dynamic shear stresses along platelet flight trajectories in MCS devices, as extracted from computational fluid dynamics (CFD) simulations (20-22). However, these approaches have been limited by the trade-off between the dynamic flow conditions and the shear stress magnitudes mimicking those found in MCS devices (23). Furthermore, there is a poor understanding of how the shear dose (i.e. product of shear stress and exposure time) affects platelet response under such dynamic conditions (24). In addition, no group has utilized a closed-loop system that exposes platelets repeatedly to very high shear stresses and very brief exposure times, thus mimicking passages through a blood recirculating device or stenosis.
In the present study, we describe a recirculating syringe-capillary viscometer device able to achieve stress levels and doses greater than prior systems. Wall shear stresses generally range from 1 to 50 dyne/cm2 in healthy blood vessels (25), with stresses approaching 350 to 1400 dyne/cm2 in pathologies such as severe stenoses (19, 26). However, exogenous devices such as MCS systems generate shear stresses that exceed those observed in cardiovascular pathologies (9). For example, numerical simulations of the MicroMed HeartAssist 5 show that a typical passage through the VAD exposes platelets to peak shear stresses of 1000-2000 dyne/cm2, with overall passage time of 100-190 ms (27). The shear dose is highest in the impeller-shroud gap, with values of approximately 9.5 dyne-s/cm2 per passage. Our syringe-capillary viscometer has been developed to allow examination of the effect of shear stresses experienced in MCS systems at extreme levels – what we have termed “hypershear”. We hypothesized that platelets activate and change shape after single and repeated brief, high shear stress exposure. This exposure includes short bursts of average shear stresses up to 1000 dyne/cm2 (wall shear stress of 2000 dyne/cm2), with durations as short as 25 ms in a cycle. For some of the experiments, we also tested the hypothesis that platelets exposed to very high shear stress for brief exposures would continue to activate despite subsequent exposure to low shear stress, such as those found downstream of MCS devices and cardiovascular pathologies, in a process we previously termed as sensitization (28). This concept of platelet memory of prior shear stress exposure has been explored by prior platelet activation and aggregation studies, where continuing or residual platelet response was present during low shear stress exposure succeeding initial high shear pulses or waveforms (24, 29, 30). The developed “platelet hammer” provides both the academic and clinical arenas a potentially useful tool to measure platelet activation responses to disturbed dynamic flow conditions present in MCS devices and a variety of cardiovascular pathologies. This may allow further investigation of thrombotic risks and antithrombotic therapy choices in order to enhance overall patient safety.
MATERIALS AND METHODS
Platelet preparation
Whole blood (30 ml) from consenting healthy adult volunteers of both sexes who had not taken aspirin or ibuprofen for two weeks was drawn via venipuncture into 3 ml acid-citrate dextrose (ACD-A), in accordance with a Stony Brook University IRB-approved protocol. Purified gel-filtered platelets (GFP) were prepared as previously described and diluted to a count of 20,000/μl in HEPES-modified Tyrode’s buffer, with 3 mM CaCl2 added 10 minutes prior to experiments (28).
Syringe-capillary viscometer – “Platelet Hammer”
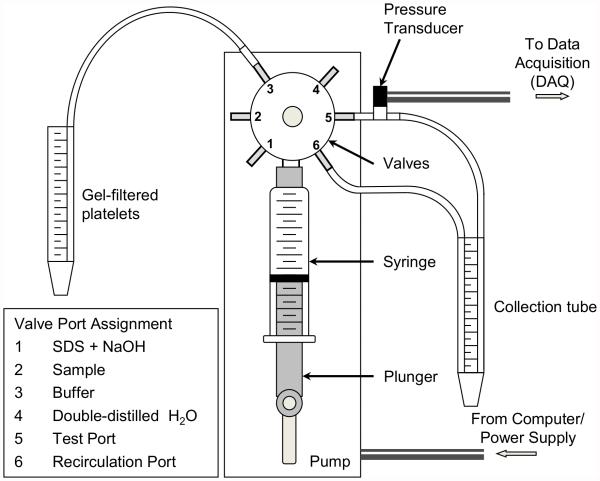
The pump (PSD/8, Hamilton, Reno, NV) is composed of 2 stepper motors, one controlling a 5 ml glass syringe with a Luer fitting (Hamilton, Reno, NV) via a connected plunger and the other controlling the opening and closing of the input and output valves (Fig. 1). The resolution of the syringe stepper motor is 24,000 steps for a full 5 ml syringe, translating to 0.208 μl per step. The motor operates with a minimum response time of 5 ms, with an acceleration resolution of 0.1 μl/s2 and minimum flow rate of 1 μl/s. With the exception of the glass syringe and the ceramic valve ports, all blood-contacting surfaces are composed of PVC or PTFE. The syringe is coated with Sigmacote (Sigma-Aldrich, St. Louis, MO) prior to experiments. Platelets were drawn from and collected in polyethylene conical tubes. The connection tubing is 1.2 or 2.0 mm in diameter, while the tubing used for high shear exposure ranged from 0.366 mm to 0.8 mm in diameter. Pump commands are programmed in LabView 2010 (National Instruments, Austin, TX) and transmitted via an RS-232 port. Syringe motion for these experiments is programmed to yield a constant flow rate, but can be adjusted with velocity and acceleration commands to produce dynamic flow patterns during the ejection phase. A pressure transducer (Omega Engineering Inc., Stamford, CT) is mounted downstream of the high shear valve to measure the pressure drop across the length of the shear exposure tubing, and pressure measurements are transmitted to LabView via a USB data acquisition (DAQ) device (National Instruments, Austin, TX). The maximum pressure drop that can be sustained by the input and output valves is 689 kPa (100 psi), and tubing geometry and flow rates were selected in order not to exceed this limit.
FIG. 1.
The platelet hammer - a syringe-capillary viscometer capable of repeatedly exposing platelets to wall shear stresses up to 2000 dyne/cm2 for brief durations.
Exposure of platelets to shear stress in syringe-capillary viscometer
GFP were exposed to high shear stresses in the syringe-capillary pump at 37°C in the following manner (Table 1):
TABLE 1.
Design and shear stress parameters for syringe-capillary viscometer experiments
| τavg
(dyne/cm2) |
texp (s) | No. of loops |
Tubing diameter (mm) |
Tubing length (cm) |
Outflow rate (ml/min) |
SA (dyne- s/cm2) |
|
|---|---|---|---|---|---|---|---|
|
a) High shear,
single passage |
250 | 0.100 | 1 | 0.366 | 22.95 | 14.44 | 25 |
| 500 | 0.050 | 1 | 0.366 | 22.95 | 28.88 | 25 | |
| 1000 | 0.025 | 1 | 0.366 | 22.95 | 57.76 | 25 | |
|
b) Low shear,
multiple passages |
15 | 80.146 | 2 | 0.366 | 1100 | 0.87 | 2412 |
| 15 | 80.146 | 4 | 0.366 | 1100 | 0.87 | 4833 | |
| 15 | 80.146 | 6 | 0.366 | 1100 | 0.87 | 7253 | |
| 15 | 80.146 | 8 | 0.366 | 1100 | 0.87 | 9674 | |
| 15 | 80.146 | 10 | 0.366 | 1100 | 0.87 | 12094 | |
|
c) Variable
shear and exposure times |
2.5 | 6.000 | 1 | 0.8 | 30 | 1.51 | 15 |
| 25 | 0.600 | 6 | 0.8 | 30 | 15.08 | 112 | |
| 50 | 0.300 | 12 | 0.8 | 30 | 30.16 | 228 | |
| 100 | 0.150 | 25 | 0.8 | 30 | 60.32 | 479 | |
| 150 | 0.100 | 37 | 0.8 | 30 | 90.48 | 711 | |
| 200 | 0.075 | 50 | 0.8 | 30 | 120.64 | 962 | |
| 250 | 0.060 | 62 | 0.8 | 30 | 150.80 | 1194 | |
| 300 | 0.050 | 74 | 0.8 | 30 | 180.96 | 1426 | |
| 350 | 0.050 | 74 | 0.8 | 35 | 211.12 | 1611 | |
| 400 | 0.050 | 74 | 0.8 | 40 | 241.27 | 1796 | |
| 500 | 0.063 | 74 | 0.38 | 30 | 32.32 | 2883 | |
| 600 | 0.053 | 120 | 0.38 | 30 | 38.79 | 4304 |
Shear stress, exposure time, tubing parameters, and stress accumulation (SA) values for all conditions.
(a) Single exposure to high average shear stresses (τav) ranging from 250 to 1000 dyne/cm2, with respective exposure times from 100 to 25 ms, corresponding to a stress accumulation (SA) of 25 dyne-s/cm2 for each condition. GFP were then subsequently exposed to 0.5 dyne/cm2 for 30 min in the HSD. This additional step was undertaken to examine whether platelets were primed for continued activation despite the low shear stress exposure, in a process termed as shear-induced platelet sensitization (28).
(b) Repeated exposure to low shear stress, 15 dyne/cm2 for 80 s, with SA ranging from 2412 to 12094 dyne-s/cm2. GFP were subsequently exposed to 0.5 dyne/cm2 for 10 min in the HSD.
(c) Repeated exposure to variable high shear stresses and exposure times to examine the effect of SA on platelet activation. SA ranged from 15 to 4304 dyne-s/cm2, with τav ranging from 2.5 to 600 dyne/cm2.
Platelets were drawn into the syringe at a low flow rate, with the volume drawn equivalent to the desired collection volume, 2.5 ml, plus the dead volume in the test tubing. Platelets were ejected into a collection tube at a flow rate that produced the desired average shear stress and exposure time for a given platelet in the tubing. The exposure time was defined as the dead volume in the tubing divided by the flow rate. For repeated passage experiments, collected platelets were drawn into the syringe at τav = 25 dyne/cm2 for an exposure time of 320 ms (experiments a-b) or τav = 5 dyne/cm2 for an exposure time of 800 ms (experiments c), and the ejection and re-entry cycle was repeated until the desired number of passages was attained. Approximately 250 μl of platelets remain in the collection tube due to the dead volume in the test tubing. Platelets were subsequently exposed to 0.5 dyne/cm2 for either 30 min (experiments a) or 10 min (experiments b) in the HSD to examine the shear-induced sensitization response (24, 28).
Prior to every experiment, the syringe and shear exposure tubing were flushed with platelet buffer to ensure that all surfaces were wetted and that no air gaps, which may perturb and activate platelets, were present. After shear stress exposure, all platelet-contacting surfaces were washed once with 0.5% SDS + 50 mM NaOH and double-distilled H2O, followed by an air purge, using LabView-programmed commands.
Platelet activation state
Samples for the modified prothrombinase-based platelet activation state (PAS) assay (31, 32) were taken at the beginning and end of high shear stress exposure, and samples for platelet sensitization analysis were drawn from the Couette region of the HSD every 5 min from 0 to 30 min (experiments a) or every 2 min from 0 to 10 min (experiments b). Briefly, platelet samples were incubated with 200 nM acetylated prothrombin and 100 pM factor Xa for 10 min at 37°C. The generated thrombin species does not participate in feedback on platelets or factor Va, resulting in linear kinetics (31, 32). The thrombin generation rate of the sample was assayed with 0.3 mM Chromozym TH (Roche Life Science, Indianapolis, IN) in a 96-well plate microplate reader at 25°C, where the absorbance rate was defined as PAS. These PAS values were normalized against the activity of fully activated platelets, obtained by sonication (10 W for 10 s, Branson Sonifier 150 with microprobe, Branson, MO). PAS values are therefore expressed as a fraction of the maximum thrombin-generating capacity, with a maximum of 1.0. The difference between the initial and post- high shear exposure PAS values, ΔPAS, was calculated for all experiments. For experiments examining post-high shear sensitization, regression lines were fit to PAS values measured for samples obtained from the HSD. Platelet activation rates (PAR) were calculated from the slopes of these regression lines.
Platelet shape change
Scanning electron microscopy (SEM) images were obtained for platelets exposed to average peak shear stresses of 50, 150, 400, and 500 dyne/cm2 to analyze the role of repeated pathological shear stress exposure on shape change. Briefly, 150 μl of shear-exposed platelets were added to 150 μl of 2% v/v glutaraldehyde in platelet buffer and simultaneously placed onto 12 mm circular glass coverslips in a 24-well plate for 15 min. Excess solution was partially aspirated, leaving approximately 50 μl of the mixture. Coverslips were washed with 25%, 50%, 75%, and 100% of double-distilled H2O in 1% glutaraldehyde. The coverslips were then dehydrated through a graded series of 0%, 25%, 50%, 75%, and 100% ethanol in double-distilled H2O. Samples were stored in 100% ethanol until drying through a series of 25%, 50%, 75%, and 100% hexamethyldisiloxane (HMDS) in ethanol. Each preparation stage required 5 min immersion at room temperature. Coverslips were then mounted on double-sided carbon tape, allowed to air dry overnight, and then sputter-coated with islanded gold in an argon chamber. Images were obtained at 30 kV and 20,000× zoom using an Inspect F50 scanning electron microscope (FEI, Hillsboro, OR). Pseudopod numbers and lengths were obtained using ImageJ (NIH).
Statistics
For all experiments, ΔPAS was compared for each unique shear stress-exposure time combination using one-way ANOVA with Tukey's post hoc test. For experiments examining the post-high shear-induced sensitization, PAR was compared using one-way ANOVA with Tukey’s post hoc test. Exposure to constant 0.5 dyne/cm2 shear stress for 10 minutes was considered the negative control for these experiments. A threshold for the repeated variable shear stress and exposure time experiments was defined as that SA at which there was a statistically significant difference between ΔPAS values for shear-exposed platelets and resting control platelets (p < 0.05). For all other experiments, the Student’s t-test was used to establish significance (p < 0.05). Results are reported as mean ± standard error of the mean. All statistical analyses were done with IBM SPSS 21.0 (IBM Corp., Armonk, NY).
RESULTS
The Platelet Hammer
We successfully fabricated and tested a controlled syringe-capillary viscometer system capable of exposing blood platelets to hypershear stress levels experienced in MCS devices. The viscometer exposed up to 5 ml of the platelet suspension to both single and repeated passages at flow rates of up to 241 ml/min and pressure drop of up to 501 kPa (72 psi). The length and diameter of the PTFE outflow tubing was adjustable, depending on the outflow shear stress and exposure time required. Furthermore, the number of passages was programmed to expose platelets to a predetermined shear stress accumulation (SA), or product of shear stress and exposure time, for which we measured the PAS, subsequent low shear PAR, and platelet morphological response. The combination of shear stresses and exposure times in the valve ports and syringe were considered negligible and were not considered in SA calculations. However, our calculations include SA for platelets returning to the syringe through the inflow tubing, yielding exposure to 6.3 dyne/cm2 for 0.69 s or 25 dyne/cm2 for 0.32 s per passage, respectively.
Single exposure to hypershear
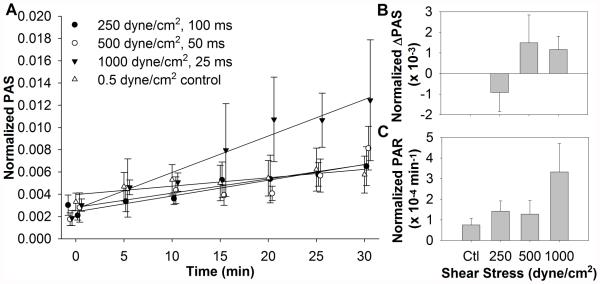
Platelets were exposed to three shear stress conditions, all with SA of 25 dyne/cm2 (Fig. 2A, n = 4). These conditions mimic the shear stresses and exposure times found in prosthetic blood-recirculating devices. No significant differences in ΔPAS were observed during the initial high shear stress exposure (Fig. 2B). The 1000 dyne/cm2, 25 ms exposure condition showed the highest PAR during the subsequent low shear stress exposure, but this was not significant compared to the 0.5 dyne/cm2 control (Fig. 2C). This indicates some dependence on the shear stress magnitude, despite the same initial SA.
FIG. 2.
(A) Platelets were exposed once to very high shear stresses for durations on the order of milliseconds, followed by subsequent 30 min exposure to 0.5 dyne/cm2 in the HSD, with samples assayed for PAS at regular intervals. (B) The initial change in the high shear PAS is negligible, with (C) a non-significant shear-dependent upward trend in the post-high shear platelet activation rate (PAR).
Repeated exposure to physiological shear and hypershear conditions
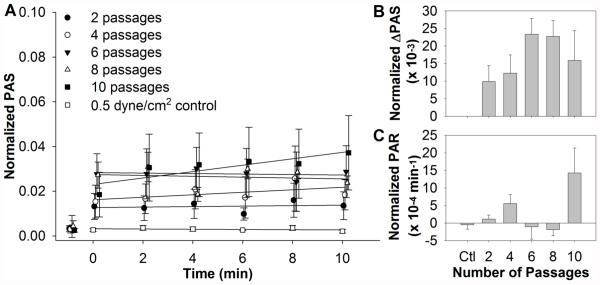
During their lifespan, platelets that are not consumed have a propensity to recirculate through the prosthetic device. Therefore, we also examined repeated exposure of platelets to shear stresses and exposure times similar to those utilized in our previously published observation of shear-induced platelet sensitization. Samples were exposed for 2 to 10 repeats of 15 dyne/cm2 for 80 s (Fig. 3A, n = 4). The 2, 4, and 10 repeat conditions followed the increasing trend previously observed for the ΔPAS, while the 6 and 8 repeat conditions had the highest ΔPAS values (Fig. 3B). However, these values were not significant when compared to the 0.5 dyne/cm2 control. Similarly, the increasing trend in post-high shear PAR was evident for the 2, 4, and 10 repeat conditions, but negative and similar to the control for the 6 and 8 repeat conditions (Fig. 3C). Significance was only noted between the 8 and 10 repeat conditions (p < 0.05).
FIG. 3.
(A) Platelets were exposed repeatedly to 15 dyne/cm2 average shear stress for 80 s, followed by subsequent 10 min exposure to 0.5 dyne/cm2 in the HSD, with samples assayed for PAS at regular intervals. For the 2, 4, and 10 passage experiments, an increasing trend is observed for both (B) initial PAS and (C) subsequent post-high shear PAR. However, the 6 and 8 passage experiments showed the highest initial PAS, but negative post-high shear PAR.
Platelets were exposed repeatedly to average shear stresses ranging from 0 to 600 dyne/cm2, with an SA range of 0 to 4304 dyne-s/cm2 (Fig. 4, n = 6). As the SA increases, an increase in the PAS value is observed. Significance compared to the control was only observed at and above an SA of 1611 dyne-s/cm2, corresponding to 74 passages at 350 dyne/cm2 and 50 ms (p < 0.001). This may represent a threshold at which platelets are irreversibly activated. Comparatively, platelets exposed to constant shear stress of 70 dyne/cm2 for 40 s (SA = 2800 dyne-s/cm2) only achieved PAS of about 0.024 (28), while exposure to triangular waveforms with peak shear stress of 70 dyne/cm2 and frequency of 6.25 Hz (4 min, SA = 2800 dyne-s/cm2) increased the mean ΔPAS more than five-fold to 0.127 (24). In this study, the 500 dyne/cm2, 50 ms exposure time condition, which has similar SA (2883 dyne-s/cm2) to conditions mentioned above, yields a ΔPAS of 0.588. This indicates that shear stress magnitude, the dynamic nature of the shear stress waveform, and frequency all play a role in shear-induced platelet activation.
FIG. 4.
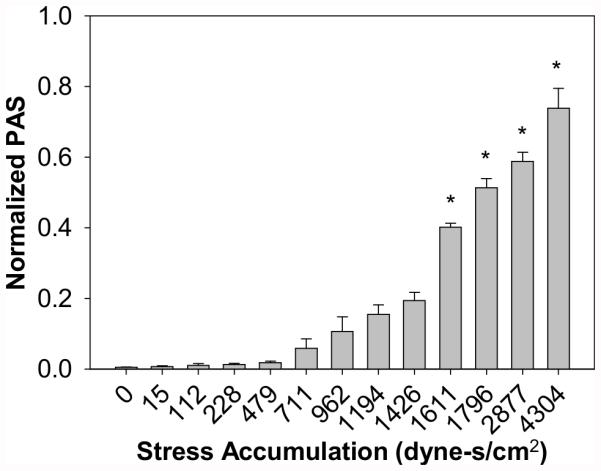
Platelets were repeatedly exposed to various shear stresses and exposure times, with PAS measured for the resulting stress accumulation. Significance was achieved for stress accumulations above 1611 dyne-s/cm2, or 74 passages at 350 dyne/cm2 for 50 ms (*p < 0.001).
Platelet morphological response to repeated shear stress exposure
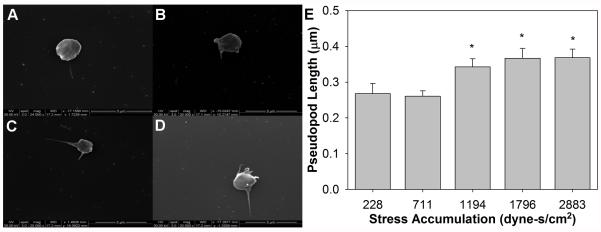
Platelets were exposed to a large range of SA values, from 228 dyne-s/cm2 (repeated exposure to 50 dyne/cm2 for 300 ms) to 2883 dyne-s/cm2 (500 dyne/cm2 for 63 ms) in order to observe platelet shape change in response to repeated passages through shear stress conditions prevalent in MCS devices. Pseudopods were few in number, but displayed a dendritic phenotype and generally increased in length as the SA increased (Fig. 5). However, the average pseudopod length did not exceed 0.4 μm. Significance in length was only detected between SA of 1194 dyne-s/cm2 (250 dyne/cm2 for 60 ms) and higher SA values (n = 20, p < 0.05).
FIG. 5.
Shape change was observed for platelets for several of the repeated shear stress and exposure time conditions. (A) Baseline platelets have a discoid shape, with little or no protrusions, which increase in length and number as the stress accumulation is increased. Conditions tested include (B) 228 dyne-s/cm2 (50 dyne/cm2 for 300 ms), (C) 1194 dyne-s/cm2 (250 dyne/cm2 for 60 ms), and (D) 2883 dyne-s/cm2 (500 dyne/cm2 for 63 ms). (E) A significant increase is noted only between the 711 dyne-s/cm2 (150 dyne/cm2 for 100 ms) condition and higher stress accumulations (p < 0.05).
DISCUSSION
In this study we describe a closed-loop recirculating syringe-capillary viscometer device, “the platelet hammer,” which allowed repeated exposure of platelets to shear stresses and durations relevant to both MCS devices and cardiovascular pathologies, such as arterial stenosis. The capability of the platelet hammer to generate repetitive passages confers the added benefit of determining how platelets’ shear stress histories affect their activation behavior due to both the high shear stress exposure and subsequent low shear exposure, as is present in the physiological circulation. Previously utilized in vitro tools are limited in the appropriate range of shear stresses and exposure times, use of only constant instead of the relevant intermittent or dynamic shear stress conditions, and the inability to use the previously-sheared sample in subsequent shear stress exposures. Herein, the platelet hammer is interfaced with LabView and equipped with a six-port ceramic valve, which allows programming and generation of multiple shear profiles in a single experiment by using different tubing diameters and lengths at each of those ports. Hence, we have developed a tool that can be used to understand how platelets respond to dynamic conditions present in the milieu of MCS devices and stenoses.
We observed that a single passage through shear stress conditions approaching τav = 1000 dyne/cm2 (wall shear stresses up to 2000 dyne/cm2) for as short as 25 ms activates platelets, using thrombin generation as a marker. We also observed that repeated passages, with stress accumulation (SA) at and beyond 1611 dyne-s/cm2, equivalent to 74 loops at τav = 350 dyne/cm2 for 50 ms, significantly increased the PAS when compared to unsheared platelets. In contrast, SA of at least 3600 dyne-s/cm2, achieved under constant shear stress exposure to 30 dyne/cm2 for 2 min, was required to establish significance in a prior study (24). The discrepancy in the SA at which platelets show significant activation indicates that the frequency of exposure and the shear stress magnitude also play a large role in the level of platelet activation. The role of frequency in platelet activation was previously explored in a study showing an increasing trend in ΔPAS with an increase in frequency of triangular stress peaks of 70 dyne/cm2, despite the SA being held constant at 2800 dyne-s/cm2 (24). The dependence of the threshold SA on the shear stress magnitude was indirectly illustrated in an earlier study utilizing a Couette viscometer for single passages of PRP, where a minimum SA range of 17.85 dyne-s/cm2 (2550 dyne/cm2 for 7 ms) to 399 dyne-s/cm2 (570 dyne/cm2 for 700 ms) was required to obtain small yet significant granular release of β-TG (14). A minimum exposure to 4500 dyne-s/cm2 (150 dyne/cm2 for 30 s) was required to induce significant serotonin release in PRP sheared in a Couette viscometer (11), while a stress accumulation of 17.5 dyne-s/cm2 (average shear stress of 3500 dyne/cm2 for 5 ms) was required for a similar release in PRP sheared in a syringe-capillary viscometer (12). Our PAS results with single exposure to average shear stress of 1000 dyne/cm2 for 25 ms (SA of 25 dyne-s/cm2), while not significant, are similar to the lower SA, shear stress magnitude, and exposure time required to measure significant β-TG and serotonin release. However, the SA (1611 dyne-s/cm2) required for significant PAS in our repeated exposure experiments is four-fold higher than required for β-TG release and three-fold lower than required for serotonin release for a shear stress of similar magnitude (i.e. 350 dyne/cm2 in this study, compared to 570 dyne/cm2 in the β-TG study and 150 dyne/cm2 in the serotonin study). It is important to note that in these prior studies, lower SA induced granular release, but the shear stresses required were generally much higher compared to our study, and only constant shear stress exposures were considered. Furthermore, granular release of β-TG and serotonin may not necessary correlate with thrombin generation, which is a platelet surface- (membrane-) based process. However, these correlations were performed due to a lack of thrombin data for such high shear stress levels. Lastly, experiments in this study were conducted with isolated platelets at a count of 20,000/μl, whereas prior studies utilized PRP with physiological platelet counts of at least 150,000/μl, which may cause a faster and non-linear platelet activation response (32).
We also examined the role of hypershear in platelet sensitization, or the priming of platelets for further activation despite exposure to a subsequent low shear environment (28). While not significant, even a single 25 ms exposure to an average shear stress of 1000 dyne/cm2 caused a marked difference in the post-high shear activation rate (PAR) compared to the control (SA = 25 dyne-s/cm2, Fig. 2). Two repeats of 15 dyne/cm2 for 80 s (SA = 2412 dyne-s/cm2, Fig. 3) resulted in a similar post-high shear PAR as a single passage at 1000 dyne/cm2 for 25 ms (SA = 25 dyne-s/cm2, Fig. 2). This may indicate that while PAS may be highly impacted by the SA of high shear stress exposure, shear-induced platelet sensitization is likely dependent on the magnitude of high shear stresses, such as those found in MCS devices. As in the case of PAS measurement, the effect on sensitization due to exposure of PRP with higher platelet counts due to the dynamic very high shear stress conditions utilized in this study needs to be examined.
Stress accumulation appears to play a role in pseudopod growth (Fig. 5). A significant increase in length is observed above a stress accumulation of 711 dyne-s/cm2 (37 passages at τav = 150 dyne/cm2 for 100 ms, p < 0.05). Several studies have correlated pseudopod length to agonist-induced platelet activation (33, 34), but no study has examined pseudopod extension in response to fluid shear stress. In a prior study of flow-induced platelet shape change, approximately 24% of washed platelets exposed to 4000 s−1 for 5 min (SA = 12,000 dyne s/cm2) in a Couette viscometer showed pseudopod extension and organelle centralization, as identified by transmission electron microscopy (TEM) (35). This was similar to platelets obtained from patients with coronary stenosis. Ultrastructural TEM investigations of platelets exposed to several shear stresses and exposure times showed that platelets are mostly rounded with several pseudopods and centralized granules after 113 ms exposure to 1700 dyne/cm2, while those exposed to higher shear stresses are ballooned and partially aggregated (14). In our study, platelets were either rounded or discoid, but no platelets analyzed displayed ballooning indicating significant damage.
While evidence showing increasing platelet activation and sensitization due to very high shear stress was observed after exposure in our syringe-capillary viscometer, there are some potential improvements for future studies. The use of isolated platelets at low counts does not fully mimic physiology, where several cell types and proteins are involved. This allowed us to use a near real-time modified prothrombinase assay to measure the direct effect of shear stress on platelet activation, as activation and sensitization due to platelet cross-talk may make it difficult to distinguish shear effects (28, 32). However, this device and procedure can be adapted for whole blood, as well as other measurement techniques, such as P-selectin expression measurement by flow cytometry, which correlates strongly to PAS measurements (36). The selection of shear stresses and exposure times was dependent on tubing length and diameter, as well as a maximum pressure drop of 689 kPa (100 psi), in order to avoid damaging pump valve components. Furthermore, tubing length and diameters had to be adjusted if flow rates exceeded 241 ml/min, beyond which tubing connectors failed. These limitations resulted in a maximum average shear stress of 1000 dyne/cm2 (wall shear stress of 2000 dyne/cm2). However, the large range of shear stresses and the ability to generate repeated pulsatile conditions mimics blood recirculating device conditions more closely than many devices, such as cone-and-plate viscometers and parallel plate flow chambers, currently used to measure shear-induced platelet response. Platelets exposed repeatedly to high shear stresses in the syringe-capillary viscometer were exposed to lower shear stresses for 230 or 690 ms between exposures. However, platelets in vivo may be exposed to a variety of low shear stresses in the vasculature, ranging from seconds to minutes, before returning to the MCS device. Thus, the level of platelet trauma encountered in vitro in this study may exceed that found in vivo. Lastly, extending this approach to the study of shear-induced thrombogenic risk and related antithrombotic therapies will require validation through the use of whole blood and platelets from patients implanted with MCS devices, as well as the complex shear stress conditions located downstream from such devices. This will allow for a better understanding of shear-induced platelet activation in MCS patients and assist in the development of approaches to mitigate the negative effects of these devices and associated pharmacotherapy.
CONCLUSION
The platelet hammer, developed and utilized in this study, presents a useful device capable of generating shear stress conditions present in MCS devices and cardiovascular pathologies. With this device, we have shown that platelets activate, sensitize, and change shape in response to both single and repeated passages through a variety of shear stresses and exposure times, and their resulting stress accumulation. These results are very similar to many shear-induced platelet activation studies performed over the last 40 years, but the stress accumulations required to reach significant platelet activation are generally lower than the vast majority of prior studies, which typically used single exposure to constant shear stress. While the platelet hammer can be used to extend analysis of shear-induced platelet activation, its range of shear stresses and exposure times may allow for the investigation of pathologies and measures associated with MCS devices and stenoses, including thrombosis, acquired von Willebrand disease, and objective pharmacotherapy management.
Acknowledgements
This work was supported by a Quantum Grant from the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health (Award No. 5U01EB012487-04).
Footnotes
Author contributions: Jawaad Sheriff designed the study, acquired and analyzed data, and drafted and critically revised the manuscript. Phat L. Tran designed the study, acquired and analyzed data, and drafted and critically revised the manuscript. Marcus Hutchinson acquired and analyzed data. Tracy DeCook acquired and analyzed data. Marvin J. Slepian designed the study and critically revised the manuscript. Danny Bluestein designed the study, critically revised the manuscript, and secured funding. Jolyon Jesty designed the study and critically revised the manuscript.
Conflict of Interest: None
REFERENCES
- 1.Stewart GC, Givertz MM. Mechanical circulatory support for advanced heart failure: patients and technology in evolution. Circulation. 2012;125:1304–15. doi: 10.1161/CIRCULATIONAHA.111.060830. [DOI] [PubMed] [Google Scholar]
- 2.INTERMACS . Intermacs Quarterly Statistical Report: 2014 Q4 [Internet] University of Alabama Birmingham School of Medicine (US); Birmingham (AL): c2007- [updated 2015 Mar 16; cited 2015 Mar 31]. Available from: http://www.uab.edu/medicine/intermacs/images/Federal_Quarterly_Report/Federal_Partners_Report_2014_Q4.pdf. [Google Scholar]
- 3.Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014;33:12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Adzic A, Patel SR, Maybaum S. Impact of adverse events on ventricular assist device outcomes. Curr Heart Fail Rep. 2013;10:89–100. doi: 10.1007/s11897-012-0127-3. [DOI] [PubMed] [Google Scholar]
- 5.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125:3038–47. doi: 10.1161/CIRCULATIONAHA.111.040246. [DOI] [PubMed] [Google Scholar]
- 6.Nishida M, Maruyama O, Kosaka R, Yamane T, Kogure H, Kawamura H, et al. Hemocompatibility evaluation with experimental and computational fluid dynamic analyses for a monopivot circulatory assist pump. Artif Organs. 2009;33:378–86. doi: 10.1111/j.1525-1594.2009.00730.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Gellman B, Koert A, Dasse KA, Gilbert RJ, Griffith BP, et al. Computational and experimental evaluation of the fluid dynamics and hemocompatibility of the CentriMag blood pump. Artif Organs. 2006;30:168–77. doi: 10.1111/j.1525-1594.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis P, Tarbell JM, Frangos JA. An in vitro evaluation of an artificial heart. ASAIO Trans. 1991;37:27–32. doi: 10.1097/00002480-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Girdhar G, Xenos M, Alemu Y, Chiu WC, Lynch BE, Jesty J, et al. Device thrombogenicity emulation: a novel method for optimizing mechanical circulatory support device thromboresistance. PLoS One. 2012;7:e32463. doi: 10.1371/journal.pone.0032463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deutsch S, Tarbell JM, Manning KB, Rosenberg G, Fontaine AA. Experimental fluid mechanics of pulsatile artificial blood pumps. Annu Rev of Fluid Mech. 2006;38:65–86. [Google Scholar]
- 11.Anderson GH, Hellums JD, Moake J, Alfrey CP., Jr Platelet response to shear stress: changes in serotonin uptake, serotonin release, and ADP induced aggregation. Thromb Res. 1978;13:1039–47. doi: 10.1016/0049-3848(78)90232-3. [DOI] [PubMed] [Google Scholar]
- 12.Colantuoni G, Hellums JD, Moake JL, Alfrey CP., Jr The response of human platelets to shear stress at short exposure times. Trans Am Soc Artif Intern Organs. 1977;23:626–31. doi: 10.1097/00002480-197700230-00169. [DOI] [PubMed] [Google Scholar]
- 13.Ramstack JM, Zuckerman L, Mockros LF. Shear-induced activation of platelets. J Biomech. 1979;12:113–25. doi: 10.1016/0021-9290(79)90150-7. [DOI] [PubMed] [Google Scholar]
- 14.Wurzinger LJ, Opitz R, Blasberg P, Schmid-Schonbein H. Platelet and coagulation parameters following millisecond exposure to laminar shear stress. Thromb Haemost. 1985;54:381–6. [PubMed] [Google Scholar]
- 15.Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007;109:566–76. doi: 10.1182/blood-2006-07-028282. [DOI] [PubMed] [Google Scholar]
- 16.Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–73. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 17.Tovar-Lopez FJ, Rosengarten G, Westein E, Khoshmanesh K, Jackson SP, Mitchell A, et al. A microfluidics device to monitor platelet aggregation dynamics in response to strain rate micro-gradients in flowing blood. Lab Chip. 2010;10:291–302. doi: 10.1039/b916757a. [DOI] [PubMed] [Google Scholar]
- 18.Reininger AJ, Heijnen HF, Schumann H, Specht HM, Schramm W, Ruggeri ZM. Mechanism of platelet adhesion to von Willebrand factor and microparticle formation under high shear stress. Blood. 2006;107:3537–45. doi: 10.1182/blood-2005-02-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108:1903–10. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nobili M, Sheriff J, Morbiducci U, Redaelli A, Bluestein D. Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO J. 2008;54:64–72. doi: 10.1097/MAT.0b013e31815d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alemu Y, Girdhar G, Xenos M, Sheriff J, Jesty J, Einav S, et al. Design Optimization of a Mechanical Heart Valve for Reducing Valve Thrombogenicity-A Case Study with ATS Valve. ASAIO J. 2010;56:389–96. doi: 10.1097/MAT.0b013e3181e65bf9. [DOI] [PubMed] [Google Scholar]
- 22.Xenos M, Girdhar G, Alemu Y, Jesty J, Slepian M, Einav S, et al. Device Thrombogenicity Emulator (DTE) - Design optimization methodology for cardiovascular devices: A study in two bileaflet MHV designs. J Biomech. 2010;43:2400–9. doi: 10.1016/j.jbiomech.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girdhar G, Bluestein D. Biological effects of dynamic shear stress in cardiovascular pathologies and devices. Expert Rev Med Devices. 2008;5:167–81. doi: 10.1586/17434440.5.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheriff J, Soares JS, Xenos M, Jesty J, Bluestein D. Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices. Ann Biomed Eng. 2013;41:1279–96. doi: 10.1007/s10439-013-0758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hathcock JJ. Flow effects on coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2006;26:1729–37. doi: 10.1161/01.ATV.0000229658.76797.30. [DOI] [PubMed] [Google Scholar]
- 26.Jackson SP, Nesbitt WS, Westein E. Dynamics of platelet thrombus formation. J Thromb Haemost. 2009;7:17–20. doi: 10.1111/j.1538-7836.2009.03401.x. [DOI] [PubMed] [Google Scholar]
- 27.Chiu WC, Girdhar G, Xenos M, Alemu Y, Soares JS, Einav S, et al. Thromboresistance comparison of the HeartMate II ventricular assist device with the device thrombogenicity emulation- optimized HeartAssist 5 VAD. J Biomech Eng. 2014;136:021014. doi: 10.1115/1.4026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheriff J, Bluestein D, Girdhar G, Jesty J. High-shear stress sensitizes platelets to subsequent low-shear conditions. Ann Biomed Eng. 2010;38:1442–50. doi: 10.1007/s10439-010-9936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JN, Bergeron AL, Yu Q, Sun C, McIntire LV, Lopez JA, et al. Platelet aggregation and activation under complex patterns of shear stress. Thromb Haemost. 2002;88:817–21. [PubMed] [Google Scholar]
- 30.Soares JS, Sheriff J, Bluestein D. A novel mathematical model of activation and sensitization of platelets subjected to dynamic stress histories. Biomech Model Mechanobiol. 2013;12:1127–41. doi: 10.1007/s10237-013-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jesty J, Bluestein D. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal Biochem. 1999;272:64–70. doi: 10.1006/abio.1999.4148. [DOI] [PubMed] [Google Scholar]
- 32.Schulz-Heik K, Ramachandran J, Bluestein D, Jesty J. The extent of platelet activation under shear depends on platelet count: differential expression of anionic phospholipid and factor Va. Pathophysiol Haemost Thromb. 2005;34:255–62. doi: 10.1159/000093104. [DOI] [PubMed] [Google Scholar]
- 33.Frojmovic M, Longmire K, van de Ven TG. Long-range interactions in mammalian platelet aggregation. II. The role of platelet pseudopod number and length. Biophys J. 1990;58:309–18. doi: 10.1016/S0006-3495(90)82378-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hantgan RR. A study of the kinetics of ADP-triggered platelet shape change. Blood. 1984;64:896–906. [PubMed] [Google Scholar]
- 35.Ajzenberg N, Talab AT, Masse JM, Drouin A, Jondeau K, Kobeiter H, et al. Platelet shape change and subsequent glycoprotein redistribution in human stenosed arteries. Platelets. 2005;16:13–8. doi: 10.1080/0953710042000267716. [DOI] [PubMed] [Google Scholar]
- 36.Claiborne TE, Girdhar G, Gallocher-Lowe S, Sheriff J, Kato YP, Pinchuk L, et al. Thrombogenic potential of Innovia polymer valves versus Carpentier-Edwards Perimount Magna Aortic Bioprosthetic Valves. ASAIO J. 2011;57:26–31. doi: 10.1097/MAT.0b013e3181fcbd86. [DOI] [PubMed] [Google Scholar]