Abstract
Objectives
Infants with gastroschisis often require long periods of gastric suctioning and hospitalization. The impact of these interventions on the intestinal microbiota and attempts to alter the microbial community have not been studied. We sought to determine how the intestinal microbiota is influenced by current treatment of gastroschisis and whether alteration of the intestinal microbiota with a probiotic microbe will influence length of hospitalization.
Methods
We performed a randomized, placebo-controlled pilot study of administration of probiotic Bifidobacterium longum subsp infantis in 24 infants with gastroschisis. The primary outcome was changes in the fecal microbiota and the secondary outcome was length of hospital stay.
Results
Administration of the probiotic or placebo was well tolerated, even during the period of gastric suctioning. The overall microbial communities were not significantly different between groups, though analysis of the final specimens by family demonstrated higher Bifidobacteriaceae, lower Clostridiaceae, and trends toward lower Enterobacteriaceae, Enterococcaceae, Staphylococcaceae, and Streptococcaceae in the probiotic group. Clinical outcomes, including length of hospital stay did not differ between groups.
Conclusions
In this pilot study there was significant dysbiosis in infants with gastroschisis that was partially attenuated by administration of Bifidobacterium longum subsp infantis.
Introduction
Gastroschisis is a ventral body wall defect that results in the evisceration of bowel in utero.(1) Gastroschisis occurs in approximately 3-4/1000 pregnancies and appears to be increasing in incidence worldwide.(2) Poor gut motility is common in these infants often leading to delayed onset of enteral feeds, prolonged administration of parenteral nutrition (with associated complications) and prolonged hospital stays.(3)
The intestinal microbiota changes significantly in the first few weeks after birth with long term consequences on development, immune function, and disease risk.(4-6) The term “dysbiosis” refers to alterations in the composition and/or diversity of the microbiota associated with disease. Mounting evidence suggests associations between dysbiosis and a wide variety of common pediatric and adult diseases including necrotizing enterocolitis, colic, atopic disease, antibiotic-associated diarrhea, metabolic syndrome, and obesity.(7)
Probiotics are dietary supplements containing live bacteria. Multiple well-designed studies have demonstrated both short and long term benefits of administration of probiotics in the perinatal period, particularly for infants at high risk for atopic disease(8) or necrotizing enterocolitis.(9) A recent meta-analysis demonstrated decreased intestinal transit time in adults receiving probiotics.(10) Furthermore, probiotic therapy has been shown to increase gut motility in preterm infants fed formula.(11) We reasoned that probiotic administration to infants with gastroschisis might improve intestinal motility. One barrier to probiotic therapy in these infants is that they are kept NPO (nil per os) with gastric decompression, often for long periods. We hypothesized that infants with gastroschisis have an intestinal microbiota that differs markedly from the breast-fed infant and that administration of probiotic Bifidobacterium longum subsp. infantis (B. infantis) would lead to more normal gut colonization in these infants. We also hypothesized that more severe dysbiosis in this population is associated with more prolonged gut dysmotility and therefore prolonged hospital stays and that administration of probiotic B. infantis would lead to shorter lengths of stay and earlier initiation of enteral feeds.
Methods
This randomized, placebo-controlled, blinded study was approved by the Institutional Review Board at UC Davis, registered at clinicaltrials.gov (NCT01316510), and performed at the UC Davis Children’s Hospital in Sacramento, CA from March 2011 to February 2015. Infants were eligible for the study if they had confirmed gastroschisis at birth and gestational age at birth > 34 weeks. For this pilot study we proposed enrollment of 24 infants based on the following assumptions: probiotic administration would decrease fecal Enterobacteriaceae to 20% compared to 80% in the placebo group with alpha = 0.05 and power = 0.76 (i.e. this pilot study was powered only to demonstrate very large differences in the microbiota, with the intent that if smaller differences were noted then these data would be useful to determine the sample size and feasibility for a future larger study). Patient enrollment is summarized in Supplemental Figure 1. Following written, informed consent from the parents, 24 enrolled infants were randomly assigned by the UCDMC investigational pharmacy to receive either B. infantis ATCC 15697 109 CFU (colony forming units) or placebo twice daily for six weeks or until hospital discharge (whichever came first).
To avoid the challenges of using over-the-counter probiotic products (eg, unknown composition and viability) the probiotic strain was grown by a food-grade commercial facility (Culture Systems, Inc, Mishawaka, Indiana) and stored at −80C. Purity and number of viable bacteria per gram of the probiotic product were confirmed by the investigators every 6 months by culture. The probiotic is not commercially available and is not approved by the FDA for prevention, treatment or mitigation of disease. The probiotic doses were prepared each day by the UC Davis investigational pharmacy by dissolving the freeze-dried powder in water. The placebo was a dilute mixture of powdered elemental formula (Pregestimil, Mead Johnson) with a similar appearance and volume to the B. infantis (caloric value was negligible). The supplementation with probiotic or placebo began following surgery as soon as consent was obtained. The supplement was instilled into the naso-gastric (Replogle) tube and then the tube was clamped for 1 hour before returning to routine gastric decompression. When the Replogle tube was removed the doses were given orally. All caregivers and parents were blinded to study group assignment. The primary outcome was the composition of the fecal microbiota and the secondary outcome was length of hospital stay.
Feces were obtained from the first soiled diaper and then weekly thereafter when possible. Fecal samples were refrigerated overnight then frozen at −40 degrees C. Upon completion of the study, the samples were transported on dry ice and stored at −80 degrees C until analysis. Several infants did not pass feces for a prolonged period of time and for some infants only a single stool specimen was obtained. DNA extraction from fecal samples and DNA library construction was performed as described previously.(12) Samples were submitted to the UC Davis Genome Center DNA Technologies Core for sequencing on an Illumina MiSeq instrument (Illumina, San Diego CA). QIIME software package (University of Colorado. Boulder CO, version1.8.0) was used for quality filtering and demultiplexing the resulting sequencing data.(13) Operational taxonomic units were assigned using UCLUST (drive5.com, Tiburon, CA) based on 97% pairwise identity(14) and taxonomic classification was based on the Ribosomal Database Project classifier (Michigan State University, East Lansing, MI) against a representative subset of the Greengenes 16S rRNA database (Second Genome, South San Francisco, CA, gg_13_8 release).(15, 16)
Statistical analysis
There was variability among infants in the number of stool samples able to be collected due to heterogeneity in length of stay and stool production. To decrease the twin drawbacks of undue influence from the infants with a high number of samples and disregarding some specimens, we analyzed the data twice, once including all data points and once including just the first and last specimens available for each infant. T-tests with assumption of unequal variance (given the small sample size) were used to compare percentages of key bacterial families and length of hospital stay. Linear and multiple regression models were developed using STATA version 12.1 (STATACorp, College Station, TX).
Results
Specimens, demographic and clinical data were included for all 24 enrolled babies on an intention- to-treat basis. A summary of patient details is provided in Supplemental Table 1; there were no significant differences in demographic characteristics between the placebo and the probiotic group. Five infants (2 in the control group, 3 in the B. infantis group) had a silo placed with subsequent reduction surgery 5-6 days later and 19 infants had the gastroschisis defect repaired primarily on the first day of life. No vomiting was noted from clamping the Replogle tube after the doses, and no infants suffered any serious adverse events (no death, sepsis, pneumonia, or obvious small bowel bacterial overgrowth). The two groups did not significantly differ in clinical outcomes (Supplemental Table 2). Of note, after being enrolled in the study, two infants in the control group were found to have atretic segments of bowel: infant 14 underwent colostomy and resection of the atretic ascending and transverse colon on day of life 1 with subsequent ileostomy and resection of the ileocecal valve and distal ileum on day of life 38, and infant 24 underwent cecostomy and resection of the ascending and transverse colon on day of life 58.
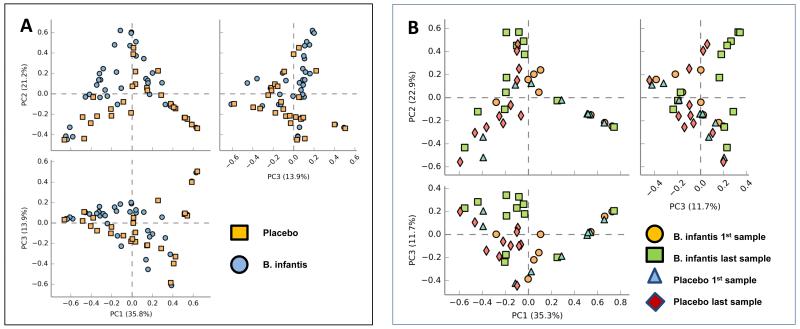
For most infants the first fecal sample was not obtained until after the probiotic or placebo was started. For three infants we were unable to collect any usable fecal samples (infants 9 and 13 in the B. infantis group and infant 8 in the placebo group). Supplemental Figure 2 is a heatmap of the relative abundance of taxa, at the family level, for all specimens analyzed. Planococcaceae, Bifidobacteriaceae, Enterobacteriaceae and Staphylococcaceae are the most abundant taxa. Figure 1 presents principal coordinate analyses for all samples (Panel A) and just the first and last sample for each infant (Panel B) with no differences between the two groups when considering the fecal microbiota as an entire community.
Figure 1.
The fecal microbiota of infants receiving placebo or B. infantis. Principal coordinate analyses for all samples (panel A) and for the first and last sample for each infant (panel B).
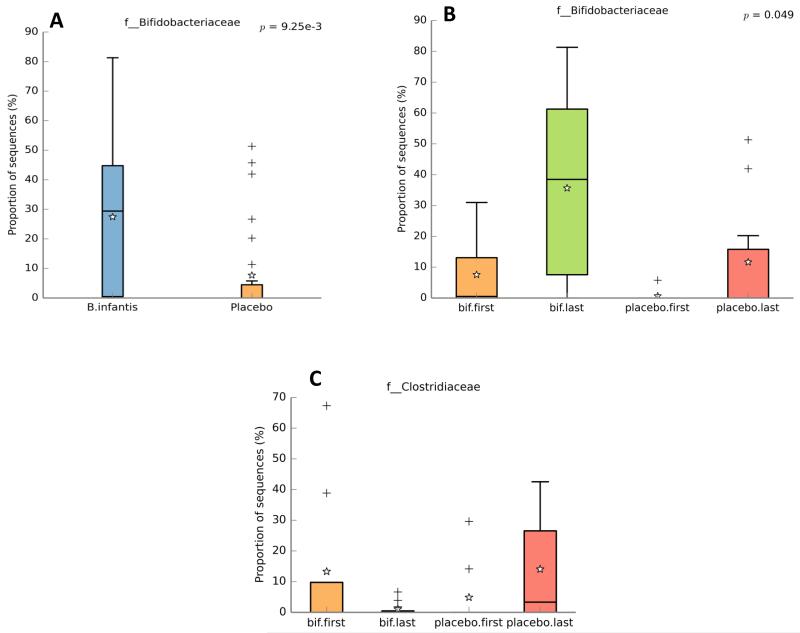
To characterize differences between groups we looked first at just the final specimen for each infant. Significantly higher percentages of Bifidobacteriaceae and lower percentages of Clostridiaceae were noted in the infants receiving the probiotic (Table 1 and Figure 2). Trends towards higher percentages of Enterobacteriaceae, Enterococcaceae, Staphylococcaceae, and Streptococcaceae in the final fecal sample of the placebo group did not reach statistical significance (Table 1). We next looked at the first specimens from all infants (except infant 6 from whom we had only one specimen). There was high variability and no significant differences between groups. Supplemental Figure 3 contains clinical and bacterial details for each individual infant in the placebo group. Supplemental Figure 4 presents similar details for the infants receiving B. infantis. For several infants colonization with bifidobacteria did not occur until after cessation of gastric suctioning (e.g. infants 4, 7, 12, 16, 22), though for two infants, > 10% of the fecal bacteria were bifidobacteria while gastric suction was ongoing (infants 17 and 20).
Table 1.
Mean percentage of bacterial families in the final stool specimen by group for all infants and excluding the infants with intestinal atresia. P values generated using t-tests; sample sizes represent the number of infants needed in each arm to confirm a difference of the observed magnitude.
| All infants | Exclude infants 14 and 24 | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (N=11) |
Probiotic (N=10) |
p | Sample size |
Placebo (N=9) |
Probiotic (N=10) |
p | Sample size |
|
| Bifidobacteriaceae | 12 | 42 | 0.009 | 48 | 14 | 42 | 0.03 | 59 |
| Enterobacteriaceae | 33 | 27 | 0.65 | 1068 | 27 | 27 | 0.98 | >400K |
| Staphylococcaeae | 3.4 | 0.71 | 0.34 | 649 | 4.1 | 0.71 | 0.28 | 481 |
| Enterococcaceae | 11 | 1.1 | 0.12 | 136 | 11 | 1.1 | 0.16 | 140 |
| Clostridiaceae | 14 | 1.1 | 0.03 | 101 | 17 | 1.1 | 0.01 | 77 |
| Streptococcaceae | 15 | 6.4 | 0.24 | 293 | 18 | 6.4 | 0.12 | 175 |
Figure 2.
Fecal Bifidobacteriaceae. Proportion of sequences of family Bifidobacteriaceae from infants receiving B. infantis or placebo among all samples collected (panel A, student’s t-test) and just the first and last samples collected (Panel B, Welch’s two sided t-test with Bonferroni correction, the p value for Student’s t-test comparing the last specimens for each infant between groups = 0.009). Panel C: proportion of sequences of family Clostridiaceae from the first and last samples collected, the p value for Student’s t-test comparing the last specimens for each infant between groups = 0.03. Boxes represent the 25th and 75th centiles with the center line the median, whiskers represent the 5th and 95th centiles, stars represent the mean and pluses represent outliers.
We hypothesized an association between dysbiosis and gut motility. To test this hypothesis we assumed that total hospital days would be a reasonable surrogate for gut motility in this population (for most infants with gastroschisis hospital discharge is primarily determined by ability to tolerate feedings). Simple linear regression of the initial stool specimen for each infant vs total hospital days showed no significant association when all available samples were included, however when we excluded the two infants with intestinal atresia, the percentage of fecal Enterobacteriaceae in the first stool specimen showed a modest positive association (R2=0.36, p=0.009). A similar approach using the final specimen for each infant showed a positive association between fecal Enterobacteriaceae and hospital days (R2=0.29, p=0.03) when all infants were included that was not significant when the two infants with atresia were excluded.
A multiple regression model of hospital length of stay including predictor variables other than the fecal microbiota (e.g. gestational age, birth weight, delivery type, feeding type, antibiotic days, morphine days, and study group) was not significant (R2=0.46, adjusted R2=0.19, p=0.18), however within that model morphine days showed an unstandardized regression coefficient of 4.9 (95% confidence intervals 0.55 and 9.3, p=0.03). Since the overall model was not significant this observation should be interpreted as useful for further hypothesis testing. Multiple regression models of hospital length of stay that included percentages of six bacterial families of potential clinical relevance (Enterobacteriaceae, Bifidobacteriaceae, Clostridiaceae, Enterococcaceae, Streptococcaceae, and Staphylococcaceae) in the initial and final samples and the number of days of administration of morphine sulfate were explored for all 21 infants for whom specimens were available (intention to treat) and again excluding the two infants with intestinal atresia. For all 21 infants, this model explained 78% of the variance in hospital days (R2=0.93, adjusted R2=0.78, p=0.017). Excluding the two infants with atresia, the same model explained 87% of the variance in hospital days (R2=0.97, adjusted R2=0.87, p=0.021). Table 2 presents both of these models with the unstandardized coefficients for the multiple regression equation (with 95% confidence intervals) for each dependent variable. Note that in the final model, excluding the two infants with intestinal atresia, percentage of Enterococcaceae in the initial specimen, morphine days, and percentage of Enterococcaceae and Clostridiaceae in the final specimen were significant predictors of length of hospital stay.
Table 2.
Multiple regression modeling with outcome of number of hospital days for all infants for whom initial and final specimens were available and excluding the infants with intestinal atresia.
| All infants (N=20) | Exclude infants 14 and 24 (N=18) |
||||
|---|---|---|---|---|---|
| Coefficient (95% CI) |
p | Coefficient (95% CI) |
p | ||
| Initial sample |
Bifidobacteriaceae | −3.5 (−5.2/−1.8) | 0.002 | −0.64 (−1.7/0.39) | 0.2 |
| Enterobacteriaceae | −1.0 (−1.6/−4.2) | 0.006 | −0.082 (−0.46/0.30) | 0.6 | |
| Staphylococcaeae | −0.61 (−1.1/−0.84) | 0.03 | −0.11 (−0.40/1.9) | 0.4 | |
| Enterococcaceae | −7.7 (−14/−1.6) | 0.02 | −3.8 (−6.7/−0.90) | 0.02 | |
| Clostridiaceae | 0.87 (−0.25/1.8) | 0.06 | 0.025 (−0.27/0.32) | 0.8 | |
| Streptococcaceae | 2.0 (0.78/3.3) | 0.007 | 0.52 (−0.12/1.2) | 0.09 | |
| Morphine days | 5.6 (0.96/10) | 0.03 | 3.9 (1.4/6.3) | 0.01 | |
| Final sample |
Bifidobacteriaceae | 0.36 (−0.68/1.4) | 0.4 | 0.16 (−0.082/0.39) | 0.1 |
| Enterobacteriaceae | 1.6 (0.70/2.4) | 0.004 | 0.35 (−0.13/0.83) | 0.1 | |
| Staphylococcaeae | 1.9 (−0.85/4.7) | 0.1 | 0.39 (−0.47/1.3) | 0.3 | |
| Enterococcaceae | 1.9 (0.083/3.6) | 0.04 | 1.0 (0.067/1.9) | 0.04 | |
| Clostridiaceae | −0.99 (−2.0/0.25) | 0.05 | −0.46 (−0.87/−0.046) | 0.04 | |
| Streptococcaceae | −1.2 (−2.7/0.25) | 0.08 | 0.024 (−0.45/0.49) | 0.9 | |
Discussion
In this pilot study of administration of a promising probiotic subspecies of Bifidobacterium, we demonstrated a feasible route of administration of the probiotic that was well tolerated (no emesis or other safety concerns). As anticipated, the infants in the placebo group became colonized with a community of microbes that differs significantly from healthy breast-fed infants: commensal microbes such as Bifidobacteriaceae, Bacteriodetes, and Lactobacillaceae were uncommon and the dominant organisms were mostly families associated with pathogens such as Enterobacteriaceae, Staphylococcaceae, Streptococcaceae, and Enterococceae. Dysbiosis in infancy has been associated with a variety of adult diseases including obesity, metabolic syndrome and atopic diseases.(17) Long term follow-up studies of infants with gastroschisis are limited but suggest that obesity and hypercholesterolemia are common.(18) While the trends toward higher percentages of several of these taxa did not reach statistical significance, these data do allow for sample size calculations to assess feasibility of a larger study (Table 1).
Many of the infants in the probiotic group became colonized with moderate numbers of Bifidobacteriaceae, but this was most common after gastric suctioning was stopped, suggesting that the twice daily 1 hour exposure of the gastric mucosa to the probiotic at this dose was not sufficient for colonization. Further pilot studies of more frequent dosing and/or higher doses of probiotic organisms would be helpful to determine whether probiotic administration during a period of gastric suctioning has any impact. An alternative explanation for this observation is that colonization with this probiotic strain is more effective in conjunction with human milk feeding, as suggested by a previous study in premature infants.(19) The small number of formula-fed infants in this study precludes analysis of this question in this study, however linear regression of percentage of human milk feeding versus percentage of Bifidobacteriaceae in the final stool sample showed a trend consistent with this hypothesis (placebo group: R2=0.02, p=0.7, B. infantis group: R2=0.25, p=0.14).
We chose to administer Bifidobacterium longum subsp. infantis for two major reasons. First B. infantis has encoded in its genome a full complement of transport proteins and glycosidases for ingestion and digestion of the full range of human milk oligosaccharides, i.e. this organism has an evolutionary advantage in that it can outcompete other gut microbes in the infant gut for the components of human milk that are not able to be utilized by the infant.(20) Second, B. infantis has demonstrated anti-inflammatory properties, particularly in the immature host.(21, 22) We hypothesized that many of the infants with gastroschisis would become colonized with Gram negative Enterobacteriaceae as is common in premature infants who also have long hospitalizations in the neonatal intensive care unit. Given the demonstrated pro-inflammatory properties of many Enterobacteriaceae, and the anti-inflammatory properties of B. infantis, we further hypothesized that increased Enterobacteriaceae and decreased Bifidobacteriaceae would correlate with higher TPN days and longer hospital stays. Linear regression of the final specimen from each infant demonstrated a modest correlation between Enterobacteriaceae and hospital days (p=0.03, R2=0.23), however this correlation was not significant when the two infants with intestinal atresia were removed from the analysis. The multiple regression models suggest that the composition of the intestinal microbiota and the number of days an infant receives morphine are significant predictors of length of hospital stay (likely related to alterations in intestinal motility).
The major weaknesses of this study are the small sample size and the large variation in number and timing of samples obtained per infant. The challenge of sampling the intestinal microbiota in infants that pass stools infrequently is significant. If future studies are undertaken, there may be some value in sampling the gastric fluid microbiota at regular intervals during the period of gastric suctioning.
Conclusion
We have demonstrated that infants with gastroschisis develop a significant dysbiosis, that the degree of dysbiosis may be associated with length of hospital stay, and that it is feasible to provide an oral probiotic to infants following gastroschisis repair, however impact on the intestinal microbiota appears to have been minimal during the period of gastric suctioning. By the time of the final stool sample there were more bifidobacteria and less clostridia in the stools of infants receiving the probiotic. That there were no obvious differences in length of hospital stay or TPN days between groups is not surprising as this pilot study was not powered to look at these outcomes. Given the observed dysbiosis in this population and the demonstrated associations between the intestinal microbiota and length of hospitalization, further studies of probiotics in this population are indicated.
Supplementary Material
Supplemental Figure 1: Patient flow chart. Summary of eligible and enrolled infants
Supplemental Figure 2: Heat map with hierarchical clustering of all samples analyzed
Supplemental Figure 3: Microbial composition of each stool specimen for each infant in the placebo group. The numbers in parentheses are the days of life on which the infant received the placebo. The numbers below each bar graph are the day of life on which the specimen was collected. *=infant receiving antibiotics on the day of this specimen, circled number= specimen obtained while the infant was undergoing gastric suctioning, double underscore = 100% human milk feeding, single underscore = >75% human milk feeding, dotted underscore = 100% formula feeding.
Supplemental Figure 4: Microbial composition of each stool specimen for each infant in the probiotic group. The numbers in parentheses are the days of life on which the infant received B. infantis. The other markings are as described for Supplemental Figure 3.
What’s known about this subject?
Infants with gastroschisis often have poor intestinal motility.
In adults, probiotics may improve intestinal motility.
What are the new findings?
The infants in this pilot study had significant dysbiosis which may be associated with length of hospital stay.
Probiotic administration altered the fecal microbiota.
Acknowledgments
Source of Funding: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health, Bethesda Maryland (grant number R01HD059127) and the National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda Maryland (grant number UL1 TR000002). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. DAM acknowledges support as the Peter J. Shields Endowed Chair in Dairy Food Science at UC Davis.
Role of authors: WP assisted with enrollment, sample collection, DNA extraction, data collection, and wrote the initial draft of the manuscript; RB assisted with study design, obtaining IRB approval and registration at clinicaltrials.gov, patient enrollment, sample collection and editing and approval of the final draft; MM assisted with patient enrollment, sample collection, data analysis and editing and approval of the final draft; KK assisted with study design, DNA extraction and sequencing, data analysis, and editing and approval of the final draft; DM assisted with study design, sample and data analysis, and editing and approval of the final draft; MU assisted with study design, obtaining IRB approval and registration at clinicaltrials.gov, patient enrollment, sample collection and wrote the final draft.
Footnotes
Conflicts of Interest: DAM is a co-founder of Evolve Biosystems, a company focused on diet-based manipulation of the gut microbiota. None of the other authors has a potential conflict of interest.
Registered at clinicaltrials.gov (NCT01316510)
References
- 1.Feldkamp ML, Carey JC, Sadler TW. Development of gastroschisis: review of hypotheses, a novel hypothesis, and implications for research. Am J Med Genet. 2007;143A:639–652. doi: 10.1002/ajmg.a.31578. [DOI] [PubMed] [Google Scholar]
- 2.Kirby RS, Marshall J, Tanner JP, et al. National Birth Defects Prevention Prevalence and correlates of gastroschisis in 15 states, 1995 to 2005. Obstet Gynecol. 2013;122:275–281. doi: 10.1097/AOG.0b013e31829cbbb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durfee SM, Benson CB, Adams SR, et al. Postnatal outcome of fetuses with the prenatal diagnosis of gastroschisis. J Ultrasound Med. 2013;32:407–412. doi: 10.7863/jum.2013.32.3.407. [DOI] [PubMed] [Google Scholar]
- 4.Cacho N, Neu J. Manipulation of the intestinal microbiome in newborn infants. Adv Nutr. 2014;5:114–118. doi: 10.3945/an.113.004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrington JE, Stewart CJ, Cummings SP, et al. The neonatal bowel microbiome in health and infection. Curr Opin Infect Dis. 2014;27:236–243. doi: 10.1097/QCO.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 6.Huda MN, Lewis Z, Kalanetra KM, et al. Stool Microbiota and Vaccine Responses of Infants. Pediatrics. 2014;134:e362–372. doi: 10.1542/peds.2013-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Underwood MA. Intestinal dysbiosis: novel mechanisms by which gut microbes trigger and prevent disease. Prev Med. 2014;65:133–137. doi: 10.1016/j.ypmed.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Foolad N, Armstrong AW. Prebiotics and probiotics: the prevention and reduction in severity of atopic dermatitis in children. Benef Microbes. 2014;5:151–160. doi: 10.3920/BM2013.0034. [DOI] [PubMed] [Google Scholar]
- 9.Alfaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;4:CD005496. doi: 10.1002/14651858.CD005496.pub4. [DOI] [PubMed] [Google Scholar]
- 10.Miller LE, Ouwehand AC. Probiotic supplementation decreases intestinal transit time: meta-analysis of randomized controlled trials. World J Gastroenterol. 2013;19:4718–4725. doi: 10.3748/wjg.v19.i29.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indrio F, Riezzo G, Raimondi F, et al. Effects of probiotic and prebiotic on gastrointestinal motility in newborns. J Physiol Pharmacol. 2009;60(Suppl 6):27–31. [PubMed] [Google Scholar]
- 12.Frese SA, Parker K, Calvert CC, et al. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome. 2015;3:28. doi: 10.1186/s40168-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Garrity GM, Tiedje JM, et al. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev. 2015;73(Suppl 1):32–40. doi: 10.1093/nutrit/nuv039. [DOI] [PubMed] [Google Scholar]
- 18.Harris EL, Minutillo C, Hart S, et al. The long term physical consequences of gastroschisis. J Pediatr Surg. 2014;49:1466–1470. doi: 10.1016/j.jpedsurg.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Underwood MA, Kalanetra KM, Bokulich NA, et al. A comparison of two probiotic strains of bifidobacteria in premature infants. J Pediatrics. 2013;163:1585–1591. doi: 10.1016/j.jpeds.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Underwood MA, German JB, Lebrilla CB, et al. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res. 2015;77:229–235. doi: 10.1038/pr.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganguli K, Meng D, Rautava S, et al. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am J Physiol Gastrointest Liver Physiol. 2013;304:G132–141. doi: 10.1152/ajpgi.00142.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Underwood MA, Arriola J, Gerber CW, et al. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr Res. 2014;76:326–333. doi: 10.1038/pr.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Patient flow chart. Summary of eligible and enrolled infants
Supplemental Figure 2: Heat map with hierarchical clustering of all samples analyzed
Supplemental Figure 3: Microbial composition of each stool specimen for each infant in the placebo group. The numbers in parentheses are the days of life on which the infant received the placebo. The numbers below each bar graph are the day of life on which the specimen was collected. *=infant receiving antibiotics on the day of this specimen, circled number= specimen obtained while the infant was undergoing gastric suctioning, double underscore = 100% human milk feeding, single underscore = >75% human milk feeding, dotted underscore = 100% formula feeding.
Supplemental Figure 4: Microbial composition of each stool specimen for each infant in the probiotic group. The numbers in parentheses are the days of life on which the infant received B. infantis. The other markings are as described for Supplemental Figure 3.