Abstract
Circulating levels of fibroblast growth factor 23 (FGF23) are elevated in patients with chronic kidney disease (CKD), but the mechanisms are poorly understood. Here we tested whether inflammation and iron deficiency regulate FGF23. In wild-type mice, acute inflammation induced by single injections of heat-killed Brucella abortus or interleukin-1β (IL-1β) decreased serum iron within 6 hours, and was accompanied by significant increases in osseous Fgf23 mRNA expression and serum levels of C-terminal FGF23, but no changes in intact FGF23. Chronic inflammation induced by repeated bacteria or IL-1β injections decreased serum iron, increased osseous Fgf23 mRNA and serum C-terminal FGF23, but modestly increased biologically active, intact FGF23 serum levels. Chronic iron deficiency mimicked chronic inflammation. Increased osseous FGF23 cleavage rather than a prolonged half-life of C-terminal FGF23 fragments accounted for the elevated C-terminal FGF23 but near-normal intact FGF23 levels in inflammation. IL-1β injection increased Fgf23 mRNA and C-terminal FGF23 levels similarly in wild-type and Col4a3KO mice with CKD, but markedly increased intact FGF23 levels only in the CKD mice. Inflammation increased Fgf23 transcription by activating Hif1α signaling. Thus, inflammation and iron deficiency stimulate FGF23 production. Simultaneous upregulation of FGF23 cleavage in osteocytes maintains near-normal levels of biologically active, intact circulating FGF23, whereas downregulated or impaired FGF23 cleavage may contribute to elevated intact serum FGF23 in CKD.
Keywords: FGF23, inflammation, anemia, bone, mineral metabolism, hypoxia
Introduction
Fibroblast growth factor 23 (FGF23) is a bone-derived hormone that is essential for maintaining normal phosphate and vitamin D homeostasis.1 FGF23 inhibits renal phosphate reabsorption by downregulating expression of the sodium-phosphate cotransporters NPT2a and NPT2c, and decreases circulating 1,25-dihydroxyvitamin D3 levels by inhibiting renal expression of CYP27B1 (1-α-hydroxylase) and stimulating expression of CYP24A1 (24-hydroxylase).2, 3 FGF23 levels rise progressively in chronic kidney disease (CKD), and higher levels are strongly associated with increased risks of CKD progression, cardiovascular events, and mortality.4-9 Experimental data suggesting that FGF23 may contribute causally to certain cardiovascular complications of CKD10 emphasize the therapeutic importance of defining the molecular mechanisms that stimulate FGF23 production beginning early in the course of CKD.
FGF23 is regulated by an incompletely understood interplay between local bone factors that modulate turnover and mineralization11-13 and systemic factors that control mineral metabolism.14, 15 High levels of parathyroid hormone (PTH), 1,25-dihydroxyvitamin D3, phosphate and calcium stimulate FGF23 production14-16 but cannot adequately explain the increases in FGF23 levels in early CKD. Indeed, FGF23 elevations usually antedate hyperparathyroidism and hyperphosphatemia,17 and CKD is characterized by low levels of 1,25 dihydroxyvitamin D3 and calcium rather than high levels.
Iron is a newly described regulator of FGF23 production. Animal and human studies demonstrate that iron deficiency stimulates Fgf23 transcription which is counterbalanced by commensurately increased cleavage of newly synthesized FGF23 within healthy osteocytes.18 This results in high circulating concentrations of FGF23 fragments that can be detected with C-terminal FGF23 (cFGF23) assays, but normal serum phosphate levels because the levels of intact, biologically active FGF23, measured by intact FGF23 assays (iFGF23), remain normal.
As CKD progresses, total FGF23 levels rise and the proportion of circulating C-terminal FGF23 fragments relative to intact hormone decreases, perhaps because FGF23 cleavage is impaired in CKD.19, 20 In the setting of reduced cleavage of newly synthesized FGF23, factors that activate FGF23 transcription, such as iron deficiency, would be expected to increase circulating levels of intact hormone, as occurs in autosomal dominant hypophosphatemic rickets, which is the prototype disease of impaired FGF23 cleavage.18 “Functional” iron deficiency is a consequence of chronic inflammation in which iron sequestration in the reticuloendothelial system decreases the amount of iron available for erythropoiesis despite adequate total body iron stores. Given the high prevalence of functional iron deficiency in CKD,21 and the association between chronic inflammation and elevated FGF23 levels observed in cross-sectional studies of CKD patients,22, 23 we hypothesized that inflammation and functional iron deficiency might be novel, interrelated mechanisms of increased FGF23 production that may contribute to elevated FGF23 levels in CKD.
Results
Iron deficiency regulates FGF23
We fed 3-week-old wild-type mice a low iron diet for 3 weeks. By 6 weeks of age, the mice developed iron-deficiency, marked by decreased serum iron and ferritin levels (p<0.05) compared to mice fed the control diet (Figure 1A-B). Iron deficiency resulted in significantly increased serum cFGF23 levels (Figure 1C) and significantly increased osseous expression of Fgf23 mRNA (Figure 1E) and protein (Figure 1F), but Fgf23 mRNA expression was not induced in the kidney. As shown in previous studies of young mice,24 iron deficiency also significantly increased iFGF23 (Figure 1D). Consistent with increased intact FGF23, renal expression of Npt2c and Cyp27b1 decreased significantly, and Cyp24a1 expression (Figure 1G) increased significantly resulting in lower 1,25(OH)2D levels (Table 1). Urinary phosphate excretion tended to increase, although this trend did not reach significance, and serum phosphate levels were unchanged. Bone expression of both the osteoblastic marker Sp7, which encodes osterix, and the osteoclastic marker Ctsk, which encodes cathepsin K, decreased significantly, (Figure 1G), consistent with decreased bone turnover.
Figure 1. Effects of diet-induced iron deficiency on FGF23 regulation.

Serum levels of iron (A); ferritin (B); cFGF23 (C); iFGF23 (D); bone and renal expression of Fgf23 mRNA (E); representative immunoblot of bone FGF23 protein expression (F); and bone mRNA expression of the osteoblastic markers, osterix (Sp7) and osteocalcin (Bglap), the osteoclastic marker, cathepsin K (Ctsk), and renal mRNA expression of the vitamin D metabolizing enzymes, Cyp27b1 and Cyp24a1, and the sodium-phosphate co-transporters, Npt2a and Npt2c, in response to low or normal iron diets for 3 weeks (G). Data are presented as mean ± SEM, n ≥ 4/group, * p<0.05 vs. control (Ctr).
Table 1. Serum and urine chemistries of experimental and control mice.
Groups of C57Bl6 mice were fed either a control or low iron diet for 3 weeks (n ≥ 5/group), and were induced to develop acute or chronic inflammation by treatment with heat-killed Brucella abortus (BA), IL-1β, or control (for BA: n ≥ 5/group at 6 hours, n ≥ 3/group at 12 days; for IL-1β: n ≥ 5/group at 6 hours and at 4 days).
| Control Diet | Low Iron Diet | Control 6 hours | BA 6 hours | Control 6 hours | Il1-β 6 hours | Control 12 days | BA 12 days | Control 4 days | IL1-β 4 days | |
|---|---|---|---|---|---|---|---|---|---|---|
| BUN, mg/dL | 21.8 ± 4.5 | 15.9 ± 2.0* | 19.8 ± 5.1 | 16.5 ± 4.1 | 20.7 ± 3.3 | 23.8 ± 1.9 | 22.8 ± 2.5 | 26.2 ± 6.8 | 17.1 ± 2.0 | 19.2 ± 2.5 |
| PTH, pg/mL | 72.1 ± 26.8 | 158.4 ± 63.2 | 98.7 ± 12.6 | 372.3 ± 27.8* | 117.3 ± 46.4 | 639.9 ± 230.5* | 108.6 ± 25.3 | 62.3 ± 3.6* | 118.6 ± 50.3 | 47.7 ± 13.5* |
| 1,25(OH)2D, pg/mL | 113.2 ± 11.8 | 80.7 ± 9.3* | 104.7 ± 31.5 | 120.4 ± 8.2 | 105.7 ± 7.3 | 180.5 ± 39.1* | 91.1 ± 15.5 | 48.4 ± 16.8* | 107.6 ± 24.6 | 44.4 ± 14.3* |
| Pi, mg/dL | 10.2 ± 1.3 | 10.1 ± 0.6 | 7.6 ± 0.8 | 8.7 ± 1.7 | 8.4 ± 0.9 | 9.5 ± 0.6 | 6.0 ± 0.4 | 6.9 ± 1.0 | 7.2 ± 0.4 | 6.2 ± 0.9 |
| Ca, mg/dL | 8.4 ± 1.0 | 8.6 ± 0.9 | 10.2 ± 0.4 | 10.1 ± 0.6 | 9.4 ± 0.4 | 9.5 ± 0.2 | 8.5 ± 0.3 | 8.4 ± 0.4 | 8.5 ± 0.5 | 9.2 ± 0.5 |
| FEPi, % | 9.9 ± 1.4 | 13.9 ± 2.5 | 18.7 ± 6.1 | 18.6 ± 9.0 | 12.4 ± 6.2 | 24.9 ± 5.4* | NM | NM | 12.3 ± 1.9 | 18.1 ± 2.5* |
| FECa, % | 4.3 ± 1.5 | 3.6 ± 1.5 | 6.6 ± 2.3 | 5.1 ± 2.6 | 10.1 ± 3.7 | 20.0 ± 11.5 | NM | NM | 11.7 ± 1.5 | 12.3 ± 1.6 |
Values are mean ± SD.
p<0.05 versus the specific experimental time-matched control
BUN, blood urea nitrogen; PTH, parathyroid hormone; 1,25(OH)2D, 1,25 dihydroxyvitamin D; Pi, serum phosphate; Ca, serum calcium; FEPi, urinary fractional excretion of phosphate; FECa, urinary fractional excretion of calcium; NM, not available for measurement
Acute inflammation induces functional iron deficiency and increases FGF23 production
To determine whether inflammation regulates FGF23, we studied the Brucella abortus (BA) mouse model 25 that develops acute and chronic inflammation starting at 3 hours and lasting through 14 days after a single intraperitoneal injection of heat-killed bacteria.26 Six hours after injection, Brucella abortus significantly decreased serum iron (Figure 2A) and increased ferritin levels compared to control (Figure 2B), consistent with the acute phase inflammatory reaction.27, 28 Serum cFGF23 levels rose significantly compared to controls, concomitant with a significant 8-fold elevation in bone expression of Fgf23 mRNA, but iFGF23 levels were unchanged (Figure 2C-E). Analysis of femoral bone protein extracts demonstrated evidence of increased FGF23 production and cleavage compared to control (Figure 2F). Consistent with the lack of increase in iFGF23 levels, there were no differences in renal mRNA expression of Cyp24a1, Npt2a or Npt2c (Figure 2G). As previously shown,29-31 acute inflammation resulted in significantly increased PTH levels, Cyp27b1 expression, (Figure 2G, Table 1), and bone mRNA expression of Sp7 and Ctsk (Figure 2G), consistent with increased bone turnover.32
Figure 2. Effects of acute inflammation on iron and FGF23 regulation.

Acute inflammation was induced by a single injection of Brucella abortus (BA) or IL1-β. Serum levels of iron (A); ferritin (B); cFGF23 (C); iFGF23 (D); bone and renal expression of Fgf23 mRNA (E); representative immunoblot of bone FGF23 protein expression (F); and bone mRNA expression of the osteoblastic markers, osterix (Sp7) and osteocalcin (Bglap), the osteoclastic marker, cathepsin K (Ctsk), and renal mRNA expression of the vitamin D metabolizing enzymes, Cyp27b1 and Cyp24a1, and the sodium-phosphate co-transporters, Npt2a and Npt2c, in response to BA (G). Serum levels of iron (H); ferritin (I); cFGF23 (J); iFGF23 (K); bone and renal expression of Fgf23 mRNA (L); representative immunoblot of bone FGF23 protein expression (M); and bone mRNA expression of Sp7, Bglap, and Ctsk, and renal mRNA expression of Cyp27b1, Cyp24a1, Npt2a and Npt2c in response to IL1-β (N). Data are presented as mean ± SEM, n ≥ 5/group, * p<0.05 vs. control (Ctr).
We also investigated FGF23 regulation in response to IL-1β injection, which is an intensely pro-inflammatory cytokine33 and an established cause of inflammation-induced, functional iron deficiency.34 Six hours after a single injection of IL-1β, serum iron decreased and ferritin increased significantly (Figure 2H-I). Longitudinal evaluation during 6 hours following IL-1β injection demonstrated that serum cFGF23 progressively increased, but iFGF23 was unchanged (Figure 2J-K). Bone expression of Fgf23 mRNA and protein increased significantly (Figure 2L-M). Renal Fgf23 mRNA expression also increased significantly in response to IL-1β, but the effect was modest compared to the dramatic increase in bone (Figure 2L).
IL1-β significantly increased renal expression of Cyp27b1 and Npt2a (Figure 2N), but the change in serum phosphate levels did not reach significance, likely due to increased renal phosphate excretion induced by significantly higher PTH levels (Table 1). Similar to the Brucella abortus model, IL-1β significantly increased osseous mRNA expression of markers of bone remodeling, including Sp7, Ctsk and Bglap, which encodes osteocalcin (Figure 2N).
Functional iron deficiency in the absence of inflammation increases FGF23 production
Hepcidin is an important molecular mediator of functional iron deficiency. Hepcidin is produced by the liver in response to inflammation, and it increases iron sequestration and decreases gastrointestinal iron absorption.35, 36 To induce a state of functional iron deficiency without superimposed inflammation, we administered 1 μg/g exogenous hepcidin to wild-type mice. Six hours after hepcidin injection, serum cFGF23 levels and bone expression of Fgf23 mRNA increased significantly, whereas iFGF23 levels remained unchanged (Supplementary Figure 1). These data suggest that functional iron deficiency alone can stimulate FGF23 production and preferentially increase circulating concentrations of C-terminal FGF23 fragments that are detected as elevated cFGF23 levels.
Chronic inflammation increases circulating levels of biologically active FGF23
Twelve days after the single Brucella abortus injection, iron levels remained significantly decreased compared to control (Figures 3A), and serum ferritin remained significantly increased (Figures 3B). At day 12, cFGF23 levels remained markedly increased compared to control, and iFGF23 levels were modestly increased (Figure 3C-D), although the result was of borderline statistical significance (p=0.051). Femoral expression of intact and cleaved FGF23 protein was also increased (Figure 3E).
Figure 3. Effects of chronic inflammation on iron and FGF23 regulation.

Serum levels of iron (A); ferritin (B); cFGF23 (C); iFGF23 (D); and representative immunoblot of bone FGF23 protein expression (E) 12 days after a single injection of Brucella abortus (BA). Data are presented as mean ± SEM, n ≥ 3/group,* p<0.05 vs. control (Ctr). Serum levels of iron (F); ferritin (G); cFGF23 (H); iFGF23 (I); representative immunoblot of bone FGF23 protein expression (J); organ-specific expression of Fgf23 mRNA (K); and bone mRNA expression of the osteoblastic markers, osterix (Sp7) and osteocalcin (Bglap), and the osteoclastic marker cathepsin K (Ctsk), and renal mRNA expression of the vitamin D metabolizing enzymes, Cyp27b1 and Cyp24a1, and the sodium-phosphate co-transporters, Npt2a and Npt2c, after 4 days of daily IL1-β injections (L). Data are presented as mean ± SEM, n ≥ 5/group, * p<0.05 vs. control (Ctr).
Four days of daily IL-1β injections significantly decreased serum iron and increased serum ferritin levels (Figure 3F-G). At day 4, cFGF23 levels were significantly elevated compared to controls, but the absolute values were substantially lower compared to 6 hours after IL-1β injection (Figures 3H, 2J) despite persistently high osseous expression of Fgf23 mRNA and protein (Figure 3J-K). Serum iFGF23 levels increased significantly (Figure 3I), suggesting that chronic inflammation altered the balance of FGF23 production and cleavage, leading to higher circulating intact hormone. Analyses of mRNA expression in the major organs confirmed that bone continued to be the primary site of FGF23 production (Figure 3K). Consistent with increased bioactive FGF23 levels, urinary fractional excretion of phosphate and Cyp24a1 mRNA expression were significantly increased, whereas Cyp27b1 and Npt2a mRNA expression were significantly decreased (Table 1, Figure 3L). This was associated with the significantly decreased serum 1,25(OH)2D and PTH levels (Table 1) that characterize chronic inflammation.37, 38 In contrast to the acute effects, repeated IL-1β administration significantly decreased expression of Sp7 and Bglap, while increasing Ctsk (Figure 3L), consistent with the uncoupling of bone formation and bone resorption that contributes to bone loss in chronic inflammatory states (Supplementary Figure 2).32, 39
Increased FGF23 cleavage maintains near-normal circulating levels of biologically active FGF23 during inflammation
To investigate further the mechanism of increased cFGF23 levels during inflammation, we first tested the effects of blocking FGF23 cleavage during acute inflammation in vivo and in vitro. Pre-treatment of 6-week old wild-type mice with a furin/furin-like protease inhibitor 40 did not affect the IL1-β-associated increase in Fgf23 mRNA or cFGF23, however it significantly increased serum iFGF23 (Figure 4A-C). In the MC3T3 osteoblast-like cells, furin inhibition also enhanced IL1-β-mediated dose-dependent induction of iFGF23 production compared to controls without significantly altering Fgf23 promoter activity (Figure 4E-F).
Figure 4. Effects of acute inflammation on FGF23 production and cleavage in vivo and in vitro.

Effects of IL1-β administration in mice pre-treated with or without furin inhibitors on bone expression of Fgf23 mRNA (A); serum levels of cFGF23 (B) and iFGF23 (C); and representative immunoblot of serum FGF23 protein probed with Immutopics (upper panel) and MyBiosource (lower panel) antibodies (D). Data are presented as mean ± SEM, n ≥ 3/group, p<0.05 * vs. untreated control, # vs. 50 ng/g IL1-β treated mice and & vs. furin treated mice. Effects of IL1-β on Fgf23 promoter activity (E); and on intracellular and extracellular FGF23 protein concentrations (F) in MC3T3-E1 cells in the presence or absence of furin inhibitor. Data are presented as mean ± SEM, n ≥ 5/group, p<0.05 * vs. untreated control and & vs. furin-treated cells.
Next, we tested whether an increase in the half-life of FGF23 fragments in circulation could explain elevated cFGF23 levels in inflammation. Co-injection of IL-1β and recombinant cFGF23 to FGF23ko mice did not increase the half-life of cFGF23 peptides compared to injection of cFGF23 alone (t1/2 IL-1β= 48 min vs. t1/2 control= 55 min; p=NS). Collectively, these data suggest that increased FGF23 cleavage maintains normal circulating levels of biologically active FGF23 in the setting of markedly increased Fgf23 transcription induced by inflammation.
Acute inflammation preferentially increases iFGF23 levels in a mouse model of CKD
To investigate whether the FGF23 response to inflammation differs in animals with impaired kidney function, we evaluated the effects of a single injection of IL-1β in Col4a3ko mice with moderate CKD,19, 41 as evidenced by significantly elevated BUN levels compared to wild-type (Figure 5A). Six hours after IL-1β injection, kidney function was unchanged (Figure 5A), but serum iron levels decreased significantly and ferritin levels increased significantly in both Col4a3ko and WT mice. Six hours post-injection, Fgf23 mRNA expression and cFGF23 levels were significantly and similarly increased in Col4a3ko and WT mice (Figure 5D, F), but IL-1β injection triggered a significantly greater increase in iFGF23 levels in the CKD mice compared to the mild elevation observed in wild-type mice (Figure 5E). Consistent with their increase in iFGF23 levels, urinary fractional excretion of phosphate increased significantly and serum phosphate trended lower in the CKD mice (Table 2).
Figure 5. Effects of acute inflammation on FGF23 production and cleavage in the Col4a3ko mouse model of CKD.

Effects of IL1-β on serum levels of BUN (A); iron (B); ferritin (C); bone expression of Fgf23 mRNA (D); and serum cFGF23 (E) and iFGF23 (F) in 6 week-old wild type (WT) and Col4a3 knockout (Col4a3ko) mice. Data are presented as mean ± SEM, n ≥ 3/group, p<0.05 *vs. age-matched untreated WT, # vs. age-matched IL1-β treated WT and & vs. age-matched untreated Col4a3ko mice.
Table 2. Serum and urine chemistries of WT and Col4a3ko mice.
WT and Col4a3ko mice were induced to develop acute inflammation by treatment with IL-1β or control for 6 hours.
| WT Ctr |
WT Il1-β |
Col4a3ko Ctr |
Col4a3ko Il1-β |
|
|---|---|---|---|---|
| Pi, mg/dL | 7.2 ± 1.6 | 8.4± 0.7 | 10.1 ± 3.3 | 9.2 ± 1.8 |
| Ca, mg/dL | 9.8 ± 0.8 | 10.5 ± 0.7 | 10.7 ± 0.3 | 11.8 ± 0.5* |
| FEPi, % | 8.8 ± 2.8 | 12.1 ± 2.6 | 15.2 ± 4.6 | 29.3 ± 6.4* |
| FECa, % | 5.2 ± 2.1 | 4.8 ± 2.3 | 8.4 ± 3.4 | 7.5 ± 4.1 |
Values are mean ± SD.
p<0.05 versus the specific experimental time-matched control
Pi, serum phosphate; Ca, serum calcium; FEPi, urinary fractional excretion of phosphate; FECa, urinary fractional excretion of calcium.
HIF1α is a mediator of inflammation- and iron deficiency-induced FGF23 production
One mechanism of how iron deficiency increases FGF23 expression is stabilization of nuclear HIF1α.24 To investigate further the cellular mechanisms of how inflammation and iron deficiency differentially stimulate bone production of FGF23, we treated MC3T3-E1 osteoblast-like cells or bone marrow stromal cells (BMSC) with the iron chelator, deferoxamine, or IL-1β for 12 hours. Treatment with 50 μM of deferoxamine increased Fgf23 mRNA expression in both bone cell lines (Figure 6A) without affecting Hif1α mRNA expression (Figure 6B), consistent with stabilization of preexisting HIF1α (Figure 6C), as previously reported.18, 24 Treatment with 10 μg/mL of IL-1β increased Fgf23 mRNA levels in both cell lines (Figure 6A), suggesting that IL-1β directly increases FGF23 production independently of systemic iron changes. In contrast to deferoxamine, the IL-1β-induced increases in Fgf23 expression were accompanied by increased cellular expression of Hif1α mRNA (Figure 6B) and nuclear HIF1α abundance (Figure 6C).
Figure 6. The role of Hif1α in the regulation of FGF23 production and cleavage in vitro and in vivo.
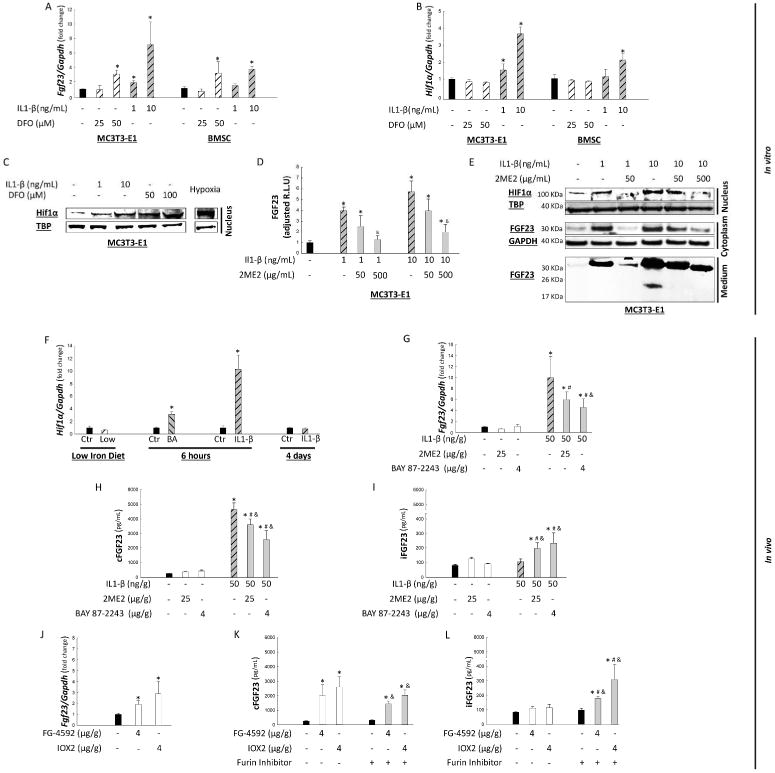
mRNA expression of Fgf23 (A) and Hif1α (B) and representative immunoblot for nuclear HIF1α protein (C) in MC3T3-E1 and BMSC cells that were stimulated to differentiate into osteoblasts and treated with IL1-β or deferoxamine (DFO). Effects of IL1-β on Fgf23 promoter activity in MC3T3-E1 cells in the presence or absence of the HIF1α inhibitor, 2ME2 (D); and representative immunoblots of nuclear HIF1α, cytoplasmic and secreted FGF23 (E). Data are presented as mean ± SEM, n ≥ 3/group, p<0.05 vs. * control (Ctr), &vs. IL-1β treated. Bone mRNA expression of Hif1α in response to iron deficiency, Brucella abortus (BA) and IL1-β injections (F). Effects of acute inflammation on bone mRNA Fgf23 expression (G) and serum levels of cFGF23 (H); iFGF23 (I) in wild-type mice injected with IL1-β or pre-treated with HIF inhibitors, 2ME2 and BAY 87-2243. Data are presented as mean ± SEM, n ≥ 3/group, p<0.05 * vs. untreated control, # vs. HIF1α inhibitor treated mice and & vs. 50 ng/g IL1-β injected mice. Effects of HIF1α induction by 2 prolyl hydroxylase inhibitors, FG-4592 and BAY 87-2243, on bone mRNA Fgf23 expression (J); serum levels of cFGF23 (K) and iFGF23 (L). Data are presented as mean ± SEM, n ≥ 3/group, p<0.05 * vs. untreated control, # vs. HIF1α agonist treated mice and & vs. furin inhibitor treated mice.
To determine if the effects of IL-1β on Fgf23 transcription were direct, we stably transfected MC3T3-E1 cells with an FGF23 reporter vector carrying a secreted luciferase expression cassette under the control of the murine 1.2 kB Fgf23 promoter. IL-1β treatment stimulated Fgf23 mRNA expression (data not shown), Fgf23 promoter activity and FGF23 protein in a dose-dependent manner (Figure 6 D, E). These effects were partially blocked in a similar dose-dependent manner by co-treatment with the HIF1α inhibitor, 2-methoxyestradiol (2ME2) (Figure 6D, E). These data are consistent with our observation of increased bone expression of Hif1α mRNA in the Brucella abortus- and IL-1β treated mice (Figure 6F), and with prior reports that IL-1β potently induces Hif1α expression.42, 43
In vivo, IL-1β injection in mice pre-treated with the HIF1α inhibitors, 2ME2 and BAY 87-2243, significantly attenuated inflammation-induced increases in Fgf23 mRNA expression and cFGF23 levels (Figure 6G, H). Interestingly, HIF1α inhibition also simultaneously resulted in increased iFGF23 (Figure 6I), suggesting that HIF1α may contribute to coordinating the concomitant increases in FGF23 production and cleavage induced by inflammation. In support of this hypothesis, treatment of mice with the prolyl-hydroxylase inhibitors, FG-4592 and IOX2, which increase nuclear HIF1α abundance, resulted in increased Fgf23 mRNA expression and cFGF23 levels (Figure 6J-K), whereas iFGF23 levels rose only with co-administration of furin inhibitors that partially inhibit FGF23 cleavage. In aggregate, these data suggest that inflammatory cytokines regulate Fgf23 transcription directly by stimulating Hif1α expression, and indirectly by inducing functional iron deficiency that stabilizes HIF1α (Figure 7).
Figure 7. Schematic representation of FGF23 regulation by inflammation.

Inflammation induces functional iron deficiency, which stabilizes HIF1α in osteocytes. Inflammatory cytokines also stimulate Hif1α transcription directly, leading to increased cytoplasmic HIF1α, which translocates to the nucleus and binds to HIF1β. Binding of HIF1 heterodimers to hypoxia response elements (HRE) on the Fgf23 promoter stimulates transcription, but the excess FGF23 protein is proteolytically cleaved within osteocytes. In the serum, physiological coupling of FGF23 production and cleavage during acute inflammatory states results in high circulating concentrations of C-terminal FGF23 fragments that can be detected with C-terminal FGF23 assays, but normal levels of biologically active FGF23 levels as demonstrated by intact FGF23 assays. In chronic inflammation, cFGF23 levels remain increased compared to baseline, but to a lesser extent than in acute inflammation. Sustained FGF23 overproduction during chronic inflammation also leads to increased serum levels of biologically active FGF23, perhaps due to saturation or partial down regulation of the FGF23 cleavage process within osteocytes.
Discussion
Osteocytes regulate circulating levels of biologically active FGF23 by controlling transcription of FGF23 and intracellular cleavage of newly synthesized FGF23 protein.44 Fgf23 transcription can be assessed directly in bone specimens and in cell culture models, whereas the relative amounts of FGF23 transcription and cleavage can be indirectly assessed in vivo by simultaneously measuring serum FGF23 with cFGF23 and iFGF23 assays in the presence and absence of inhibitors of FGF23 cleavage.11, 45 Using these tools, we demonstrate that acute inflammation markedly increases Fgf23 expression, and that bone is the predominant source of increased Fgf23 transcription relative to other organs, including the kidney, which may be a secondary source of FGF23 in kidney disease.46 In the setting of acute inflammation-induced increases in Fgf23 transcription, osteocytes maintain normal serum levels of biologically active FGF23 by commensurately increasing FGF23 cleavage. This results in marked increases in circulating cFGF23 levels but normal iFGF23 levels.
In contrast to acute inflammation, chronic inflammation also increases biologically active iFGF23 levels, albeit to a lesser extent than cFGF23. We can speculate that sustained periods of FGF23 overproduction could overwhelm the capacity of the FGF23 cleavage apparatus in osteocytes. Alternatively, chronic inflammation may reduce the activity of the FGF23 cleavage enzymes or enhance post-translational O-glycosylation of FGF23 by GALNT3 that protects FGF23 from cleavage.47, 48 Although we did not find any differences in total Galnt3 or Furin mRNA expression in our acute and chronic inflammation models (data not shown), further research is needed to investigate whether the activity of these proteins is altered by inflammation.
Our results suggest that increased activity of HIF1α is one mechanism through which inflammation stimulates Fgf23 transcription. HIFs are members of the heterodimeric basic helix–loop–helix family of transcription factors, and consist of an oxygen-sensitive α-subunit and a constitutively expressed β-subunit.49 HIF heterodimers regulate gene expression by binding to hypoxia response elements on the promoters of target genes.49 Degradation and activation of HIF1α is iron-dependent. Iron depletion and iron chelators stabilize HIF1α,49 whereas iron overload shortens HIF1α half-life, even in hypoxic conditions.50 We confirm prior reports that implicated HIF1α stabilization as a mechanism of increased FGF23 production in iron deficiency,51 and extend this line of investigation to demonstrate that inflammatory stimuli directly increase transcription of Hif1α, independent of iron deficiency. Furthermore, we observed in vivo and in vitro that inhibition of HIF1α partially attenuates the acute inflammation-induced increases in both FGF23 production and FGF23 cleavage. These data suggest that activation of HIF1α may be one mechanism that governs the coordinated response within osteocytes that increases both FGF23 production and cleavage and results in preferential increases in circulating cFGF23 levels in iron deficiency and inflammation.
Why the osteocyte produces FGF23 only to immediately degrade it in specific settings is unclear, but we can speculate certain possibilities. Full-length FGF23 is biologically active, but the effects of FGF23 fragments are uncertain.52, 53 FGF23 fragments may exert endocrine or paracrine functions that necessitate increased FGF23 production in order to generate adequate amounts of fragments in the settings of iron deficiency and inflammation. Alternatively, perhaps continuous FGF23 production and cleavage provide the osteocyte with an ability to increase secretion of biologically active FGF23 more rapidly than could be supported by increased de novo transcription, for example, by acutely decreasing cleavage. This mechanism could contribute to the rapid increase in iFGF23 we previously reported in acute kidney injury.54 It is also possible that the isolated rapid increase in cFGF23 levels we observed only 3 hours after IL-1β injection or even sooner after onset of acute kidney injury reflects enhanced cleavage of FGF23 that was already synthesized and stored in osteocytes, which we did not study.
In our analyses of 6-week old mice exposed to 3 weeks of a low iron diet, both cFGF23 and iFGF23 levels increased. These results are qualitatively similar to those reported in even younger iron deficient mice,24 but differ from the results we reported in otherwise healthy iron deficient women, in whom cFGF23 levels were elevated but iFGF23 was normal. 45 Perhaps there are age- or growth-dependent differences in the threshold of FGF23 production above which the metabolic coupling of FGF23 cleavage can no longer match production. Thus, younger mice may be less capable of cleaving the excess FGF23 in the setting of markedly increased FGF23 production than fully-grown animals. Whether neonates or growing children also demonstrate a differential FGF23 response to iron deficiency than adult humans requires further study.
Reports of intravenous iron-induced hypophosphatemia due to increased iFGF23 levels55-58 may appear to contradict our current data and prior studies that demonstrate that iron deficiency stimulates FGF23 production.18, 24, 45 However, the ability to transiently increase iFGF23 levels appears to be a specific effect of certain intravenous iron preparations. For example, ferric carboxymaltose lowered cFGF23 in previously iron deficient women, consistent with the expected effect of correcting iron deficiency, but raised iFGF23 levels temporarily, perhaps because the carbohydrate moiety simultaneously inhibited FGF23 cleavage to a greater extent than the reduction in FGF23 transcription.45 In contrast, iron dextran lowered cFGF23 without altering iFGF23 levels. Thus, the seeming paradox appears to be a byproduct of the unique effects of specific iron formulations rather than a global effect of iron itself.
Our findings suggest novel potential mechanisms of elevated FGF23 levels in CKD. CKD is an inflammatory state and higher FGF23 levels are independently associated with higher levels of inflammatory markers in patients with CKD.22, 23 Our results suggest that direct effects of inflammation to raise FGF23 levels may underlie these associations. Furthermore, our finding that acute inflammation dramatically increased iFGF23 levels to a much greater extent in the Col4a3ko mouse model of CKD versus wild-type mice suggests that FGF23 cleavage is relatively downregulated or impaired in CKD, as suggested by previous studies.19, 20 Thus, we propose that a combination of increased FGF23 production and relatively decreased FGF23 cleavage contributes to the markedly elevated FGF23 levels observed in murine CKD. Further research is needed to investigate the roles of true iron deficiency, functional iron deficiency and inflammation in the regulation of FGF23 levels in human CKD.
Materials and Methods
Animals
Six-week-old C57Bl6, Col4a3ko and FGF23ko mice were maintained on a standard diet (Teklad 7912, 0.82% calcium and 0.53% phosphorus; Harlan Teklad, USA) except when otherwise specified. Newly weaned, 3 week-old mice were fed a control diet that contained 380 ppm Fe or a low iron diet that contained 2-6 ppm Fe for 3 weeks (Harlan Teklad). All studies were approved by Institutional Animal Care and Use Committees at the University of Miami, Northwestern University and Massachusetts General Hospital.
Experimental procedures
Intra-peritoneal injections included heat-killed Brucella abortus strain 1119-3, 5 × 106 particles (US Department of Agriculture); recombinant IL1-β, 50 ng/g (Cell Signaling Technology, MA, USA); 2-ME2, 25 μg/g; 4 μg/g of BAY87-2243, FG-4592 or IOX2 (Selleckchem-Pfizer, TX, USA); irreversible furin/furin-like pro-protein convertase inhibitor 1, 7.5 μg/g (EMD Millipore, MA, USA); hepcidin, 1 μg/g (PLP-4434-s, Peptide international); and recombinant human C-terminal FGF23 peptides, 50 ng/g (courtesy of M. Mohammadi and R. Goetz).52
Biochemistry
Serum samples were collected by tail-bleeding or intracardiac exsanguination, and urine by spot collection at sacrifice. FGF23 levels were measured using both an iFGF23 ELISA that measure the intact active protein exclusively (Kainos Laboratories, Tokyo, Japan), and a murine cFGF23 ELISA that recognizes the full-length protein and its C-terminal cleavage fragments (Immutopics, Carlsbad, CA, USA). For other assays, calcium was measured by CPC Liquicolor Kit (Stanbio Laboratories, Boerne, TX, USA); phosphate by the phosphomolybdylate-ascorbic acid method;14 iron, BUN and creatinine using Pointe Scientific kits (Canton, MI, USA); ferritin by mouse ELISA (abcam, Cambridge, MA, USA); PTH by mouse intact ELISA (Immutopics); and 1,25(OH)2D by immunoassay (Immunodiagnostic Systems, Gaithersburg, MD, USA).
RT-PCR
Total RNA was isolated using TRI-reagent, and first-strand cDNA was synthesized from the entire femur.14 The iCycler iQ real-time PCR detection system and iQ SYBR Green supermix (Bio-Rad Laboratories, USA) were used for real-time quantitative PCR analysis. Primer pairs are shown in the Supplemental Table. The expression was normalized to glyceraldehyde-3-phosphate dehydrogenase in the same sample and expressed as fold-change versus wild-type.
Cell culture
BMSC cultures were prepared, as previously described.59, 60 BMSC were plated at 10 × 104 cells/well and cultured for 3 weeks in an osteoblast-differentiating medium (αMEM, 10% FBS, 10 U/ml penicillin, 100 μg/ml streptomycin, 10 mM β-glycerophosphate, and 50 μg/ml ascorbic acid; Sigma-Aldrich). MC3T3-E1 osteoblast precursor cells were cultured according to ATCC guidelines.14 Briefly, 5 × 104 cells were stably transfected with the pLuc-Fgf23 promoter plasmid carrying a secreted luciferase expression cassette under the control of the proximal Fgf23 promoter, a secreted alkaline phosphatase (SEALP) under the control of the CMV-promoter, and a puromycin resistance cassette (Genecopoeia,, MD, USA). Stably transfected cells were plated at a concentration of 10 × 104 cells/well and cultured for 12 days in the osteoblast-differentiating medium. Cells and cell-culture media were collected after 12 hours during the last day of culture. Luciferase assays were performed according to the manufacturer's instructions (Turner Biosystems, USA). Promoter activity is represented by RLU normalized to pSEALP-CMV control. All experiments were conducted in triplicate.
Protein extraction and immunoblotting
Whole femurs were analyzed as previously described.12, 59 Cells were cultured in 75 cm2 flasks for 12 days before treatment. 4 mL culture medium was filtered through a 50KDa ultra centrifugation column (EMD Millipore) and dried overnight in a Savant SpeedVac system (Thermo Fisher Scientific, MA, USA). Whole cell lysates were extracted using T-Per lysis buffer containing protease inhibitors cocktail, and nuclear and cytoplasmic extracts using NEPER™ Nuclear and Cytoplasmic Extraction kit (Thermo Fisher Scientific). Whole cell extracts and cytoplasmic lysates were filtered through a 50KDa ultra centrifugation size exclusion column and dried overnight.
Proteins were fractionated on 4-12 Bis-Tris minigels (Thermo Fisher Scientific), transferred on 0.22 PVDF membranes (Bio-Rad Laboratories) and probed with goat polyclonal anti-FGF23 (Immutopics), rabbit polyclonal MBS854462 anti-FGF23 (MyBioSource, CA, USA), rabbit polyclonal ab9485 anti-GAPDH, rabbit polyclonal ab2185 anti-HIF1α, rabbit polyclonal ab63766 anti-TATA binding protein TBP, and goat polyclonal ab8229 anti- β-actin antibodies (abcam). Antigen antibody complexes were visualized by immunoblotting using anti-goat and anti-rabbit IR fluorescent antibodies on an ODYSSEY® CLx system (LI-COR, NE, USA).
Statistics
Data are presented as mean ± SD or SEM. One-way ANOVA followed by Fisher and t tests were used for statistical inference using Statistica software (Statsoft, OK, USA). P values < 0.05 were considered statistically significant.
Supplementary Material
Supplementary Figure 1: Effects of recombinant hepcidin administration on FGF23 production
Effects of recombinant hepcidin administration on FGF23 production, Functional iron deficiency was induced by a single injection of 1 μg/g recombinant murine hepcidin. Serum levels of cFGF23 (A); iFGF23 (B); bone expression of Fgf23 mRNA measured 6 hours after hepcidin administration. Data are presented as mean ± SEM, n ≥ 3/group,* p<0.05 vs. control (Ctr).
Supplementary Figure 2: Effects of iron deficiency, acute and chronic inflammation on bone resorption
Representative sections of femur TRAcP staining in response to low or normal iron diets for 3 weeks (A), six hours after a single injection of (B) Brucella abortus (BA), (C) IL1-β, or after 4 days of daily IL1-β injections (D). Bone trabeculae are shown by methyl-blue counterstaining and osteoclasts by red-purple staining and indicated by black arrows.
Supplementary Table: Sequences of primers used for RT-PCR
Acknowledgments
This study was supported by grants R01DK076116 and K24DK093723 to MW and R01DK087727 to JLB from the National Institutes of Health, and by a Howard Goodman Fellowship Award from the Massachusetts General Hospital to JLB.
Footnotes
Conflict of interest: The authors declare that they do not have any conflict of interest regarding the data published in this manuscript.
References
- 1.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiological reviews. 2012;92:131–155. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David V, Quarles LD. Chapter 42 - FGF23/Klotho New Regulators of Vitamin D Metabolism. In: Adams, F D, P WS, editors. Vitamin D. Third. Academic Press; San Diego: 2011. pp. 747–761. [Google Scholar]
- 3.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. The New England journal of medicine. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isakova T, Houston J, Santacruz L, et al. Associations between fibroblast growth factor 23 and cardiac characteristics in pediatric heart failure. Pediatric nephrology (Berlin, Germany) 2013;28:2035–2042. doi: 10.1007/s00467-013-2515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovesdy CP, Quarles LD. The Role of Fibroblast Growth Factor-23 in Cardiorenal Syndrome. Nephron Clinical practice. 2013;123:194–201. doi: 10.1159/000353593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prie D, Forand A, Francoz C, et al. Plasma fibroblast growth factor 23 concentration is increased and predicts mortality in patients on the liver-transplant waiting list. PloS one. 2013;8:e66182. doi: 10.1371/journal.pone.0066182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touchberry CD, Green TM, Tchikrizov V, et al. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. American journal of physiology Endocrinology and metabolism. 2013;304:E863–873. doi: 10.1152/ajpendo.00596.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. Journal of the American Society of Nephrology : JASN. 2011;22:956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin A, David V, Li H, et al. Overexpression of the DMP1 C-terminal fragment stimulates FGF23 and exacerbates the hypophosphatemic rickets phenotype in Hyp mice. Mol Endocrinol. 2012;26:1883–1895. doi: 10.1210/me.2012-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin A, Liu S, David V, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:2551–2562. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng JQ, Ward LM, Liu S, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nature genetics. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David V, Dai B, Martin A, et al. Calcium regulates FGF-23 expression in bone. Endocrinology. 2013;154:4469–4482. doi: 10.1210/en.2013-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. Journal of the American Society of Nephrology : JASN. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 16.Lopez I, Rodriguez-Ortiz ME, Almaden Y, et al. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011;80:475–482. doi: 10.1038/ki.2011.107. [DOI] [PubMed] [Google Scholar]
- 17.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrow EG, Yu X, Summers LJ, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E1146–1155. doi: 10.1073/pnas.1110905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stubbs JR, He N, Idiculla A, et al. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27:38–46. doi: 10.1002/jbmr.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada T, Urakawa I, Isakova T, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. The Journal of clinical endocrinology and metabolism. 2010;95:578–585. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishbane S, Pollack S, Feldman HI, et al. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988-2004. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:57–61. doi: 10.2215/CJN.01670408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manghat P, Fraser WD, Wierzbicki AS, et al. Fibroblast growth factor-23 is associated with C-reactive protein, serum phosphate and bone mineral density in chronic kidney disease. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21:1853–1861. doi: 10.1007/s00198-009-1142-4. [DOI] [PubMed] [Google Scholar]
- 23.Munoz Mendoza J, Isakova T, Ricardo AC, et al. Fibroblast growth factor 23 and Inflammation in CKD. Clinical journal of the American Society of Nephrology : CJASN. 2012;7:1155–1162. doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinkenbeard EL, Farrow EG, Summers LJ, et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29:361–369. doi: 10.1002/jbmr.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasu BJ, Cooke KS, Arvedson TL, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115:3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 26.Kim A, Fung E, Parikh SG, et al. A mouse model of anemia of inflammation: complex pathogenesis with partial dependence on hepcidin. Blood. 2014;123:1129–1136. doi: 10.1182/blood-2013-08-521419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locatelli F, Nissenson AR, Barrett BJ, et al. Clinical practice guidelines for anemia in chronic kidney disease: problems and solutions. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2008;74:1237–1240. doi: 10.1038/ki.2008.299. [DOI] [PubMed] [Google Scholar]
- 28.Kdoqi, National Kidney F. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006;47:S11–145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Bikle DD. Vitamin D regulation of immune function. Vitamins and hormones. 2011;86:1–21. doi: 10.1016/B978-0-12-386960-9.00001-0. [DOI] [PubMed] [Google Scholar]
- 30.Liu NQ, Kaplan AT, Lagishetty V, et al. Vitamin D and the regulation of placental inflammation. Journal of immunology. 2011;186:5968–5974. doi: 10.4049/jimmunol.1003332. [DOI] [PubMed] [Google Scholar]
- 31.Toribio RE, Kohn CW, Hardy J, et al. Alterations in serum parathyroid hormone and electrolyte concentrations and urinary excretion of electrolytes in horses with induced endotoxemia. Journal of veterinary internal medicine / American College of Veterinary Internal Medicine. 2005;19:223–231. doi: 10.1892/0891-6640(2005)19<223:aispha>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Hardy R, Cooper MS. Bone loss in inflammatory disorders. The Journal of endocrinology. 2009;201:309–320. doi: 10.1677/JOE-08-0568. [DOI] [PubMed] [Google Scholar]
- 33.Shito M, Wakabayashi G, Ueda M, et al. Interleukin 1 receptor blockade reduces tumor necrosis factor production, tissue injury, and mortality after hepatic ischemia-reperfusion in the rat. Transplantation. 1997;63:143–148. doi: 10.1097/00007890-199701150-00026. [DOI] [PubMed] [Google Scholar]
- 34.Gordeuk VR, Prithviraj P, Dolinar T, et al. Interleukin 1 administration in mice produces hypoferremia despite neutropenia. J Clin Invest. 1988;82:1934–1938. doi: 10.1172/JCI113812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;55:726–741. doi: 10.1053/j.ajkd.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babitt JL, Lin HY. Mechanisms of anemia in CKD. Journal of the American Society of Nephrology : JASN. 2012;23:1631–1634. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinheiro da Silva F, Zampieri FG, Barbeiro HV, et al. Decreased parathyroid hormone levels despite persistent hypocalcemia in patients with kidney failure recovering from septic shock. Endocrine, metabolic & immune disorders drug targets. 2013;13:135–142. doi: 10.2174/1871530311313020001. [DOI] [PubMed] [Google Scholar]
- 38.af Ekenstam E, Benson L, Hallgren R, et al. Impaired secretion of parathyroid hormone in patients with rheumatoid arthritis: relationship to inflammatory activity. Clinical endocrinology. 1990;32:323–328. doi: 10.1111/j.1365-2265.1990.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 39.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang YH, Lin KH, Liao CH, et al. Furin overexpression suppresses tumor growth and predicts a better postoperative disease-free survival in hepatocellular carcinoma. PloS one. 2012;7:e40738. doi: 10.1371/journal.pone.0040738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai B, David V, Martin A, et al. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PloS one. 2012;7:e44161. doi: 10.1371/journal.pone.0044161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thornton RD, Lane P, Borghaei RC et al. Interleukin 1 induces hypoxia-inducible factor 1 in human gingival and synovial fibroblasts. The Biochemical journal. 2000;350 Pt 1:307–312. [PMC free article] [PubMed] [Google Scholar]
- 43.Westra J, Brouwer E, Bos R, et al. Regulation of cytokine-induced HIF-1alpha expression in rheumatoid synovial fibroblasts. Annals of the New York Academy of Sciences. 2007;1108:340–348. doi: 10.1196/annals.1422.035. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharyya N, Wiench M, Dumitrescu C, et al. Mechanism of FGF23 processing in fibrous dysplasia. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27:1132–1141. doi: 10.1002/jbmr.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28:1793–1803. doi: 10.1002/jbmr.1923. [DOI] [PubMed] [Google Scholar]
- 46.Spichtig D, Zhang H, Mohebbi N, et al. Renal expression of FGF23 and peripheral resistance to elevated FGF23 in rodent models of polycystic kidney disease. Kidney Int. 2014;85:1340–1350. doi: 10.1038/ki.2013.526. [DOI] [PubMed] [Google Scholar]
- 47.Topaz O, Shurman DL, Bergman R, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nature genetics. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 48.Kato K, Jeanneau C, Tarp MA, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. The Journal of biological chemistry. 2006;281:18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 49.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knowles HJ, Raval RR, Harris AL, et al. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer research. 2003;63:1764–1768. [PubMed] [Google Scholar]
- 51.Clinkenbeard EL, Farrow EG, Summers LJ, et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013 doi: 10.1002/jbmr.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goetz R, Nakada Y, Hu MC, et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:407–412. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goetz R, Beenken A, Ibrahimi OA, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Molecular and cellular biology. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christov M, Waikar SS, Pereira RC, et al. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int. 2013;84:776–785. doi: 10.1038/ki.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schouten BJ, Doogue MP, Soule SG, et al. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Annals of clinical biochemistry. 2009;46:167–169. doi: 10.1258/acb.2008.008151. [DOI] [PubMed] [Google Scholar]
- 56.Schouten BJ, Hunt PJ, Livesey JH, et al. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. The Journal of clinical endocrinology and metabolism. 2009;94:2332–2337. doi: 10.1210/jc.2008-2396. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu Y, Tada Y, Yamauchi M, et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone. 2009;45:814–816. doi: 10.1016/j.bone.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 58.Takeda Y, Komaba H, Goto S, et al. Effect of intravenous saccharated ferric oxide on serum FGF23 and mineral metabolism in hemodialysis patients. American journal of nephrology. 2011;33:421–426. doi: 10.1159/000327019. [DOI] [PubMed] [Google Scholar]
- 59.David V, Martin A, Hedge AM, et al. Matrix extracellular phosphoglycoprotein (MEPE) is a new bone renal hormone and vascularization modulator. Endocrinology. 2009;150:4012–4023. doi: 10.1210/en.2009-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin A, David V, Laurence JS, et al. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology. 2008;149:1757–1772. doi: 10.1210/en.2007-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Effects of recombinant hepcidin administration on FGF23 production
Effects of recombinant hepcidin administration on FGF23 production, Functional iron deficiency was induced by a single injection of 1 μg/g recombinant murine hepcidin. Serum levels of cFGF23 (A); iFGF23 (B); bone expression of Fgf23 mRNA measured 6 hours after hepcidin administration. Data are presented as mean ± SEM, n ≥ 3/group,* p<0.05 vs. control (Ctr).
Supplementary Figure 2: Effects of iron deficiency, acute and chronic inflammation on bone resorption
Representative sections of femur TRAcP staining in response to low or normal iron diets for 3 weeks (A), six hours after a single injection of (B) Brucella abortus (BA), (C) IL1-β, or after 4 days of daily IL1-β injections (D). Bone trabeculae are shown by methyl-blue counterstaining and osteoclasts by red-purple staining and indicated by black arrows.
Supplementary Table: Sequences of primers used for RT-PCR


