Abstract
The anti-neoplastic, pro-differentiative effects of bromodomain and extra-terminal (BET) bromodomain (BRD) inhibitors were initially discovered in NUT midline carcinoma (NMC), an aggressive subtype of squamous cancer driven by the BRD4-NUT fusion oncoprotein. BRD4-NUT blocks differentiation and maintains tumor growth through a potent chromatin modifying mechanism. OTX015/MK-8628, a novel oral BET inhibitor, targets BRD2/3/4/T with preclinical activity in NMC and several other tumor types, and is currently in clinical development. Antitumor activity was evaluated in four advanced stage NMC patients with confirmed BRD4-NUT fusions who were treated with 80 mg OTX015/MK-8628 once daily in a compassionate-use context. Two patients responded rapidly with tumor regression and symptomatic relief, and a third had meaningful disease stabilization with a minor metabolic response. The main side effects were mild to moderate gastrointestinal toxicity and fatigue, and reversible grade 3 thrombocytopenia. This is the first proof-of-concept evidence of clinical activity of a bromodomain inhibitor in targeting BRD4-NUT.
Keywords: BRD4-NUT, NUT midline carcinoma, BET inhibitor, OTX015/MK-8628
INTRODUCTION
The role of BRD4 in cancer and the potential of bromodomain and extra-terminal (BET) inhibitors to therapeutically target it, was first identified in NUT midline carcinoma (NMC) (1, 2), a disease harboring BRD4-NUT, the only known oncogene form of BRD4. NMC is a recently described poorly differentiated squamous cell carcinoma (SCC), typically affecting head and neck and thoracic structures. It is observed equally in males and females, and over all ages (range 0-78 years) (3). While considered rare, this tumor is likely underdiagnosed due to the need for a specific (100%) and sensitive (87%) immunohistochemistry (IHC) test for nuclear NUT expression (4). Nonetheless NMC should be considered in the differential diagnosis of any aggressive poorly differentiated carcinoma in the absence of a viral or other known etiology. With a median survival of only 6.7 months, NMC is a very aggressive solid tumor, with most patients having locally advanced or metastatic disease at diagnosis (5). While response to chemotherapy occurs, patients invariably relapse rapidly and are refractory to subsequent treatments (5).
NMC is defined genetically by chromosomal rearrangements involving the NUT gene on chromosome 15q14. In approximately two-thirds of cases, NUT is fused to BET gene BRD4 on chromosome 19p13.1, creating an in-frame BRD4-NUT oncogene driven by the BRD4 promoter, or less commonly to other genes including BRD3 and NSD3 (1, 5-7). BRD4 is a member of the BET protein family, including BRD2, BRD3 and BRDT. BET proteins are components of transcription factor complexes and are thought to be determinants of epigenetic memory (8). The BRD-NUT oncoprotein is considered a major pathogenetic driver of cellular transformation (6). Consistent with its oncogenic function to block differentiation, siRNA knockdown of BRD4-NUT in NMC cells results in differentiation and growth arrest (6), providing a strong rationale for targeting these proteins.
BRD4-NUT function is dependent upon the acetyl-histone-binding of the BRD4 bromodomains (6, 9, 10). BET inhibitors are acetyl-histone mimetic compounds that target BRD4 by competitively inhibiting its binding to chromatin. Indeed, treatment with the paradigm BET inhibitor, JQ1 (an OTX015/MK-8628 analog), depletes BRD4-NUT from chromatin and induces rapid growth arrest and differentiation of NMC cells in vitro and in vivo (2, 10). BRD4 has been shown to play a role in many cancers (11, 12), and at least six clinical trials using BET inhibitors have been initiated (NCT01713582, NCT02259114, NCT02296476, NCT01587703, NCT01987362, NCT02158858).
OTX015/MK-8628, a novel synthetic small molecule targeting BRD2, BRD3 and BRD4, inhibits proliferation in a wide range of hematologic malignancies and solid tumor cell lines (13, 14). In vivo, OTX015/MK-8628 inhibited tumor growth in the Ty82 NMC model harboring a BRD4-NUT fusion (15). We report here on the first NMC patients treated with OTX015/MK-8628 on a compassionate basis.
RESULTS
Patient 1
A 39-year-old female was diagnosed in February 2012 with grade 3 invasive ductal carcinoma of the left breast, positive for hormone receptors and HER2/neu. She received neoadjuvant epirubicin and cyclophosphamide followed by paclitaxel and herceptin. Herceptin and hormone therapy were administered after surgery/radiotherapy. In June 2013, a CT scan showed a 2×3 cm nodule on the left lower lobe of the lung, with increased uptake on an FDG-PET scan (SUVmax, 8.8). A percutaneous core biopsy was initially interpreted as adenosquamous carcinoma. In August 2013 a left lobectomy with mediastinal lymph node dissection revealed a poorly differentiated carcinoma, with extensive necrosis and angio-invasion, morphologically different from the previous breast carcinoma. IHC revealed nuclear NUT protein expression in all tumor cells that were negative for hormone receptors and Her2/neu (Fig. 1A). Fluorescence in situ hybridization (FISH) was positive for BRD4-NUT fusion confirming the NMC diagnosis (Fig. 1B). Molecular profiling confirmed the BRD4-NUT fusion and revealed an activating NOTCH mutation (c.4793G>A (p.R1598H), exon 26) previously described in adenoid cystic carcinoma (16). The tumor lacked additional known oncogenic driving mutations, rearrangements, or copy number variation (Supplementary Tables S1-2). No adjuvant therapy was given after surgery. In February 2014, a lower dorsal subcutaneous nodule was confirmed as NMC (FISH- and IHC-positive). A PET-CT showed extensive metastatic disease involving the left longissimus muscle at the fifth dorsal vertebra, bone (several costal arc and vertebral metastases) and mediastinal lymph nodes. Palliative radiotherapy (20 Gy) was administered to the third right costovertebral articulation.
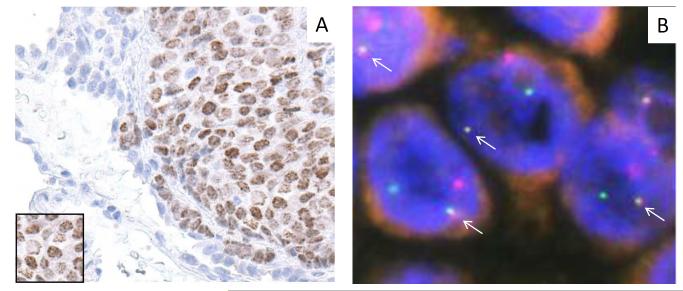
Figure 1. Molecular pathologic features of Patient 1.
A) Diagnostic nuclear, speckled (inset) IHC staining of a biopsy with NUT antibody. Adjacent lung parenchyma is visible. B) Fusion (arrows) of BRD4 (red) and NUT (green) loci by dual color fluorescent in situ hybridization of tumor tissue from Patient 1.
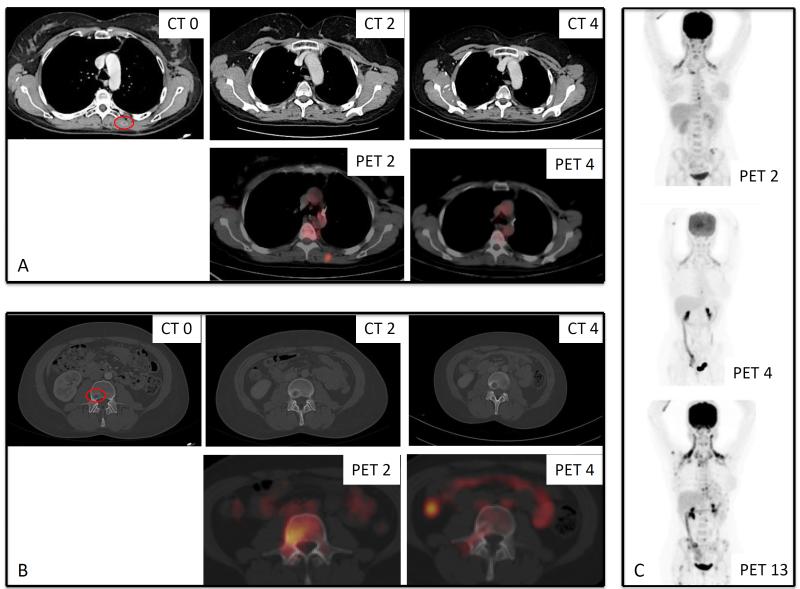
No further treatments were administered after palliative radiotherapy, and the patient was referred for OTX015/MK-8628 treatment , initiated on April 2014. The patient had back pain despite opioids, a dorsal subcutaneous nodular lesion (2 cm diameter) by physical examination. A rapid clinical response was observed, along with symptomatic improvement allowing cessation of opioids during the second week of treatment. After 3 weeks, the soft tissue nodule had disappeared (physical exam). A PET-CT performed at the end of cycle 2 showed metabolic activity in several bone lesions, in lymph nodes, and within a lesion of the left longissimus muscle. On the CT scan, all PET-positive lesions were stable compared with baseline, while increased peripheral density was observed in some bone lesions (Fig 2A-B: CT 0, CT2, PET 2). A bone biopsy was thus performed during cycle 3 (lesion of the third lumbar vertebrae), which showed extensive medullary fibrosis and focal granulomatous reaction around foci of keratinization. A few residual tumor cells were seen with NUT IHC. This was interpreted as a possible treatment effect and minimal residual disease. Based on the clear clinical improvement and stable findings on CT, treatment was continued. After four cycles, PET-CT showed normalization of metabolic activity in all previously PET-positive lesions together with a reduction in the size on CT scan of a retrocardiac lymph node, a lesion in the right costovertebral articulation, and disappearance of the lesion in the left longissimus muscle (Fig 2A-B: CT 4, PET 4; Fig 2C: PET 2 and PET 4). All other lesions were stable on CT. These findings were consistent with a partial remission based on CT. OTX015/MK-8628 was well tolerated. The main side effects included fatigue and headache (grade 1) and reversible thrombocytopenia (grade 3), the latter necessitating ad hoc treatment interruptions (1 to 2 weeks) from cycle 3, and dose reduction to 60 mg QD from cycle 9. In December 2014, at the end of cycle 13, a PET-CT showed disease progression with lung and diffuse bone metastases (Fig 2C: PET 13). A lung biopsy confirmed NMC relapse. The patient developed fever, pain and symptoms of spinal cord compression. Lactate dehydrogenase (LDH) levels increased significantly (4.7x upper normal limit [ULN]). No further systemic therapy was given and the patient died from progressive disease in February 2015, 19 months following her initial NMC diagnosis.
Figure 2. Radiologic response of Patient 1 to OTX015/MK-8628.
A) Lesion in the left longissimus muscle on CT and PET scans over 4 cycles. B) Lesion on the third lumbar vertebra by PET/CT over 4 cycles. A biopsy of this lesion during cycle 3 was consistent with residual NMC. C) Longitudinal assessments and comparison of PET over 13 cycles. CT 0: at baseline, CT 2: after two cycles, CT 4: after 4 cycles, PET 2: after two cycles, PET 4: after 4 cycles, PET 13: after 13 cycles.
Patient 2
A 22-year-old male, was diagnosed in May 2014 with a poorly differentiated carcinoma of the nasopharynx with extensive bone metastases. This diagnosis was subsequently revised to NMC (Fig. 3A), confirmed by the presence of a BRD4-NUT fusion by FISH (data not shown). Molecular profiling showed no additional oncogenic mutations, copy number variation, or rearrangements other than the BRD4-NUT (Supplementary Tables S1-2). He received two cycles of docetaxel, cisplatin and 5-fluorouracil. Despite an initial brief clinical response, his disease progressed rapidly after the second cycle. Two cycles of cisplatin and etoposide were administered and a decrease in volume on facial lesions was observed during the week that followed cisplatin/etoposide, administered as a 21-day cycle, with a new increase in tumor growth starting around day 15. Finally, a CT scan after the second cycle of cisplatin/etoposide confirmed disease progression.
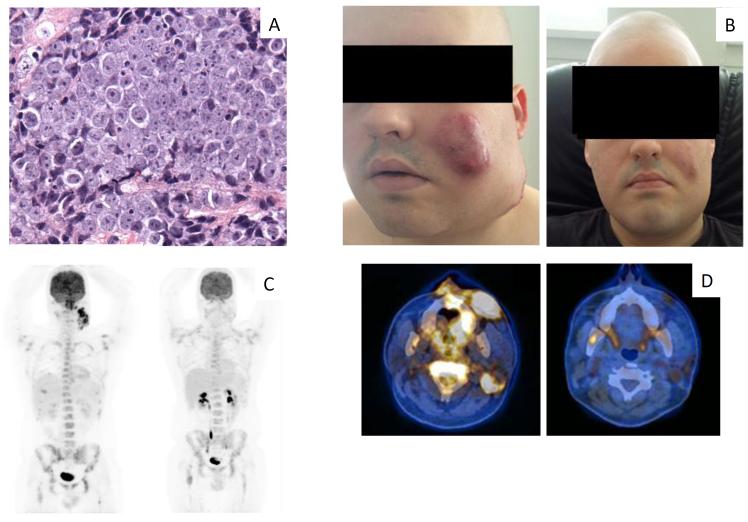
Figure 3. Histopathologic features at baseline and clinical and radiological features at baseline and in response to treatment with OTX015/MK-8628 in Patient 2.
A) Pretreatment biopsy with H&E staining, characterized by undifferentiated malignant epithelioid cells. B) Clinical findings at baseline (on the left) and response observed at day 8 of the first cycle of treatment with OTX015/MK-8628 (on the right). C) Longitudinal assessment of PET at baseline (left) and PET after two cycles (right). D) PET-CT of the primary tumor shown at baseline (left) and after two cycles (right). Photos in panel B published with permission of the patient, given at the time the photos were taken.
The patient was referred in August 2014 with severe dorsolumbar pain due to extensive bone metastases despite opioids and NSAIDs. Laboratory analyses revealed increased LDH (2.3x ULN) and alkaline phosphatase (3.3x ULN). Physical examination showed a bulky tumor involving the left maxillary and mandibular region extending to the ipsilateral cervical lymph nodes. OTX015/MK-8628 was initiated and a dramatic clinical response was achieved with almost complete disappearance of the bulky facial tumor by physical examination after 8 days (Fig. 3B). Pain also improved rapidly, and after an initial increase on day 8, LDH levels were normal by day 15. At the end of cycle 2, a PET-CT showed partial response (Fig. 3C-D).
Treatment was complicated by nausea, dysgueusia, anorexia, hyperglycemia (all grade 2) and thrombocytopenia (grade 3). OTX015/MK-8628 was interrupted during cycle 2 for non-hematologic toxicity and the dose was reduced to 60 mg QD from cycle 3. Clinical evidence of local tumor progression was apparent at the end of cycle 3 (Fig 4A). A tumor biopsy from a local tumor relapse at the mandibular region confirmed NMC progression, with a greater degree of squamous differentiation (Fig. 4B). Analysis of a post-tumor biopsy confirmed decreased NUT expression in areas of differentiation, whereas NUT expression was unchanged in undifferentiated cells (Fig. 4C). The differentiation seen here may be evidence of the on-target pro-differentiative effects of BRD4-NUT inhibition (2). The molecular profile was identical to that of the pretreatment biopsy (Supplementary Tables S1-2). The patient received a fourth cycle ending in October 2014, at which time he deteriorated rapidly and died the following month, seven months following his initial diagnosis.
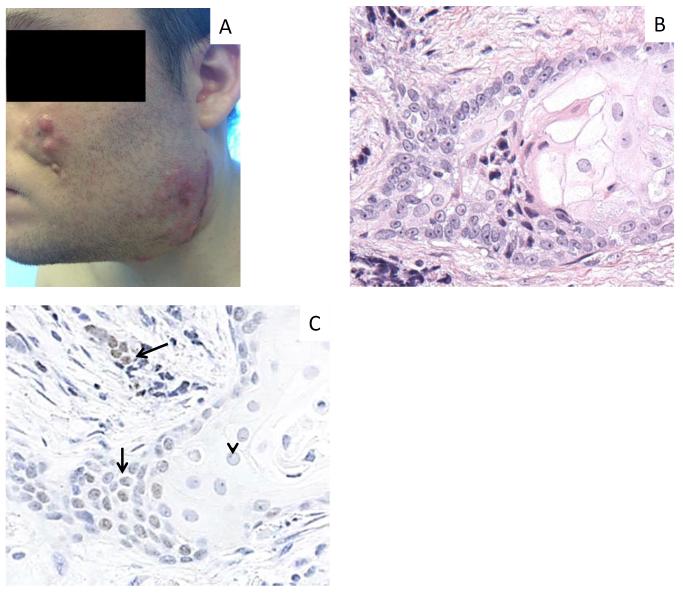
Figure 4. Clinical and histopathologic changes in Patient 2 during tumor progression.
A) Signs of local disease progression at the end of cycle 3. B) Post-treatment biopsy of recurrent tumor with H&E staining reveals a markedly greater degree of squamous differentiation, evidenced by accumulation of abundant pink cytoplasm, and nuclei with pale, open chromatin. C) IHC for NUT expression remains intact in undifferentiated cells (arrows), but is decreased in areas of differentiation (arrowhead). Photo in panel A published with permission of the patient, given at the time the photo was taken.
Patient 3
A 66-year-old female was diagnosed with locally advanced small cell lung carcinoma in May 2014 and received four cycles of carboplatin/etoposide. After review, diagnosis of NMC was established based on IHC and FISH. Molecular profiling was not possible due to insufficient tissue.
OTX015/MK-8628 was initiated in September 2014. The patient had grade 2 dyspnea due to a bulky tumor (8×6.5 cm) on the right lung invading the superior lobe bronchus, pulmonary artery, and posterior wall of the superior vena cava, along with increased LDH (1.6x ULN). Treatment was interrupted for 5 days after the first two weeks due to nausea and fatigue (grade 2) with worsening dyspnea. Nausea and fatigue improved to grade 1 while dyspnea returned to baseline levels. A thorax CT after cycle 1 showed stable disease. Treatment was resumed at 60 mg QD. Stable disease was seen after cycle 2, along with a limited SUVmax reduction (7 vs 10 at baseline). Cycle 3 was marked by grade 2 nausea and fatigue and treatment was discontinued at the end of this cycle (September 2014) when a PET-CT showed progression. Radiotherapy (45 Gy) to the lung achieved a partial response which was later followed by disease progression during administration of paclitaxel. Treatment with paclitaxel continued until September 2015, and the patient died in November 2015, 18 months following her initial diagnosis.
Patient 4
A 20-year-old male with no previous medical history, was diagnosed with FISH-positive t(15;19)(BRD4/NUT) translocation NMC in May 2014, presenting with bulky mediastinal lymphadenopathy and a pulmonary embolus. Molecular profiling of his tumor showed no additional oncogenic mutations, copy number variation, or rearrangements (Tables S1-2). Four cycles of cyclophosphamide, cisplatin, and doxorubicin resulted in partial remission, and was consolidated with radiotherapy (60 Gy) with concomitant cisplatin (one dose). A PET-CT scan performed after radiotherapy showed extensive disease progression with diffuse massive lymphadenopathy in the mediastinum and abdomen, as well as new cerebral and bone metastases.
At referral, the patient was in very poor general condition (ECOG performance status 4), with severe abdominal pain. His LDH levels were increased (2.2x ULN). OTX015/MK-8628 was started in October 2014, but after 2 weeks treatment was complicated by grade 4 thrombocytopenia, requiring treatment interruption and a platelet transfusion. Despite an initial stabilization of pain, during the treatment interruption there was rapid disease progression, and the patient died of massive mediastinal compression in November 2014, five months following his initial diagnosis.
Discussion
Recent findings have improved understanding of how BRD4-NUT drives growth and blocks differentiation. BRD4 tethers NUT to the chromatin (6), where the NUT portion recruits the histone acetyl-transferase, p300. The resultant increased histone acetylation recruits further BRD4-NUT and p300 in a feed-forward mechanism forming large contiguous expanses measuring up to 2 megabases co-occupied by BRD4-NUT, p300, and active histone acetylation (histone H3 acetyl lysine 27 (H3K27Ac)). These regions, termed ‘megadomains’, are cell lineage specific and drive expression of key pro-growth genes such as c-MYC and TP63 in NMC. Megadomains are depleted by BET inhibitors, resulting in tumor differentiation and growth arrest, providing a mechanistic rationale for their use in NMC (10).
The molecular profiles of the patients' tumors presented here suggest that BRD4-NUT may possibly be an all-in-one oncoprotein, potent enough to drive malignancy alone or with minimal additional genetic aberrations. The profiles were remarkable for the absence of inactivating mutations almost universally seen in SCC (TP53 and CDKN2A (17)) of SCC (the panel includes 9 of the 19 most common mutations in SCC, Supplementary Methods), and the lack of additional oncogenic mutations, rearrangements, or copy number variations. These findings support the concept that BRD4-NUT provides a 'short-cut' to SCC, or requires non-canonical pathways to transform and drive SCC growth (3). Finally, it is noteworthy that no additional mutations were seen in the NMC recurrence in Patient 2 during OTX015/MK-8628 treatment.
About two-thirds of NMCs harbor BRD4-NUT fusions. An important concern is thus whether BET inhibitors arrest growth of non-BRD4-NUT NMCs, such as those harboring BRD3-NUT and NSD3-NUT fusions. BRD3 dual bromodomains share high amino acid sequence similarity with those of BRD4, and like BRD4, BRD3 tethers NUT to chromatin (6). Moreover, BRD3-NUT, like BRD4-NUT, forms megadomains on chromatin, indicating similar active chromatin binding activity of its bromodomains to those of BRD4 (10). Finally, studies have demonstrated comparable binding of both domains of BRD3 to the BET inhibitor, JQ1, as with BRD4, predictive of similar pharmacodynamics (2). NSD3-NUT is a recently described fusion in NMC (7). NSD3 is a histone H3 lysine 36 methyl-transferase that does not possess chromatin-binding bromodomains, however it does interact with BRD4, and the blockade of differentiation and maintenance of growth in NSD3-NUT+ NMC cells is dependent upon NSD3-NUT interacting with BRD4 (7). NSD3-NUT is thought to form a BRD4-NUT-like complex that is tethered to chromatin via its association with BRD4. In support of this, NSD3-NUT+ NMC cells differentiate and arrest growth in the presence of JQ1 (7). Thus there is mechanistic rationale for use of BET inhibitors in patients with BRD3-NUT+ or NSD3-NUT+ NMC.
The role of wild-type BRD4 in other cancers is related to its pro-transcriptional function to facilitate transcriptional elongation of coding and enhancer RNAs (18). In the non-NMC cancer context, it is a key component of cell-type specific super-enhancers that drive expression of genes promoting growth and/or block differentiation, including, in many but not all cases, c-MYC (19). Despite these differences in BRD4-NUT and BRD4 enrichment within chromatin, in vitro and in vivo findings demonstrate exquisite sensitivity of BRD4 super-enhancer-dependent growth to BET inhibitors in a wide range of non-NMC cancers (11, 19). By contrast, regular enhancers are relatively resistant to BET inhibitors (19). Thus, BET inhibition is also a valid targeting approach in a broad range of non-NMC cancers.
While there are several ongoing phase I BET inhibitor trials in patients with solid and hematopoietic tumors, with preliminary evidence of clinical activity in acute leukemia and lymphoma (20, 21), no solid tumor studies have been published. We present the first mechanistic clinical proof of concept for a specific BET inhibitor OTX015/MK-8628, in three of four pretreated NMC cases, reporting dramatic clinical and radiologic responses in two patients and stable disease in another. The responses were rapid and associated with improvement of disease-related symptoms during the first weeks. Two of our patients (Patients 1 and 3) achieved overall survivals (19 and 18 months respectively) notably longer than the median survival of 6.7 months reported in the largest retrospective series of NMC patients (N = 54) published to date (5). Moreover, Patient 3 is amongst only two of 31 NMC patients treated non-surgically to survive longer than 17 months (5). While it is not possible to establish based on comparison of this small number of patients with historical data, whether OTX015/MK-8628 can increase survival in NMC patients, the present data provide rationale supporting further clinical development of BET inhibitors in this indication. Studies are needed to establish the impact of BET inhibitors on survival in advanced NMC patients.
Despite the impressive clinical activity observed, disease often progressed transiently after relatively short interruptions, suggesting a need for continuous effective levels of drug exposure to obtain antitumor pharmacodynamic activity in this aggressive disease. The progressive disease seen after several months of treatment in the three clinical responders begs the question of secondary resistance mechanisms. Two recent studies have found that BRD4-dependence can be bypassed through WNT signaling to restore MYC transcription as a possible resistance mechanism (22, 23) in acute leukemia. In Patient 2, although immunohistochemical staining for MYC remained intact in OTX015-resistant tumor cells, the WNT pathway was not activated, as determined by cytoplasmic localization of beta-catenin in both pre-treatment and post-OTX015 tumor tissue (Supplementary Fig. S1). Alternatively, it was recently shown that triple negative breast cancer can overcome BET inhibition through BRD4 phosphorylation, allowing it to remain associated with chromatin through interaction with MED1 (24). In our NMC patients, BRD4-NUT may be bypassed by similar or squamous-cell specific pathways to activate MYC expression. These findings consequently support the need for additive (or synergistic) combination treatment strategies in future BET inhibitor clinical development. Reversible side effects were seen in all patients, including thrombocytopenia, diarrhea, fatigue, nausea, dysgeusia and hyperglycemia that were similar to the main adverse events observed in the phase I study in patients with hematologic malignancies treated with OTX015/MK-8628 (20, 21). An ongoing phase Ib study evaluating OTX015/MK-8628 in advanced solid tumors including NMC (NCT02259114) may establish a more tolerable dose and/or administration schedule.
NMC is one of the most aggressive solid tumors known, affects people of all ages, most commonly teens to patients in their thirties, and can arise from a broad range of not exclusively midline primary sites (3). With the emergence of promising therapies such as BET inhibitors targeting this often undiagnosed cancer, there is a need for increased recognition of NMC. Patient 3 is a pointed example of how NMC can be mistaken clinically for other more common cancers, in this case small cell carcinoma due to its rapid disseminating biological behavior. For the oncologist, NMC should be considered and the pathologist asked to perform NUT IHC on any rapidly growing and/or metastasizing non-cutaneous carcinoma, particularly in patients in their second to fourth decade. Being a small round blue cell tumor, NMC can resemble small cell carcinoma histologically and share some immunohistochemical features, as for Patient 3. Histologically, NMC is a poorly differentiated carcinoma that often but not always exhibits squamous differentiation, and has no pathognomic histopathologic features. As such, the pathologist should evaluate any poorly differentiated carcinoma without specific etiology (e.g. HPV, EBV, or long term heavy smoking) by performing IHC for NUT.
The disappointing results obtained to date with standard chemotherapy or chemo-radiotherapy, along with mounting preclinical evidence that BRD-NUT fusion oncoproteins drive oncogenic growth and that bromodomain inhibition can cause NMC growth arrest, provide a strong rationale for further clinical evaluation of BET inhibitors in NMC. These four cases present the first clinical evidence of activity of BET inhibition in NMC and are a unique example of small molecule SCC therapy directly targeting the causative oncoprotein. The findings thus establish a new precedent for the development of this novel targeted approach, alone or in combination with other therapeutic agents, in NMC and other cancers.
METHODS
Patients and Treatment
Four patients with advanced NMC failing previous therapy were treated with OTX015/MK-8628 on a single-patient compassionate-use basis at the Oncology Institute of Southern Switzerland. Treatment was given in accordance with the Declaration of Helsinki under investigator responsibility. All patients gave written informed consent prior to treatment. OTX015/MK-8628 (supplied by Oncoethix SA) was administered orally at 80 mg once daily (QD) with 3-week cycles, a safe and pharmacodynamically active dose based on an ongoing first-in-human Phase I study in hematologic malignancies (20, 21). Dosing was interrupted and/or reduced according to toxicity.
Fluorescence In Situ Hybridization (FISH)
BRD4 and NUT dual color split-apart and bring-together FISH on formalin-fixed, paraffin-embedded sections was performed as described (7), using NUT 3′ telomeric probes, RP11-1H8 and RP11-64o3 (digoxigenin labeled, green), and BRD4 5′ centromeric probes, RP11-207i16 and RP11-3055m5 (biotin labeled, red). 200 nuclei were counted in four different areas of each tumor. Eighty percent positive interpretable nuclei were defined as positive for a rearrangement.
Immunohistochemistry
IHC for NUT was performed on 5-micron-thick, formalin-fixed, paraffin-embedded sections. Slides underwent heat-induced epitope retrieval in citrate buffer and were incubated with primary rabbit monoclonal anti-NUT (clone C52B1,1:50) (Cell Signaling Technology, Danvers, MA) and visualized using Bond Polymer Refine Detection (Leica Microsystems, Buffalo Grove, IL).
OncoPanel Gene Sequencing
Targeted exome sequencing was performed for Patients 1, 2 and 4 on a panel of 300 genes (see Supplementary Methods) using a customized next-generation cancer sequencing platform, the OncoPanel, developed at Brigham and Women's Hospital Dana-Farber Cancer Institute, as described (25). Somatic DNA mutations, copy number variations, and gene rearrangements can be detected from formalin-fixed paraffin embedded tumor sections. DNA was isolated from tissue containing at least 20% tumor nuclei and analyzed by massively parallel sequencing using a solution-phase Agilent SureSelect hybrid capture kit and an Illumina HiSeq 2500 sequencer. Exonic sequences of 300 genes and sequences of 113 introns across 35 genes were included (see Supplementary Methods).
Supplementary Material
STATEMENT OF SIGNIFICANCE We present the first clinical proof-of-concept that targeting BRD4-NUT with a BET inhibitor results in impressive and rapid antitumor activity in NMC. It offers strong potential for future clinical application in this rare patient population as either single agent or in combination.
Acknowledgements
We thank the patients and their families, as well as Prof. Esteban Cvitkovic (Oncoethix SA) for guidance and constructive discussion throughout the treatment of these patients, and Dr Sarah MacKenzie (Oncology Therapeutic Development) for manuscript editing.
Financial support: This work was supported by grants from the NIH 2R01CA124633 (C.A. French) and the Samuel Waxman Cancer Research Foundation (C.A. French).
Anastasios Stathis and Jean-Pierre Delord received institutional funding from Oncoethix for a phase I clinical trial of OTX015/MK-8628 in hematologic malignancies (NCT01713582) that was active during the compassionate treatment of these patients, and for the clinical trial of OTX015/MK-8628 in solid tumors (NCT02259114) that is currently ongoing. Mohamed Bekradda is an employee of Oncology Therapeutic Development (OTD, France), the CRO managing the two clinical studies with OTX015/MK-8628.
Footnotes
Declaration of potential conflicts of interest
All other authors declare no competing interests.
REFERENCES
- 1.French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–7. [PubMed] [Google Scholar]
- 2.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–73. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol. 2012;7:247–65. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 4.Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–91. doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, Luer SC, et al. Clinicopathologic Features and Long-Term Outcomes of NUT Midline Carcinoma. Clin Cancer Res. 2012;18:5773–9. doi: 10.1158/1078-0432.CCR-12-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French CA, Ramirez CL, Kolmakova J, Hickman TT, Cameron MJ, Thyne ME, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–42. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 7.French CA, Rahman S, Walsh EM, Kuhnle S, Grayson AR, Lemieux ME, et al. NSD3-NUT Fusion Oncoprotein in NUT Midline Carcinoma: Implications for a Novel Oncogenic Mechanism. Cancer Discov. 2014;4:928–41. doi: 10.1158/2159-8290.CD-14-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dey A, Nishiyama A, Karpova T, McNally J, Ozato K. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol Biol Cell. 2009;20:4899–909. doi: 10.1091/mbc.E09-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grayson AR, Walsh EM, Cameron MJ, Godec J, Ashworth T, Ambrose JM, et al. MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene. 2014;33(13):1736–42. doi: 10.1038/onc.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alekseyenko AA, Walsh EM, Wang X, Grayson AR, Hsi PT, Kharchenko PV, et al. The oncogenic BRD4-NUT chromatin regulator drives aberrant transcription within large topological domains. Genes Dev. 2015;29(14):1507–23. doi: 10.1101/gad.267583.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–82. doi: 10.1038/nature13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–8. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boi M, Gaudio E, Bonetti P, Kwee I, Bernasconi E, Tarantelli C, et al. The BET bromodomain inhibitor OTX015 affects pathogenetic pathways in preclinical B-cell tumor models and synergizes with targeted drugs. Clin Cancer Res. 2015;21:1628–38. doi: 10.1158/1078-0432.CCR-14-1561. [DOI] [PubMed] [Google Scholar]
- 14.Coude MM, Braun T, Berrou J, Dupont M, Bertrand S, Masse A, et al. BET inhibitor OTX015 targets BRD2 and BRD4 and decreases c-MYC in acute leukemia cells. Oncotarget. 2015;6(19):17698–712. doi: 10.18632/oncotarget.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noel JK, Iwata K, Ooike S, Sugahara K, Nakamura H, Daibata M. Development of the BET bromodomain inhibitor OTX015 (Abstract) Mol Cancer Ther. 2013;12:C244. [Google Scholar]
- 16.Stoeck A, Lejnine S, Truong A, Pan L, Wang H, Zang C, et al. Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discov. 2014;4:1154–67. doi: 10.1158/2159-8290.CD-13-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence MS, Sougnez C, Lichtenstein L, Cibulskis K, Lander E, Gabriel S, et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanno T, Kanno Y, LeRoy G, Campos E, Sun HW, Brooks SR, et al. BRD4 assists elongation of both coding and enhancer RNAs by interacting with acetylated histones. Nat Struct Mol Biol. 2014;21:1047–57. doi: 10.1038/nsmb.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–34. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berthon C, Raffoux E, Thomas X, Vey N, Gomez-Roca C, Yee K, et al. Bromodomain and extraterminal inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Hematology. 2016 doi: 10.1016/S2352-3026(15)00247-1. in press. [DOI] [PubMed] [Google Scholar]
- 21.Amorim S, Stathis A, Gleeson M, Iyengar S, Magarotto V, Leleu X, et al. A phase 1 dose-finding and pharmacokinetic study of the BET bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma. Lancet Hematology. 2016 doi: 10.1016/S2352-3026(16)00021-1. in press. [DOI] [PubMed] [Google Scholar]
- 22.Fong CY, Gilan O, Lam EY, Rubin AF, Ftouni S, Tyler D, et al. BET inhibitor resistance emerges from leukaemia stem cells. Nature. 2015;525:538–42. doi: 10.1038/nature14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathert P, Roth M, Neumann T, Muerdter F, Roe JS, Muhar M, et al. Transcriptional plasticity promotes primary and acquired resistance to BET inhibition. Nature. 2015;525:543–7. doi: 10.1038/nature14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu S, Lin CY, He HH, Witwicki RM, Tabassum DP, Roberts JM, et al. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature. 2016;529:413–7. doi: 10.1038/nature16508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagle N, Berger MF, Davis MJ, Blumenstiel B, Defelice M, Pochanard P, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93. doi: 10.1158/2159-8290.CD-11-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.