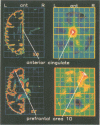
Abstract
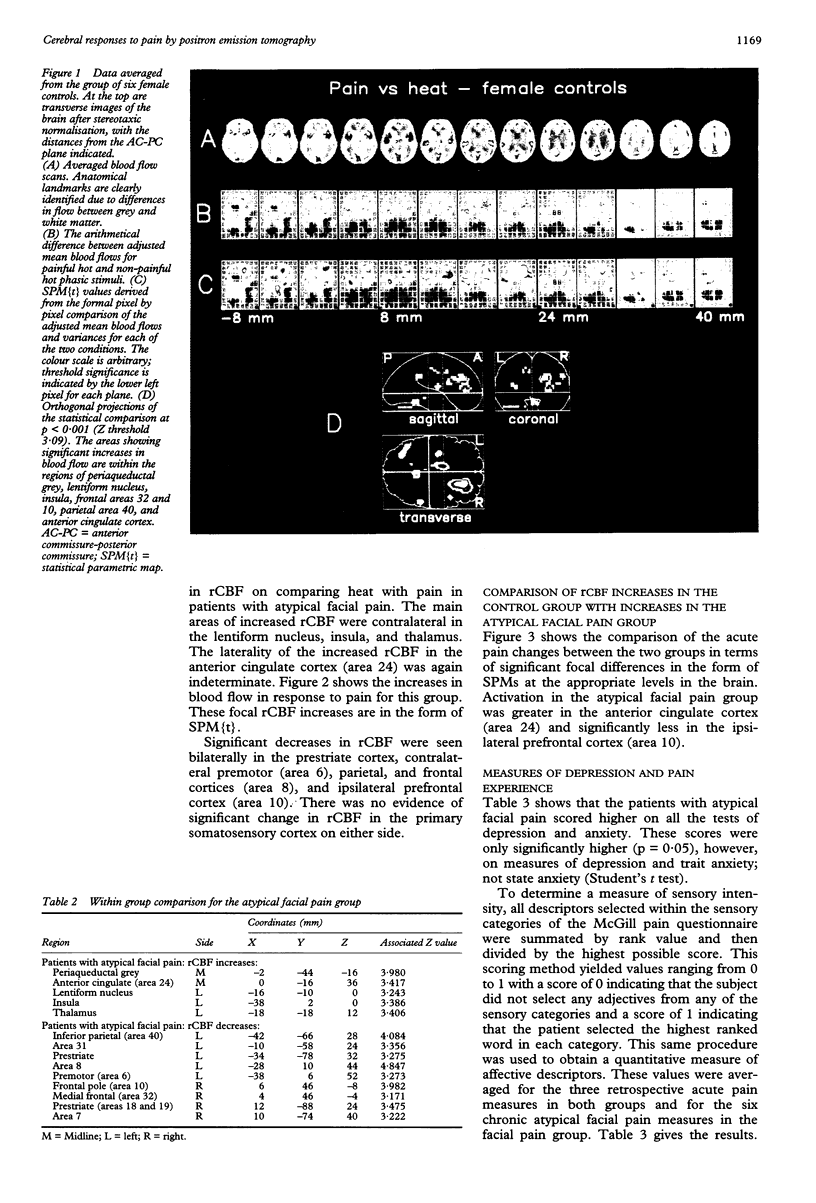
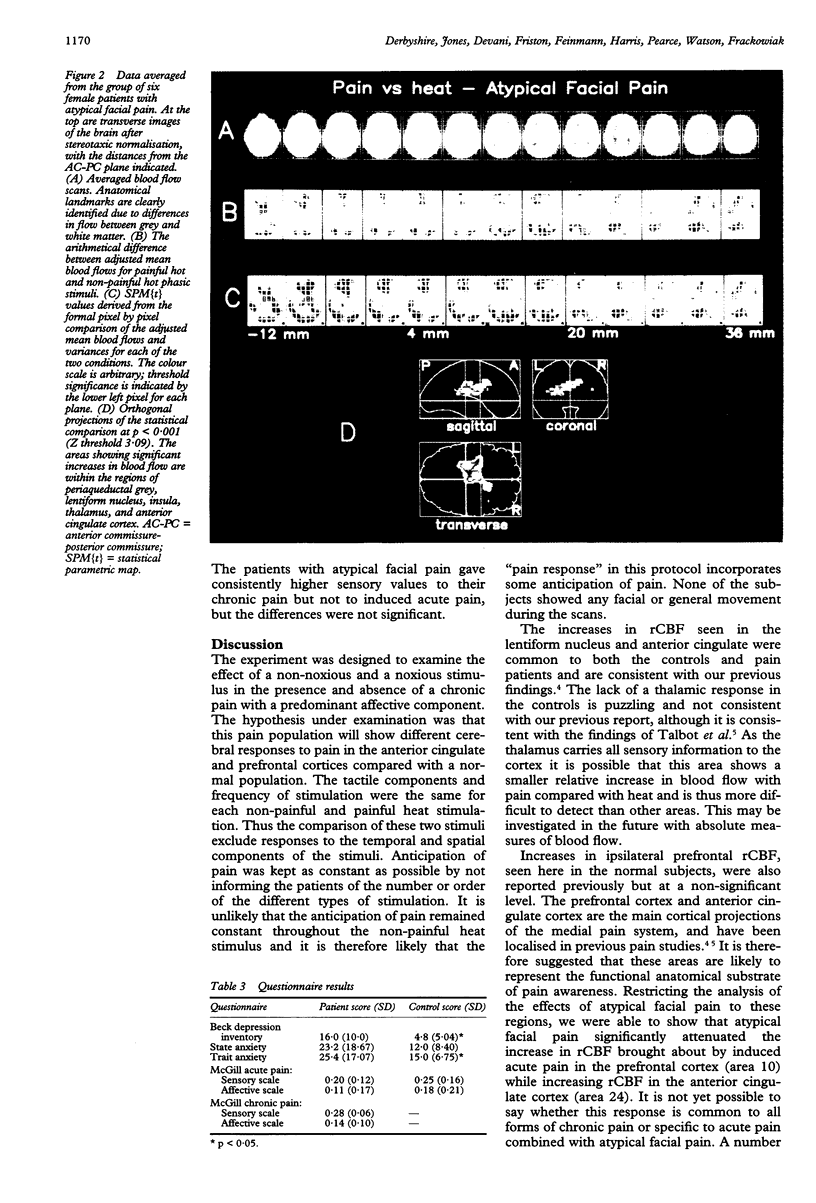
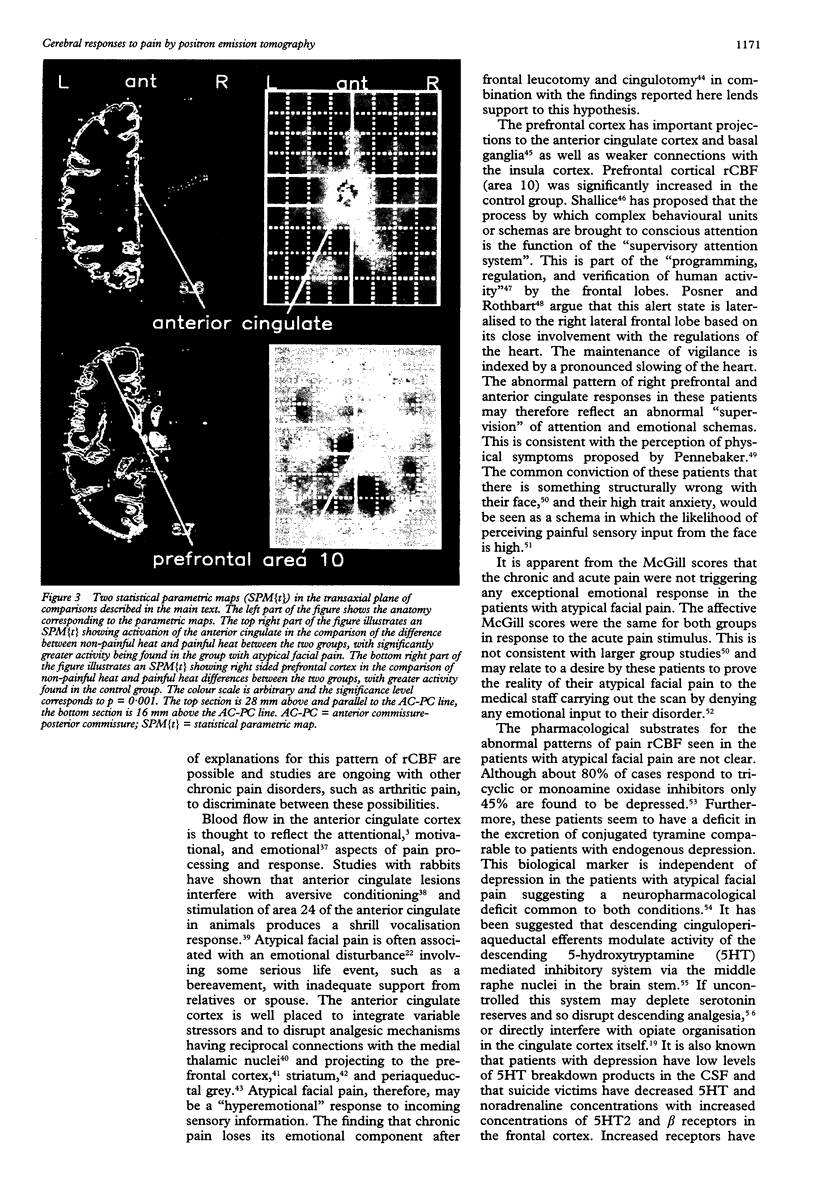
The localised PET cerebral correlates of the painful experience in the normal human brain have previously been demonstrated. This study examined whether these responses are different in patients with chronic atypical facial pain. The regional cerebral responses to non-painful and painful thermal stimuli in six female patients with atypical facial pain and six matched female controls were studied by taking serial measurements of regional blood flow by PET. Both groups displayed highly significant differences in responses to painful heat compared with non-painful heat in the thalamus, anterior cingulate cortex (area 24), lentiform nucleus, insula, and prefrontal cortex. These structures are closely related to the "medial pain system". The atypical facial pain group had increased blood flow in the anterior cingulate cortex and decreased blood flow in the prefrontal cortex. These findings show the importance of the anterior cingulate cortex and the reciprocal (possibly inhibitory) connections with the prefrontal cortex in the processing of pain in patients with this disorder. A hypothesis is proposed to explain the mechanisms of cognitive and pharmacological manipulation of these pain processes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agerberg G., Carlsson G. E. Functional disorders of the masticatory system. I. Distribution of symptoms according to age and sex as judged from investigation by questionnaire. Acta Odontol Scand. 1972 Dec;30(6):597–613. doi: 10.3109/00016357209019791. [DOI] [PubMed] [Google Scholar]
- Aghabeigi B., Feinmann C., Glover V., Goodwin B., Hannah P., Harris M., Sandler M., Wasil M. Tyramine conjugation deficit in patients with chronic idiopathic temporomandibular joint and orofacial pain. Pain. 1993 Aug;54(2):159–163. doi: 10.1016/0304-3959(93)90204-3. [DOI] [PubMed] [Google Scholar]
- BECK A. T., WARD C. H., MENDELSON M., MOCK J., ERBAUGH J. An inventory for measuring depression. Arch Gen Psychiatry. 1961 Jun;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bailey D. L., Jones T., Spinks T. J. A method for measuring the absolute sensitivity of positron emission tomographic scanners. Eur J Nucl Med. 1991;18(6):374–379. doi: 10.1007/BF02258426. [DOI] [PubMed] [Google Scholar]
- Domesick V. B. Projections from the cingulate cortex in the rat. Brain Res. 1969 Feb;12(2):296–320. doi: 10.1016/0006-8993(69)90002-x. [DOI] [PubMed] [Google Scholar]
- Duncan G. H., Bushnell M. C., Talbot J. D., Evans A. C., Meyer E., Marrett S. Pain and activation in the thalamus. Trends Neurosci. 1992 Jul;15(7):252–253. doi: 10.1016/0166-2236(92)90062-d. [DOI] [PubMed] [Google Scholar]
- Duncan G. H., Bushnell M. C., Talbot J. D., Evans A. C., Meyer E., Marrett S. Pain and activation in the thalamus. Trends Neurosci. 1992 Jul;15(7):252–253. doi: 10.1016/0166-2236(92)90062-d. [DOI] [PubMed] [Google Scholar]
- Eysenck M. W., MacLeod C., Mathews A. Cognitive functioning and anxiety. Psychol Res. 1987;49(2-3):189–195. doi: 10.1007/BF00308686. [DOI] [PubMed] [Google Scholar]
- FOLTZ E. L., WHITE L. E., Jr Pain "relief" by frontal cingulumotomy. J Neurosurg. 1962 Feb;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- Feinmann C., Harris M. Psychogenic facial pain. Part 1: The clinical presentation. Br Dent J. 1984 Mar 10;156(5):165–168. doi: 10.1038/sj.bdj.4805298. [DOI] [PubMed] [Google Scholar]
- Feinmann C., Harris M. Psychogenic facial pain. Part 2: Management and prognosis. Br Dent J. 1984 Mar 24;156(6):205–208. doi: 10.1038/sj.bdj.4805304. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Mintun M. A. Noninvasive functional brain mapping by change-distribution analysis of averaged PET images of H215O tissue activity. J Nucl Med. 1989 Feb;30(2):141–149. [PubMed] [Google Scholar]
- Friston K. J., Frith C. D., Liddle P. F., Dolan R. J., Lammertsma A. A., Frackowiak R. S. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990 Jul;10(4):458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- Friston K. J., Frith C. D., Liddle P. F., Dolan R. J., Lammertsma A. A., Frackowiak R. S. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990 Jul;10(4):458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- Friston K. J., Frith C. D., Liddle P. F., Frackowiak R. S. Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab. 1991 Jul;11(4):690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H., Lindblom U., Schmidt W. C. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976 Nov;39(11):1071–1075. doi: 10.1136/jnnp.39.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. K., Brown W. D., Friston K. J., Qi L. Y., Frackowiak R. S. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc Biol Sci. 1991 Apr 22;244(1309):39–44. doi: 10.1098/rspb.1991.0048. [DOI] [PubMed] [Google Scholar]
- Jones A. K., Friston K. J., Qi L. Y., Harris M., Cunningham V. J., Jones T., Feinman C., Frackowiak R. S. Sites of action of morphine in the brain. Lancet. 1991 Sep 28;338(8770):825–825. doi: 10.1016/0140-6736(91)90717-4. [DOI] [PubMed] [Google Scholar]
- Kupers R. C., Konings H., Adriaensen H., Gybels J. M. Morphine differentially affects the sensory and affective pain ratings in neurogenic and idiopathic forms of pain. Pain. 1991 Oct;47(1):5–12. doi: 10.1016/0304-3959(91)90004-H. [DOI] [PubMed] [Google Scholar]
- Leventhal H., Brown D., Shacham S., Engquist G. Effects of preparatory information about sensations, threat of pain, and attention on cold pressor distress. J Pers Soc Psychol. 1979 May;37(5):688–714. doi: 10.1037//0022-3514.37.5.688. [DOI] [PubMed] [Google Scholar]
- Lueck C. J., Zeki S., Friston K. J., Deiber M. P., Cope P., Cunningham V. J., Lammertsma A. A., Kennard C., Frackowiak R. S. The colour centre in the cerebral cortex of man. Nature. 1989 Aug 3;340(6232):386–389. doi: 10.1038/340386a0. [DOI] [PubMed] [Google Scholar]
- MELZACK R., STOTLER W. A., LIVINGSTON W. K. Effects of discrete brainstem lesions in cats on perception of noxious stimulation. J Neurophysiol. 1958 Jul;21(4):353–367. doi: 10.1152/jn.1958.21.4.353. [DOI] [PubMed] [Google Scholar]
- Mazziotta J. C., Huang S. C., Phelps M. E., Carson R. E., MacDonald N. S., Mahoney K. A noninvasive positron computed tomography technique using oxygen-15--labeled water for the evaluation of neurobehavioral task batteries. J Cereb Blood Flow Metab. 1985 Mar;5(1):70–78. doi: 10.1038/jcbfm.1985.10. [DOI] [PubMed] [Google Scholar]
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975 Sep;1(3):277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- Müller-Preuss P., Jürgens U. Projections from the 'cingular' vocalization area in the squirrel monkey. Brain Res. 1976 Feb 13;103(1):29–43. doi: 10.1016/0006-8993(76)90684-3. [DOI] [PubMed] [Google Scholar]
- Pardo J. V., Pardo P. J., Janer K. W., Raichle M. E. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990 Jan;87(1):256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky I., Bassett D., Barrett R., Petrovic L., Minniti R. The Illness Behavior Assessment Schedule: reliability and validity. Int J Psychiatry Med. 1983;13(1):11–28. doi: 10.2190/lddl-r28d-t39h-w3dy. [DOI] [PubMed] [Google Scholar]
- Roland P. Cortical representation of pain. Trends Neurosci. 1992 Jan;15(1):3–5. doi: 10.1016/0166-2236(92)90337-8. [DOI] [PubMed] [Google Scholar]
- Royce G. J. Laminar origin of cortical neurons which project upon the caudate nucleus: a horseradish peroxidase investigation in the cat. J Comp Neurol. 1982 Feb 10;205(1):8–29. doi: 10.1002/cne.902050103. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 25;298(1089):199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Sikes R. W., Vogt B. A. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol. 1992 Nov;68(5):1720–1732. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- Stea R. A., Apkarian A. V. Pain and somatosensory activation. Trends Neurosci. 1992 Jul;15(7):250–253. doi: 10.1016/0166-2236(92)90061-c. [DOI] [PubMed] [Google Scholar]
- Talbot J. D., Marrett S., Evans A. C., Meyer E., Bushnell M. C., Duncan G. H. Multiple representations of pain in human cerebral cortex. Science. 1991 Mar 15;251(4999):1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Vogt B. A., Rosene D. L., Pandya D. N. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979 Apr 13;204(4389):205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- Woods R. P., Cherry S. R., Mazziotta J. C. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992 Jul-Aug;16(4):620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]