Abstract
Essential fatty acids can serve as important regulators of inflammation. A new window into mechanisms for the resolution of inflammation was opened with the identification and structural elucidation of mediators derived from these fatty acids with pro-resolving capacity. Inflammation is necessary to ensure the continued health of the organism after an insult or injury; however, unrestrained inflammation can lead to injury “from within” and chronic changes that may prove both morbid and fatal. The resolution phase of inflammation, once thought to be a passive event, is now known to be a highly regulated, active, and complex program that terminates the inflammatory response once the threat has been contained. Specialized pro-resolving mediators (SPMs) are biosynthesized from omega-3 essential fatty acids to resolvins, protectins, and maresins and from omega-6 fatty acids to lipoxins. Through cell-specific actions mediated through select receptors, these SPMs are potent regulators of neutrophil infiltration, cytokine and chemokine production, and clearance of apoptotic neutrophils by macrophages, promoting a return to tissue homeostasis. This process appears to be defective in several common human lung diseases, such as asthma and COPD, which are characterized by chronic unrestrained inflammation and significant associated morbidity. Here, we highlight translational research in animal models of disease and with human subjects that sheds light on this rapidly evolving area of science and review the molecular and cellular components of the resolution of lung inflammation.
Keywords: Fatty acids, resolution, inflammation, lung
1. Introduction
The hallmarks of acute inflammation include specific cellular events, including increased permeability of the endothelium and epithelium, infiltration of polymorphonuclear leukocytes, inflammatory macrophages, and lymphocytes to sites of infection or injury, and subsequent tissue edema (Cotran, 2009). The cellular events of resolution oppose inflammation and in a process known as catabasis return the host tissues to a non-inflammatory state (Serhan et al., 2004). Barrier integrity is restored and the permeability of endothelium and epithelium is reduced; neutrophils cease trafficking to sites of inflammation; macrophages clear the inflammatory milieu by phagocytosis of microbes and apoptotic neutrophils in a process termed efferocytosis (Savill et al., 1989; Schwab et al., 2007); and neutrophils at the mucosal interface are released by CD55 from the apical surface of epithelial cells for luminal clearance (Campbell et al., 2007). As tissue leukocytes recede from sites of acute inflammation, levels of proinflammatory cytokines and chemokines also decrease. At this turning point, metabolism of polyunsaturated fatty acids switches from conversion to pro-inflammatory mediators, such as leukotrienes and prostaglandins, to pro-resolving mediators, such as lipoxins (Levy et al., 2001). These pro-resolving mediators act as agonists at specific receptors to contribute to the restoration of host tissues to a homeostatic state (Serhan, 2014).
Excessive acute inflammation and chronic unresolved inflammation have been linked to important and morbid human lung diseases, including acute respiratory distress syndrome (ARDS), asthma, cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD). Current research is determining whether the ungoverned inflammation in these diseases reflects a failure of the healthy resolution program in the lungs. This review will examine research in animal models of disease in which SPMs have been shown to increase survival and promote resolution of inflammation as well as several human diseases that are associated with dysregulation of SPM pathways, including SPMs generated from docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA).
2. Polyunsaturated fatty acids are available during acute inflammation
Polyunsaturated fatty acids (PUFAs) are known to be essential nutrients that are not produced by humans to any significant extent. The omega 6 PUFA arachidonic acid is incorporated into cellular phospholipids and on cellular activation is liberated by phospholipase A2 enzymes for enzymatic conversion to prostaglandins, leukotrienes, or lipoxins (Samuelsson et al., 1987; Serhan, 2007). Omega 3 fatty acids are found primarily in dietary fish oils (Burr, 1929; Calder, 2013). The omega 3 PUFAs eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3) are detectable in whole blood from healthy individuals and comprise a small proportion of total fatty acids. EPA levels in blood range from 0.5 to 2.8% of total fatty acids and DHA ranges from 1.3 to 5% (Albert et al., 2002; Gong et al., 1992; Kew et al., 2004; Newcomer et al., 2001; Wakai et al., 2005) varying based on differences in dietary intake. DHA and EPA have long been known to have beneficial health effects including anti-inflammatory, anti-thrombotic, and immuno-regulatory properties (De Caterina, 2011; Iigo et al., 1997). DHA and EPA are concentrated in neural tissues including brain and retina as well as in human milk, plasma, and sperm (Bazan, 2003; Calder, 2013; Crawford et al., 2013). The airway mucosa is also enriched with DHA in health (Freedman et al., 2004). Mechanisms for dietary enrichment of airway mucosal PUFA remain to be determined. These essential omega 3 PUFAs can serve important roles in regulating acute inflammation through their conversion to potent lipid derived pro-resolving mediators (reviewed in (Serhan, 2014)).
DHA and EPA are now known to be available at sites of acute inflammation through several distinct mechanisms. In particular, circulating DHA and EPA in plasma can be detected in inflammatory exudates at the site of tissue injury within minutes of initial injury (Kasuga et al., 2008) (Fig. 1A). DHA and EPA are carried via plasma edema into the inflammatory arena at the tissue level where they are made available for rapid enzymatic conversion into resolvins, protectins, and maresins that are bioactive for tissue inflammation and organ injury (Serhan, 2011). Additionally, on cell activation, DHA and EPA are rapidly released from cell membranes through the activity of select secretory phospholipase A2 enzymes that hydrolyze phospholipids to release free fatty acids (Murakami et al., 2011) (Fig. 1B).
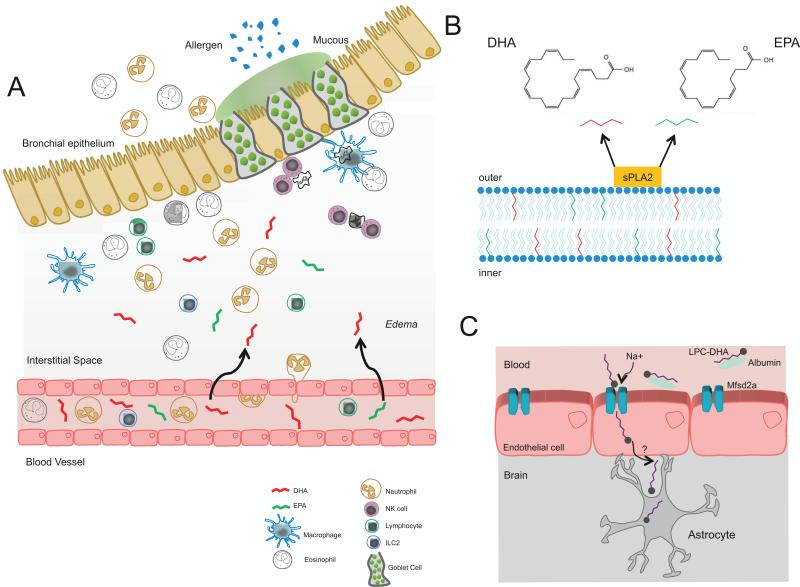
Fig. 1.
EPA and DHA are available at sites of acute inflammation. A) The omega-3 fatty acids EPA and DHA circulate in plasma. On injury they move with edema into the tissue sites of acute inflammation where they are converted to exudate SPMs to interact with local immune cells. Shown is a cartoon representation of acute lung inflammation in the setting of allergic asthma. B) DHA and EPA can also be released from the cell membranes through the actions of secretory phospholipase A2 (sPLA2). C) DHA (as LPC) is transported across the blood brain barrier via the transporter Mfsd2a.
DHA is highly enriched in brain phospholipids and is an essential component for brain growth; however, DHA cannot be synthesized in vivo and must be transported across the blood brain barrier. Recently, a new mechanism for DHA transport across the blood brain barrier was identified in mice where the orphan receptor Mfsd2a is engaged to transport DHA (in the form of lysophosphatidylcholine – LPC) across the blood brain barrier in a Na+-dependent fashion (Nguyen et al., 2014) (Fig. 2C).
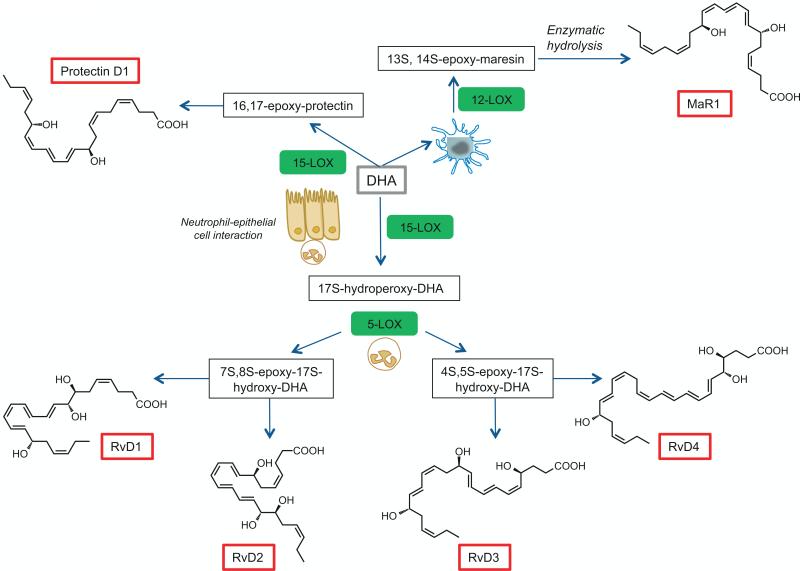
Fig. 2.
Biosynthesis of D-series resolvins, maresins, and protectins. Docosahexaenoic acid (DHA; C22:6n-3; grey box) is converted by 15-LOX to the intermediate 17S-hydroperoxy-DHA which is then further converted by neutrophil derived 5-LOX to the D-series resolvins, RvD1 through RvD4. DHA can also be converted via 15-LOX into a 16, 17-epoxy-protectin intermediate and then onto to protectin D1. DHA is converted via 12-LOX in macrophages to a 13S, 14S-epoxy-maresin and then through enzymatic hydrolysis to MaR1.
3. DHA and EPA are enzymatically transformed to SPMs
Essential PUFAs serve an important role in regulating the acute inflammatory response as substrates for enzymatic conversion to potent lipid-derived mediators (Samuelsson et al., 1987). During resolution of a self-limited experimental model of acute inflammation in vivo, liquid chromatography tandem mass spectrometry (LC-MS/MS)-based lipid mediator metabolomic profiling revealed that EPA and DHA are enzymatically converted into specific bioactive compounds that display protective anti-inflammatory and pro-resolving activities (reviewed in (Serhan, 2014)). The biosynthetic pathways for conversion of DHA to the D-series resolvins, protectins, and maresins is shown in Fig. 2 and the pathways for conversion of EPA to the E-series resolvins are shown in Fig. 3.
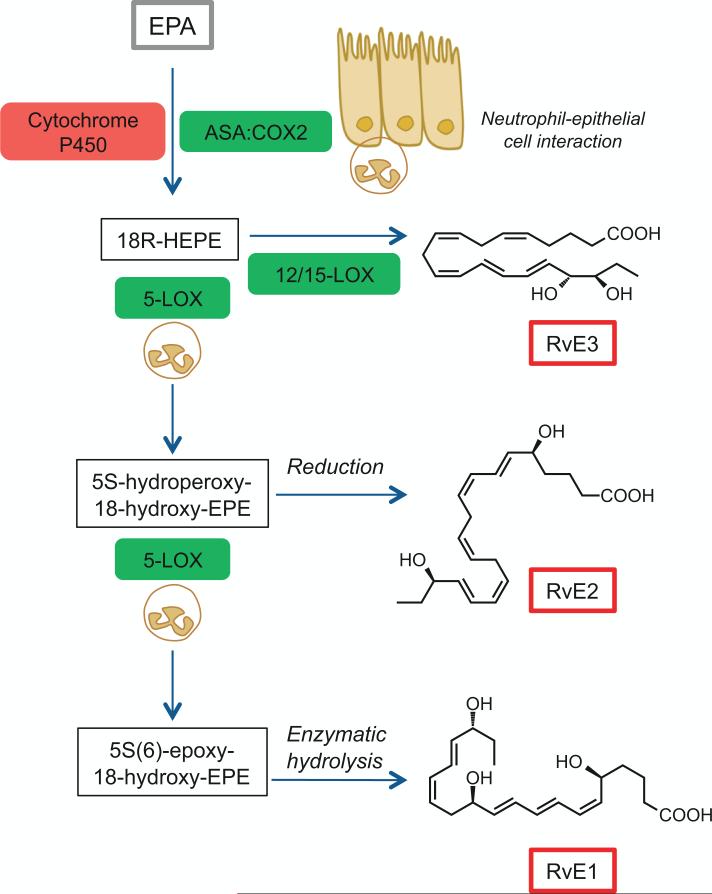
Fig. 3.
Biosynthesis of E-series resolvins. Eicosapentaenoic acid (EPA; C20:5n-3; grey box) is converted via aspirin acetylated COX-2 or by cytochrome p450 in microbes to the intermediate 18R-hydroperoxy-EPE (18R-HEPE). The intermediate 18R-HEPE can be further transformed via neutrophil 5-LOX to an intermediate for subsequent enzymatic conversion to RvE1 or via reduction to RvE2. RvE3 is generated directly from 18R-HEPE via 12/15-LOX.
3.1. D-series Resolvins
DHA (C22:6) is enzymatically converted to the D-series resolvins (RvD1-4) through trans-cellular biosynthesis (Fig. 2). During cell-cell interactions in airway inflammation, 15-lipoxygenase (15-LOX) in epithelial cells converts DHA to 17S-hydroperoxy-DHA, which can be subsequently converted by 5-LOX (in neutrophils) to RvD1 through RvD4 via epoxide-containing biosynthetic intermediates (Hong et al., 2003; Serhan et al., 2002) (Fig. 2A). The aspirin triggered resolvins (AT-RvD1-4) are produced after initial conversion of DHA by aspirin-acetylated COX-2 in vascular endothelial cells to 17R-hydroxy-DHA, which can be transformed via neutrophil-derived 5-LOX to the epimeric forms of the resolvins (Serhan et al., 2002). The stereochemistry of some of the D-series resolvins has been established (RvD1 (Sun et al., 2007), RvD2 (Spite et al., 2009), RvD3 (Dalli et al., 2013a), and additional members (RvD4 through RvD6) have been detected. The D-series resolvins have been detected in blood from healthy individuals and increase with omega 3 fatty acid dietary supplementation (Colas et al., 2014; Oh et al., 2012; Psychogios et al., 2011).
3.2. Protectins
DHA can also be enzymatically converted through 15-LOX action via an epoxide-containing intermediate to the protectin family (also known as neuroprotectins in neural tissues) (Hong et al., 2003; Serhan et al., 2006). Protectin D1 is present in mouse inflammatory exudates, mouse lung, human blood, and human exhaled breath condensates in asthmatic and healthy individuals (Hong et al., 2003; Levy et al., 2007; Serhan et al., 2006).
3.3 Maresins
Macrophage mediators in resolving inflammation, or maresins, are a third major family of DHA-derived SPMs. Maresins are primarily produced in macrophages (Serhan et al., 2009) wherein phagocytosis of apoptotic neutrophils generates SPMs (Freire-de-Lima et al., 2006; Schwab et al., 2007), as well as 14-hydroxy-DHA (14-HDHA) (Serhan et al., 2009). Maresin 1 (MaR1) is generated through conversion of 14-HDHA, via 12-LOX, through a 13,14-epoxide intermediate by human macrophages (Dalli et al., 2013b). MaR1 is also generated during human platelet-neutrophil interaction via platelet 12-LOX conversion of DHA to 13S, 14S-epoxy-maresin followed by neutrophil conversion to MaR1 (Abdulnour et al., 2014).
3.4 Sulfido Conjugates
The DHA metabolome also includes sulfido-conjugates of maresin, protectin, and the D-series resolvins that are bioactive (Dalli et al., 2014; Dalli et al., 2015). These sulfido-conjugate compounds of SPMs are generated by conversion of DHA by activate phagocytes and are active in stimulating the resolution of acute inflammation and promoting tissue regeneration.
3.5 E-series Resolvins
EPA (C20:5) can be enzymatically converted into the E-series resolvins family of SPMs through trans-cellular mechanisms (Fig. 3). EPA is converted to 18R-hydroxyeicosapentaenoic acid (18R-HEPE) by vascular endothelial cells expressing COX-2, which is acetylated in the presence of aspirin during inflammation. 18R-HEPE is then released from endothelial cells and converted via neutrophil-derived 5-LOX through a common epoxy intermediate to RvE1 and RvE2 (Arita et al., 2005a; Ogawa et al., 2009; Oh et al., 2012; Serhan et al., 2000a; Tjonahen et al., 2006). In the absence of aspirin, RvE1 biosynthesis can be initiated through cytochrome p450 enzymatic conversion of EPA (Serhan et al., 2000b). RvE1 has been detected in plasma, sera, and sputum (Arita et al., 2005a; Colas et al., 2014; Yang et al., 2012). RvE3 is distinct from the other E-series resolvins in its biosynthesis, as it is generated via the actions of 12/15-LOX derived from mouse eosinophils (Isobe et al., 2012) rather than neutrophil 5-LOX.
4. Cellular actions of SPMs
Acute inflammation is essential for host defense and survival. Controlling and resolving inflammation to protect vital tissues and organs from collateral damage and further injury is equally as important. The acute inflammatory response resolves in an active way that engages specific cellular events. Neutrophils begin to die via induction of cytokines and T cells undergo activation-induced programmed cell death. SPMs induce the expression of CCR5 on T cells, which scavenges residual proinflammatory chemokines. Macrophages subsequently engulf apoptotic leukocytes clearing the tissue of inflammatory cells and mediators. Efferocytosis also leads to SPM production, which positively feeds back to limit neutrophil migration into the tissues and also enhances further macrophage phagocytosis of apoptotic neutrophils.
In addition to these innate immune effectors, SPMs have actions on multiple lymphocyte subsets. They enhance B cell differentiation into antibody-secreting cells (Ramon et al., 2012), direct NK cell-mediated apoptosis of neutrophils and eosinophils (Barnig et al., 2013) as well as NK cytotoxicity (Haworth et al., 2011), modulate pro-inflammatory cytokine production by innate lymphoid cells (ILCs) (Barnig et al., 2013) and engage regulatory T cells to restrict cytokine production by ILCs (Krishnamoorthy et al., 2014). These findings uncover an interesting and pivotal role for SPMs at the bridge between innate and adaptive immunity during an inflammatory response.
SPMs are active in picogram to nanogram doses and have varied actions that promote control of inflammation, shorten the time interval to resolution, promote healing, and alleviate pain (reviewed in (Serhan, 2014)). The SPMs display stereoselective and cell-type-specific actions, which are discussed below and shown in Table 1.
Table 1.
Cell-based actions of resolvins, protectins and maresins.
| SPM | Cell Type | Action | Reference |
|---|---|---|---|
| Resolvin E1 | T cells | Increases CCR5 expression; Regulates Th17 cells | Ariel et al 2006; Haworth et al 2008 |
| Neutrophils | Inhibits trans-migration and promotes clearance | Campbell et al 2007 | |
| Macrophages | Enhances phagocytosis of apoptotic neutrophils | Schwab et al 2007 | |
| NK Cells | Promotes clearance of eosinophils and neutrophils | Haworth et al 2011 | |
| Dendritic Cells | Inhibits IL-23 release; Reduces migration and IL-12 production | Haworth et al 2008; Arita et al 2005 | |
| Platelets | Reduces activation and aggregation | Dona et al 2008 | |
| Vascular smooth muscle | Confers a protective phenotype switch by decreasing PDGF-stimulated migration | Ho et al 2010 | |
| Resolvin D1 | Neutrophils | Inhibits trans-migration; Stimulates rapid shape change and halts chemotaxis; Blocks adhesion molecules |
Sun et al 2007; Kasuga 2008; Krishnamoorthy et al 2010 |
| Macrophages | Enhances phagocytosis of apoptotic neutrophils; Enhances allergen clearance | Krishnamoorthy et al 2010; Rogerio et al 2012 | |
| B cells | Promotes IgM and IgG production | Ramon et al 2012 | |
| Resolvin D2 | Neutrophils | Reduces recruitment by altering adhesion receptor expression | Spite et al 2009 |
| Macrophages | Enhances phagocytosis of apoptotic neutrophils | Spite et al 2009 | |
| Endothelial cells | Reduces leukocyte: endothelial interactions through NO production | Spite et al 2009 | |
| Protectin D1 | T cells | Blocks T cell migration; Increases CCR5 expression; Promotes T cell apoptosis; Inhibits TNF-α and IFN-γ production |
Ariel et al 2005; Ariel et al 2006 |
| Neutrophils | Inhibits trans-endothelial migration | Schwab et al 2007; Serhan et al 2011 | |
| Macrophages | Promotes efferocytosis of apoptotic neutrophils | Schwab et al 2007; Serhan et al 2011 | |
| Epithelial cells | Protects against oxidative-stress induced apoptosis (retinal pigment epithelium) | Mukherjee et al 2004 | |
| Glial cells | Reduces cytokine production | Hong et al 2003 | |
| Maresin 1 | Macrophages | Stimulates phagocytosis of apoptotic neutrophils | Serhan et al 2009 |
| Platelet-neutrophil aggregates | Decreases lung neutrophils, edema, tissue hypoxia, and prophlogistic mediators | Abdulnour et al 2014 | |
| Innate lymphoid cells (type 2) | Reduces expression of IL-5 and IL-13; increases amphiregulin | Krishnamoorthy et al 2014 | |
| Regulatory T cells (Tregs) | Augments Treg generation leading to ILC2 effects | Krishnamoorthy et al 2014 | |
4.1. D-series Resolvins
RvD1 has been shown to prevent transendothelial migration of neutrophils in a murine model of peritonitis (Sun et al., 2007) and to block integrin adhesion molecules on human neutrophils (Krishnamoorthy et al., 2010). RvD1 also promotes macrophage engulfment and phagocytosis of apoptotic neutrophils (Krishnamoorthy et al., 2010). RvD1 can influence the humoral immune response by increasing production of IgM and IgG from activated B cells (Ramon et al., 2012). RvD1 also enhances macrophage phagocytosis and clearance of allergen in a murine model of allergic airways disease (Rogerio et al., 2012).
RvD2 increases survival in a mouse model of sepsis (Spite et al., 2009). In this model, RvD2 decreases endothelial-leukocyte interactions via nitric oxide production as well as modifying adhesion receptors on leukocytes (Spite et al., 2009). In addition, RvD2 decreases local and systemic bacterial burden, blunts cytokine production and neutrophil recruitment, and increases peritoneal mononuclear cell and macrophage phagocytosis (Spite et al., 2009), all of which contribute to regulation of excessive inflammation.
4.2. Protectins
Protectin D1 is produced from DHA by T helper type 2-skewed PBMCs. Protectin D1 promotes human T cell apoptosis via lipid raft clustering and blocks T cell migration in vivo (Ariel et al., 2005). Protectin D1 promotes upregulation of CCR5 expression on apoptotic T cells leading to termination of chemokine signaling during the resolution phase of inflammation (Ariel et al., 2006). Protectin D1 also inhibits tumor necrosis factor alpha (TNF-[.Delta]) and interferon-gamma (IFN-[.theta1]) secretion from T cells (Ariel et al., 2005). Protectin D1 is active in human retinal pigment epithelial cells to protect against oxidative stress-induced apoptosis (Mukherjee et al., 2004) and in glial cells to block cytokine production (Hong et al., 2003). Finally, like the D-series and E-series resolvins, protectin D1 has also been described to inhibit transendothelial migration of neutrophils and enhance macrophage efferocytosis of apoptotic neutrophils (Schwab et al., 2007; Serhan et al., 2011).
4.3. Maresins
During the resolution phase of murine peritonitis, maresins are a potent stimulus for macrophage uptake and phagocytosis of apoptotic neutrophils (Serhan et al., 2009). MaR1 acts directly on planaria tissue to promote regeneration and in mice MaR1 reduces neuropathic pain (Serhan et al., 2012). MaR1 reduces lung inflammation and ILC type 2 (ILC2) production of the type 2 cytokines IL-5 and IL-13 (Krishnamoorthy et al., 2014). Regulation of ILC2s is selective as MaR1-mediated inhibition of type 2 cytokines was accompanied by increased production of amphiregulin, which is important in tissue repair (Krishnamoorthy et al., 2014). MaR1 also induces regulatory T cell (Treg)-based suppressive activity for ILC2 and Th2 cells (Krishnamoorthy et al., 2014).
4.4. E-series Resolvins
In a mouse model of peritonitis, apoptotic T cells upregulated CCR5 expression in the presence of RvE1 (Ariel et al., 2006). RvE1 also acts at the mucosal surface to reduce transendothelial migration of neutrophils as well as promote luminal clearance of neutrophils by apical CD55 expression at mucosal sites of acute inflammation (Campbell et al., 2007). RvE1 has activity that promotes a reduction in neutrophils by regulating tissue infiltration, increasing macrophage ingestion of apoptotic neutrophils, and enhancing lymphatic clearance of phagocytes (Schwab et al., 2007). RvE1 dramatically reduces dermal inflammation, peritonitis, dendritic cell migration, and IL-12 production (Arita et al., 2005a) and blocks platelet activation and aggregation (Dona et al., 2008). RvE1 modulates vascular smooth muscle cells towards a more protective phenotype not associated with peripheral arterial disease and atherosclerosis (Ho et al., 2010). RvE1 promotes NK cell mediated clearance of eosinophils and antigen specific T cells (Haworth et al., 2011) and regulates pro-inflammatory cytokine production by dendritic cells and Th17 cells in allergic inflammation (Haworth et al., 2008).
5. SPMs act through specific receptors (Fig. 4)
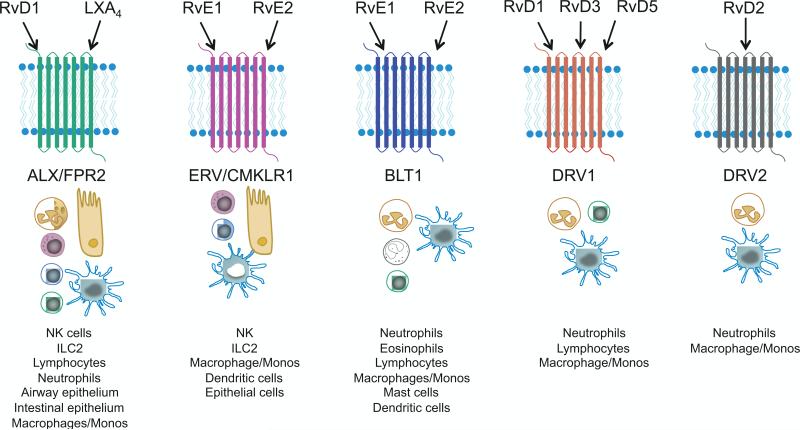
Fig. 4.
Cellular targets and receptors for resolvins, protectins, and maresins. SPMs signal through specific 7-transmembrane G-protein coupled receptors. Lipoxin A4 (LXA4) and the D-series resolvins, in particular RvD1 are agonists for the ALX/FPR2 receptor that is present on a wide variety of leukocytes and other cells. RvE1 and RvE2 are agonists for the CMKLR1/ERV receptor, which is widely expressed on immune cells, and antagonists at the BLT1 receptor. The D-series resolvins are also agonists at the DRV1 and DRV2 receptors. The receptors for protectins and maresins have yet to be molecularly characterized. SPMs signal through these specific receptors to exert influence on leukocytes including halting neutrophil trafficking, promoting macrophage phagocytosis of apoptotic cells and microbes, and blocking further pro-inflammatory cytokine and chemokine production.
5.1. ALX/FPR2 Receptor
The ALX/FRP2 receptor is present on human neutrophils, eosinophils, airway epithelium, monocytes, macrophages, T cells, synovial fibroblasts, and intestinal epithelial cells (Bonnans et al., 2006; Chiang et al., 2006; Fiore et al., 1994; Maddox et al., 1997) as well as human NK cells and ILCs (Barnig et al., 2013). Orthologs of the ALX receptor are expressed in mice (Takano et al., 1997) and rats (Chiang et al., 2003) with preserved structure and function. The ALX receptor is a 7-transmembrane G-protein coupled receptor that interacts with and transmits intracellular signals from LXA4, 15-epi-LXA4, RvD1, aspirin-triggered RvD1, and the corticosteroid-induced protein annexin A1 (Chiang et al., 2006; Fiore et al., 1994; Krishnamoorthy et al., 2012; Krishnamoorthy et al., 2010). ALX receptor expression on cells is regulated by inflammatory mediators, transcription factors, and epigenetic mechanisms. LXA4 itself increases ALX expression by activating its promoter in a positive-feedback fashion (Simiele et al., 2012). Intriguingly, single-nucleotide polymorphisms in the ALX receptor promoter decrease its activity and are associated with increased cardiovascular risk (Simiele et al., 2012). Additionally, in severe asthma there is a deficit in generation of LXA4 and granulocytes have decreased expression of surface ALX (Levy et al., 2012), suggesting an association with defective signaling through ALX and chronic lung inflammation.
5.2. ERV (CMKLR1) Receptor
The chemokine receptor-like 1 (CMKLR1; also known as ChemR23 and now E-series resolvin (ERV) receptor) is expressed in brain, kidney, cardiovascular, GI, and myeloid tissues (Arita et al., 2005a) and in particular on NK cells, ILCs, macrophages, dendritic cells, and epithelial cells (Barnig et al., 2013; Campbell et al., 2007; Cash et al., 2008; Du and Leung, 2009; Parolini et al., 2007). The ERV receptor is also a 7-transmembrane G-protein coupled receptor that interacts with and transmits intracellular signals from the peptide mediator chemerin as well as RvE1. RvE2 binds to human neutrophils with strong affinity and likely shares receptors with RvE1 (Oh et al., 2012).
ERV is expressed in the lung by both leukocytes and structural cells and signaling through ERV plays pivotal roles in the regulation of innate and adaptive immune cell activation in the lung. In particular, ERV may play a significant role in anti-viral immunity. ERV knock out mice have increased mortality, delayed viral clearance, and increased neutrophilic infiltration and worse lung function compared to controls in a murine model of viral pneumonia (Bondue et al., 2011).
5.3. BLT1 Receptor
The leukotriene B4 receptor 1 (BLT1) is expressed on human neutrophils, eosinophils, monocytes, macrophages, mast cells, dendritic cells, and T cells (Yokomizo et al., 1997). RvE1 and RvE2 are antagonists at the BLT1 receptor, and have counterregulatory effects that lead to inhibition of neutrophil chemotaxis, calcium mobilization, and NF-kB activation (Arita et al., 2005a; Arita et al., 2007).
5.4. DRV1 Receptor
The G-protein coupled receptor 32 (GPR32; now known as the RvD1 receptor—DRV1) is expressed on human neutrophils, lymphocytes, macrophages, and monocytes as well as vascular tissues (Krishnamoorthy et al., 2010). RvD1, its aspirin triggered epimer AT-RvD1, RvD3, and RvD5 have all been shown to bind and signal through the DRV1 receptor (Chiang et al., 2012; Dalli et al., 2013a; Krishnamoorthy et al., 2012; Krishnamoorthy et al., 2010; Sun et al., 2007). It appears that RvD1 differentially interacts with the DRV1 receptor during periods of homeostasis and the ALX receptor during the resolution phase of inflammation (Krishnamoorthy et al., 2012).
5.5. DRV2 Receptor
The G-protein coupled receptor 18 (GPR18; known now as the RvD2 receptor—DRV2) is expressed on human and murine neutrophils, monocytes, and macrophages (Chiang et al., 2015). RvD2 binds to and signals through the DRV2 receptor to limit neutrophil infiltration, enhance phagocyte clearance of bacteria, and promote tissue resolution in a murine model of acute inflammation (Chiang et al., 2015).
5.6. Other Receptors
The molecular identification of the receptors through which protectin D1 signals has not yet been elucidated. Protectin D1 activity is cell-type specific, with a structure activity relationship suggestive of the presence of one or more receptors. Protectin D1 binds to neutrophils at high- and low-affinity binding sites as well as retinal epithelial cells (Marcheselli et al., 2010). The molecular identification of the receptors through which MaR1 signals has also not yet been elucidated; however, MaR1 can block the transient receptor potential V1 (TRPV1) signaling in primary sensory neurons thus reducing inflammatory and neuropathic pain in vivo in mice (Serhan et al., 2012). Studies are ongoing to further define the receptors through with the maresins exert their effects.
6. Human lung diseases are associated with decreased SPMs
In humans, the actions of the resolvins, protectins, and maresins in vivo are just beginning to be investigated. RvD1, RvD2, and RvE1 can be detected in samples of peripheral venous blood from healthy individuals and their levels are modified by omega-3 fatty acid dietary supplementation (Colas et al., 2014; Mas et al., 2012; Psychogios et al., 2011). A recent study has described the upregulation of RvD1 and RvD2 in the plasma of patients recently diagnosed with pulmonary tuberculosis (Frediani et al., 2014). Importantly, RvE1 has also been detected in human milk (Weiss et al., 2013). This conveyance of SPMs from mother to child is strong suggestive evidence of their protective activities. SPMs are also detectable in serum and CSF from patients with multiple sclerosis (Pruss et al., 2013), plasma of patients with IgA nephropathy (Zivkovic et al., 2012), and in sputum from adults with cystic fibrosis (Yang et al., 2012). Protectin D1 is present in serum and cerebrospinal fluid of patients with multiple sclerosis (Pruss et al., 2013), exhaled breath condensates from asthmatics (Levy et al., 2007) and embryonic stem cells (Yanes et al., 2010). Finally, MaR1 has been detected in synovial fluid of patients with rheumatoid arthritis (Giera et al., 2012).
Common lung diseases such as asthma and COPD are characterized in part by unrestrained and chronic inflammation that fails to resolve. Several studies, which we review in this section and Table 2, have identified deficiencies in levels of SPMs in the setting of common human inflammatory lung diseases, suggesting that an insufficiency in the activity of these SPMs in the resolution phase of acute inflammation may perpetuate the chronic inflammation and underlie the pathobiology of these diseases.
Table 2.
Human lung diseases associated with decreased levels of SPMs
| SPM | Disease | Finding | Reference |
|---|---|---|---|
| Resolvin E1 | Cystic Fibrosis | Decreased RvE1 in CF patients with lower lung function | Yang et al 2012 |
| Protectin D1 | Asthma Exacerbation | Decreased PD1 in uncontrolled asthma Decreased PD1 in eosinophils in severe asthma |
Levy et al 2007
Miyata et al 2013 |
| Lipoxin A4 | Severe Asthma | Decreased LXA4 biosynthesis in severe asthma (blood, sputum, BAL); Lower LXA4 levels in exhaled breath condensates correlates with worse lung function |
Levy et al 2005; Vachier et al 2005; Celik et al 2007; Planaguma et al 2008; Bhavsar et al 2010; We et al 2010; Fritscher et al 2012; Kazani et al 2013. |
| Asthma Exacerbation | Decreased LXA4 in exhaled breath condensates during exacerbation | Hasan et al 2012 | |
| Exercise-induced Asthma | Decreased LXA4 in plasma of children with exercise induced bronchospasm | Tahan et al 2008 | |
| Aspirin-intolerant asthma | Decreased lipoxins in aspirin-intolerant asthmatics compared to aspirin tolerant asthmatics |
Sanak et al 2000; Celik et al 2007; Yamaguchi et al 2011 |
|
| COPD | Reduced LXA4 detected in exhaled breath condensates; serum amyloid A opposes LXA4 at ALX |
Fritscher et al 2012; Bozinovski et al 2012 |
|
| Cystic Fibrosis | Decreased LXA4 production and defective lipoxin activity |
Karp et al 2004; Chiron et al 2008; Mattoscio et al 2010; Yang et al 2012 |
|
| Pleural Effusions | LXA4 present in exudative effusions and correlates with neutrophils | Levy et al 2001 | |
| Lung Transplant | LXA4 present in BAL fluid during rejection | Levy et al 2011 | |
| Scleroderma | Decreased LXA4 levels in BAL of patients with scleroderma lung disease | Kowal-Bielecka et al 2005 | |
Severe asthmatic patients have decreased levels of arachidonic acid (i.e., omega-6 PUFA) dervied LXA4 in blood, induced sputum, bronchoalveolar lavage fluid, and exhaled breath condensates that in some cases has correlated with severity of disease (i.e., FEV1) (Bhavsar et al., 2010; Kazani et al., 2013) (Celik et al., 2007; Fritscher et al., 2012; Levy et al., 2005; Planaguma et al., 2008; Vachier et al., 2005). LXA4 is reduced in the exhaled breath condensates of children with status asthmaticus in the pediatric intensive care unit compared to controls (Hasan et al., 2012). Children with exercise-induced asthma have lower levels of LXA4 in plasma after exercise challenge compared to those asthmatics not triggered by exertion (Tahan et al., 2008). Asthmatic individuals who are aspirin-intolerant have a more frequent and severe respiratory symptoms and a lower biosynthetic capacity to generate lipoxins compared to aspirin-tolerant asthmatic patients or controls, which may contribute to their more severe clinical phenotype (Celik et al., 2007; Sanak et al., 2000; Yamaguchi et al., 2011). In addition to asthmatic individuals, moderate to severe COPD patients also have reduced levels of LXA4 in exhaled breath condensates (Fritscher et al., 2012). During exacerbations in glucocorticoid-resistant COPD patients, serum amyloid A levels are markedly increased and because serum amyloid A can pirate ALX/FPR2 receptors to promote inflammation, it opposes the counter-regulatory mechanisms of LXA4 at the ALX receptor, which leads to steroid-refractory inflammation in pre-clinical murine models of airway inflammation (Bozinovski et al., 2012).
LXA4 is present in exudative pleural effusions from human subjects with pleural disease and is associated with pleural exudates that are rich in neutrophils (Levy et al., 2001). Endogenous levels of LXA4 in BAL fluid from human lung transplant recipients correlates with the severity of both airway and vascular rejection, suggesting a role for SPMs in regulating immune-mediated mechanisms of organ rejection (Levy et al., 2011).
In health, the airway mucosa is enriched with DHA. Of interest, mucosal levels of DHA decrease in asthma and cystic fibrosis (Freedman et al., 2004), which would adversely impact the generation of DHA-derived SPMs such as D-series resolvins, maresins, and protectins. Levels of EPA-derived RvE1 in respiratory secretions from adults with CF correlated with better lung function (i.e., FEV1) compared to those patients with undetectable levels of RvE1 (Yang et al., 2012). Lipoxin levels in airway secretions (Karp et al., 2004; Yang et al., 2012) and platelets (Mattoscio et al., 2010) from patients with CF are significantly suppressed compared with controls, and can be increased by antibiotic therapy (Chiron et al., 2008). In a mouse model of CF, exogenous administration of a lipoxin analog suppresses neutrophilic inflammation, decreases pulmonary bacterial burden and attenuates disease severity (Karp et al., 2004).
Protectin D1 is an anti-inflammatory and pro-resolving molecule synthesized by human eosinophils; however, levels of protectin D1 are significantly lower in exhaled breath condensates from subjects with asthma exacerbations (Levy et al., 2007), and eosinophils from patients with severe asthma have decreased protectin D1 production, even in presence of exogenous DHA (Miyata et al., 2013).
Patients with scleroderma lung disease have an upregulation of pro-inflammatory molecules such as leukotrienes in the lungs that are not balanced by a concomitant upregulation of anti-inflammatory or pro-resolving molecules such as LXA4 (Kowal-Bielecka et al., 2005).
Additional studies assessing the presence and activity of SPMs in other human pulmonary diseases such as viral infection, acute respiratory distress syndrome, interstitial lung disease, idiopathic fibrosis, lung cancer and tuberculosis as well as diseases of the pulmonary vasculature such as pulmonary hypertension are warranted.
7. SPMs in animal models of disease
7.1. Allergic asthma
In mouse models of allergic asthma/airway inflammation, SPMs display potent immunomodulatory actions (Table 3). In acute and chronic allergen-driven models, RvE1 promotes the resolution of inflammatory airway responses by inhibiting airway eosinophil and lymphocyte recruitment, suppressing the production of IL-6, IL-13, and IL-23, promoting LXA4 and IFN-γ production, and decreasing airway hyperresponsiveness to inhaled methacholine (Flesher et al., 2014; Haworth et al., 2008). When administered prior to allergen challenge, RvE1 also has anti-inflammatory effects, including decreasing airway eosinophilia and lymphocyte recruitment and decreasing type 2 cytokine secretion (Aoki et al., 2008). RvD1 markedly decreases airway eosinophilia and mucus metaplasia, shortens the resolution interval for lung eosinophilia, significantly enhances macrophage phagocytosis of allergen from the airways, and potently regulates NF-kB activity (Rogerio et al., 2012).
Table 3.
Actions in vivo for SPMs in animal models of disease
| SPM | Disease Model | In vivo action | Reference |
|---|---|---|---|
| Resolvin E1 | Pneumonia | Enhances bacterial clearance; Decreases lung neutrophils; Improves survival | Seki et al 2010 |
| Allergic Airway Inflammation | Enhances T cells and eosinophil clearance; Abrogates airway hyperresponsiveness; Improves mucous metaplasia; Prevents Th2 cytokine release; Enhances IFN-γ production; Suppresses macrophage cytokine production |
Haworth et al 2008; Aoki et al 2008; Flesher et al 2014 | |
| Acid-induced acute lung injury | Decreases neutrophil trafficking to lung; Attenuates pro-inflammatory mediators | Seki et al 2010 | |
| Colitis | Decreases neutrophil recruitment; Down-regulates pro-inflammatory gene expression; Improves survival |
Arita et al 2005b; Ishida et al 2010 |
|
| Peritonitis | Inhibits neutrophil recruitment; Promotes lymphatic removal of phagocytes; Regulates chemokine/cytokine production |
Arita et al 2005a; Bannenberg et al 2005 |
|
| Retinopathy | Protects against pathological neovascularlization | Connor et al 2007 | |
| Heart ischemia | Protects against reperfusion injury after hypoxia | Keyes et al 2010 | |
| Solid organ transplant | Preserves organ function; prolongs survival of allograft | Levy et al 2011 | |
| Resolvin D1 | Acute lung injury (acid-induced)* | Decreases PMNs; Reduces pro-inflammatory cytokines; Improves barrier integrity; Reduces airway resistance |
Eickmeier et al 2013 |
| Acute lung injury (LPS-induced) | Decreases PMN recruitment; reduces TNF-α and IL-6 production; Reduces mortality | Wang et al 2011 | |
| Allergic airway inflammation | Decreases airway eosinophilia and mucus metaplasia; Enhances macrophage phagocytosis of allergen from airways | Rogerio et al 2012 | |
| Secondary lung injury | Reduces second organ injury in ischemia/reperfusion models by inhibiting neutrophil migration | Kasuga et al 2008 | |
| Cigarette induced lung inflammation | Promotes M2 macrophages and neutrophil efferocytosis; Accelerates resolution of inflammation |
Hsiao et al 2013 | |
| Septic Shock | Suppresses release of inflammatory cytokines | Murakami et al 2011 | |
| Alzheimer's disease | Stimulates phagocytosis of amyloid-β by macrophages | Mizwicki et al 2013 | |
| Resolvin D2 | Sepsis | Reduces inflammatory cytokine release; Enhances bacterial clearance; Improves survival | Spite et al 2009 |
| Secondary lung injury | Reduces second organ injury in ischemia/reperfusion models by inhibiting neutrophil migration | Chiang et al 2015 | |
| Burn wound | Prevents secondary thrombosis and necrosis | Bohr et al 2013; Kurihara et al 2013 | |
| Protectin D1 | Influenza (H1N1) infection | Inhibits viral replication and reduces severity of disease | Morita et al 2013 |
| Allergic airway inflammation | Enhances eosinophil and T cell clearance from lung | Levy et al 2007 | |
| Maresin 1 | ARDS | Decreases lung neutrophils, edema, tissue hypoxia, and inflammatory mediators; Dependent on platelet/neutrophil interactions | Abdulnour et al 2014 |
| Allergic airway inflammation | Restrains airway inflammation through Treg suppression of ILC2 cytokine production | Krishnamoorthy et al 2014 | |
| Peritonitis | Reduces neutrophil recruitment | Serhan et al 2009 | |
| Colitis | Promotes macrophage M2 phenotype; Decreases inflammatory cytokines | Marcon et al 2013 | |
AT-RvD1
When protectin D1 is administered before aeroallergen challenge in a murine asthma model, airway eosinophil and T cell recruitment is decreased, airway mucous and levels of pro-inflammatory mediators (including IL-13, cysteinyl leukotrienes and PGD2) are decreased, and airway hyperresponsiveness to inhaled methacholine is dampened (Levy et al., 2007). When protectin D1 is given later, after aeroallergen challenge, the resolution phase of inflammation is markedly accelerated (Levy et al., 2007). MaR1 decreases ILC2 cytokine production and augments the generation of regulatory T cells for anti-inflammatory and pro-resolving actions, respectively (Krishnamoorthy et al., 2014).
7.2. Pneumonia/Acute Lung Injury
In mouse models of pneumonia and acute lung injury, SPMs also display protective actions (Table 3). In a model of aspiration pneumonia where mice receive intratracheal hydrochloric acid followed by Escherichia coli, RvE1 was important in resolving sterile and infectious lung inflammation (Seki et al., 2010). Animals who received RvE1 had significantly decreased lung levels of pro-inflammatory cytokines, including IL-1[.Epsilon], enhanced bacterial clearance, decreased lung neutrophil trafficking, and improved survival (Seki et al., 2010).
In a mouse model of LPS-induced acute lung injury, pretreatment of animals with RvD1 prior to LPS exposure is protective (Wang et al., 2011). RvD1 exposure decreases mortality, improves lung pathological changes, inhibits LPS-induced increases in neutrophil and mononuclear leukocytes recruitment, and inhibits TNF-α and IL-6 production in the BAL fluid (Wang et al., 2011). The RvD1 epimer AT-RvD1 is protective in an acid-injury model of acute lung injury. AT-RvD1 reduces BAL fluid neutrophils by ~75% and inhibits neutrophil-platelet interactions by downregulating both P-selectin and its ligand CD24 (Eickmeier et al., 2013). Animals treated with AT-RvD1 had improved epithelial and endothelial barrier integrity and decreased airway resistance associated with increased BAL fluid levels of epinephrine (Eickmeier et al., 2013). AT-RvD1 also significantly decreased levels of BAL fluid pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α (Eickmeier et al., 2013). Second organ lung injury following reperfusion after ischemia is mediated in part by activated neutrophils that have difficulty navigating the pulmonary capillary bed, leading to local release of several potentially injurious molecules (e.g., proteases, reactive oxygen species and lipases). In murine hind-limb ischemia/reperfusion models, RvD1 analogues and RvD2 provide significant protection against secondary lung damage by controlling leukocyte activation and infiltration by approximately 50% (Chiang et al., 2015; Kasuga et al., 2008).
A mouse model of lethal influenza infection has shown that protectin D1 is a strong inhibitor of viral replication and leads to improved survival in those animals with severe disease (Morita et al., 2013). In a murine model of ARDS, early MaR1 production occurs in the vascular bed and is dependent on platelet-neutrophil interactions (Abdulnour et al., 2014). MaR1 production is associated with lung protective effects, including decreased neutrophil trafficking to lung, decreased production of inflammatory mediators, and reduced lung edema and tissue hypoxia (Abdulnour et al., 2014).
7.3. Other models of disease
In mouse models of peritonitis, RvE1 (Arita et al., 2005a; Bannenberg et al., 2005), RvD1 (Norling et al., 2012; Recchiuti et al., 2011), and MaR1 (Serhan et al., 2009) have all been demonstrated to limit recruitment of neutrophils to site of inflammation, promote lymphatic removal of phagocytes, inhibit pro-inflammatory cytokine production and generally accelerate the resolution of inflammation. In a murine model of colitis, maresin 1 decreases inflammatory cytokines and promotes the M2 phenotype of macrophages (Marcon et al., 2013).
RvD1 (Murakami 2011) and RvD2 (Spite 2009) both reduce the release of pro-inflammatory cytokines and reducing neutrophil trafficking to sites of inflammation in models of septic shock and RvD2 protects against death (Spite 2009). In murine models of significant trauma from burns, RvD2 administered after a burn prevents secondary thrombosis and necrosis of tissue (Bohr et al., 2013) and improves survival (Kurihara et al., 2013).
RvE1 improves survival in models of colitis (Arita et al., 2005b; Ishida et al., 2010), protects against neovascularization in a model of retinopathy (Connor et al., 2007), protects against reperfusion injury in a model of heart ischemia (Keyes et al., 2010), and leads to prolonged survival of allograft in a model of solid organ transplant (Levy et al., 2011).
In a mouse model of cigarette exposure RvD1 promotes differentiation of alternatively activated (M2) macrophages and neutrophil efferocytosis and accelerates the resolution of lung inflammation (Hsiao et al., 2013). RvD1 also improves macrophage phagocytosis of amyloid beta protein in a murine model of Alzheimer's disease (Mizwicki et al., 2013).
8. Conclusions
In order to maintain health, the body must respond to a wide array of insults including injury, infection, and noxious stimuli. In particular, the lung is exposed to this milieu of agents potentially through each breath. The host needs to respond to respiratory pathogens and allergens as well as toxins and irritants by mounting an acute inflammatory response to resolve the insult in a timely manner and restore homeostasis. If there is a failure of catabasis to resolve the acute immune response, then overly exuberant acute inflammation or conversion to chronic inflammation may ensue. The pathobiology of several common lung diseases, such as asthma, COPD, and cystic fibrosis, is linked to a chronic inflammatory state that fails to resolve. Similarly, unrestrained acute lung inflammation, as in acute respiratory distress syndrome, may also result from impaired resolution pathways.
The omega-3 fatty acids DHA and EPA and the omega-6 fatty acid arachidonic acid are available at sites of acute inflammation where they are converted into bioactive SPMs. These SPMs are comprised of several distinct families, including the omega-6 PUFA derived lipoxins, and the omega-3 PUFA derived D-series resolvins, E-series resolvins, protectins, and maresins. The SPMs act at subnanogram doses on specific receptors on diverse cells of the immune system, including neutrophils, macrophages, endothelial and epithelial cells, and lymphocytes. The actions of SPMs direct key features of inflammation resolution including inhibition of neutrophil migration, enhancement of macrophage phagocytosis of apoptotic neutrophils, and suppression of pro-inflammatory cytokines and chemokines. There are several reports in preclinical animal models of disease that demonstrate important roles for SPMs in modulating pathobiology and promoting a return to homeostasis after infection or injury leading to improved outcomes, including survival. Common and important human diseases are associated with decreased levels of SPMs including uncontrolled asthma, COPD, and cystic fibrosis. Of great interest, a recent clinical trial of SPMs in humans showed that topical 15-epi-LXA4 significantly reduced skin inflammation and symptoms in patients with infantile eczema with efficacy that was at least equivalent to the current clinical gold standard therapy topical steroids (Wu et al., 2013), supporting the therapeutic potential of SPMs. Further study of human diseases in the context of inflammation resolution is likely to inform potential new avenues for therapeutic intervention in several common, morbid, and sometimes fatal diseases.
Acknowledgements
This work was funded in part by US National Institutes of Health grants R01-HL122531, U01-HL108712, U10-HL109172, U24-AI118656 and P01-GM095467.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict Of Interest
B.D.L. is an inventor on patents assigned to Brigham and Women's Hospital; some of these patents (those pertaining to Rvs) are licensed to Resolvyx Pharmaceuticals. The interests of B.D.L. were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. M.G.D. has no conflict of interest to disclose.
References
- Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16526–16531. doi: 10.1073/pnas.1407123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochemical and biophysical research communications. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nature immunology. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. The Journal of biological chemistry. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. The Journal of experimental medicine. 2005a;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. Journal of immunology. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proceedings of the National Academy of Sciences of the United States of America. 2005b;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. Journal of immunology. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Science translational medicine. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- Bhavsar PK, Levy BD, Hew MJ, Pfeffer MA, Kazani S, Israel E, Chung KF. Corticosteroid suppression of lipoxin A4 and leukotriene B4 from alveolar macrophages in severe asthma. Respiratory research. 2010;11:71. doi: 10.1186/1465-9921-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondue B, Vosters O, de Nadai P, Glineur S, De Henau O, Luangsay S, Van Gool F, Communi D, De Vuyst P, Desmecht D, Parmentier M. ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS pathogens. 2011;7:e1002358. doi: 10.1371/journal.ppat.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. The American journal of pathology. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, Merritt AS, Wark PA, Hutchinson A, Irving LB, Levy BD, Anderson GP. Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:935–940fff. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr GB, MM A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 1929;82:345–367. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. The Journal of experimental medicine. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik GE, Erkekol FO, Misirligil Z, Melli M. Lipoxin A4 levels in asthma: relation with disease severity and aspirin sensitivity. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2007;37:1494–1501. doi: 10.1111/j.1365-2222.2007.02806.x. [DOI] [PubMed] [Google Scholar]
- Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. The Journal of experimental medicine. 2015;212:1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacological reviews. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Chiang N, Takano T, Arita M, Watanabe S, Serhan CN. A novel rat lipoxin A4 receptor that is conserved in structure and function. British journal of pharmacology. 2003;139:89–98. doi: 10.1038/sj.bjp.0705220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron R, Grumbach YY, Quynh NV, Verriere V, Urbach V. Lipoxin A(4) and interleukin-8 levels in cystic fibrosis sputum after antibiotherapy. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2008;7:463–468. doi: 10.1016/j.jcf.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. American journal of physiology. Cell physiology. 2014;307:C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr., Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature medicine. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran RVK, Collins T. Robbins Pathologic Basis of Disease. Saunders; Philadelphia: 2009. [Google Scholar]
- Crawford MA, Broadhurst CL, Guest M, Nagar A, Wang Y, Ghebremeskel K, Schmidt WF. A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution. Prostaglandins, leukotrienes, and essential fatty acids. 2013;88:5–13. doi: 10.1016/j.plefa.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Dalli J, Chiang N, Serhan CN. Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4753–4761. doi: 10.1073/pnas.1415006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Ramon S, Norris PC, Colas RA, Serhan CN. Novel proresolving and tissue-regenerative resolvin and protectin sulfido-conjugated pathways. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:2120–2136. doi: 10.1096/fj.14-268441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, Petasis NA, Serhan CN. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chemistry & biology. 2013a;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, Serhan CN. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013b;27:2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XY, Leung LL. Proteolytic regulatory mechanism of chemerin bioactivity. Acta biochimica et biophysica Sinica. 2009;41:973–979. doi: 10.1093/abbs/gmp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, Croze RH, Carlo T, Pfeffer MA, Levy BD. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal immunology. 2013;6:256–266. doi: 10.1038/mi.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. The Journal of experimental medicine. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesher RP, Herbert C, Kumar RK. Resolvin E1 promotes resolution of inflammation in a mouse model of an acute exacerbation of allergic asthma. Clin Sci (Lond) 2014;126:805–814. doi: 10.1042/CS20130623. [DOI] [PubMed] [Google Scholar]
- Frediani JK, Jones DP, Tukvadze N, Uppal K, Sanikidze E, Kipiani M, Tran VT, Hebbar G, Walker DI, Kempker RR, Kurani SS, Colas RA, Dalli J, Tangpricha V, Serhan CN, Blumberg HM, Ziegler TR. Plasma metabolomics in human pulmonary tuberculosis disease: a pilot study. PloS one. 2014;9:e108854. doi: 10.1371/journal.pone.0108854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, Alvarez JG, O'Sullivan BP. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. The Journal of biological chemistry. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- Fritscher LG, Post M, Rodrigues MT, Silverman F, Balter M, Chapman KR, Zamel N. Profile of eicosanoids in breath condensate in asthma and COPD. Journal of breath research. 2012;6:026001. doi: 10.1088/1752-7155/6/2/026001. [DOI] [PubMed] [Google Scholar]
- Giera M, Ioan-Facsinay A, Toes R, Gao F, Dalli J, Deelder AM, Serhan CN, Mayboroda OA. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochimica et biophysica acta. 2012;1821:1415–1424. doi: 10.1016/j.bbalip.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Rosner B, Rees DG, Berson EL, Weigel-DiFranco CA, Schaefer EJ. Plasma docosahexaenoic acid levels in various genetic forms of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1992;33:2596–2602. [PubMed] [Google Scholar]
- Hasan RA, O'Brien E, Mancuso P. Lipoxin A(4) and 8-isoprostane in the exhaled breath condensate of children hospitalized for status asthmaticus. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012;13:141–145. doi: 10.1097/PCC.0b013e3182231644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. Journal of immunology. 2011;186:6129–6135. doi: 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nature immunology. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, Creager MA, Serhan CN, Conte MS. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. The American journal of pathology. 2010;177:2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. The Journal of biological chemistry. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PloS one. 2013;8:e58258. doi: 10.1371/journal.pone.0058258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iigo M, Nakagawa T, Ishikawa C, Iwahori Y, Asamoto M, Yazawa K, Araki E, Tsuda H. Inhibitory effects of docosahexaenoic acid on colon carcinoma 26 metastasis to the lung. Br J Cancer. 1997;75:650–655. doi: 10.1038/bjc.1997.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, Mizuno S, Takagawa T, Morita Y, Kutsumi H, Inokuchi H, Serhan CN, Blumberg RS, Azuma T. Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflammatory bowel diseases. 2010;16:87–95. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. The Journal of biological chemistry. 2012;287:10525–10534. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y, Xu Y, Whitsett JA, Accurso FJ, Wills-Karp M, Petasis NA. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nature immunology. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. Journal of immunology. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazani S, Planaguma A, Ono E, Bonini M, Zahid M, Marigowda G, Wechsler ME, Levy BD, Israel E. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. The Journal of allergy and clinical immunology. 2013;132:547–553. doi: 10.1016/j.jaci.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew S, Mesa MD, Tricon S, Buckley R, Minihane AM, Yaqoob P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am J Clin Nutr. 2004;79:674–681. doi: 10.1093/ajcn/79.4.674. [DOI] [PubMed] [Google Scholar]
- Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. American journal of physiology. Heart and circulatory physiology. 2010;299:H153–164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- Kowal-Bielecka O, Kowal K, Distler O, Rojewska J, Bodzenta-Lukaszyk A, Michel BA, Gay RE, Gay S, Sierakowski S. Cyclooxygenase- and lipoxygenase-derived eicosanoids in bronchoalveolar lavage fluid from patients with scleroderma lung disease: an imbalance between proinflammatory and antiinflammatory lipid mediators. Arthritis and rheumatism. 2005;52:3783–3791. doi: 10.1002/art.21432. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, Phipps RP, Petasis NA, Kuchroo VK, Serhan CN, Levy BD. Cutting Edge: Maresin-1 Engages Regulatory T Cells To Limit Type 2 Innate Lymphoid Cell Activation and Promote Resolution of Lung Inflammation. Journal of immunology. 2014 doi: 10.4049/jimmunol.1402534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. The American journal of pathology. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Jones CN, Yu YM, Fischman AJ, Watada S, Tompkins RG, Fagan SP, Irimia D. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:2270–2281. doi: 10.1096/fj.12-219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E, Severe Asthma Research Program, N.H.L., Blood, I. Diminished lipoxin biosynthesis in severe asthma. American journal of respiratory and critical care medicine. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nature immunology. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. Journal of immunology. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Vachier I, Serhan CN. Resolution of inflammation in asthma. Clinics in chest medicine. 2012;33:559–570. doi: 10.1016/j.ccm.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Zhang QY, Bonnans C, Primo V, Reilly JJ, Perkins DL, Liang Y, Amin Arnaout M, Nikolic B, Serhan CN. The endogenous pro-resolving mediators lipoxin A4 and resolvin E1 preserve organ function in allograft rejection. Prostaglandins, leukotrienes, and essential fatty acids. 2011;84:43–50. doi: 10.1016/j.plefa.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. The Journal of biological chemistry. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, Sheets K, Winkler JW, Petasis NA, Serhan CN, Bazan NG. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins, leukotrienes, and essential fatty acids. 2010;82:27–34. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcon R, Bento AF, Dutra RC, Bicca MA, Leite DF, Calixto JB. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. Journal of immunology. 2013;191:4288–4298. doi: 10.4049/jimmunol.1202743. [DOI] [PubMed] [Google Scholar]
- Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clinical chemistry. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- Mattoscio D, Evangelista V, De Cristofaro R, Recchiuti A, Pandolfi A, Di Silvestre S, Manarini S, Martelli N, Rocca B, Petrucci G, Angelini DF, Battistini L, Robuffo I, Pensabene T, Pieroni L, Furnari ML, Pardo F, Quattrucci S, Lancellotti S, Davi G, Romano M. Cystic fibrosis transmembrane conductance regulator (CFTR) expression in human platelets: impact on mediators and mechanisms of the inflammatory response. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:3970–3980. doi: 10.1096/fj.10-159921. [DOI] [PubMed] [Google Scholar]
- Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, Takihara T, Tomomatsu K, Suzuki Y, Oguma T, Sayama K, Arai H, Betsuyaku T, Arita M, Asano K. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. The Journal of allergy and clinical immunology. 2013;131:353–360. e351–352. doi: 10.1016/j.jaci.2012.07.048. [DOI] [PubMed] [Google Scholar]
- Mizwicki MT, Liu G, Fiala M, Magpantay L, Sayre J, Siani A, Mahanian M, Weitzman R, Hayden EY, Rosenthal MJ, Nemere I, Ringman J, Teplow DB. 1alpha,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-beta phagocytosis and inflammation in Alzheimer's disease patients. Journal of Alzheimer's disease : JAD. 2013;34:155–170. doi: 10.3233/JAD-121735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, Kadowaki A, Ohto T, Nakanishi H, Taguchi R, Nakaya T, Murakami M, Yoneda Y, Arai H, Kawaoka Y, Penninger JM, Arita M, Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Taketomi Y, Sato H, Yamamoto K. Secreted phospholipase A2 revisited. J Biochem. 2011;150:233–255. doi: 10.1093/jb/mvr088. [DOI] [PubMed] [Google Scholar]
- Newcomer LM, King IB, Wicklund KG, Stanford JL. The association of fatty acids with prostate cancer risk. Prostate. 2001;47:262–268. doi: 10.1002/pros.1070. [DOI] [PubMed] [Google Scholar]
- Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Urabe D, Yokokura Y, Arai H, Arita M, Inoue M. Total synthesis and bioactivity of resolvin E2. Org Lett. 2009;11:3602–3605. doi: 10.1021/ol901350g. [DOI] [PubMed] [Google Scholar]
- Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. Resolvin E2 formation and impact in inflammation resolution. Journal of immunology. 2012;188:4527–4534. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, Communi D, Parmentier M, Majorana A, Sironi M, Tabellini G, Moretta A, Sozzani S. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109:3625–3632. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, Castro M, Chung KF, Gaston B, Jarjour NN, Busse WW, Wenzel SE, Levy BD. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. American journal of respiratory and critical care medicine. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H, Rosche B, Sullivan AB, Brommer B, Wengert O, Gronert K, Schwab JM. Proresolution lipid mediators in multiple sclerosis - differential, disease severity-dependent synthesis - a clinical pilot trial. PloS one. 2013;8:e55859. doi: 10.1371/journal.pone.0055859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PloS one. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. Journal of immunology. 2012;189:1036–1042. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. Journal of immunology. 2012;189:1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Sanak M, Levy BD, Clish CB, Chiang N, Gronert K, Mastalerz L, Serhan CN, Szczeklik A. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. The European respiratory journal. 2000;16:44–49. doi: 10.1034/j.1399-3003.2000.16a08.x. [DOI] [PubMed] [Google Scholar]
- Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. The Journal of clinical investigation. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. Journal of immunology. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annual review of immunology. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. The Journal of experimental medicine. 2000a;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Gronert K, Chiang N. Anti-microinflammatory lipid signals generated from dietary N-3 fatty acids via cyclooxygenase-2 and transcellular processing: a novel mechanism for NSAID and N-3 PUFA therapeutic actions. J Physiol Pharmacol. 2000b;51:643–654. [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, Zhu M, Winkler JW, Petasis NA. Novel proresolving aspirin-triggered DHA pathway. Chemistry & biology. 2011;18:976–987. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins & other lipid mediators. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. Journal of immunology. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. The Journal of experimental medicine. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. The Journal of experimental medicine. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simiele F, Recchiuti A, Mattoscio D, De Luca A, Cianci E, Franchi S, Gatta V, Parolari A, Werba JP, Camera M, Favaloro B, Romano M. Transcriptional regulation of the human FPR2/ALX gene: evidence of a heritable genetic variant that impairs promoter activity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:1323–1333. doi: 10.1096/fj.11-198069. [DOI] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. The Journal of biological chemistry. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- Tahan F, Saraymen R, Gumus H. The role of lipoxin A4 in exercise-induced bronchoconstriction in asthma. The Journal of asthma : official journal of the Association for the Care of Asthma. 2008;45:161–164. doi: 10.1080/02770900701847068. [DOI] [PubMed] [Google Scholar]
- Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epilipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. The Journal of experimental medicine. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chemistry & biology. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Vachier I, Bonnans C, Chavis C, Farce M, Godard P, Bousquet J, Chanez P. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. The Journal of allergy and clinical immunology. 2005;115:55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Wakai K, Ito Y, Kojima M, Tokudome S, Ozasa K, Inaba Y, Yagyu K, Tamakoshi A, Group JS. Intake frequency of fish and serum levels of long-chain n-3 fatty acids: a cross-sectional study within the Japan Collaborative Cohort Study. J Epidemiol. 2005;15:211–218. doi: 10.2188/jea.15.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, Min S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulmonary pharmacology & therapeutics. 2011;24:434–441. doi: 10.1016/j.pupt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Weiss GA, Troxler H, Klinke G, Rogler D, Braegger C, Hersberger M. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids in health and disease. 2013;12:89. doi: 10.1186/1476-511X-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br J Dermatol. 2013;168:172–178. doi: 10.1111/j.1365-2133.2012.11177.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Higashi N, Mita H, Ono E, Komase Y, Nakagawa T, Miyazawa T, Akiyama K, Taniguchi M. Urinary concentrations of 15-epimer of lipoxin A(4) are lower in patients with aspirin-intolerant compared with aspirin-tolerant asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2011;41:1711–1718. doi: 10.1111/j.1365-2222.2011.03839.x. [DOI] [PubMed] [Google Scholar]
- Yanes O, Clark J, Wong DM, Patti GJ, Sanchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G. Metabolic oxidation regulates embryonic stem cell differentiation. Nature chemical biology. 2010;6:411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free radical biology & medicine. 2012;53:160–171. doi: 10.1016/j.freeradbiomed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- Zivkovic AM, Yang J, Georgi K, Hegedus C, Nording ML, O'Sullivan A, German JB, Hogg RJ, Weiss RH, Bay C, Hammock BD. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics : Official journal of the Metabolomic Society. 2012;8:1102–1113. doi: 10.1007/s11306-012-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]