Abstract
The prevalence of diabetes-related cataracts during childhood is less than 1%. When cataracts occur, it is often in adolescent females with prolonged symptoms and significant hyperglycemia. Cataracts are not a classic feature of monogenic diabetes. We report a case of a six year old previously healthy Caucasian male who presented with bilateral acquired cataracts and was subsequently diagnosed with new onset diabetes. Additional symptoms at presentation included a several year history of polyuria and polydipsia, mild hepatomegaly, and short stature. Pertinent negatives include acanthosis nigricans, lipoatrophy, deafness, muscle weakness, or neuropathy. HbA1c was significantly elevated at diagnosis (>14%, 129.5mmol/mol) without evidence of ketosis. Autoantibody testing was negative. Features of Mauriac Syndrome (short stature, hepatomegaly) as well as acquired cataracts indicated long standing hyperglycemia with sufficient insulin production to prevent ketone production and development of diabetic ketoacidosis. Whole exome sequencing was conducted and a de novo heterozygous mutation in the INS gene (c.94G>A; p.Gly32Ser) was identified. INS gene mutations are common causes of permanent neonatal diabetes but rare causes of antibody-negative diabetes in children. Importantly, INS gene mutations have not been previous associated with acquired cataracts. Knowledge of a monogenic cause of diabetes allows clinicians to tailor counseling and screening of diabetes related co-morbidities. In summary, this case highlights the need to consider testing for monogenic diabetes, specifically INS gene mutations, in pediatric patients with antibody-negative diabetes, especially if complications of prolonged hyperglycemia are present at diagnosis.
Keywords: cataract, insulin gene, exome sequencing
Introduction
Although cataracts are a complication of long-standing hyperglycemia, cataracts in children with diabetes are uncommon with a prevalence of less than 1% (1, 2). It is rarer still for cataracts to be present at the initial presentation of diabetes in children. Previously published reports suggest that gender (female), adolescence, long standing history of symptoms or poorly controlled diabetes, and a high HbA1c level at diagnosis are all associated with an increased risk of cataract formation during childhood (3). Hereby, we report a case of a previously healthy 6-year-old male who presented with bilateral acquired cataracts and hyperglycemia leading to the diagnosis of insulin-dependent diabetes. The atypical clinical features in this case led to the diagnosis of an insulin gene (INS) deficiency with a de novo mutation (c.94G>A; p.Gly32Ser) as the cause of his diabetes. This case highlights the importance of evaluating for monogenic forms of diabetes in pediatric patients when complications of chronic hyperglycemia are present at diagnosis and antibody testing is negative.
Case Report
A previously healthy Caucasian male, presented at 6 years 2 months of age to ophthalmology after failing a school vision screen. The family reported progressive deterioration in near and distance vision over the prior year; he was no longer able to see the ball while playing baseball and had difficulty reading text in school. Eye exam noted visual acuity of 20/125 in the right eye and 20/200 in the left eye, a significant change from prior vision testing at 3 years of age where acuity was 20/25 bilaterally. Anterior segment examination noted dense bilateral cataracts involving the lens nucleus with cortical vacuoles and posterior subcapsular opacification. Fundoscopic exam was unremarkable. The patient was subsequently referred for evaluation for metabolic disorders.
Upon evaluation by endocrinology, further history uncovered symptoms of polyuria, polydipsia, and poor linear growth over the last three years; however, there was no prior history of cataracts, hearing loss, weight loss, or muscle weakness and no family history of cataracts or diabetes. Physical exam revealed a well-appearing prepubertal male with a height of 105.7 cm (1st percentile, mid-parental height 50th percentile), a body mass index of 14.27 kg/m2 (15th percentile), and mild liver enlargement. No acanthosis nigricans or lipoatrophy were detected. Initial laboratory evaluation was notable for long standing and severe hyperglycemia without evidence of ketosis (Table 1).
Table 1.
Presenting Metabolic Labs
| Lab | Result |
|---|---|
| Serum Glucose | >700 mg/dL |
| Bicarbonate (RR: 16–29 mmol/L) | 23 mmol/L |
| Urine Glucose | >1000 mg/dL |
| Urine Ketones | Negative |
| HbA1c (3.5–6.3%) | >14% (129.5 mmol/mol) |
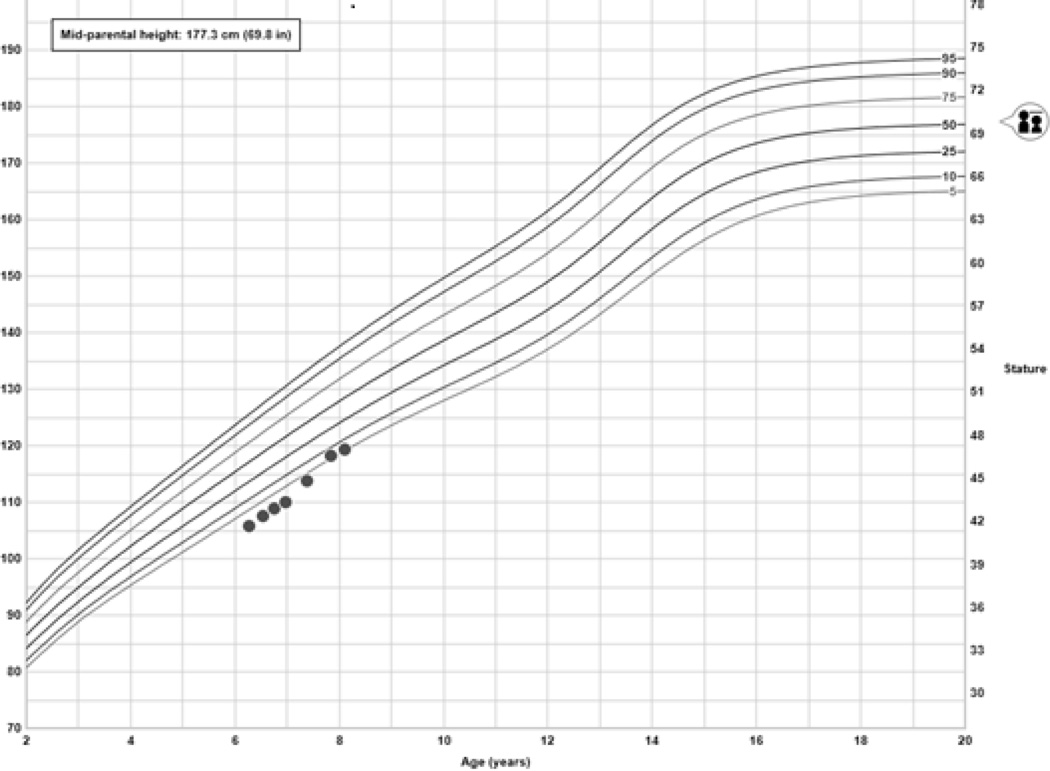
Insulin therapy was initiated at 0.4 u/kg/day with rapid improvement of hyperglycemia. Autoantibody testing for glutamic acid decarboxylase (GAA), insulin antigen -2 (IA-2), insulin autoantibodies (IAA) and zinc transporter 8 (ZnT8) proved negative (Table 2). Glycemic control rapidly improved with a HbA1c of 7.6% (59.6 mmol/mol) four months after diagnosis. His hepatomegaly resolved and weight gain normalized. The cataracts failed to improve with glycemic control; thus, cataract surgery with bilateral intraocular lens placement was performed consecutively. Importantly, there was no evidence of diabetic retinopathy or cystoid macular edema. Six weeks post-operatively, visual acuity was 20/30 in both eyes. He remains below his mid-parental target height; however, his growth velocity has improved (Figure 1). He continues to require full insulin replacement of 0.5 u/kg/day.
Table 2.
Islet Cell Antibody testing
| Antibody | Result |
|---|---|
| GAA (<20) | 0 |
| IA-2 (<5) | 0 |
| IAA (<0.011) | 0.001 |
| ZnT8 (<0.021) | 0.002 |
Figure 1.
Improvement in growth velocity after starting insulin
Given this patient’s unusual presentation suggestive of long standing hyperglycemia (clinical features of Mauriac Syndrome with hepatomegaly and short stature, cataracts, and elevated HbA1c) without ketosis, genetic defects that lead to impairment in insulin secretion or activity were considered. Lack of family history, early childhood age, and presence of long-standing complications at presentation diminished the likelihood of common forms of monogenic diabetes (HNF1A, GCK, and HNF4BA). Whole exome sequencing, of the patient and his parents, was pursued to detect an unknown and potentially novel underlying genetic explanation for the highly unusual phenotype of childhood onset antibody-negative diabetes, bilateral cataracts, short stature, and hepatomegaly. This was performed in the clinical molecular genetics laboratory at Cincinnati Children’s Hospital Medical Center as previously described (4).
A de novo heterozygous change in the INS gene (c.94G>A; p.Gly32Ser) was found and confirmed by Sanger sequencing. The c.94G>A mutation, located in the insulin domain, was predicted to result in the substitution of serine for glycine at amino acid 32. This mutation is listed in the human gene mutation database (HGMD) as a disease causing mutation (CM074280). In addition, this mutation was identified in the heterozygous state in seven families with permanent neonatal diabetes and one patient with type 1b diabetes mellitus (5–8). Thus, the identification of this mutation provides a genetic explanation for our patient’s diabetes. Whole exome sequencing did not reveal any other mutation associated with cataract formation.
Discussion
INS gene mutation and diabetes
The INS gene encodes for a 110 amino acid preproinsulin molecule that undergoes subsequent cleavage of the signal sequence in the endoplasmic reticulum of pancreatic beta cells forming proinsulin. Disulfide bridge formation between the A and B insulin chains folds proinsulin for transport to the golgi apparatus where cleavage to insulin and C-peptide in maturing secretory granules takes place (9). Pathogenic mutations in the INS gene, like in our patient, frequently occur at locations that disrupt the normal folding and disulfide bridge formation. Specific to p.Gly32Ser found in this patient, Rajan et al demonstrated that this mutation may prevent the insulin protein from being efficiently secreted from the endoplasmic reticulum by affecting its folding (10). Prior studies demonstrate that impaired folding leads to accumulation of proinsulin, protein degradation within the endoplasmic reticulum, severe stress response, and ultimate apoptosis of the pancreatic beta cell (5, 11).
INS gene mutations are the second most common cause of permanent neonatal diabetes (PND), accounting for 15–20% of cases (5, 6, 12). Patients with PND due to INS gene mutations tend to present at an older age then those with KCNJ11 or ABCC8 gene mutations (mean age 13 weeks vs 5 weeks vs 7 weeks, respectively) suggesting a progressive destruction of pancreatic beta cells (5). Indeed, several studies have identified INS mutations in a small fraction of cases diagnosed with diabetes between 6–12 months of age, whereas only very rarely have INS mutations been found in those diagnosed with diabetes after a year of age (6–8, 12). As part of a larger study to identify INS gene mutations in other forms of diabetes, Edghill et al performed DNA sequencing on 296 patients with the classic “MODY” phenotype (non-obese, diabetes diagnosis before 25 years of age, family history of diabetes suggestive of autosomal dominant inheritance) but negative for mutations in HNF4A, GCK and HNF1A. Only one patient was found to have an INS gene mutation. This study also found that 80% of all INS gene mutations are de novo, as found in this patient; unlike more common forms of monogenic diabetes there is not a history of first degree relatives affected with diabetes (6). Mutations in INS were also identified in two of seven patients with antibody-negative Type 1 diabetes (GAD, IAA, IA-2, ZnT8) and no other comorbidities (7). One child, diagnosed at age 2 years 10 months, had the same mutation as our patient. While Rubio-Cabezas et al found INS gene mutations in 8% of 25 children with antibody-negative diabetes, both children were diagnosed in the first year of life (8). Molven et al. conducted a study of 124 patients with antibody-negative diabetes (GAD and IA-2) from the Norwegian Childhood Diabetes Registry and identified one case of an INS gene mutation (12). Most recently, INS mutations were identified in 5 of 34 Japanese children with antibody-negative diabetes (GAD and IA-2) diagnosed before five years of age; however, only two of the five children were diagnosed after their first birthday (13).Therefore, while diabetes secondary to INS gene mutations typically presents before 12 months of life and are rare causes of monogenic diabetes in early childhood, our case as well as the other reports of childhood onset antibody-negative diabetes suggests that testing for INS gene mutations should be included in any comprehensive panel of monogenic diabetes. It is important for clinicians to be aware that many commercially available panels for monogenic diabetes outside the neonatal period do not include INS gene mutations; thus, this diagnosis could be missed on a routine MODY panel. With advancing technology reducing time and cost for genetic testing, next-generation sequencing is advocated as a comprehensive, though efficient, approach to screen for genes associated with monogenic diabetes (14, 15).
Type 1 diabetes mellitus and cataracts
Longstanding uncontrolled diabetes is a known risk factor for cataract development. In the modern era, the prevalence of cataracts in adults with longstanding Type 1 diabetes is 19.8% (16). However, the prevalence of diabetes –related cataracts during childhood is only approximately 1% (1, 2). Data from case series suggest that the risk for cataract formation in children is higher in female adolescence with long standing, severe, hyperglycemia; however, there are rare cases of cataracts present at or shortly after the diagnosis of diabetes (3, 17–19). Neither family history of diabetes nor antibody status is reported. It is also important to note that these cases occurred before an era of high intensity glucose management. More recent data from Iafusco et al found 6 cases of cataracts in a cohort of 826 children with antibody mediated Type 1 diabetes, the youngest of which being a 5.5 year old male. All patients presented in severe diabetic ketoacidosis and had a mean duration of diabetes symptoms of 3.75 months (range 2.5–6 months) (2). Our patient reported symptoms of diabetes for several years and did not present with ketosis, suggesting a more gradual destruction of beta cells with some preservation of insulin secretion.
Cataracts secondary to hyperglycemia are usually bilateral and characterized by “snowflake” deposits in the posterior subcapsular region. In this case, the cataracts were not classic and involved the lens nucleus, cortex, and posterior capsule. Vacuoles were present initially in the cortex that coalesced to cortical opacification. Cataracts may be transient, resolving with improvement in glycemic control. However, most cataracts are permanent, resulting in the need for surgical intervention as in our patient. The exact etiology of cataract formation in diabetes remains to be elucidated. In animal models, excess glucose in the lens is converted to sorbitol by aldose reductase (20). Accumulation of sorbitol leads to osmotic stress, however in humans the sorbitol levels do not rise high enough to cause significant damage. Additional proposed mechanisms include NADPH depletion leading to oxidative stress or non-enzymatic glycation of protein in the lens (21).
Cataracts secondary to monogenic diabetes
The most common forms of monogenic diabetes are mutations in HNF4A (MODY1), GCK (MODY2), and HNF1A (MODY 3). These forms of diabetes typically do not present with severe hyperglycemia in early childhood. Cataracts are also not a classic feature of monogenic diabetes and have not been previously described as a feature of INS mutations. The overall prevalence of cataract formation in any form of monogenic diabetes has not been previously reported, however one case of bilateral cataracts at presentation was reported in an adolescent female with an HNF1B (MODY5) mutation (22). Therefore, not only is this case unique in the presentation of diabetes-related cataracts in a young male with a significantly elevated HbA1c without ketosis, it is also describes a rare presentation of monogenic diabetes that would have been missed on a typical “MODY” panel. By utilizing whole exome sequencing, we were not only able to identify a causative gene mutation for diabetes, but also rule out other genetic causes of acquired cataracts in childhood.
Conclusion
Identifying the exact genetic cause of a patient’s diabetes is worthwhile as it directs future monitoring and treatment. Testing for monogenic diabetes is important and should be considered in any pediatric patient with antibody negative diabetes. Also, as diabetes-related complications at diagnosis are unusual in pediatric patients, this too should trigger consideration of genetic testing. When complications such as cataracts are present at diagnosis of monogenic diabetes, monitoring for other chronic complications (retinopathy, neuropathy, and proteinuria) should be considered given the possibility of a long prodrome of unrecognized hyperglycemia. On the other hand, routine screening for other antibody-mediated comorbidities of Type 1 diabetes such as hypothyroidism and celiac disease are not necessary in these patients. As science advances, we hypothesize patients like the child presented here, may be ideal candidates for beta cell transplantation as the risk for islet cell antibody mediated attack would be negligible.
In summary, we recommend testing for monogenic diabetes, specifically INS mutations, in any child diagnosed with antibody-negative diabetes at a young age, especially if presenting atypically with complications associated with chronic hyperglycemia.
Acknowledgements
The authors would like to thank Chao Wei, PhD for his work on the genomic analysis. Funding support for this work was provided to Halley Wasserman, MD, through the NIH T32-ES10957.
References
- 1.Falck A, Laatikainen L. Diabetic cataract in children. Acta Ophthalmol Scand. 1998;76:238–240. doi: 10.1034/j.1600-0420.1998.760223.x. [DOI] [PubMed] [Google Scholar]
- 2.Iafusco D, Prisco F, Romano MR, Dell'omo R, Libondi T, Costagliola C. Acute juvenile cataract in newly diagnosed type 1 diabetic patients: a description of six cases. Pediatr Diabetes. 2011;12:642–648. doi: 10.1111/j.1399-5448.2010.00749.x. [DOI] [PubMed] [Google Scholar]
- 3.Datta V, Swift PG, Woodruff GH, Harris RF. Metabolic cataracts in newly diagnosed diabetes. Arch Dis Child. 1997;76:118–120. doi: 10.1136/adc.76.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valencia A. Next generation sequencing technologies in medical genetics. Springer Press; 2013. [Google Scholar]
- 5.Stoy J, Edghill EL, Flanagan SE, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edghill EL, Flanagan SE, Patch AM, et al. Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008;57:1034–1042. doi: 10.2337/db07-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonfanti R, Colombo C, Nocerino V, et al. Insulin gene mutations as cause of diabetes in children negative for five type 1 diabetes autoantibodies. Diabetes Care. 2009;32:123–125. doi: 10.2337/dc08-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubio-Cabezas O, Edghill EL, Argente J, Hattersley AT. Testing for monogenic diabetes among children and adolescents with antibody-negative clinically defined Type 1 diabetes. Diabet Med. 2009;26:1070–1074. doi: 10.1111/j.1464-5491.2009.02812.x. [DOI] [PubMed] [Google Scholar]
- 9.Stoy J, Steiner DF, Park SY, Ye H, Philipson LH, Bell GI. Clinical and molecular genetics of neonatal diabetes due to mutations in the insulin gene. Rev Endocr Metab Disord. 2010;11:205–215. doi: 10.1007/s11154-010-9151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajan S, Eames SC, Park SY, et al. In vitro processing and secretion of mutant insulin proteins that cause permanent neonatal diabetes. Am J Physiol Endocrinol Metab. 2010;298:E403–E410. doi: 10.1152/ajpendo.00592.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes. 2003;52:409–416. doi: 10.2337/diabetes.52.2.409. [DOI] [PubMed] [Google Scholar]
- 12.Molven A, Ringdal M, Nordbo AM, et al. Mutations in the insulin gene can cause MODY and autoantibody-negative type 1 diabetes. Diabetes. 2008;57:1131–1135. doi: 10.2337/db07-1467. [DOI] [PubMed] [Google Scholar]
- 13.Moritani M, Yokota I, Tsubouchi K, et al. Identification of INS and KCNJ11 gene mutations in type 1B diabetes in Japanese children with onset of diabetes before 5 years of age. Pediatr Diabetes. 2013;14:112–120. doi: 10.1111/j.1399-5448.2012.00917.x. [DOI] [PubMed] [Google Scholar]
- 14.Ellard S, Lango Allen H, De Franco E, et al. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia. 2013;56:1958–1963. doi: 10.1007/s00125-013-2962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnefond A, Philippe J, Durand E, et al. Highly sensitive diagnosis of 43 monogenic forms of diabetes or obesity through one-step PCR-based enrichment in combination with next-generation sequencing. Diabetes Care. 2014;37:460–467. doi: 10.2337/dc13-0698. [DOI] [PubMed] [Google Scholar]
- 16.Podgor MJ, Kannel WB, Cassel GH, Sperduto RD. Lens changes and the incidence of cardiovascular events among persons with diabetes. Am Heart J. 1989;117:642–648. doi: 10.1016/0002-8703(89)90740-0. [DOI] [PubMed] [Google Scholar]
- 17.Ehrlich RM, Kirsch S, Daneman D. Cataracts in children with diabetes mellitus. Diabetes Care. 1987;10:798–799. doi: 10.2337/diacare.10.6.798. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery EL, Batch JA. Cataracts in insulin-dependent diabetes mellitus: sixteen years' experience in children and adolescents. J Paediatr Child Health. 1998;34:179–182. doi: 10.1046/j.1440-1754.1998.00195.x. [DOI] [PubMed] [Google Scholar]
- 19.Lang-Muritano M, La Roche GR, Stevens JL, Gloor BR, Schoenle EJ. Acute cataracts in newly diagnosed IDDM in five children and adolescents. Diabetes Care. 1995;18:1395–1396. doi: 10.2337/diacare.18.10.1395. [DOI] [PubMed] [Google Scholar]
- 20.Varma SD. Aldose reductase and the etiology of diabetic cataracts. Curr Top Eye Res. 1980;3:91–155. [PubMed] [Google Scholar]
- 21.Obrosova IG, Chung SS, Kador PF. Diabetic cataracts: mechanisms and management. Diabetes Metab Res Rev. 2010;26:172–180. doi: 10.1002/dmrr.1075. [DOI] [PubMed] [Google Scholar]
- 22.Raile K, Klopocki E, Holder M, et al. Expanded clinical spectrum in hepatocyte nuclear factor 1b-maturity-onset diabetes of the young. J Clin Endocrinol Metab. 2009;94:2658–2664. doi: 10.1210/jc.2008-2189. [DOI] [PubMed] [Google Scholar]