Abstract
Objective
To describe the incidence of, and clinical and neurobiological risk factors for, new-onset impulse control disorder (ICD) symptoms and related behaviours in early Parkinson disease (PD).
Methods
The Parkinson's Progression Markers Initiative is an international, multicenter, prospective study of de novo patients with PD untreated at baseline and assessed annually, including serial dopamine transporter imaging (DAT-SPECT) and ICD assessment (Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease short form, QUIP). Participants were included if they screened negative on the QUIP at baseline. Kaplan-Meier curves and generalised estimating equations examined frequency and predictors of incident ICD symptoms.
Results
Participants were seen at baseline (n=320), year 1 (n=284), year 2 (n=217) and year 3 (n=96). Estimated cumulative incident rates of ICD symptoms and related behaviours were 8% (year 1), 18% (year 2) and 25% (year 3) and increased each year in those on dopamine replacement therapy (DRT) and decreased in those not on DRT. In participants on DRT, risk factors for incident ICD symptoms were younger age (OR=0.97, p=0.05), a greater decrease in right caudate (OR=4.03, p=0.01) and mean striatal (OR=6.90, p=0.04) DAT availability over the first year, and lower right putamen (OR=0.06, p=0.01) and mean total striatal (OR=0.25, p=0.04) DAT availability at any post-baseline visit.
Conclusions
The rate of incident ICD symptoms increases with time and initiation of DRT in early PD. In this preliminary study, a greater decrease or lower DAT binding over time increases risk of incident ICD symptoms, conferring additional risk to those taking DRT.
Clinical trial registration
Introduction
Impulse control disorders (ICDs) and related behaviours commonly occur in Parkinson disease (PD), with a prevalence of disorders or symptoms ranging from 14% to 43%.1,2,3 The primary ICDs in PD are compulsive gambling, eating, buying and sexual behaviours. While rates of ICD behaviours are similar in patients with PD prior to initiation of dopamine replacement therapy (DRT) compared with healthy controls,4,5 these behaviours increase with DRT, in particular dopamine agonists.1 ICDs are associated with greater functional impairment and comorbid psychiatric symptoms in PD.6 Treatment is challenging, as patients may experience dopamine agonist withdrawal syndrome (DAWS).7,8,9 It is critical to identify predictive factors to minimise this complication.
Clinical correlates of ICDs include depression,6,3,10,11 anxiety,6,12 personal or family history of alcohol abuse or gambling,1 increased impulsivity and novelty-seeking,6 younger age,1,3,12 early PD onset,3 unmarried status1 and smoking.1,13,14 Since these risk factors were identified in cross-sectional studies, longitudinal studies need to distinguish associated factors from true risk factors.
Neurobiological risk factors for ICDs might be identified through imaging of the dopamine system, which may play a role in ICD pathogenesis.15,16,17 Dopamine transporter (DAT) imaging may reveal early biomarkers predictive of ICD risk, as decreased DAT availability has been associated with both prevalent17 and incident18 ICDs in small, preliminary studies.
We examined clinical and DAT imaging risk factors as predictors of incident ICD behaviour symptoms in a prospective, early PD cohort. We hypothesised that changes in DAT availability over time, reflecting integrity of the dopamine system, would predict incident ICD behaviour symptoms.
Methods
Participants
The Parkinson Progression Markers Initiative (PPMI) is an observational, international, multicenter (21 US and 12 international sites) cohort study of newly diagnosed, untreated (at enrolment) patients with PD and healthy controls.19 A public-private partnership, it is funded by the Michael J Fox Foundation for Parkinson's Research and funding partners, including AbbVie, Avid Radiopharmaceuticals, Biogen, Bristol-Meyers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche, Servier and UCB. The aims/methodology of the study have been published19 and are available on the PPMI website (http://www.ppmi-info.org/study-design). Inclusion criteria included meeting established diagnostic criteria for PD, and a DAT imaging deficit at enrolment. Exclusion criteria included taking medications for PD, and a diagnosis of dementia based on the clinical assessment of the site investigator. At baseline, mean disease duration was 6.6 months.4
Data were obtained from PPMI database (http://www.ppmi-info.org/data, accessed 16 December 2014, medication information updated 26 March 2015). PD participants were included if they completed baseline (BL) and at least year 1 ICD behaviour screening. Participants were excluded if they screened positive for ICD behaviours at baseline.
At the time of the original data download, there were 418 PD participants with baseline assessments of ICD behaviours. Eighty four participants who screened positive for ICD behaviours at baseline were excluded. Fourteen participants who did not have at least one post-baseline ICD behaviour assessment were excluded. Our cohort therefore included baseline data for 320 PD participants. DAT imaging occurred at baseline, year 1 and year 2. Participants started DRT (defined here as levodopa, dopamine agonist, amantadine or monoamine oxidase-B inhibitor20) as clinically indicated. Participants were classified as on DRT at a given visit if they were reported to be taking DRT at least 1 day prior to the visit. Levodopa equivalent daily dosage (LEDD) was calculated for each post-baseline visit.21 For analyses, we selected the LEDD either at the time of the initial QUIP conversion from negative to positive, or at the last available visit for those remaining QUIP-negative.
Clinical measures
ICD behaviours were assessed with the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's disease (QUIP)-short form. The QUIP is designed as a screening instrument for ICD behaviours based on any single positive response for the four major ICDs (gambling, eating, buying, sexual behaviours), hobbyism, simple motor activities (ie, punding), or walkabout items.22 A positive QUIP was defined as a positive response for any of these disorders, as previously described.4 Other neuropsychiatric assessments included baseline 15-item Geriatric Depression Scale (GDS-15),23 State-Trait Anxiety Inventory (STAI)24 and Montreal Cognitive Assessment (MoCA).25 Motor severity at baseline was assessed with the Movement Disorders Society-Unified Parkinson's Disease Rating Scale Part III (MDS-UPDRS III).
DAT imaging
DaTscan SPECT imaging was completed at the various PPMI sites, after a standardisation procedure for equipment calibration and data acquisition (full protocol can be found at http://www.ppmi-info.org/study-design/research-documents-and-sops/). Processing and reconstruction was verified, and data analysed at a central imaging core by experienced nuclear medicine experts to ensure consistency. Regions of interest were placed on right and left caudate and putamen, and occipital cortex (reference tissue) to obtain striatal binding ratios. For our study, we also calculated mean total striatal binding ratios (the average of the four aforementioned regions).
Standard protocol approvals, registrations and patient consents
The study was approved by the institutional review board at each participating site and written informed consent was obtained from all study participants prior to enrolment.
Statistical analysis
Kaplan-Meier analysis was used to estimate the rate of initiation of PD medication and to estimate the cumulative frequency of incident ICD behaviours in the full cohort. Kaplan-Meier method uses all participants in the sample and can appropriately account for censored (missing) observations. Observed ICD incidence rates by DRT status (on vs off) were calculated at each visit and compared with Fisher's exact test. Generalised estimating equation26 (GEE) was used to examine predictors of incident ICD behaviour. The logit link was applied to the binary outcome of ICD events over time in the GEE model, and OR was reported to quantify the association between predictors and the risk of developing ICD. GEE analysis accounts for correlations among repeated measures of ICD behaviour and incomplete follow-up (ie, missing data). Individual GEE models were created for each clinical predictor of interest, controlling for baseline age and sex in models. DAT binding ratios for right and left caudate and putamen, and mean total striatal ratio, were analysed as predictors of incident ICD using three separate approaches: (1) baseline DAT binding; (2) change in DAT binding from baseline to year 1; and (3) time-dependent post-baseline DAT binding (assessing the relationship between absolute DAT binding at each post-baseline visit and incident ICD symptoms at that same visit). All models controlled for visit, baseline age and sex. Sensitivity analyses controlling for LEDD were performed. Subgroup analyses were performed including only those participants taking DRT at the time they were assessed for ICD behaviours. Further sensitivity analyses were performed assessing interactions between age and DAT binding and between DRT use and DAT binding.
All statistical tests were 2-sided. Statistical significance was set at p≤0.05. Since our study was exploratory rather than confirmatory, multiple testing adjustment was not performed.27 Analyses were conducted with IBM SPSS Statistics (V.22.0).
Results
Participant characteristics
A total of 320 participants with PD provided baseline clinical and imaging data. A total of 284, 217 and 96 participants with PD provided data at years 1, 2 and 3. Baseline demographic and clinical characteristics are provided in table 1. Twenty-nine and 87 assessed participants were missing DAT imaging data at years 1 and 2. Mean follow-up time from baseline was 1.97 years (SD=0.85). Across the entire PPMI study, the retention rate at the time of analysis was 93%. The majority of participants without year 2 or 3 data remains active study participants but has not yet reached the second or third year of participation.
Table 1. Cohort characteristics.
| Characteristic (N=320) | Number (%) or mean (SD) |
|---|---|
| Sex (male) | 210 (65.6%) |
| Baseline age (years) | 61.7 (9.5) |
| Education (years) | 15.6 (3.0) |
| Baseline MoCA score | 27.2 (2.3) |
| Baseline GDS-15 score | 5.2 (1.3) |
| Baseline STAI score | 93.3 (7.8) |
| Baseline UPDRS III score | 21.2 (9.1) |
| LEDD* | 361.4 (289.4) |
LEDD at time of incident ICD or last follow-up.
GDS-15, Geriatric Depression Scale-15; LEDD, levodopa equivalent daily dosage; MoCA, Montreal Cognitive Assessment; STAI, State-Trait Anxiety Inventory; UPDRS, Unified Parkinson's disease rating scale.
The estimated cumulative rates of DRT initiation were 59.4%, 85.1% and 93.9% at years 1, 2 and 3 (figure 1). A similar proportion of participants were taking levodopa and dopamine agonists at year 1 (see etable 1). The percentages of participants taking levodopa, dopamine agonists, MAO-B inhibitors and amantadine increased over time (see etable 1).
Figure 1.
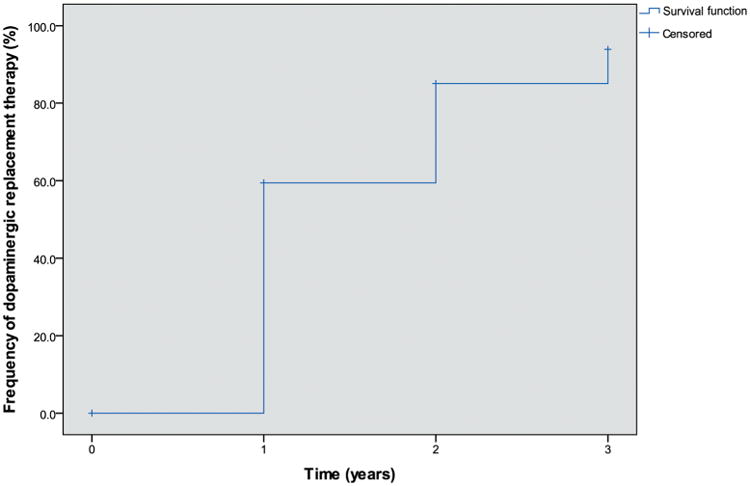
Estimated cumulative rate of initiation of dopamine replacement therapy. Rate of dopamine replacement therapy (DRT) initiation over time determined by Kaplan-Meier method. Censored data indicates that some participants at each visit had not initiated DRT by that visit and have not yet provided subsequent medication information.
Incident ICD behaviours
Fifty-four participants developed incident ICD or related behaviour symptoms during follow-up. The overall number of positive screens per ICD behaviour were as follows: gambling (n=3), sex (n=13), buying (n=4), eating (n=9), hobbyism (n=9), simple motor activities (n=7), walkabout (n=2), multiple ICD behaviours (n=7). The estimated cumulative rate of incident ICD symptoms was 7.8%, 18.1% and 25.1% at years 1, 2 and 3 (figure 2). The observed cumulative incidence rate of ICD symptoms increased numerically over time in participants on DRT, and decreased over time in those not on DRT, though this difference did not reach statistical significance at any time point (table 2). At the time of the positive screen for incident ICD behaviour, 20 of the 54 participants were on levodopa (37%), 23 on a dopamine agonist (43%), 11 on other DRT (20%) and 7 on no DRT (13%). Mean total LEDD at the time incident ICD symptoms were reported was 300.9 mg (SD 210.1), and mean dopamine agonist LEDD was 151.2 mg (SD 65.3).
Figure 2.
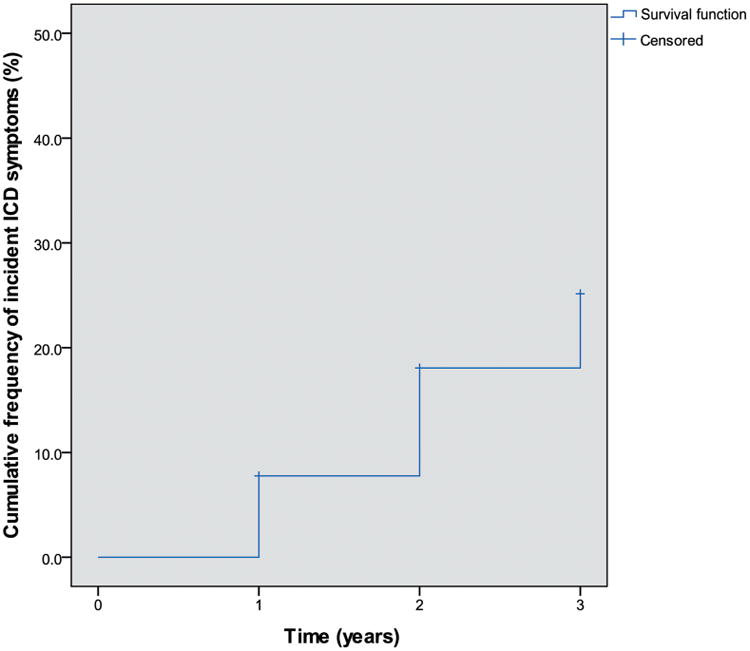
Estimated cumulative rate of incident impulse control disorder (ICD) symptoms. Rate of incident ICD or related behaviour symptoms determined by Kaplan-Meier method. Censored data indicates that some participants at each visit had not developed ICD symptoms and have not yet completed subsequent ICD assessment.
Table 2. Observed cumulative rate of incident ICD symptoms by DRT use.
| Year 1 (N=284) | Year 2 (N=217) | Year 3 (N=96) | |
|---|---|---|---|
| Total participants on DRT (n, %) | 183 (64.4%) | 188 (86.6%) | 85 (88.5%) |
| Incident ICD on DRT (n, %) | 17 (9.3%) | 28 (14.9%) | 16 (18.8%) |
| Total participants not on DRT (n, %) | 101 | 29 | 11 |
| Incident ICD: not on DRT n, (%) | 7 (6.9%) | 1 (3.7%) | 0 (0.0%) |
| p Value* | 0.66 | 0.14 | 0.20 |
Fishers exact test comparing incident ICD rates at each visit on versus off DRT. DRT, dopamine replacement therapy; ICD, impulse control disorder.
Clinical and demographic predictors
Younger age at baseline was significantly associated with higher risk of incident ICD symptoms (OR=0.97, p=0.02). Every year decrease in baseline age was associated with an approximately 3% increase in the risk of developing ICD symptoms. Sex, education and baseline global cognitive performance, anxiety symptoms, depressive symptoms and motor severity were not significantly associated with incident ICD symptoms (table 3). Similar results were obtained in the subgroup analysis that included only participants on DRT at the time of a given ICD assessment.
Table 3. Baseline clinical and demographic predictors of incident ICD symptoms.
| 95% CI | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | Wald* | df | OR | Lower | Upper | p Value |
| ALL | ||||||
| Age | 5.07 | 1 | 0.97 | 0.94 | 1.00 | 0.02 |
| Sex | 0.23 | 1 | 1.17 | 0.62 | 2.22 | 0.63 |
| Education | 1.31 | 1 | 1.07 | 0.96 | 1.19 | 0.25 |
| MoCA | 0.24 | 1 | 0.97 | 0.85 | 1.10 | 0.63 |
| STAI | 0.07 | 1 | 1.01 | 1.00 | 1.05 | 0.79 |
| GDS | 2.23 | 1 | 1.18 | 0.95 | 1.47 | 0.14 |
| UPDRS III | 1.72 | 1 | 1.03 | 0.99 | 1.07 | 0.19 |
| LEDD | 2.95 | 1 | 1.00 | >0.99 | 1.00 | 0.09 |
| ON DRT | ||||||
| Age | 3.72 | 1 | 0.97 | 0.94 | 1.00 | 0.05 |
| Sex | 0.05 | 1 | 1.08 | 0.55 | 2.14 | 0.82 |
| Education | 1.67 | 1 | 1.08 | 0.96 | 1.21 | 0.20 |
| MoCA | 0.46 | 1 | 1.05 | 0.92 | 1.20 | 0.50 |
| STAI | 0.03 | 1 | 1.00 | 0.96 | 1.04 | 0.87 |
| GDS | 1.66 | 1 | 1.17 | 0.92 | 1.49 | 0.20 |
| UPDRS III | 0.84 | 1 | 1.02 | 0.98 | 1.07 | 0.36 |
| LEDD | 2.92 | 1 | 1.00 | >0.99 | 1.00 | 0.09 |
Generalised estimating equations models for each variable controlled for baseline age and sex.
DRT, dopamine replacement therapy; GDS, Geriatric Depression Scale; LEDD, levodopa equivalent daily dosage; MoCA, Montreal Cognitive Assessment; STAI, State-Trait Anxiety Inventory; UPDRS III, Unified Parkinson's Disease Rating Scale III.
Baseline and serial DAT imaging as risk factor
Mean total striatal DAT availability decreased from baseline to 1 year (mean=0.17, SD=0.19), with no significant difference between participants on DRT and those not on DRT at this time point (mean difference=−0.001, SE=0.014 (95% CI (−0.03 to 0.03)), p=0.92). This argues against a direct impact of DRT itself on DAT imaging results.
Baseline DAT availability was not a significant predictor of incident ICD symptoms, either in the full cohort or in the DRT subgroup (table 4). For the change in DAT availability from baseline to 1 year, our analysis of the full cohort suggested that a greater decrease in right caudate binding is associated with an increased risk of incident ICD symptoms (OR (95% CI) =2.75 (0.90 to 8.41), p=0.08). In the DRT subgroup only, a greater decrease in right caudate (OR=4.03 (1.39 to 11.68), p=0.01) and total mean striatal (OR=6.90 (1.09 to 43.50), p=0.04) binding was significantly associated with increased risk of incident ICD symptoms.
Table 4. DAT imaging predictors of incident ICD symptoms.
| All participants | Participants on DRT | |||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI)* | p Value | OR (95% CI) | p Value | |
| Baseline DAT binding | ||||
| Right caudate | 1.07 (0.62 to 1.85) | 0.82 | 1.12 (0.61 to 2.06) | 0.71 |
| Left caudate | 0.91 (0.55 to 1.50) | 0.70 | 0.94 (0.54 to 1.67) | 0.84 |
| Right putamen | 0.77 (0.31 to 1.91) | 0.58 | 0.99 (0.35 to 2.83) | 0.99 |
| Left putamen | 0.55 (0.23 to 1.31) | 0.18 | 0.78 (0.29 to 2.14) | 0.63 |
| Mean total striatal | 0.82 (0.36 to 1.86) | 0.64 | 0.99 (0.38 to 2.57) | 0.98 |
| Change in DAT binding (baseline-year 1) | ||||
| Right caudate | 2.75 (0.90 to 8.41) | 0.08 | 4.03 (1.39 to 11.68) | 0.01 |
| Left caudate | 1.58 (0.60 to 4.12) | 0.35 | 1.78 (0.65 to 4.88) | 0.26 |
| Right putamen | 2.37 (0.42 to 13.20) | 0.33 | 3.28 (0.44 to 24.55) | 0.25 |
| Left putamen | 1.66 (0.41 to 6.69) | 0.48 | 2.52 (0.54 to 11.73) | 0.24 |
| Mean total striatal | 4.04 (0.64 to 25.37) | 0.14 | 6.90 (1.09 to 43.50) | 0.04 |
| DAT binding (post-baseline)† | ||||
| Right caudate | 0.66 (0.30 to 1.47) | 0.31 | 0.47 (0.21 to 1.08) | 0.07 |
| Left caudate | 0.66 (0.30 to 1.46) | 0.31 | 0.62 (0.25 to 1.58) | 0.32 |
| Right putamen | 0.17 (0.03 to 0.88) | 0.04 | 0.06 (0.01 to 0.45) | 0.01 |
| Left putamen | 0.17 (0.03 to 0.87) | 0.03 | 0.15 (0.02 to 1.18) | 0.07 |
| Mean total striatal | 0.36 (0.11 to 1.16) | 0.09 | 0.25 (0.07 to 0.92) | 0.04 |
Generalised estimating equations models for each variable controlled for visit, baseline age and sex.
Any post-baseline DAT binding value (time-dependent), assessed for association with incident ICD at the corresponding visit.
DAT, dopamine replacement therapy; ICD, impulse control disorder.
In addition, post-baseline DAT availability was analysed as a time-dependent predictor. In the full cohort, lower right (OR=0.17 (0.03 to 0.88), p=0.04) and left (OR=0.17 (0.03 to 0.87), p=0.03) putamen DAT binding ratios at a given post-baseline visit were associated with a higher risk of incident ICD symptoms at that visit. There was a suggestion that lower total mean striatal DAT binding at a given post-baseline visit was associated with increased ICD risk at that visit in the full cohort, and this reached significance in the DRT subgroup (OR=0.25 (0.07 to 0.92), p=0.04). In the DRT subgroup, right putamen DAT binding at a given post-baseline visit was also significantly associated with incident ICD symptoms at that visit (OR=0.06 (0.01 to 0.45), p=0.01).
There was no significant interaction between DAT availability and baseline age on prediction of incident ICD symptoms. Sensitivity analysis evaluating interaction between DRT and the DAT binding variables in the entire cohort revealed similar associations as in the DRT subgroup analysis reported above (data not shown).
Controlling for LEDD did not substantially alter the results of these models (table 5). Lower DAT binding in several regions and mean total striatum at a given post-baseline visit remained predictive of ICD symptoms at that visit in the full cohort and the DRT subgroup, while baseline and change from baseline to year 1 DAT binding ratios were not significant predictors.
Table 5. DAT imaging predictors of incident ICD symptoms controlling for LEDD.
| All participants | Participants on DRT | |||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI)* | p Value | OR (95% CI) | p Value | |
| Baseline DAT binding | ||||
| Right caudate | 0.83 (0.42 to 1.64) | 0.60 | 0.87 (0.44 to 1.75) | 0.71 |
| Left caudate | 0.85 (0.48 to 1.52) | 0.58 | 0.86 (0.47 to 1.59) | 0.64 |
| Right putamen | 0.49 (0.13 to 1.87) | 0.30 | 0.57 (0.15 to 2.16) | 0.41 |
| Left putamen | 0.62 (0.21 to 1.77) | 0.37 | 0.63 (0.21 to 1.88) | 0.40 |
| Mean total striatal | 0.64 (0.24–1.74) | 0.38 | 0.69 (0.25 to 1.94) | 0.48 |
| Change in DAT binding (baseline-year 1) | ||||
| Right caudate | 1.58 (0.58 to 4.31) | 0.38 | 1.67 (0.61 to 4.55) | 0.32 |
| Left caudate | 0.98 (0.38 to 2.53) | 0.96 | 1.00 (0.39 to 2.58) | 1.00 |
| Right putamen | 0.66 (0.10 to 4.25) | 0.66 | 0.75 (0.12 to 4.76) | 0.76 |
| Left putamen | 1.44 (0.25 to 8.27) | 0.68 | 1.54 (0.26 to 9.01) | 0.64 |
| Mean total striatal | 1.29 (0.25 to 6.58) | 0.76 | 1.43 (0.28 to 7.33) | 0.67 |
| DAT binding (post-baseline)† | ||||
| Right caudate | 0.41 (0.17 to 0.95) | 0.04 | 0.47 (0.19 to 1.19) | 0.11 |
| Left caudate | 0.52 (0.20 to 1.37) | 0.18 | 0.61 (0.22 to 1.73) | 0.35 |
| Right putamen | 0.02 (0.002 to 0.21) | 0.001 | 0.02 (0.001 to 0.18) | 0.001 |
| Left putamen | 0.07 (0.01 to 0.56) | 0.01 | 0.05 (0.01 to 0.40) | 0.005 |
| Mean total striatal | 0.15 (0.04 to 0.56) | 0.01 | 0.17 (0.04 to 0.71) | 0.02 |
Generalised estimating equations models for each variable controlled for baseline age and sex.
Any post-baseline DAT binding value (time-dependent), assessed for association with incident ICD at the corresponding visit.
DAT, dopamine replacement therapy; ICD, impulse control disorder; LEDD, levodopa equivalent daily dosage.
Discussion
Incident ICD and related behaviour symptoms were common in this prospective cohort of early patients with PD followed from time of diagnosis, and increased in frequency over time and with initiation of DRT. Younger age was the only clinical predictor of incident ICD symptoms. DAT availability was associated both with incident ICD symptoms at the time of imaging, and with future risk of developing ICD symptoms. Though the specific DAT binding ratios that reached significance varied by statistical approach, the direction of the association was consistent across all analyses, with lower DAT binding (ie, a greater decrease in DAT availability) associated with increased risk of developing ICD symptoms. There may be a synergistic effect of DRT and DAT availability on the development of ICD in patients with PD, since DAT availability was a stronger predictor of ICD development in participants on DRT.
Our study expands the knowledge on the natural history of incident ICD behaviours in this well-characterised cohort. Only one prior study to our knowledge has evaluated ICD incidence,13 reporting incidence of 40% during 21 months of dopamine agonist treatment. However, this study was limited by small sample size, single site and retrospective collection for some data. Our results are consistent with this study and others that suggest ICDs are closely linked to DRT. It has previously been reported in the PPMI cohort that at baseline, the frequency of ICD behaviours is similar in PD and healthy controls.4 We now report that over the first 3 years of follow-up, the frequency of ICD symptoms continues to rise in those on DRT only. Continued follow-up will be required to confirm this finding, and determine if this difference becomes significant over time.
Identifying clinical predictors may guide treatment decisions regarding dopamine agonist use. We found that younger age is a predictor of ICD risk, which is consistent with prior studies. Although depressive and anxiety symptoms have been identified as associated clinical factors, these symptoms were not predictive of ICD development in our study. This difference in findings may be due to our prospective study design, which suggests that depressive and anxiety symptoms are secondary to the concomitant ICD and the associated psychosocial distress. The use of rating scales (GDS-15, STAI) to assess neuropsychiatric symptoms instead of a structured diagnostic interview may have affected our results, though these rating scales are recommended for use in PD.
Bastiaens et al13 also found that motor complications were associated with ICDs. In our study, baseline motor severity did not predict future ICD development, not surprising given these were de novo patients, although motor complications were not specifically assessed. Previous literature regarding the association between cognition and ICDs has been mixed. Our results suggest global cognitive performance in non-demented PD is not a risk factor for ICD development. However, further studies should evaluate if performance on specific relevant tasks predict ICD risk (eg, delayed discounting tasks, response inhibition tasks) in addition to their association with ICDs in cross-sectional studies.28
Dopamine imaging, using multiple modalities, has been proposed as a possible biomarker for ICDs. The majority of prior studies have included only prevalent ICD cases, and imaging at only a single time point. In PD with ICD, ((11)C) raclopride PET binding is reduced in ventral striatum, indicating decreased D2/3 receptor binding and increased dopamine release.15,29 DAT imaging studies have consistently reported decreased striatal DAT availability in patients with PD with ICDs compared with those without ICDs.16,17
Right striatal DAT availability has been found to be more consistently reduced in patients with PD with ICDs.16,17,18 Though the pathophysiology underlying this laterality difference is unknown, the result is consistent with evidence that hypomania/mania, which is a common symptom in the ICD-related dopamine dysregulation syndrome (DDS),30 is associated with right hemisphere abnormalities.31 In Vriend et al,18 reduced right ventral and anterodorsal striatal DAT availability was associated with incident ICD after PD medication initiation.
Our study is consistent with these preliminary findings that reduced DAT availability may be a biomarker for ICD. Importantly, ours is the first study to our knowledge to evaluate incident ICD symptoms in a large, multisite, international de novo cohort, and the first to utilise serial DAT imaging. We were therefore able to evaluate several different statistical methods for using DAT availability as a predictor of incident ICD symptoms over time. Controlling for LEDD, we found that measurements that included baseline DAT binding ratios were less predictive of incident ICD symptoms compared with post-baseline values. Our results suggest that changes in DAT availability even early in the disease course may be more predictive than baseline values for determining future ICD risk.
The relationship between reduced DAT availability and ICD neurobiology is not entirely clear. One hypothesis is that a combination of pre-existing biological and genetic risk factors contributes to DAT functional downregulation. An alternate hypothesis is that striatal DAT availability is decreased due to disease-related dopaminergic neuron degeneration, since DAT availability is a marker of overall dopaminergic synaptic integrity. Specifically, degeneration of dopaminergic projections to the ventral striatum has been posited to result in impaired reward-based learning and perhaps contribute to development of ICDs in patients with PD,18 although we were not able to assess ventral striatal DAT availability here. Since decreased DAT function leads to increased dopamine neurotransmission, this may result in excessive activity in ventral striatum. This imbalance may be exacerbated by introduction of DRT, particularly dopamine agonists, which may overdose the relatively preserved mesolimbic dopamine system compared to the more impaired nigrostriatal system.32 The ventral striatum regulates task shifting and sustained attention, and increased dopamine in this system may lead to perseveration on a task and lack of appropriate response to prefrontal cortex goal-directed cues.28 D2 and D3 receptors are thought to play a major role in this system, and D2/3 receptor specific ligand imaging studies have supported impaired regulation of these receptors in patients with PD with ICDs.33,34
A major challenge in studying DAT availability in participants with PD on DRT has been distinguishing potential changes in DAT availability directly related to DRT use from changes related to ICD behaviours. We sought to address this by using a de novo PD cohort and assessing change in DAT availability as participants initiated DRT. Change in DAT availability over the first year, during which over half of participants initiated DRT, was not significantly different between those who did and did not initiate DRT. This supports previous literature that DAT differences observed between those with and without an ICD is not simply an epiphenomenon of DRT use.17 Furthermore, we performed additional analyses controlling for LEDD. In these models, changes from baseline to year 1 DAT availability in right caudate and mean total striatum were no longer significantly predictive of incident ICDs, suggesting that DRT initiation helped explain these relationships. On the other hand, the models using post-baseline DAT availability did not change substantially after controlling for LEDD. This highlights the importance of absolute DAT availability or changes in DAT availability over longer periods of time in the development of ICDs.
In addition to dopamine neuroimaging and DRT, genetic risk factors may also contribute to ICDs. For instance, polymorphisms in dopamine receptor (DRD3), glutamate NMDA receptor (GRIN2B) and serotonin receptor (HTR2A) genes were associated with PD+ICD in a Korean cohort.35,36 Future analyses of the PPMI cohort can examine genetic predictors, including interaction with DAT imaging and DRTuse, on ICD development in early PD.
Strengths of this study include the large sample size of de novo PD, and prospective serial ICD screening and DAT imaging. Our statistical analysis approach controlled for potential confounders (baseline age and sex) in all models, and accounted for variable duration of follow-up in this ongoing cohort study. In analysis of DAT availability, several complementary statistical approaches were utilised, and the results were consistent overall in terms of direction of association.
Limitations include the relatively small absolute number of participants who developed incident ICD symptoms, which should increase over time in the PPMI cohort as more participants are exposed to DRT for a longer duration and at higher doses. This may explain why no statistically significant difference was found between those on versus not on DRT in rates of incident ICD symptoms. The small sample size limited our ability to assess the differential role of dopamine agonists versus other DRT, as well as differences in DRT and DAT availability between the four major ICDs and punding/hobbyism/walkabout behaviours. In addition, since the QUIP was designed as a screening instrument with high sensitivity (94%) and lower specificity (72%),22 some participants' ICD symptoms may have been false positives or not clinically relevant. Our results remain preliminary, and should be confirmed over a longer duration of follow-up in PPMI and similar early PD cohorts. Future studies should also use more detailed diagnostic assessments to verify clinical ICD diagnosis and allow determination of the severity and impact of ICD symptoms.
In summary, decreased DAT availability, particularly ongoing loss over time, may predict risk of future ICD behaviours in early PD with initiation of DRT. Both neurobiological and clinical variables are risk factors for development of ICD symptoms in the face of DRT. Further study to elucidate the interaction of different risk factors is warranted to improve our understanding of the neurobiology of ICDs in PD, minimise the risk to patients and develop novel therapeutic approaches.
Supplementary Material
Acknowledgments
Funding Supported by the Michael J Fox Foundation for Parkinson's Research and funding partners: Abbott, Avid Radiopharmaceuticals, Biogen Idec, Covance, Bristol-Myers Squibb, Meso Scale Discovery, Piramal, Eli Lilly and Co, F. Hoffman-La Roche Ltd, GE Healthcare, Genentech, GlaxoSmithKline, Merck and Co, Pfizer Inc, and UCB Pharma SA.
Competing interests KMS has received an unrestricted educational grant from Medtronic Inc. SXX is currently funded by NIH grants P30-AG010124, P50-NS053488, P01-AG032953, P01-AG017586, R01-AG040271, U01-NS082134. DW has received research funding or support from Michael J Fox Foundation for Parkinson's Research, National Institutes of Health, Novartis Pharmaceuticals, Department of Veterans Affairs, Avid Radiopharmaceuticals and Alzheimer's Disease Cooperative Study; honoraria from AbbVie, Biotie, Teva Pharmaceuticals, Otsuka, UCB, Clintrex LLC and the CHDI Foundation; license fee payments from the University of Pennsylvania for the QUIP and QUIP-RS; royalties from Wolters Kluweland; and fees for legal consultation for lawsuit related to antipsychotic prescribing in a patient with Parkinson's disease.
Footnotes
Additional material is published online. To view please visit the journal (http://dx.doi.org/10.1136/jnnp-2015-311827)
Contributors KMS contributed to the study concept and design, writing of the manuscript, statistical analysis and interpretation of data. SXX contributed to the interpretation of data, statistical analysis and revision of the manuscript. DW contributed to the study concept and design, analysis and interpretation of data and revision the manuscript for content.
Ethics approval The study was approved by the institutional review board at each participating site.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–95. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Goyal V, Behari M, et al. Impulse control disorders and related behaviours (ICD-RBs) in Parkinson's disease patients: assessment using “Questionnaire for impulsive-compulsive disorders in Parkinson's disease” (QUIP) Ann Indian Acad Neurol. 2015;18:49–59. doi: 10.4103/0972-2327.144311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callesen MB, Weintraub D, Damholdt MF, et al. Impulsive and compulsive behaviors among Danish patients with Parkinson's disease: prevalence, depression, and personality. Parkinsonism Relat Disord. 2014;20:22–6. doi: 10.1016/j.parkreldis.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Weintraub D, Simuni T, Caspell-Garcia C, et al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson's disease. Mov Disord. 2015;30:919–27. doi: 10.1002/mds.26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonini A, Siri C, Santangelo G, et al. Impulsivity and compulsivity in drug-naive patients with Parkinson's disease. Mov Disord. 2011;26:464–8. doi: 10.1002/mds.23501. [DOI] [PubMed] [Google Scholar]
- 6.Voon V, Sohr M, Lang AE, et al. Impulse control disorders in Parkinson disease: a multicenter case—control study. Ann Neurol. 2011;69:986–96. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- 7.Rabinak CA, Nirenberg MJ. Dopamine agonist withdrawal syndrome in Parkinson disease. Arch Neurol. 2010;67:58–63. doi: 10.1001/archneurol.2009.294. [DOI] [PubMed] [Google Scholar]
- 8.Pondal M, Marras C, Miyasaki J, et al. Clinical features of dopamine agonist withdrawal syndrome in a movement disorders clinic. J Neurol Neurosurg Psychiatry. 2013;84:130–5. doi: 10.1136/jnnp-2012-302684. [DOI] [PubMed] [Google Scholar]
- 9.Cunnington AL, White L, Hood K. Identification of possible risk factors for the development of dopamine agonist withdrawal syndrome in Parkinson's disease. Parkinsonism Relat Disord. 2012;18:1051–2. doi: 10.1016/j.parkreldis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Phu AL, Xu Z, Brakoulias V, et al. Effect of impulse control disorders on disability and quality of life in Parkinson's disease patients. J Clin Neurosci. 2014;21:63–6. doi: 10.1016/j.jocn.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Joutsa J, Martikainen K, Vahlberg T, et al. Impulse control disorders and depression in Finnish patients with Parkinson's disease. Parkinsonism Relat Disord. 2012;18:155–60. doi: 10.1016/j.parkreldis.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Hurt CS, Alkufri F, Brown RG, et al. Motor phenotypes, medication and mood: further associations with impulsive behaviours in Parkinson's disease. J Parkinsons Dis. 2014;4:245–54. doi: 10.3233/JPD-130314. [DOI] [PubMed] [Google Scholar]
- 13.Bastiaens J, Dorfman BJ, Christos PJ, et al. Prospective cohort study of impulse control disorders in Parkinson's disease. Mov Disord. 2013;28:327–33. doi: 10.1002/mds.25291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valenca GT, Glass PG, Negreiros NN, et al. Past smoking and current dopamine agonist use show an independent and dose-dependent association with impulse control disorders in Parkinson's disease. Parkinsonism Relat Disord. 2013;19:698–700. doi: 10.1016/j.parkreldis.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Steeves TD, Miyasaki J, Zurowski M, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–85. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cilia R, Ko JH, Cho SS, et al. Reduced dopamine transporter density in the ventral striatum of patients with Parkinson's disease and pathological gambling. Neurobiol Dis. 2010;39:98–104. doi: 10.1016/j.nbd.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Voon V, Rizos A, Chakravartty R, et al. Impulse control disorders in Parkinson's disease: decreased striatal dopamine transporter levels. J Neurol Neurosurg Psychiatry. 2014;85:148–52. doi: 10.1136/jnnp-2013-305395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vriend C, Nordbeck AH, Booij J, et al. Reduced dopamine transporter binding predates impulse control disorders in Parkinson's disease. Mov Disord. 2014;29:904–11. doi: 10.1002/mds.25886. [DOI] [PubMed] [Google Scholar]
- 19.Marek K, Jennings D, Lasch S. The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95:629–35. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de la Riva P, Smith K, Xie SX, et al. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurology. 2014;83:1096–103. doi: 10.1212/WNL.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–85. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub D, Hoops S, Shea JA, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson's disease. Mov Disord. 2009;24:1461–7. doi: 10.1002/mds.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weintraub D, Oehlberg KA, Katz IR, et al. Test characteristics of the 15-item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry. 2006;14:169–75. doi: 10.1097/01.JGP.0000192488.66049.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 25.Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–25. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 26.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 27.Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54:343–9. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 28.Napier TC, Corvol JC, Grace AA, et al. Linking neuroscience with modern concepts of impulse control disorders in Parkinson's disease. Mov Disord. 2015;30:141–9. doi: 10.1002/mds.26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Sullivan SS, Wu K, Politis M, et al. Cue-induced striatal dopamine release in Parkinson's disease-associated impulsive-compulsive behaviours. Brain. 2011;134:969–78. doi: 10.1093/brain/awr003. [DOI] [PubMed] [Google Scholar]
- 30.Giovannoni G, O'Sullivan JD, Turner K, et al. Hedonistic homeostatic dysregulation in patients with Parkinson's disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry. 2000;68:423–8. doi: 10.1136/jnnp.68.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson RG, Starkstein SE. Mood disorders following stroke: new findings and future directions. J Geri Psych. 1989;22:1–15. [PubMed] [Google Scholar]
- 32.Cools R, Barker RA, Sahakian BJ, et al. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–43. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 33.Ray NJ, Miyasaki JM, Zurowski M, et al. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson's patients with medication-induced pathological gambling: a [11C] FLB-457 and PET study. Neurobiol Dis. 2012;48:519–25. doi: 10.1016/j.nbd.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Payer DE, Guttman M, Kish SJ, et al. [(1)(1)C]-(+)-PHNO PET imaging of dopamine D(2/3) receptors in Parkinson's disease with impulse control disorders. Mov Disord. 2015;30:160–6. doi: 10.1002/mds.26135. [DOI] [PubMed] [Google Scholar]
- 35.Lee JY, Jeon BS, Kim HJ, et al. Genetic variant of HTR2A associates with risk of impulse control and repetitive behaviors in Parkinson's disease. Parkinsonism Relat Disord. 2012;18:76–8. doi: 10.1016/j.parkreldis.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Lee JY, Lee EK, Park SS, et al. Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson's disease. Mov Disord. 2009;24:1803–10. doi: 10.1002/mds.22678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


