Abstract
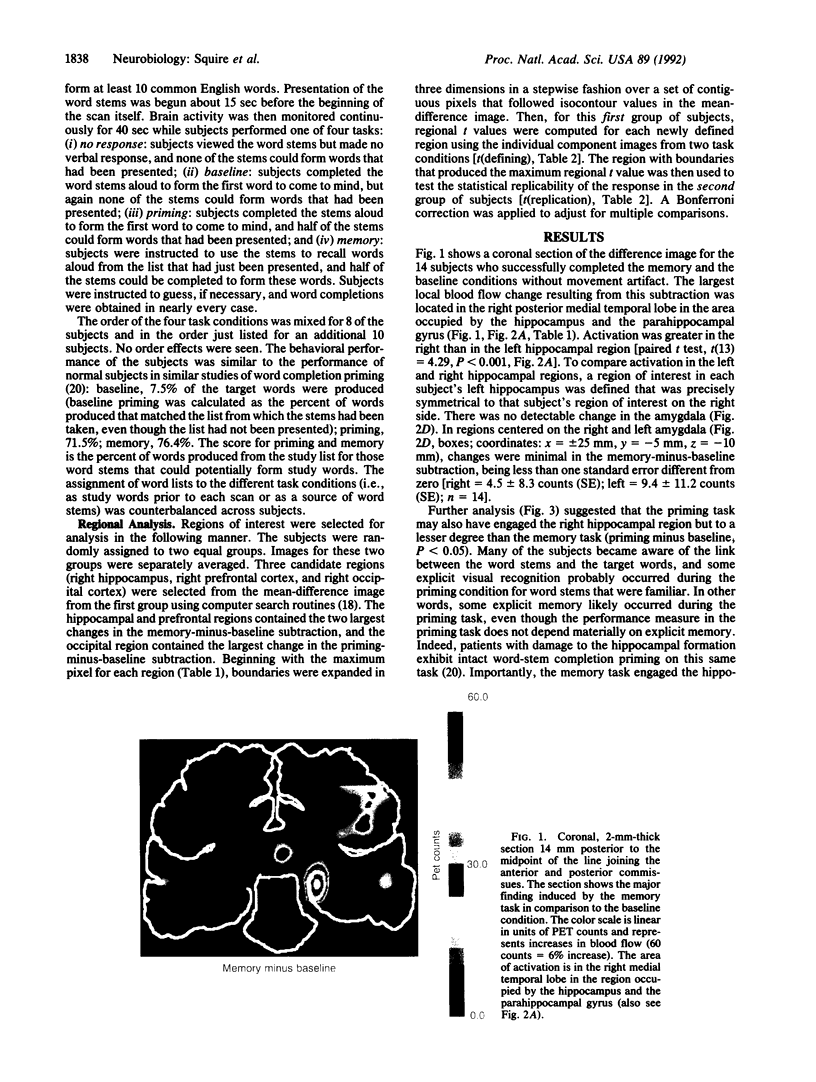
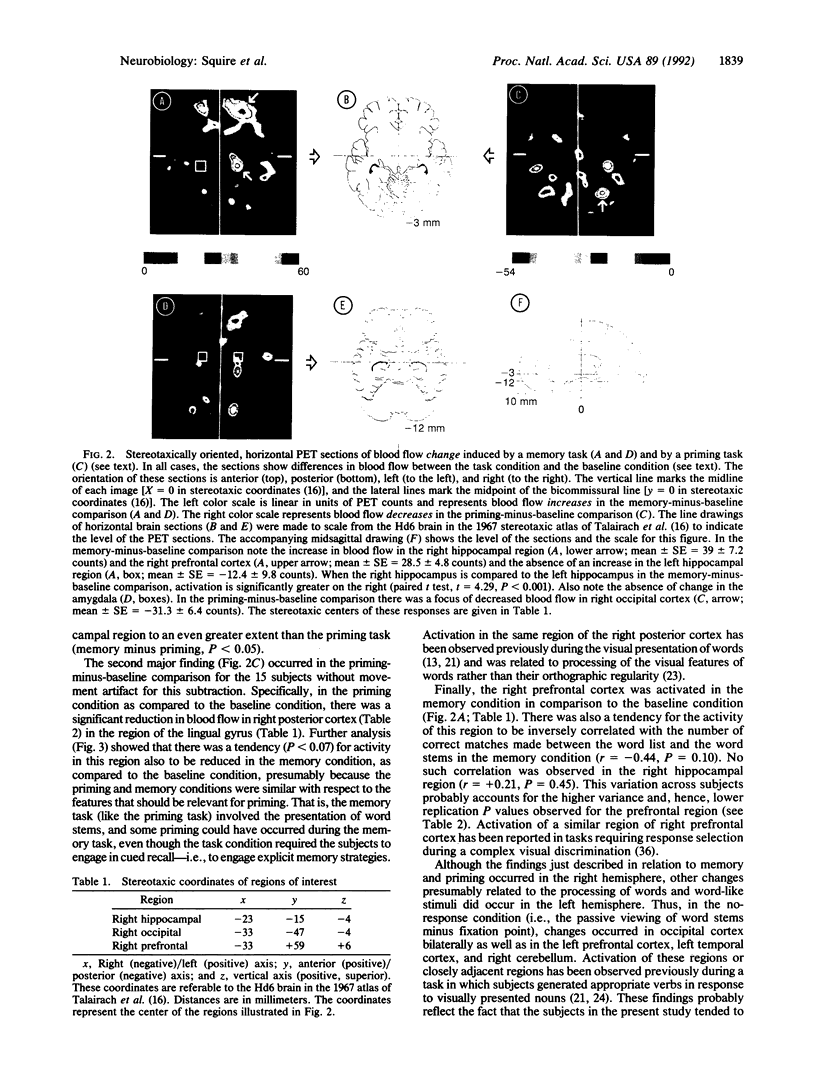
We studied regional cerebral blood flow using the H2(15)O method while normal subjects performed four similar tasks involving three-letter word beginnings (stems). Prior to each task, subjects studied a list of words. Local blood flow was then monitored during a 40-sec period while subjects (i) silently viewed word stems, (ii) completed stems to form the first words to come to mind, but the stems were not the beginnings of any study words (baseline), (iii) completed stems and half of them could form study words (priming), or (iv) tried to recall study words, and half of the stems could form these words (memory). There were three major findings. (i) The memory task engaged the right hippocampal region when the memory task was compared to either the baseline or the priming condition. The right hemispheric locus suggests that performance is driven by the visual characteristics of the words rather than by semantic or phonetic analysis. (ii) In the priming-minus-baseline comparison, there was reduction in blood flow in the right posterior cortex. (iii) Right prefrontal cortex was activated in the memory-minus-baseline condition. The results provide evidence for selective activation of the human hippocampal region in association with memory function. The results also lead to a suggestion about the neural basis of repetition priming: following presentation of a stimulus, less neural activity is required to process the same stimulus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger T. W., Thompson R. F. Identification of pyramidal cells as the critical elements in hippocampal neuronal plasticity during learning. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1572–1576. doi: 10.1073/pnas.75.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Miezin F. M., Dobmeyer S., Shulman G. L., Petersen S. E. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991 Aug;11(8):2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. T., Mintun M. A. Noninvasive functional brain mapping by change-distribution analysis of averaged PET images of H215O tissue activity. J Nucl Med. 1989 Feb;30(2):141–149. [PubMed] [Google Scholar]
- Fox P. T., Mintun M. A., Raichle M. E., Herscovitch P. A noninvasive approach to quantitative functional brain mapping with H2 (15)O and positron emission tomography. J Cereb Blood Flow Metab. 1984 Sep;4(3):329–333. doi: 10.1038/jcbfm.1984.49. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Mintun M. A., Reiman E. M., Raichle M. E. Enhanced detection of focal brain responses using intersubject averaging and change-distribution analysis of subtracted PET images. J Cereb Blood Flow Metab. 1988 Oct;8(5):642–653. doi: 10.1038/jcbfm.1988.111. [DOI] [PubMed] [Google Scholar]
- Friedman H. R., Goldman-Rakic P. S. Activation of the hippocampus and dentate gyrus by working-memory: a 2-deoxyglucose study of behaving rhesus monkeys. J Neurosci. 1988 Dec;8(12):4693–4706. doi: 10.1523/JNEUROSCI.08-12-04693.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf P., Squire L. R., Mandler G. The information that amnesic patients do not forget. J Exp Psychol Learn Mem Cogn. 1984 Jan;10(1):164–178. doi: 10.1037//0278-7393.10.1.164. [DOI] [PubMed] [Google Scholar]
- Herscovitch P., Markham J., Raichle M. E. Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis. J Nucl Med. 1983 Sep;24(9):782–789. [PubMed] [Google Scholar]
- Levy J., Trevarthen C. Perceptual, semantic and phonetic aspects of elementary language processes in split-brain patients. Brain. 1977 Mar;100(Pt 1):105–118. doi: 10.1093/brain/100.1.105. [DOI] [PubMed] [Google Scholar]
- Miller E. K., Li L., Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991 Nov 29;254(5036):1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- Mintun M. A., Fox P. T., Raichle M. E. A highly accurate method of localizing regions of neuronal activation in the human brain with positron emission tomography. J Cereb Blood Flow Metab. 1989 Feb;9(1):96–103. doi: 10.1038/jcbfm.1989.13. [DOI] [PubMed] [Google Scholar]
- Petersen S. E., Fox P. T., Posner M. I., Mintun M., Raichle M. E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988 Feb 18;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petersen S. E., Fox P. T., Snyder A. Z., Raichle M. E. Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science. 1990 Aug 31;249(4972):1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- Riches I. P., Wilson F. A., Brown M. W. The effects of visual stimulation and memory on neurons of the hippocampal formation and the neighboring parahippocampal gyrus and inferior temporal cortex of the primate. J Neurosci. 1991 Jun;11(6):1763–1779. doi: 10.1523/JNEUROSCI.11-06-01763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland P. E., Gulyás B., Seitz R. J., Bohm C., Stone-Elander S. Functional anatomy of storage, recall, and recognition of a visual pattern in man. Neuroreport. 1990 Sep;1(1):53–56. doi: 10.1097/00001756-199009000-00015. [DOI] [PubMed] [Google Scholar]
- Schacter D. L. Perceptual representation systems and implicit memory. Toward a resolution of the multiple memory systems debate. Ann N Y Acad Sci. 1990;608:543–571. doi: 10.1111/j.1749-6632.1990.tb48909.x. [DOI] [PubMed] [Google Scholar]
- Shimamura A. P. Priming effects of amnesia: evidence for a dissociable memory function. Q J Exp Psychol A. 1986 Nov;38(4):619–644. doi: 10.1080/14640748608401617. [DOI] [PubMed] [Google Scholar]
- Squire L. R., Zola-Morgan S. The medial temporal lobe memory system. Science. 1991 Sep 20;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Tulving E., Schacter D. L. Priming and human memory systems. Science. 1990 Jan 19;247(4940):301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S. M., Squire L. R. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science. 1990 Oct 12;250(4978):288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Alvarez-Royo P., Clower R. P. Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991 Apr;1(2):207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Amaral D. G. Lesions of the amygdala that spare adjacent cortical regions do not impair memory or exacerbate the impairment following lesions of the hippocampal formation. J Neurosci. 1989 Jun;9(6):1922–1936. doi: 10.1523/JNEUROSCI.09-06-01922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Amaral D. G., Suzuki W. A. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 1989 Dec;9(12):4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]