Abstract
Species of the fungal genus Candida, can cause oral candidiasis especially in immunosuppressed patients. Many studies have investigated the use of photodynamic therapy (PDT) to kill fungi in vitro, but this approach has seldom been reported in animal models of infection. This study investigated the effects of PDT on Candida albicans as biofilms grown in vitro and also in an immunosuppressed mouse model of oral candidiasis infection. We used a luciferase-expressing strain that allowed noninvasive monitoring of the infection by bioluminescence imaging. The phenothiazinium salts, methylene blue (MB) and new methylene blue (NMB) were used as photosensitizers (PS), combined or not with potassium iodide (KI), and red laser (660 nm) at four different light doses (10J, 20J, 40J and 60J). The best in vitro log reduction of CFU/ml on biofilm grown cells was: MB plus KI with 40J (2.31 log; p < 0.001); and NMB without KI with 60J (1.77 log; p < 0.001). These conditions were chosen for treating the in vivo model of oral Candida infection. After 5 days of treatment the disease was practically eradicated, especially using MB plus KI with 40J. This study suggests that KI can potentiate PDT of fungal infection using MB (but not NMB) and could be a promising new approach for the treatment of oral candidiasis.
Keywords: Bioluminescence imaging, Luciferase, Photodynamic therapy, Oral candidiasis, Candida albicans, Biofilm
Graphical Abstract
1. INTRODUCTION
A leading cause of opportunistic infections are fungal species belonging to the Candida genus, with C. albicans ranking as the most prevalent causative agent of candidiasis and candidemia around the world [1-6]. This trend has been observed over the past decade and is still the case, even in developed countries such as the United States, Denmark, Norway, and Finland [3]. C. albicans is a human commensal organism, and can colonize the skin, mucosal surfaces, gastrointestinal tract and the female genitourinary tract [7]. One important factor that contributes to the pathogenesis of candidiasis is biofilm formation, as C. albicans has the ability to form biofilms on both inert and biological surfaces [8]. These biofilms are typically surrounded by an extracellular polymeric substance [9] which effectively shelters the fungal cells against adverse environmental conditions, including host defense mechanisms and the action of antifungal drugs [10]. Research and development of new antifungal agents is complicated by the paucity of selective microbial targets, since fungi are also eukaryotic cells[11]. In addition, drug resistance of C. albicans against antifungals such as azoles and echinocandins represents an increasing problem [12].
Therefore, novel antifungal and antibiofilm drugs against these unmanageable infections are urgently needed [10,11]. Photodynamic therapy (PDT) has emerged as a promising modality due to its effectiveness against a broad range of species of microorganisms regardless of drug resistance [13]. In this approach, a photosensitizing agent or dye, which is activated by a light source at a specific wavelength in the presence of oxygen, resulting in the production of reactive oxygen species (ROS) and free radicals, is administered into the infected area. These photogenerated ROS disrupt the Candida cytoplasmic membrane and cause an increase in cellular permeability and subsequent damage to intracellular targets. It has been suggested that this oxidative stress might compromise virulence factors of the microorganism, such as the capacity to adhere to host epithelial cells, production of proteinases, reduction of biofilms and formation of germ tubes [14-20].
Mice have been used as models to study oral C. albicans infections and offer some advantages over a rat model in that mice do not harbor Candida spp. in their buccal cavity and therefore do not elicit an adaptive immune response against this yeast [21,22]. Optimally, the detection of light from small animals containing bioluminescent cells can be achieved using a charge-coupled device (CCD) based imaging system [23] allowing real-time non-invasive monitoring of the infection.
We have found no studies in the literature that monitored oral C. albicans infections in mice by bioluminescence imaging after use of photodynamic therapy, which could be a therapeutic option for the treatment of oral candidiasis. PDT could be a sole treatment option, or could be used as a coadjuvant to antifungal chemotherapy, improving the treatment of oral infections by increasing therapeutic efficacy and by reducing costs.
For these reasons the objective of the present study was to monitor oral candidiasis produced by a luciferase-expressing Candida albicans strain (CEC 749) in immunosuppressed mice using bioluminescence imaging before and after PDT using four different doses of red light (10J, 20J, 40J and 60J) with methylene blue or new methylene blue combined or not with potassium iodide to possibly potentiate the PDT effect. We have recently reported that addition of KI could potentiate antimicrobial PDT in vitro and in vivo mediated by MB [24], and also PDT mediated by cationic fullerenes in a mouse model of a wound abrasion infected by Acinetobacter baumannii [25].
2. MATERIALS AND METHODS
2.1 Ethics Committee
All animal procedures in this study were approved by the Subcommittee on Research Animal Care (IACUC) of the Massachusetts General Hospital under the protocol (2015N000099) and were in accordance with the guidelines of the National Institutes of Health (NIH).
2.2 Cells and culture conditions
A luciferase-expressing Candida albicans strain (CEC 749) was used in this study [26]. The luciferase reporter was constructed by fusing a synthetic, codon-optimized version of the Gaussia princeps luciferase gene (gLUC59) to C. albicans PGA59, which encodes a glycosylphosphatidylinositol linked cell wall protein. Luciferase expressed from this PGA59-gLUC fusion was localized at the C. albicans cell surface [26], where it can easily come into contact with its substrate coelenterazine (Gold Biotechnology, Inc., Charlestown, MA, USA) in intact cells. This system allows bioluminescence imaging of C. albicans infections after simple addition of coelenterazine solution to the surface of the infected tissue [26]. This approach also allows the detection of luciferase in both hyphae and yeast cells [26,27], given the central importance of the yeast-hyphal transition in C. albicans virulence [28].
C. albicans was routinely grown at 30°C on yeast extract-peptone-dextrose (YPD) agar and subcultured in YPD medium. The inoculum was prepared by growing the C. albicans cells in 3 ml YPD broth overnight at 30°C with shaking at 130 rpm in a C24 incubator/shaker (New Brunswick Scientific, Edison, NJ). The broths were then centrifuged at 12,000 rpm for 3 min (centrifuge 5417 C; Eppendorf, Hamburg, Germany) and resuspended in 0.5 ml of phosphate-buffered saline (PBS) or YPD. The concentrations were then adjusted to the appropriate values for the experiment by measuring the optical density at 570 nm (OD570) of the C. albicans suspensions, using an Evolution 300 UV-Vis spectrophotometer (Thermo Scientific). An OD570 of 0.65 correspond to a fungal concentration of 107 CFU/ml in PBS (in vivo test) or in YPD media (in vitro test).
2.3 Photosensitizers and light source
The phenothiazinium salts methylene blue (MB) (M9140-25G; Sigma), and new methylene blue (NMB) (556416-1G; Sigma) with or without potassium iodide (KI) were used as PSs in the study. The dye contents of MB and NMB were approximately 85% and 70%, respectively. These photosensitizers were selected by a preliminary study based on the criterion that the concentration produced an effective PDT effect with minimal dark toxicity [29]. Stock PS solutions (10 mM) were prepared by dissolution of the PS powders in sterilized water and were stored in the dark at 4°C for no longer than 14 days.
The light source used in this study was emitted by a diode laser (Arroyo Instruments, LLC, San Luis Obispo, CA, USA) at a wavelength of 660 nm, at a power of 100 mW, delivering 10 J, or 20 J, or 40 J, or 60 J. All parameters are given in table 1. For in vitro experiments, the laser beam was expanded by a lens (model LM2-B) to form a spot diameter 4.7 cm delivered without contact with the plates (distance of 7 cm). For in vivo experiments, the same laser beam was delivered using an optic fiber without a lens, without contact with mouse tongue (distance of 5 cm), covering a spot area of 0.38 cm2.
Table 1.
Parameters of light for photodynamic therapy (PDT)
| In vitro | In vivo |
|---|---|
| Wavelength (nm): 660 | Wavelength (nm): 660 |
| Pulse frequency: continuous | Pulse frequency: continuous |
| Laser power (mW): 100 | Laser power (mW): 100 |
| Irradiation time (sec): 100 / 200 / 400 / 600 | Irradiation time (sec): 400 / 600 |
| Total energy (J): 10 / 20 / 40 / 60 | Total energy (J): 40 / 60 |
| Energy density (J/cm2): 3.18 / 6.36 / 12.73 / 19.10 | Energy density (J/cm2): 105.26 / 157.89 |
| Power density (mW/cm2): 31.84 | Power density (mW/cm2): 263.15 |
| Application mode: without contact | Application mode: without contact |
Both the best sets of conditions from in vitro experiments using PDT with NMB (NMB 60 J) and MB (MB/KI 40 J) were chosen to be applied on the in vivo model of oral candidiasis. It is important to highlight that the light parameters were kept the same from the in vitro experiments (wavelength, total power and irradiation time). The optical power reaching the plates (in vitro) and the mouse tongue (in vivo) was measured using an optical energy meter PM100D Thorlabs® fitted with a sensor S310C (3.14 cm2).
2.4 In vitro photodynamic therapy
In order to grow the biofilms a 250 μl aliquot of the suspension (107 CFU/ml in YPD) was pipetted into each well of a 96-well flat-bottom microtiter plate. The plate was incubated for 1.5 h at 30°C in a shaker at 75 rpm for the initial adhesion phase. After this period, the wells were washed with 250 μl of PBS to remove loosely adhered cells. A 250 μl aliquot of YPD was then pipetted into each washed well, and the plate was incubated at 30°C in a shaker at 75 rpm for 48 h. The broth was changed after 24 h. The plate with biofilms formed by C. albicans was then washed with 250 μl of PBS to remove loosely adherent cells.
The biofilm formed by each strain was immersed in 100 μl of a solution of each photosensitizer (PS) or PBS for 10 min (pre-irradiation time). Subsequently, the suspended plate was irradiated according to the groups:
- P-L−: received PBS in absence of light. (n=6).
- P-L+: the effect of the light source only with different doses 10, 20, 40 and 60J. (n=6 for each dose).
- P+L−: the effect of the PS only [MB (100 μM) or NMB (100 μM) and these PSs combined with KI (100 mM)]. (n=6 for each PS).
- P+L+: treatment with each PS [MB (100 μM) or NMB (100 μM) and these PSs combined with KI (100 mM)] and each dose of light (10, 20, 40 and 60J). (n=6 for each PS and dose of light).
After this, each well received 100 μl of PBS and the biofilm was disrupted by homogenizing for 1 min using an ultrasonic homogenizer. The suspensions in the wells were considered to be a dilution factor of 10−1. 5 serial dilutions were then made using each original 10−1 dilution, and aliquots of 10 μl were seeded onto YPD agar plates [30] that were then incubated at 30°C for 24 h. After the incubation period, the CFU/ml values of each plate were determined.
2.5 Induction of experimental candidiasis
A total of 15 adult male BALB/c mice (Charles River Laboratories, Wilmington, MA), 7 to 8 weeks old, weighing 17 to 21 g and with no Candida in their buccal cavities, were used in all experiments. The animals were housed with access to food and water ad libitum and were maintained on a 12 h light/dark cycle under a room temperature of 21°C.
The methodology described by Takakura et al. [31] was used to induce experimental candidiasis with some modifications. Briefly, the animals were pre-treated with two separate doses of methylprednisolone actetate (Depo-Medrol, McKesson Medical-Surgical, Inc., Northborgh, Boston) in order to create a temporary state of immunosuppression. 150 mg kg−1 of Depo-Medrol were injected intramuscular (i.m.) 1 day before infection with Candida, followed by a second dose of 150 mg kg−1 injected i.m. 3 days after infection with Candida. Tetracycline chloride (Terramicina, Laboratórios Pfizer Ltda., Guarulhos, SP, Brazil) was administered in the drinking water at a concentration of 0.83 mg/ml beginning 1 day before infection and maintained throughout the experiment. Before C. albicans infection, the mice were anesthetized by intraperitoneal (i.p.) injection of a ketamine-xylazine cocktail.
A sterile swab soaked in the C. albicans suspension (luciferase-expressing C. albicans concentration of 107 CFU/ml in PBS) was used to inoculate the sedated mice by rubbing the swab for 1 minute on the tongue dorsum.
2.6 In vivo photodynamic therapy
Twenty-four hours after the second injection of Depo-Medrol, PDT was conducted on the animals under anesthesia produced by intraperitoneal injection of a mixture of ketamine/xylazine (100 mg/kg; 10:1 ratio). The mice were divided into three groups: a) MB/KI+40J+: sensitization with MB (1 mM) with KI (1 M) and 40J of light irradiation (n=5); b) NMB+60J+: sensitization with NMB (1 mM) without KI combined with 60J of light irradiation (n=5); and c) P-L−: control group not subjected to sensitization with PS or light irradiation (n=5). The groups “a” and “b” were chosen according to the best results on the in vitro tests.
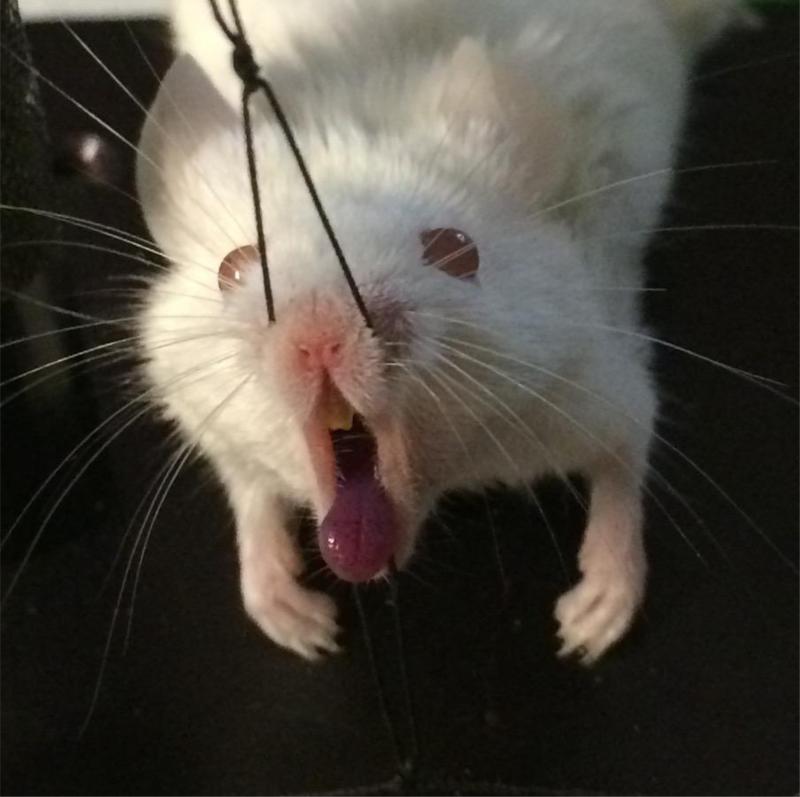
Before light irradiation, a 10 μl volume of respective PS (1mM) solution were pipetted onto the tongue dorsum and PS was then incubated for 5 min in the dark. After this time, the group “a” received 5 μl of KI solution (1M) onto the tongue. The solution of the PS was liquid and although a small amount of the PS had trickled down, the majority of the relatively small drop adhered to the mouse tongue that turned blue before the application of light (figure 1). The in vivo dark toxicity of the dye was quantified by bioluminescence imaging. To take these images, 10 μl of coelenterazine (500 μg/ml in 1:9 methanol-PBS; Gold Biotechnology, Inc., St. Louis, MO, USA) [26] were topically smeared onto the surface of each infected tongue. The 10% methanol present in the solvent was not toxic to the Candida cells. Mice received light exposure of 40J or 60J, according to the group, with bioluminescence imaging taking place after each exposure of light. The light was delivered at an irradiance of 263.15 mW/cm2 (groups “a” and “b”). The control group (“c”) received only coelenterazine in order to carry out the bioluminescence imaging.
Figure 1.
Mouse tongue turned blue after the application of photosensitizer
To record the time course of the extent of fungal infection, fungal bioluminescence images from the mouse tongue infection was measured daily after each PDT during the 5 days of daily treatment. Euthanasia was performed with an overdose of i.p. injection of a ketamine-xylazine cocktail. The tongues were longitudinally cut in half, and one-half of each organ was fixed in 10% neutral buffered formalin for 48 h, embedded in paraffin, sectioned (5 to 7 micrometer thick), and stained with hematoxylin and eosin (HE) and Periodic Acid-Schiff (PAS) for histologic analysis.
2.7 Bioluminescence imaging
The IVIS® Lumina Series III (PerkinElmer, Inc., Waltham, MA, USA) was used for bioluminescence imaging. Using photon counting mode, an image can be obtained by detecting and integrating individual photons emitted by the fungal cells. Prior to PDT and imaging, mice were anesthetized by i.p. injections of ketamine-xylazine cocktail. Mice were then placed on an adjustable stage in the imaging chamber, and the tongue infected was positioned directly under the camera. A grayscale background image of each tongue was made, and this was followed by a bioluminescence image of the same region displayed in a false-color scale ranging from red (most intense) to blue (least intense) and superimposed on the grayscale image. The signal from the bioluminescence image was quantified as region of interest (ROI) with absolute calibrated data in: photons s−1 cm−2 sr −1, using the IVIS software.
2.8 Statistical analysis
The data for CFU/ml were converted to logarithmic form and analyzed by oneway analysis of variance (ANOVA) and Tukey HSD post hoc test. The CFU/ml reduction for C. albicans biofilms were calculated comparing each group (P+L−; P-L+; P+L+) and its subgroups, with the control group (P-L−).
In a two-dimensional coordinate system, the region of interest (ROI) data, which represent the time courses of fungal luminescence of the tongue infected were calculated using numerical integration. Differences in ROI among the control, NMB 60 J and MB/KI 40 J groups in the in vivo study were compared day by day using also ANOVA and Tukey HSD post hoc test. Significance was set at P < 0.05.
3. RESULTS
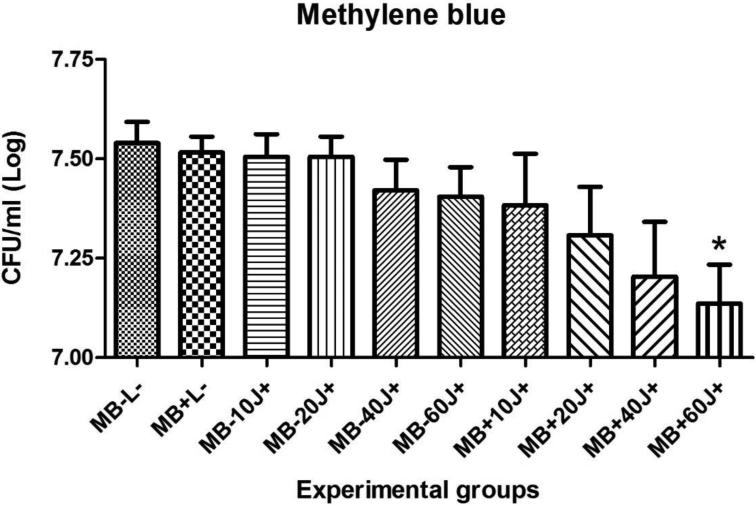
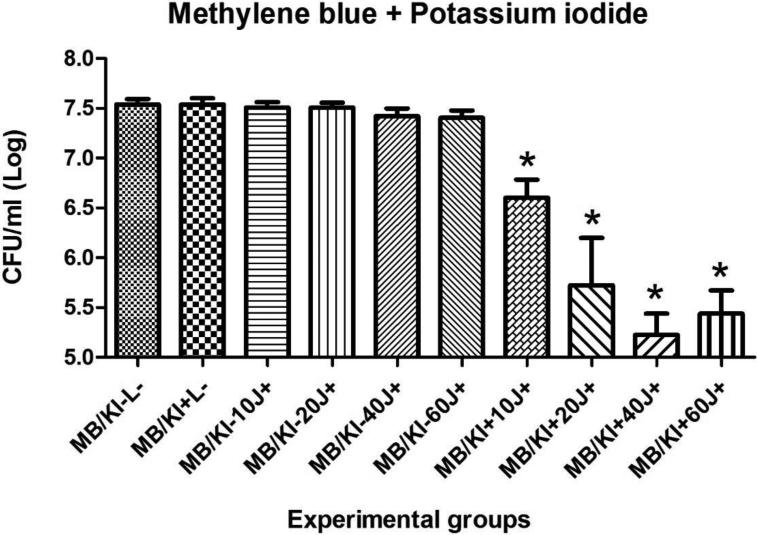
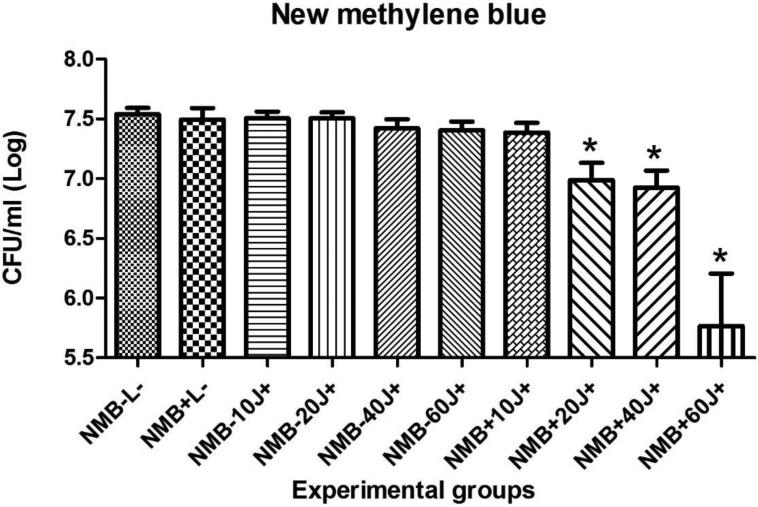
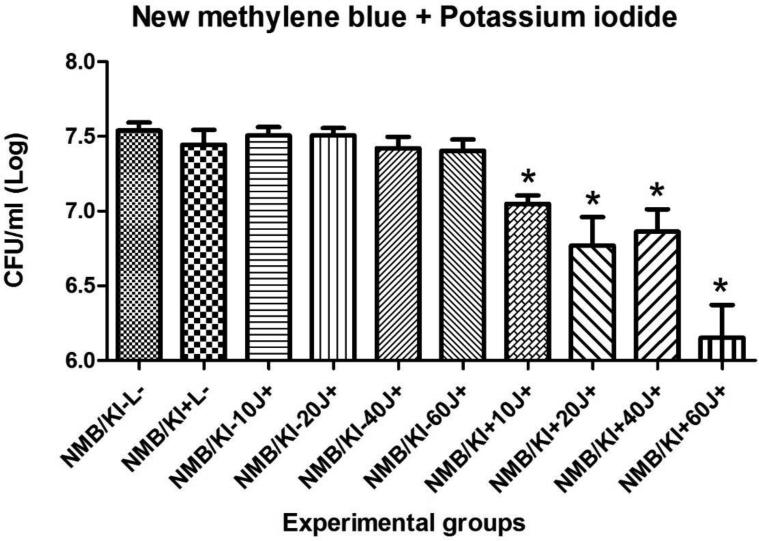
Results for the PDT of C. albicans biofilms in vitro using MB or NMB as PSs, with or without addition of KI and the different light doses are shown in figures 2, 3, 4 and 5. Only four of twenty groups was not effective (MB+10J, MB+20J, MB+40J and NMB+10J). In all other groups we had a significant reduction. It should be noted that for planktonic cells an antimicrobial effect requires at least 99.9% of Candida killing. However for biofilms less than 3 logs of killing is needed to be antimicrobial. The best log reductions of colony-forming units per milliliter (CFU/ml) were: MB (100 μM) combined with KI (100 mM) and 40J; and NMB (100 μM) without KI with 60J of irradiation, with log reductions (p < 0.001) of 2.31 and 1.77, respectively. These two sets of conditions were used in the in vivo infection model.
Figure 2.
Mean values and standard deviation of CFU/ml (log10) of C. albicans biofilms exposed to the following treatments: MB (100 μM) with 10, 20, 40 or 60J of laser doses in comparison with their respective controls, the effect of light alone, the effect of PS alone, and absolute control (no PS, no light). To analyze significance, one-way analysis of variance (ANOVA) and Tukey HSD post hoc test. The CFU/ml reduction for C. albicans biofilms were calculated comparing each group (P+L−; P-L+; P+L+) and its subgroups, with the control group (P-L-). Values followed by asterisk differed significantly among the experimental conditions (P<0.05).
Figure 3.
Mean values and standard deviation of CFU/ml (log10) of C. albicans biofilms exposed to the following treatments: MB (100 μM) combined with KI (100 mM) and 10, 20, 40 or 60J of laser doses in comparison with their respective controls, the effect of light alone, the effect of PS alone, and absolute control (no PS, no light). To analyze significance, one-way analysis of variance (ANOVA) and Tukey HSD post hoc test. The CFU/ml reduction for C. albicans biofilms were calculated comparing each group (P+L−; P-L+; P+L+) and its subgroups, with the control group (P-L−). Values followed by asterisk differed significantly among the experimental conditions (P<0.05).
Figure 4.
Mean values and standard deviation of CFU/ml (log10) of C. albicans biofilms exposed to the following treatments: NMB (100 μM) with 10, 20, 40 or 60J of laser doses in comparison with their respective controls, the effect of light alone, the effect of PS alone, and absolute control (no PS, no light). To analyze significance, one-way analysis of variance (ANOVA) and Tukey HSD post hoc test. The CFU/ml reduction for C. albicans biofilms were calcuated comparing each group (P+L−; P-L+; P+L+) and its subgroups, with the control group (P-L-). Values followed by asterisk differed significantly among the experimental conditions (P<0.05).
Figure 5.
Mean values and standard deviation of CFU/ml (log10) of C. albicans biofilms exposed to the following treatments: NMB (100 μM) combined with KI (100 mM) and 10, 20, 40 or 60J of laser doses in comparison with their respective controls, the effect of light alone, the effect of PS alone, and absolute control (no PS, no light). To analyze significance, one-way analysis of variance (ANOVA) and Tukey HSD post hoc test. The CFU/ml reduction for C. albicans biofilms were calculated comparing each group (P+L−; P-L+; P+L+) and its subgroups, with the control group (P-L−). Values followed by asterisk differed significantly among the experimental conditions (P<0.05).
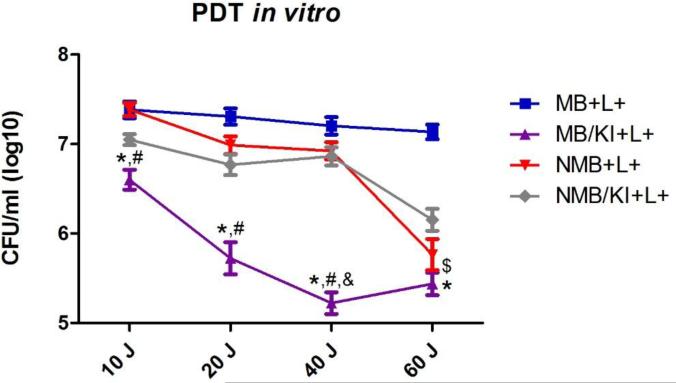
The figure 6 shows a comparison among the four treatments used in this study. The group MB plus KI showed significantly more fungal killing compared to MB alone at all doses of light utilized (10, 20, 40 and 60J) and with NMB alone with 10, 20 and 40J of light irradiation. MB plus KI also had a significant difference compared to NMB plus KI with 40J, and MB alone had significantly more killing compared to NMB alone when these groups were irradiated with 60J.
Figure 6.
Mean values and standard deviation of CFU/ml (log10) of C. albicans biofilms exposed to the following treatments: MB (100 μM), MB (100 μM) combined with KI (100 mM), NMB (100 μM) and NMB (100 μM) combined with KI (100 mM); all treated with 10, 20, 40 or 60J of laser light. To analyze significance, a ANOVA and Tukey HSD post hoc test were used. Values followed by asterisk had difference between MB and MB plus KI, values followed by hash had difference between NMB and MB plus KI, values followed by “&” had difference between MB plus KI and NMB plus KI and values followed by “$” had difference between MB and NMB. (P<0.05).
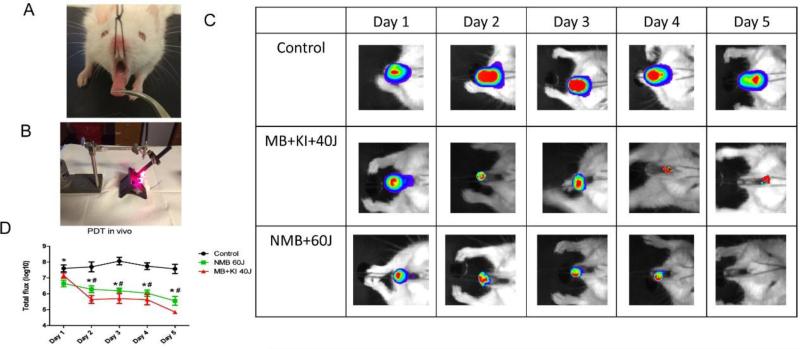
After 4 days of Candida inoculation (day 1 of treatment) a strong positive Candida bioluminescence signal was achieved with the peak occurring after 6 days for the control group (figure 7C). In the majority of animals subjected to immunosuppression and Candida inoculation, white lesions typical of pseudomembranous candidiasis were observed (figure 7A).
Figure 7.
(A) White patches observed on the tongue dorsum of a mouse without treatment (B) Representative image during the applying of PDT (C) Successive bioluminescence images of three representative mice tongues infected with 107 CFU/ml of C. albicans and treated with PDT during 5 days, starting on day 4 after fungal inoculation, in comparison with the control group that did not receive light and PS. PDT was carried out with a combination of MB (1 mM) together with KI (1 M) and 40 J of red laser; and using NMB (1 mM) with 60J of light. (D) Mean values and standard deviation of total photon flux (log10) of bioluminescent C. albicans infection exposed to the following treatments daily during 5 days: MB (1 mM) combined with KI (1 M) and 40 J of red laser; and NMB (1 mM) with 60J of light in comparison with the control group with absence of light and PS. To analyze significance, a ANOVA and Tukey HSD post hoc test were used comparing day by day of treatment. Values followed by hash had difference statistic between MB/KI+40J+ and the control group and values followed by asterisk had significant difference between NMB+60J+ and the control group (P<0.05).
Total loss of bioluminescence signal was seen in two animals on the fifth day of treatment (figure 7B) with NMB (1 mM), as can be seen in the example in figure 7C. Figure 7D shows that both PSs were effective on the treatment of oral candidiasis. No difference was found between the two PSs (P > 0.05 for all days of treatment).
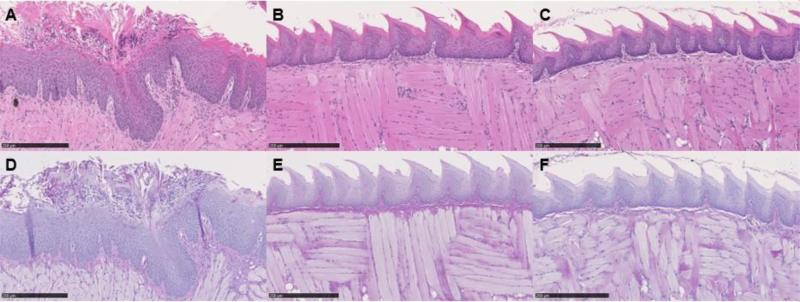
A representative set of periodic-acid-Schiff (PAS) and hematoxylin and eosin (HE) stained images from the infected tongue on day 5 post infection are shown in figure 8. These images demonstrated the presence of numerous C. albicans cells in the form of yeast and multicellular hyphal filaments in the control group. While all yeast cells were located on the surface of the keratinized epithelial layer of the tongue, the hyphal filaments branched out in every direction into the tissue. A host response to C. albicans infection, characterized by an inflammatory infiltrate, was also observed. Several lesions in the epithelial tissue were also observed, including epithelial hyperplasia, basal cell layer disorganization, exocytosis, loss of filiform papillae, hyperkeratosis and intraepithelial micro abscesses development. The figure also demonstrated that in the PDT treated groups the surfaces of the tongue appeared intact showing well-defined layers without visible yeasts or hyphal filaments, and without any lesions in the epithelial tissue.
Figure 8.
Representative hematoxylin and eosin (8A, 8B and 8C) and periodic acid-Schiff (8D, 8E and 8F) tongue biopsy specimen taken from a mouse tongue infected with 107 CFU/ml of C. albicans, showing the presence of lesions in the epithelial tissue including epithelial hyperplasia, basal cell layer disorganization, exocytosis, loss of filiform papillae, and hyperkeratosis (8A) and the presence of yeasts and hyphal filaments (8D); in comparison with the groups that received treatment with NMB (100 mM) plus 60J light dose (8B and 8E); and MB (100 mM) combined with KI (1 M) plus 40J light dose (8C and 8F) that shows the tissues to be in intact condition. Biopsies were taken on day 5 post-infection. Bar, 250 μm.
4. DISCUSSION
Candida albicans is the most frequent fungal species isolated from superficial and invasive fungal infections and systemic infections are associated with high rates of mortality. Since there is an increasing number of strains of this microorganism that are resistant to antifungal agents, studies are needed to evaluate the effect of photodynamic therapy on oral candidiasis infections. In this study, we used a low light imaging charge-coupled device (CCD) camera to non-invasively monitor the results in real time. The treatment of candidiasis continues to be a challenge in dentistry. In this respect, photodynamic therapy has shown promising results as a coadjuvant treatment for this disease.
Schelenz et al. [32] isolated 269 strains of Candida spp. from the buccal cavity of patients with hematological malignancies or head and neck solid tumors. The authors evaluated the sensitivity profile of these strains to antifungal agents and found resistance to fluconazole (4.5%), itraconazole (11.7%), ketoconazole (11.3%), voriconazole (0.75%), and caspofungin (41.1%); however, all strains were susceptible to amphotericin B. Thus, amphotericin B has been used in treatment of Candida infections caused by fluconazole-resistant strains; however, amphotericin B can have substantial side effects, and a few cases of resistance have been reported [33]. For these reasons PDT is a new approach being developed for the treatment of candidiasis and the aim of this study was to study the effects of PDT both in vitro and in vivo.
Zhang et al. [25] synthesized C60-fullerene (LC16) bearing a deca-quaternary chain and deca-tertiary-amino groups that facilitated electron-transfer reactions via the photoexcited fullerene. Addition of the harmless salt, potassium iodide (KI) (10 mM) potentiated the ultraviolet A (UVA) or white light-mediated killing of Gram-negative bacteria Acinetobacter baumannii, Gram-positive methicillin-resistant Staphylococcus aureus and fungal yeast Candida albicans by 1-2+ logs. A mouse model of a skin abrasion infected with bioluminescent Acinetobacter baumannii gave increased loss of bioluminescence when iodide (10 mM) was combined with LC16 and UVA/white light. In a study from the same group, Vecchio et al. [24] added KI to MB and observed a consistent increase of red light-mediated bacterial killing of Gram-positive and Gram-negative species in vitro and in vivo. In vivo, they also observed less bacterial recurrence in wounds in the days post-treatment. They proposed that the mechanism of action was probably due to formation of reactive iodine species that are produced quickly with a short lifetime. In our study MB (100 μM) was combined with KI (100 mM) and four different doses of light (10, 20, 40 and 60 J) against Candida albicans biofilms grown in vitro. When MB was used without KI the best log reduction was found with 60 J of light energy while the MB/KI solution achieved 2.31 of log reduction with 40 J of irradiation. This last result was the best one in all in vitro tests of our study and was chosen to apply to the oral Candida infection.
Freire et al. [34] compared Rose Bengal (RB) with Eosin Y (2,4,5,7 tetrabromofluorescein) assessing their potency for photoinactivation of Candida albicans biofilms. After culturing these biofilms for 48 h, treatment was done with 200 μM RB or Eosin Y for 5 min as a pre-irradiation period and subsequent illumination with a green LED (90mW; 532±10nm) for 180s (light dose: 42.63J/cm2; applied energy: 16.2J), which resulted in inactivation rates of 0.22 and 0.45 log10 for RB and Eosin Y, respectively. In our study MB had much better log reduction on CFU/ml of Candida albicans biofilms as described above. We also tested another phenothiazinium dye NMB (100 μM) combined or not with KI (100 mM) with four doses of red laser (10, 20, 40 and 60J). When NMB was combined with KI the highest log reduction was 1.38 with 60 J of light energy, but the best log reduction was found with this PS without any added KI (1.77 log10) and with 60 J of irradiation as well. This group (NMB+60J+) was also chosen to use in the in vivo study, which showed promising results. A possible explanation for the observation that KI potentiated PDT with MB but not with NMB lies with consideration of the photochemical mechanisms carried out by the two dyes. The production of iodine radicals from iodide anion represents a one-electron transfer reaction, and it is possible that MB carries out more electron transfer reactions (Type I photochemical mechanism), while NMB may carry out more singlet oxygen generation (Type II photochemical mechanism). This hypothesis would require further investigation to confirm it.
Khademi et al. [35] induced oral candidiasis, using C. albicans isolated from an azole-resistant patient, with a similar protocol to ours and at 2 days after the last immunosuppression injection, PDT was carried out on the tongue dorsum by use of different concentrations of MB or poly-L-lysine-chlorine (e6) conjugate (pL-ce6) as PSs, followed by a 10 min diode laser illumination at 660 nm (n = 6 each). The most effective PS found in the animals treated with PDT mediated by MB at a concentration of 450 mg/L with reduction from 3.43 log10 to 0 (CFU/ml) and it was the highest C. albicans eradication recorded among all groups in this study.
Costa et al. [36] and Dovigo et al. [37] also worked with a model of oral candidiasis using PDT treatment post infection. The first study used erythrosine (400 μmol/L) followed by exposure to a green LED (14.34 J cm−2) and the quantified (CFU/ml) yeasts recovered from the mice were significantly reduced by 0.73 log10. This group also analyzed the tongues microscopically and found the presence of yeasts and hyphae that were limited to the keratinized layer on the tongue dorsum, which was primarily localized on simple conic papillae. Several lesions in the epithelial tissue were also observed and in the areas with tissue lesions, the lamina propria exhibited a mild inflammatory infiltrate. The groups that received PDT demonstrated a reduction of yeasts, hyphae, lesions and inflammatory infiltrate. The second study used curcumin (20, 40 and 80 μM) and illumination with LED light and all animals exposed to curcumin with LED light exhibited a significant reduction in C. albicans viability after PDT, but the use of 80 μM curcumin and light was able to induce the highest log10 reduction in colony counts (4 logs) and no evidence of fungi was found on histological examination of the tongues in four animals treated with PDT, with 80 μM curcumin.
In contrast to the last 3 studies cited, the aim of our study was to evaluate PDT of oral candidiasis using real-time bioluminescence monitoring. The use of a luciferase-expressing strain of C. albicans allowed this real-time monitoring using bioluminescence imaging with an IVIS® Lumina Series III. In our study we found that 5 days of daily PDT treatment with MB (1 mM) combined with KI (1 M) and 40 J of light irradiation and NMB with 60J of light energy almost eradicated the infection (figures 7C and 7D). On day 1 of treatment only the group NMB+60J+ was significantly reduced in comparison with the control group (p = 0.012). On day 2 however both PDT groups were significantly reduced, p = 0.006 for NMB and p < 0.001 for MB. From the third day both PSs had p < 0.001 when the treatment with these PSs was compared with the control group. During the 5 days of treatment no difference was found between the treatments with MB and NMB (p > 0.05 for all days). Observing figures 6 and 7D we can see that the treatment with MB plus KI was the best one amongst the four treatments tested in this study, and this combination with 40J of irradiation was chosen for the animal experimental group.
Under optical microscopy, many yeasts and hyphae, epithelial lesions such as epithelial hyperplasia, basal cell layer disorganization, exocytosis, loss of filiform papillae, hyperkeratosis and intraepithelial micro abscess development, and mild inflammatory infiltrate with a predominance of neutrophils were observed on the tongue dorsum of the animals without treatment with MB or NMB (figures 8A and 8D). In some cases, moderate inflammatory infiltrate and mononuclear cells in the lamina propria and basal layer were observed, and congested vessels were occasionally noted. On the tongues of the animals that received PDT treatment, we found only occasional yeasts, hyphae, lesions and inflammatory infiltrate. Most of the tongues of the animals that were treated were uninjured (figures 8B, 8C, 8E and 8F) demonstrating that PDT with MB (1 mM) combined with KI (1 M) and 40 J of light irradiation and NMB with 60J of light energy is effective.
Judging by the results obtained in the present study, both NMB-PDT and MBPDT combined with KI represents two potential antifungal strategies to treat oral candidiasis in an immunosuppressed murine model. A single application of PDT was sufficient to produce elimination of C. albicans from some of the evaluated mice (NMB+60J+) but the most promising results were observed with the use of 5 days of daily treatment using either PS, suggesting that PDT using our protocols is a promising approach for treatment of oral candidiasis. Considering the non-toxic nature of KI (FDA approved and generally available as a health supplement to avoid iodine deficiency) clinical trials studies using PDT combined with KI could be undertaken to translate our results into clinical practice.
Highlights.
Methylene blue PDT of Candida biofilms is potentiated by non-toxic potassium iodide
New methylene blue PDT is not potentiated by potassium iodide
PDT of oral candidiasis in a mouse model using real time bioluminescence imaging.
Total loss of bioluminescence on 5th day of treatment with new methylene blue PDT.
ACKNOWLEDGEMENTS
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (Scholarship 2014/25772-9). CF was supported by CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico (Scholarship 202313/2014-0) and Lemann Foundation; MR Hamblin was supported by US NIH grant R01AI050875.
Abbreviation
- PDT
photodynamic therady
- MB
methylene blue
- NMB
new methylene blue
- PS
photosensitizers
- KI
potassium iodide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, Horn D. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, 2004-2008. Diagnostic microbiology and infectious disease. 2012;74(4):323–331. doi: 10.1016/j.diagmicrobio.2012.10.003. doi:10.1016/j.diagmicrobio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Ebbers J, Geurtz L, Stefanik D, Major Y, Edmond MB, Wenzel RP, Seifert H. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. International journal of antimicrobial agents. 2014;43(1):78–81. doi: 10.1016/j.ijantimicag.2013.09.005. doi:10.1016/j.ijantimicag.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Guinea J. Global trends in the distribution of Candida species causing candidemia. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(Suppl 6):5–10. doi: 10.1111/1469-0691.12539. doi:10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 4.Bassetti M, Merelli M, Ansaldi F, de Florentiis D, Sartor A, Scarparo C, Callegari A, Righi E. Clinical and therapeutic aspects of candidemia: a five year single centre study. PloS one. 2015;10(5):e0127534. doi: 10.1371/journal.pone.0127534. doi:10.1371/journal.pone.0127534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nucci M, Queiroz-Telles F, Alvarado-Matute T, Tiraboschi IN, Cortes J, Zurita J, Guzman- Blanco M, Santolaya ME, Thompson L, Sifuentes-Osornio J, Echevarria JI, Colombo AL. Epidemiology of candidemia in Latin America: a laboratory-based survey. PloS one. 2013;8(3):e59373. doi: 10.1371/journal.pone.0059373. doi:10.1371/journal.pone.0059373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das I, Nightingale P, Patel M, Jumaa P. Epidemiology, clinical characteristics, and outcome of candidemia: experience in a tertiary referral center in the UK. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2011;15(11):e759–763. doi: 10.1016/j.ijid.2011.06.006. doi:10.1016/j.ijid.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nature reviews Immunology. 2014;14(6):405–416. doi: 10.1038/nri3684. doi:10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seddiki SM, Boucherit-Otmani Z, Boucherit K, Kunkel D. [Fungal infectivities of implanted catheters due to Candida sp. Biofilms formation and resistance]. Journal de mycologie medicale. 2015;25(2):130–135. doi: 10.1016/j.mycmed.2015.03.003. doi:10.1016/j.mycmed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. Candida biofilms: an update. Eukaryotic cell. 2005;4(4):633–638. doi: 10.1128/EC.4.4.633-638.2005. doi:10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierce CG, Lopez-Ribot JL. Candidiasis drug discovery and development: new approaches targeting virulence for discovering and identifying new drugs. Expert opinion on drug discovery. 2013;8(9):1117–1126. doi: 10.1517/17460441.2013.807245. doi:10.1517/17460441.2013.807245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce CG, Srinivasan A, Uppuluri P, Ramasubramanian AK, Lopez-Ribot JL. Antifungal therapy with an emphasis on biofilms. Current opinion in pharmacology. 2013;13(5):726–730. doi: 10.1016/j.coph.2013.08.008. doi:10.1016/j.coph.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taff HT, Mitchell KF, Edward JA, Andes DR. Mechanisms of Candida biofilm drug resistance. Future microbiology. 2013;8(10):1325–1337. doi: 10.2217/fmb.13.101. doi:10.2217/fmb.13.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections--state of the art. Photodiagnosis and photodynamic therapy. 2009;6(3-4):170–188. doi: 10.1016/j.pdpdt.2009.10.008. doi:10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dovigo LN, Pavarina AC, Mima EG, Giampaolo ET, Vergani CE, Bagnato VS. Fungicidal effect of photodynamic therapy against fluconazole-resistant Candida albicans and Candida glabrata. Mycoses. 2011;54(2):123–130. doi: 10.1111/j.1439-0507.2009.01769.x. doi:10.1111/j.1439-0507.2009.01769.x. [DOI] [PubMed] [Google Scholar]
- 15.Martins Jda S, Junqueira JC, Faria RL, Santiago NF, Rossoni RD, Colombo CE, Jorge AO. Antimicrobial photodynamic therapy in rat experimental candidiasis: evaluation of pathogenicity factors of Candida albicans. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2011;111(1):71–77. doi: 10.1016/j.tripleo.2010.08.012. doi:10.1016/j.tripleo.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Munin E, Giroldo LM, Alves LP, Costa MS. Study of germ tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT). Journal of photochemistry and photobiology B, Biology. 2007;88(1):16–20. doi: 10.1016/j.jphotobiol.2007.04.011. doi:10.1016/j.jphotobiol.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Cormick MP, Alvarez MG, Rovera M, Durantini EN. Photodynamic inactivation of Candida albicans sensitized by tri- and tetra-cationic porphyrin derivatives. European journal of medicinal chemistry. 2009;44(4):1592–1599. doi: 10.1016/j.ejmech.2008.07.026. doi:10.1016/j.ejmech.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Giroldo LM, Felipe MP, de Oliveira MA, Munin E, Alves LP, Costa MS. Photodynamic antimicrobial chemotherapy (PACT) with methylene blue increases membrane permeability in Candida albicans. Lasers in medical science. 2009;24(1):109–112. doi: 10.1007/s10103-007-0530-2. doi:10.1007/s10103-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 19.Soares BM, da Silva DL, Sousa GR, Amorim JC, de Resende MA, Pinotti M, Cisalpino PS. In vitro photodynamic inactivation of Candida spp. growth and adhesion to buccal epithelial cells. Journal of photochemistry and photobiology B, Biology. 2009;94(1):65–70. doi: 10.1016/j.jphotobiol.2008.07.013. doi:10.1016/j.jphotobiol.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Costa AC, de Campos Rasteiro VM, Pereira CA, da Silva Hashimoto ES, Beltrame M, Jr., Junqueira JC, Jorge AO. Susceptibility of Candida albicans and Candida dubliniensis to erythrosine- and LED-mediated photodynamic therapy. Archives of oral biology. 2011;56(11):1299–1305. doi: 10.1016/j.archoralbio.2011.05.013. doi:10.1016/j.archoralbio.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Naglik JR, Fidel PL, Jr., Odds FC. Animal models of mucosal Candida infection. FEMS microbiology letters. 2008;283(2):129–139. doi: 10.1111/j.1574-6968.2008.01160.x. doi:10.1111/j.1574-6968.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samaranayake YH, Samaranayake LP. Experimental oral candidiasis in animal models. Clinical microbiology reviews. 2001;14(2):398–429. doi: 10.1128/CMR.14.2.398-429.2001. doi:10.1128/CMR.14.2.398-429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. Journal of photochemistry and photobiology B, Biology. 2005;81(1):15–25. doi: 10.1016/j.jphotobiol.2005.05.007. doi:10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vecchio D, Gupta A, Huang L, Landi G, Avci P, Rodas A, Hamblin MR. Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide. Antimicrobial agents and chemotherapy. 2015;59(9):5203–5212. doi: 10.1128/AAC.00019-15. doi:10.1128/AAC.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Dai T, Wang M, Vecchio D, Chiang LY, Hamblin MR. Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: in vitro and in vivo studies. Nanomedicine (Lond) 2015;10(4):603–614. doi: 10.2217/nnm.14.131. doi:10.2217/nnm.14.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enjalbert B, Rachini A, Vediyappan G, Pietrella D, Spaccapelo R, Vecchiarelli A, Brown AJ, d'Enfert C. A multifunctional, synthetic Gaussia princeps luciferase reporter for live imaging of Candida albicans infections. Infection and immunity. 2009;77(11):4847–4858. doi: 10.1128/IAI.00223-09. doi:10.1128/IAI.00223-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.d'Enfert C, Vecchiarelli A, Brown AJ. Bioluminescent fungi for real-time monitoring of fungal infections. Virulence. 2010;1(3):174–176. doi: 10.4161/viru.1.3.11119. doi:10.4161/viru.1.3.11119. [DOI] [PubMed] [Google Scholar]
- 28.Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiology and molecular biology reviews : MMBR. 2007;71(2):348–376. doi: 10.1128/MMBR.00009-06. doi:10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai T, Bil de Arce VJ, Tegos GP, Hamblin MR. Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrobial agents and chemotherapy. 2011;55(12):5710–5717. doi: 10.1128/AAC.05404-11. doi:10.1128/AAC.05404-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S, Chibli H, Nadeau J. Solubilization and bio-conjugation of quantum dots and bacterial toxicity assays by growth curve and plate count. Journal of visualized experiments : JoVE. 2012;(65):e3969. doi: 10.3791/3969. doi:10.3791/3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takakura N, Sato Y, Ishibashi H, Oshima H, Uchida K, Yamaguchi H, Abe S. A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiology and immunology. 2003;47(5):321–326. doi: 10.1111/j.1348-0421.2003.tb03403.x. [DOI] [PubMed] [Google Scholar]
- 32.Schelenz S, Abdallah S, Gray G, Stubbings H, Gow I, Baker P, Hunter PR. Epidemiology of oral yeast colonization and infection in patients with hematological malignancies, head neck and solid tumors. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2011;40(1):83–89. doi: 10.1111/j.1600-0714.2010.00937.x. doi:10.1111/j.1600-0714.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 33.Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontology 2000. 2009;49:39–59. doi: 10.1111/j.1600-0757.2008.00291.x. doi:10.1111/j.1600-0757.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 34.Freire F, Costa AC, Pereira CA, Beltrame Junior M, Junqueira JC, Jorge AO. Comparison of the effect of rose bengal- and eosin Y-mediated photodynamic inactivation on planktonic cells and biofilms of Candida albicans. Lasers in medical science. 2014;29(3):949–955. doi: 10.1007/s10103-013-1435-x. doi:10.1007/s10103-013-1435-x. [DOI] [PubMed] [Google Scholar]
- 35.Khademi H, Torabinia N, Allameh M, Jebreilamtigh HR. Comparative evaluation of photodynamic therapy induced by two different photosensitizers in rat experimental candidiasis. Dental research journal. 2014;11(4):452–459. [PMC free article] [PubMed] [Google Scholar]
- 36.Costa AC, Campos Rasteiro VM, da Silva Hashimoto ES, Araujo CF, Pereira CA, Junqueira JC, Jorge AO. Effect of erythrosine- and LED-mediated photodynamic therapy on buccal candidiasis infection of immunosuppressed mice and Candida albicans adherence to buccal epithelial cells. Oral surgery, oral medicine, oral pathology and oral radiology. 2012;114(1):67–74. doi: 10.1016/j.oooo.2012.02.002. doi:10.1016/j.oooo.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Dovigo LN, Carmello JC, de Souza Costa CA, Vergani CE, Brunetti IL, Bagnato VS, Pavarina AC. Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis. Medical mycology. 2013;51(3):243–251. doi: 10.3109/13693786.2012.714081. doi:10.3109/13693786.2012.714081. [DOI] [PubMed] [Google Scholar]