Abstract
Bone marrow mononuclear cell (BMMNC) transplantation is a promising therapy for brain ischemia. However, BMMNCs are few in number, and a limited time window is available during which they can penetrate the blood–brain barrier (BBB) and migrate to the brain. We investigated whether vascular endothelial growth factor (VEGF) can facilitate BMMNC migration into the ischemic brain and enhance their therapeutic effect in a rat model of chronic cerebral hypoperfusion. First, we assessed the impact of VEGF on the BBB of rats that had undergone permanent bilateral occlusion of the common carotid arteries (2VO). Then, we transplanted BMMNCs into 2VO rats pretreated with intracerebroventricular VEGF or vehicle. We examined cognitive function with the Morris water maze test, BMMNC migration by immunofluorescence analysis, and cytokine levels in the peripheral blood by enzyme-linked immunosorbent assay (ELISA). Angiogenesis and neural degeneration were evaluated by staining tissue with Ki67/lectin or Fluoro-Jade B. We found that at a dose of 0.2 μg/rat, VEGF significantly increased BBB permeability without causing brain edema in 2VO rats. VEGF+BMMNC-treated rats had more BMMNC migration in the ischemic brain, better learning and memory, greater proliferation of vessels, and fewer degenerating neurons than did BMMNC-treated rats. Pretreatment with VEGF receptor inhibitor SU5416 significantly decreased BMMNC migration and abolished the therapeutic effect of BMMNC transplantation. We conclude that preconditioning with an appropriate dose of VEGF can enhance the therapeutic efficacy of BMMNC transplantation in 2VO rats, possibly by facilitating BMMNC migration into the ischemic brain.
Keywords: Bone marrow mononuclear cells, Blood brain barrier, Cell transplantation, Chronic cerebral hypoperfusion, VEGF
Introduction
The Chinese population is aging rapidly. It is estimated that by 2050, the elderly will account for 30.4 % of the total population in China [1]. This aging will be accompanied by an increased incidence of chronic disease such as vascular dementia (VD), which is associated with disability, institutionalization, and mortality among elderly individuals. Jia and colleagues [2] recently reported a prevalence rate of 1.5 % for VD among the elderly in China, significantly higher than the 1.1 % reported in 1997 [3]. Chronic cerebral hypoperfusion is a main contributor to the cognitive deficiency seen in patients with VD [4]. Although VD is a common type of dementia, no specific pharmacologic agents have been approved for its treatment or prevention [5].
Cellular therapy is an emerging investigational approach for cerebral ischemia. Among many cell types, bone marrow mononuclear cells (BMMNCs) are particularly attractive for acute ischemic stroke treatment because they are composed of different kinds of stem cells, can be rapidly isolated without cultivation, and can be used in autologous applications [6]. Several research groups have shown that BMMNC transplantation can significantly improve neurologic outcome from ischemic stroke in animal models by cell differentiation [7, 8], secretion of trophic factors [9], and possible immunomodulation [10]. Some phase I/II clinical trials also have shown that BMMNC transplantation can improve clinical outcomes of patients with middle cerebral artery occlusion (MCAO) [11–14]. In fact, several independent groups have reported that BMMNC transplantation significantly reduces ischemia-induced impairment and increases vascular density and blood flow in both acute and chronic ischemic disorders, such as cardiovascular disease [15], peripheral arterial disease [16], and diabetic foot [17]. However, little research has examined the effects and possible mechanisms of BMMNC transplantation in chronic cerebral hypoperfusion.
Bilateral carotid artery occlusion (two-vessel occlusion (2VO)) is a classic and commonly used animal model of chronic cerebral hypoperfusion that mimics the pathologic condition of clinical VD [18]. We showed previously that transplantation of BMMNCs can promote therapeutic angiogenesis via upregulation of the vascular endothelial growth factor (VEGF)-VEGF receptor 2 (VEGFR2) signaling pathway in 2VO rats. This finding suggests that VEGF upregulation in the ischemic brain may be a key mechanism by which BMMNC transplantation provides a therapeutic effect [19]. Miki and colleagues [20] found that VEGF gene-transferred bone marrow mesenchymal stem cells (MSCs), which are in BMMNCs, improved neurologic deficits and reduced infarction volume in rats that underwent MCAO, compared with those in rats that received non-transferred bone marrow MSCs. Chang and colleagues [21] indicated that the VEGF secreted by human umbilical cord blood-derived MSCs is the main mechanism by which those cells protect against neonatal hyperoxic lung injury. Horie and colleagues [22] reported that the VEGF secreted from transplanted human central nervous system cells grown as neurospheres affects post-stroke recovery, inflammation, and vascular repair in rats with permanent MCAO. Thus, VEGF seems to have a critical role in the benefits provided by stem cell therapy in ischemic diseases.
The therapeutic efficacy of VEGF is associated with its angiogenic and neuroprotective effects [23]. Zechariah and colleagues [24] found that mice that were pretreated with intracerebroventricular (icv) VEGF (0.02 μg/day) daily for 21 days before 90-min MCAO had enhanced post-ischemic blood–brain barrier (BBB) integrity and increased cerebral blood flow in the ischemic brain areas, highlighting the critical role of VEGF in cerebral ischemia. However, treatment of cerebral ischemia with VEGF may also have severe side effects. Many studies have documented that VEGF may actually increase BBB permeability and induce brain edema in cerebral ischemia, especially in the acute injury phase [23, 25].
The BBB restricts molecules larger than 500 Da from reaching the central nervous system, making it a barrier to many neurologic therapies, including cell transplantation [26]. BBB leakage, however, may also make it easier for BMMNCs to enter the ischemic brain. Previous studies have shown that BMMNCs could not penetrate the healthy rat brain, but were able to migrate into the infarct area after MCAO [7, 9]. We hypothesized that supplying exogenous VEGF would facilitate the migration of BMMNCs into the chronically ischemic brain of 2VO rats, thus enhancing their therapeutic efficacy.
Materials and Methods
Animals and Ethics Statement
We purchased 372 adult male Sprague–Dawley (SD) rats from the Animal Experimental Center of Zhengzhou University and 30 adult male transgenic SD rats with enhanced green fluorescent p rotein (eGFP) from the Xingming Biotechnology Company (Shanghai, China). All rats were 11–12 weeks old (260–300 g) at arrival, were housed in plastic cages (5 per cage) with free access to food and water, and were maintained on a 12-h light/dark cycle at a constant temperature of 22±1 °C. All animal procedures were conducted in accordance with the Guidelines on the Care and Use of Animals for Scientific Purposes (National Advisory Committee for Laboratory Animal Research) and approved by the Animal Care and Use Committee of Zhengzhou University. All efforts were made to minimize the number of animals used and their suffering.
Chronic Cerebral Hypoperfusion Model
Rats were subjected to a previously reported chronic cerebral hypoperfusion model in which bilateral common carotid arteries (CCAs) are permanently occluded [19]. Briefly, we anesthetized rats with 10 % chloral hydrate (400 mg/kg) by intraperitoneal injection and made a midline incision in the ventral side of the neck to expose the CCAs. We gently separated the arteries from their sheaths and adjacent vagus nerves, and then permanently occluded them with 5–0 silk suture. The neck wound was sutured closed. We defined successful 2VO as an approximately 70 % decrease in central blood flow as measured by laser-Doppler flowmetry [27]. Sham-operated controls underwent the same surgical procedures but without carotid artery ligation.
Preparation of BMMNCs
Fresh BMMNCs were collected from femurs and tibias of the transgenic SD rats with eGFP (n = 30) and purified with Percoll gradient centrifugation as previously reported [28]. The rats were anesthetized with an overdose of chloral hydrate and then sacrificed. Bilateral femurs and tibias were aseptically dissected and cut at both ends. Bone marrow was extruded with serum-free Dulbecco’s modified Eagle’s medium (DMEM/F12, Hyclone, Logan, UT). The extracted bone marrow was subjected to density gradient centrifugation (160×g, 25 min) in 1.083 g/mL Histopaque 1083 (Sigma-Aldrich, St. Louis, MO). The mononuclear cell layer was recovered from the gradient interface and washed three times through suspension in DMEM/F12 followed by 5-min centrifugation. We verified the concentration of the cells in a Neubauer counting chamber and determined the number of viable cells with trypan blue exclusion.
Treatment and Groups
We first examined the side effects of administering an icv injection of 0.2 μg recombinant rat VEGF (rrVEGF; MultiSciences Biotech, Hangzhou, China) to 2VO rats. The dose of rrVEGF was chosen based on a previous study, which showed that at a dose of 0.2–0.6 μg kg−1 day−1 (0.05 μg–0.15 μg/rat), VEGF continuously infused via the icv route was well tolerated and did not induce edema, leakage, or an immune response in the brains of SD rats [29]. In this procedure, rats were randomly assigned to three groups: shamoperated rats treated with 2 μL phosphate-buffered saline (PBS) via icv injection (sham, n=40), 2VO rats treated with 2 μL PBS via icv injection (vehicle, n=43), and 2VO rats treated with 0.2 μg rrVEGF (diluted in 2 μL PBS) via icv injection (VEGF, n=46).
In the next procedure, we observed the therapeutic efficacy of a combination of VEGF and BMMNCs in 2VO rats. Rats were randomly assigned to six groups: sham-operated rats treated with 2 μL PBS via icv injection (sham+vehicle, n= 37), sham-operated rats treated with 2 μL PBS via icv injection and BMMNC transplantation via the tail vein (sham+ BMMNC, n=36), 2VO rats treated with 2 μL PBS via icv injection and 300 μL DMEM via the tail vein (2VO+vehicle, n=42), 2VO rats treated with 2 μL PBS via icv injection and BMMNC transplantation via the tail vein (2VO+BMMNC, n=44), 2VO rats treated with 0.2 μg rrVEGF via icv injection and BMMNC transplantation via the tail vein (2VO+VEGF+ BMMNC, n=43), and 2VO rats treated with 4 nM SU5416 (VEGFR inhibitor, diluted in 2 μL PBS) via icv injection and BMMNC transplantation via the tail vein (2VO+SU5416+ BMMNC, n=41). The 4-nM SU5416 dose is widely used to research the role of VEGF in the brains of rats [30]. All groups, except for vehicle-treated groups, received 3×106 BMMNCs diluted in 300 μL of DMEM via tail vein infusion 6 h after icv injection. The cell dose was chosen because we [19] and Yang et al. [31] have shown that systemic treatment with 1×107 cells/kg effectively promotes recovery in a rat ischemic stroke model. The mortality of each group was recorded. Body weights were measured weekly through day 28 after BMMNC transplantation and expressed as percent change as follows: (body weight at each time point−body weight before surgery)/body weight before surgery×100 %.
Intracerebroventricular Delivery of VEGF and SU5416
Using a 5-μL Hamilton microinjection syringe (Shanghai Difa Instrument Company, Shanghai, China), we injected rrVEGF or SU5416 (2 μL) into the left lateral ventricle of SD rats according to the method described by Chang et al. [32] with modifications based on the rat brain map. The location of each injection was 0.8 mm posterior and 1.5 mm lateral, relative to the bregma, and 3.5 mm below the brain surface. The duration of each injection was 30 min. Each rat received VEGF, SU5416, or PBS via icv delivery on day 7 after the 2VO procedure. This time point was chosen because the cerebral blood flow most resembles that of humans with VD beginning at 4 days after acute occlusion [33].
Western Blot Analysis of ZO-1 and Claudin-5
We killed six rats per group (sham, vehicle, and VEGF) at 6 and 24 h after VEGF injection (Fig. 1a) to measure striatal levels of ZO-1 and claudin-5 protein by Western blot analysis. Briefly, rats were deeply anesthetized and decapitated. The left striatum of brain was quickly dissected out, homogenized, and centrifuged (12,000×g, 5 min). Supernatants were collected for separation of proteins by 12 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After separation, the proteins were transferred onto polyvinylidene fluoride membranes (Millipore). Membranes were blocked with 5 % nonfat milk for 1 h at 4°C and then incubated with one of the following primary antibodies: goat anti-ZO-1 (1:200, Santa Cruz Biotechnology, Dallas, TX), goat anti-claudin-5 (1:200, Santa Cruz Biotechnology), or rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:2000, Hangzhou Goodhere Biotechnology, Zhejiang, China). After three washes, membranes were incubated with appropriate horse-radish peroxidase (HRP)-conjugated secondary antibodies. All protein bands were visualized by enhanced chemiluminescence detection kit (Beyotime Institute of Biotechnology, Beijing, China) and quantified by Gel Analysis V 2.02 software (Clinx Science Instruments, Beijing, China).
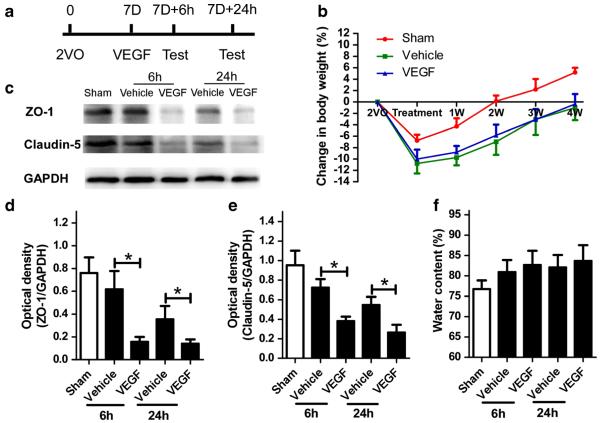
Fig. 1.
VEGF decreases the levels of ZO-1 and claudin-5 in the ischemic brain but does not affect body weight changes or brain water content. a Schematic representation of experimental design for c–f. 7D 7 days after 2VO procedure. b Changes in rat body weight over 4 weeks (n=6/group). No significant difference was found between the two 2VO groups. p>0.05. c Western blot analysis of ZO-1 and claudin-5 at 6 and 24 h after VEGF injection. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. d, e Quantification of band densities showed that ZO-1 and claudin-5 expression levels were significantly decreased in the VEGF-treated group. *p<0.05 compared with vehicle. n=8/group. f No difference was found in the brain water content among the three groups at 6 or 24 h after icv injection. p>0.05, n=8/group. Values are mean±SD
Brain Water Content
We measured the brain water content to examine the effects of rrVEGF on cerebral edema. Six rats per group (sham, vehicle, and VEGF) were killed at 6 and 24 h after the icv injection. Their brains were rapidly removed, and the hemispheres were divided, weighed, and dried at 95 °C for 48 h to obtain the dry weight. The brain water content was calculated as follows: (wet weight−dry weight)/wet weight×100 % [34].
Morris Water Maze
Ten rats from each group (sham+vehicle, sham+BMMNC, 2VO + vehicle, 2VO + BMMNC, 2VO + VEGF + BMMNC, 2VO+SU5416+BMMNC) were tested for spatial memory in the Morris water maze on day 28 as previously described [19]. We used an SLY-WMS Water Maze automatic control recorder (Beijing Sunny Instruments Co. Ltd, Beijing, China), which consisted of a black circular pool (150 cm in diameter, 50 cm high) filled with water (23–24 °C, 28 cm in depth). An escape platform 12 cm in diameter was fixed in the middle of the northeast quadrant and placed 2 cm below the surface of the water. In the reference memory protocol, rats received five training sessions (1 session/day) that each consisted of three trials 15 min apart. Each rat was placed in the pool and released facing the side wall at one of three randomly chosen starting positions; none of which were repeated. The rat was allowed to swim until it found the hidden platform. We would guide the rat to the platform and let it stay for 10 s if it did not succeed within 120 s, and record its escape latency as 120 s. The mean escape latency and swimming speed of daily trials were recorded. On the sixth day, we performed a probe trial that consisted of one 2-min trial with the platform removed. The mean time that each rat spent swimming in the northeast quadrant was recorded [35].
Histologic Analysis
BBB Integrity
Six rats from the three groups (sham, vehicle, and VEGF) were used to detect the effects of VEGF on BBB integrity in 2VO rats. Six hours after the icv injection, rats were deeply anesthetized with an overdose of 10 % chloral hydrate and transcardially perfused with 0.01 mol/L PBS followed by 4 % paraformaldehyde in 0.01 mol/L PBS (pH 7.4). The brain of each rat was removed, post-fixed in 4 % paraformaldehyde overnight at 4 °C, stored in 30 % sucrose 0.01 mol−1 L−1 PBS until it sank, and then cut into 25 μm sections by cryoultramicrotomy (CM1100, Leica Biosystems, Germany). The sections were incubated with PBS containing 0.25 % Triton X-100 (PBST) for 10 min, washed in PBS three times for 5 min each, and then incubated with 1 % bovine serum albumin in PBST for 30 min. After 1 h of incubation in fluorescein lycopersicon esculentum (tomato) lectin (1:1000, Vector Laboratories, Burlingame, CA), goat anti-ZO-1 antibody (1:200, Santa Cruz Biotechnology), or goat anti-albumin antibody (1:100, Santa Cruz Biotechnology) at room temperature [19], the sections were washed three times in PBS for 5 min, and then incubated with secondary antibody (CL555-donkey anti-goat, 1:200, Santa Cruz Biotechnology) for 1 h at room temperature. Finally, all sections were washed three times with PBS for 5 min in the dark, and then mounted on a coverslip with a drop of mounting medium (Santa Cruz Biotechnology). We also assessed BBB permeability with Evans Blue staining [36]. Six hours after icv injection, six rats each from the sham, vehicle, and VEGF groups were randomly chosen and injected with 2 % Evans Blue (in normal saline, 4 mL/kg of body weight) via the tail vein. Thirty minutes later, all rats were deeply anesthetized with an overdose of 10 % chloral hydrate and then transcardially perfused with 0.01 mol/L PBS. The Evans Blue stain in the left brain hemisphere was measured by spectrophotometer at 610 nm and quantified according to a standard curve as previously reported [36]. The results are presented as micrograms of Evans Blue per gram of tissue.
BMMNC Migration
We killed eight rats per group from the six groups (sham+ vehicle, sham +BMMNC, 2VO +vehicle, 2VO +BMMNC, 2VO+VEGF+BMMNC, 2VO+SU5416+BMMNC) to assess BMMNC migration on day 1 after BMMNC transplantation. The brains and left lower lungs of each rat were cut into 25 μm sections and incubated with anti-eGFP antibody (1:1000, Abcam, Cambridge, MA, USA) via the methods described above. All sections were mounted on coverslips with a drop of mounting medium containing 1.5 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; Santa Cruz Biotechnology).
Angiogenesis
Eight rats from each of the six groups were also killed for angiogenesis analysis on day 14 after BMMNC transplantation. We obtained 25-μm coronal sections as described above and stained them with a mixture of fluorescein lycopersicon esculentum (tomato) lectin (1:1000; Vector Laboratories) and anti-Ki67 (1:200, Santa Cruz Biotechnology), as previously described [37].
Neural Degeneration
Brain sections used for angiogenesis were also used to assess neural degeneration. We stained the 25 μm coronal sections with Fluoro-Jade B (FJB) according to standard protocol [38].
All of the stained sections were mounted and observed under a fluorescence microscope (ZEISS ScopeA1, ZEISS, Germany). We counted eGFP-positive cells and ZO-1/lectin- and Ki-67/lectin-positive vessels in the striatum under needle trauma (a lectin-positive vessel separated from adjacent vessels was counted as one vessel. The number of these vessels was added to the number of vascular branch points to yield the total number of vessels). FJB-positive neurons in the ipsilateral cortex were analyzed by an investigator blinded to animal group.
ELISA Analysis
Eight rats from each of the six groups were used to detect changes in cytokine level in the peripheral blood. We measured the concentrations of interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), and IL-10 in plasma in triplicate using the appropriate enzyme-linked immunosorbent assay (ELISA) kits (Boster, Wuhan, China) according to the manufacturer’s protocols.
Statistical Analysis
Statistical analysis was carried out with SPSS version 13.0. Results are expressed as mean±SD. We used Fisher’s exact test to examine differences in mortality among the groups and one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test to analyze histologic and ELISA differences. Repeated measures ANOVA followed by the LSD test was used to determine changes in reference memory protocol performance and differences in body weight between groups. p<0.05 was considered statistically significant.
Results
Effect of icv VEGF Injection on BBB Integrity
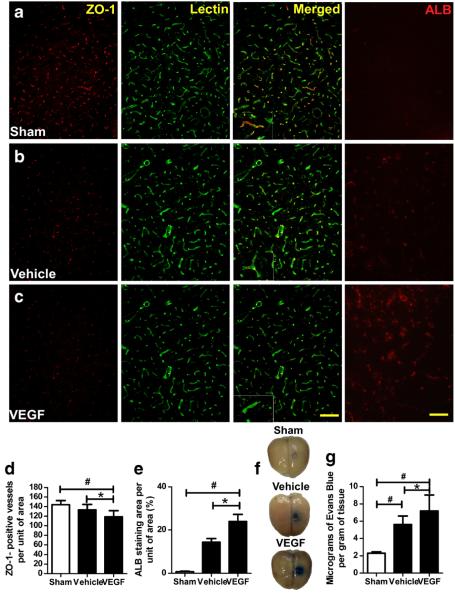
Nineteen of 129 rats died during icv injection. The mortality of each group was 4/40 (10 %) in the sham group, 7/43 (16.3 %) in the vehicle group, and 10/46 (21.7 %) in the VEGF group. Neither mortality rate nor body weight was significantly different between the two 2VO groups (Fig. 1b, p > 0.05). Compared with the sham group, rats that underwent 2VO had lower ZO-1 (n=6, p<0.05; Fig. 1c, d) and claudin-5 protein levels (n=6, p<0.05; Fig. 1c, e), fewer ZO-1-positive vessels (n=6, p<0.05; Fig. 2a–d), more albumin staining (n=6, p<0.05; Fig. 2a–c, e), and higher levels of Evans Blue staining (n=6, p<0.05; Fig. 2f, g) in the brain. Rats injected with VEGF had lower levels of ZO-1 and cluadin-5 (Fig. 1c–e), fewer ZO-1-positive vessels (Fig. 2c, d), more albumin staining (Fig. 2a–c, e), and higher levels of Evans Blue staining (Fig. 2f, g) than did 2VO rats treated with vehicle. No significant difference in brain water content was present among the three groups (n=6, p<0.05; Fig. 1f).
Fig. 2.
VEGF increases blood–brain barrier permeability. a–c Immunofluorescence staining of ZO-1-positive vessels and albumin (ALB) in the striatum 6 h after VEGF injection. Images are shown at ×200 magnification. Scale bar=50 μm. d–e Quantification showed that VEGF-treated 2VO rats had fewer ZO-1-positive (red) vessels (green; d) and higher albumin staining (e) than did sham and vehicle-treated groups. *p<0.05 vs. vehicle group; #p<0.05 vs. sham group; n=8/group. f Representative rat brains from each group 30 min after Evans Blue administration. g Quantification showed that VEGF significantly increased the amount of extravasated Evans Blue dye compared with that in the other two groups. *p<0.05 vs. vehicle; #p<0.05 versus sham. n=8/group. Values are mean±SD
Cognitive Function
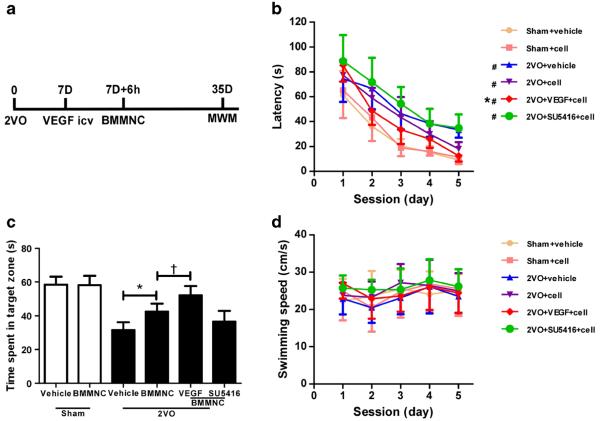
The mortality of each group was 3/37 (8.1 %) in the sham+ vehicle group, 2/36 (5.6 %) in the sham+BMMNC group, 8/42 (19 %) in the 2VO+vehicle group, 10/44 (22.7 %) in the 2VO+BMMNC group, 9/43 (20.9 %) in the 2VO+VEGF+ BMMNC group, and 7/41 (17.1 %) in the 2VO+SU5416+ BMMNC group. Mortality rates did not differ significantly among the 2VO groups. Rats in the four 2VO groups exhibited significant cognitive impairment in the Morris water maze test compared with that of sham groups (n =10, p < 0.05; Fig. 3a–c). The LSD test showed that rats that received BMMNC transplantation had better performance than those in the vehicle group. Latency to find the platform was shorter in the 2VO + VEGF + BMMNC group than in the 2VO + BMMNC and 2VO +SU5416+BMMNC groups (Fig. 3b). Pretreatment with SU5416 reversed the improvement seen with BMMNC transplantation, such that no differences were observed between the 2VO+SU5416+BMMNC and 2VO+ vehicle group. Swimming speeds did not differ among the six groups (n=10, p>0.05; Fig. 3d). One-way ANOVA showed that in the probe trial, rats in the 2VO group spent less time in the target zone than did rats in the sham group (n=10, p<0.05; Fig. 3c). A follow-up LSD test revealed that rats in the 2VO+ VEGF+BMMNC group spent more time in the target zone (52.1±5.5 s) than did the other three 2VO groups (2VO+ vehicle, 31.6 ± 4.7 s; 2VO + BMMNC, 42.5 ± 4.8 s; 2VO + SU5416 + BMMNC, 36.6 ± 6.3 s; p < 0.05). The 2VO + BMMNC group also spent more time in the target zone than either the 2VO + vehicle group or the 2VO + SU5416 + BMMNC group.
Fig. 3.
VEGF enhances the effect of BMMNC transplantation on learning and memory at day 28 after treatment. a Schematic representation of experimental design for a–c. 7D 7 days after 2VO procedure. b Time latency to find the platform in the Morris water maze (MWM) test. The performance of 2VO rats was worse than that of the sham groups. 2VO rats treated with BMMNCs (cell) exhibited decreased latency to find the platform compared with the vehicle-treated group. This therapeutic effect was enhanced by VEGF but abolished by SU5416. #p<0.05 vs. other two sham groups; *p<0.05 vs. 2VO + BMMNC group. c Quantification showed that the 2VO + BMMNC group spent more time in the target zone during the probe trial than the vehicle group, but less time than the 2VO + VEGF + BMMNC group. No time difference was found between the 2VO + vehicle and 2VO + SU5416 + BMMNC groups. *p <0.05 vs. 2VO+ vehicle group; †p < 0.05 vs. 2VO + BMMNC group. n = 10/group. d Swimming speed did not differ among the six groups during the five sessions
BMMNC Migration to the Brain
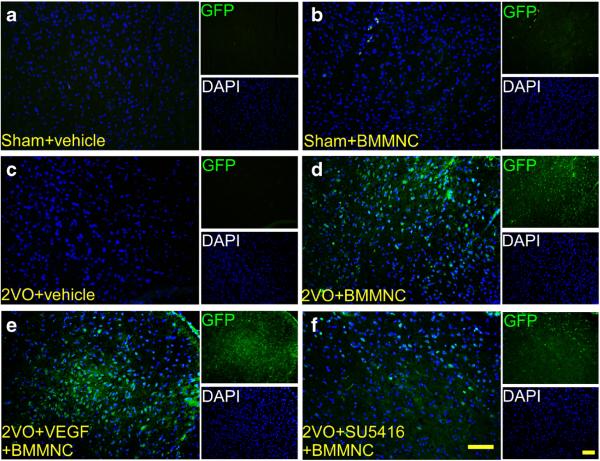
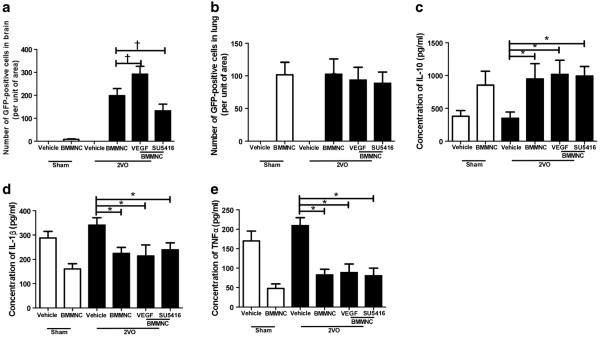
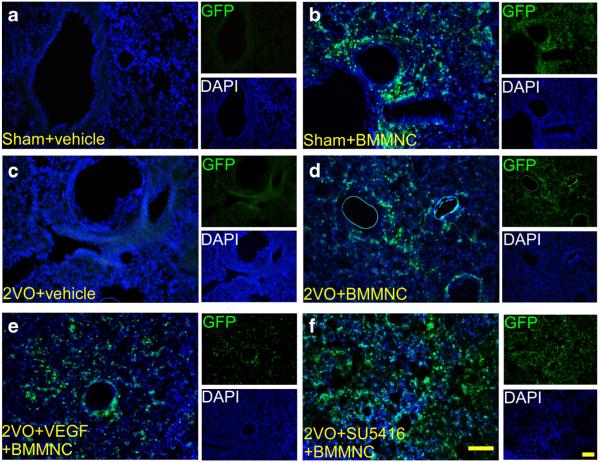
Analysis by ANOVA followed by the LSD test revealed that the number of GFP-positive cells was significantly higher in the 2VO + VEGF + BMMNC group than in the 2VO + BMMNC group (2VO+VEGF+BMMNC: 292±34; 2VO+ BMMNC: 199 ± 31, n =8, p < 0.05) and was higher in the 2VO + BMMNC group than in the 2VO + SU5416 + BMMNC group (133±28; n=8, p<0.05 vs. 2VO+BMMNC group; Figs. 4 and 6a). No GFP-positive cells were observed in the sham+vehicle or 2VO+vehicle groups.
Fig. 4.
VEGF facilitates BMMNC migration to the brain. a–f Immunofluorescence staining of GFP-positive BMMNCs in the striatum on day 1 after administration of BMMNCs or vehicle. Images are shown at ×200 magnification. Scale bar=50 μm. n=8/group
Fig. 6.
VEGF facilitates BMMNC migration to the brain without altering IL-1, IL-10, or TNF-α in peripheral blood. a Quantification of GFP-positive cells in the brain on day 1 after BMMNC transplantation showed that VEGF significantly increased BMMNC migration to brain. Preconditioning with SU5416 significantly decreased the migration of BMMNCs. †p<0.05 vs. 2VO+BMMNC; n=8/group. b All rats that received BMMNC transplantation had similar numbers of GFP-positive BMMNCs in the lungs. p>0.05 vs. 2VO+BMMNC, n=8/group. c In the 2VO groups, BMMNC administration significantly increased IL-10 level in peripheral blood compared with that in the vehicle-treated group. IL-10 level in peripheral blood did not differ significantly among the 2VO groups that received BMMNCs. *p < 0.05 vs. 2VO + vehicle, n =8/group. d, e In the 2VO groups, BMMNC administration significantly decreased IL-1β (d) and TNF-α (e) levels in peripheral blood compared with levels in the vehicle-treated group. IL-1β and TNF-α levels in peripheral blood did not differ among the 2VO groups that received BMMNCs. *p<0.05 vs. 2VO+vehicle, n=8/group. Values are mean±SD
BMMNC Migration to the Lung
One-way ANOVA analysis showed that the number of GFP-positive cells in lung did not differ among the four groups that received BMMNC transplantation (n=8; p>0.05; Figs. 5 and 6b). No GFP-positive cells were found in the control groups that received vehicle.
Fig. 5.
VEGF had no effect on the biodistribution of BMMNCs to the lung. a–f Immunofluorescence staining of GFP-positive BMMNCs in the lung on day 1 after administration of BMMNCs or vehicle. Images are shown at ×200 magnification. Scale bar=50 μm. n=8/group
Cytokines in the Peripheral Blood
One-way ANOVA revealed that the sham+BMMNC group had significantly lower levels of IL-1β and TNF-α and higher levels of IL-10 in peripheral blood than did the sham+vehicle group. The 2VO+vehicle group had the highest levels of IL-1β and TNF-α and the lowest level of IL-10 among the four 2VO groups. However, no differences were found among the 2VO + BMMNC, 2VO + VEGF + BMMNC, and 2VO + SU5416+BMMNC groups (n=8; p>0.05; Fig. 6c–e).
Angiogenesis
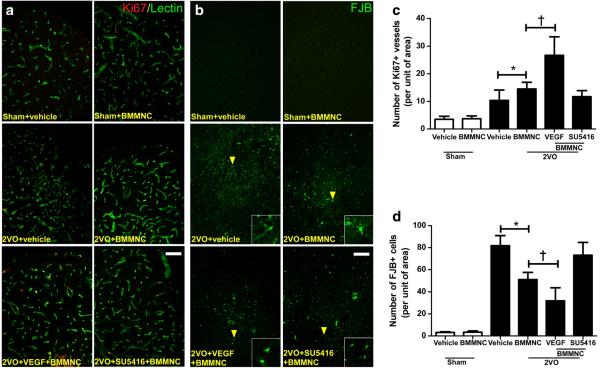
Rats that underwent the 2VO procedure had more Ki67-positive vessels in the striatum under needle trauma than did rats in the two sham groups (n=8; F=48; p<0.05). 2VO+ VEGF+BMMNC rats had more Ki67-positive vessels than did the other three 2VO groups (26.7±6.7; p<0.05 vs. other three 2VO groups (Fig. 7a, c). 2VO rats that received BMMNC transplantation had more Ki67-positive vessels than did the 2VO + vehicle group (2VO + BMMNC, 14.5 ± 2.4; 2VO+vehicle, 10.3±3.7; p<0.05), but showed no difference compared with the 2VO+SU5416+BMMNC group (11.7±2.1; p>0.05 vs. 2VO+BMMNC group).
Fig. 7.
VEGF enhances the angiogenic and neuroprotective effects of BMMNC transplantation. a Immunofluorescence staining of proliferative (Ki67-positive, red) vessels (green) in the striatum on day 14 after administration of BMMNCs. b Fluoro-Jade B (FJB, green) staining of degenerating neurons in the cortex on day 14 after administration of BMMNCs. The yellow arrowheads indicate FJB-positive cells. c Quantification showed that 2VO groups had significantly more Ki67-positive vessels than did the sham groups. In addition, the number of Ki67-positive vessels was significantly greater in the 2VO +BMMNC group than in the 2VO+ vehicle group. This angiogenic effect was accelerated by VEGF but abolished by SU5416. *p<0.05 vs. 2VO+vehicle; †p<0.05 vs. 2VO+BMMNC. n=8/group. d Quantification showed that 2VO groups had significantly more FJB-positive cells than did the sham groups. In the 2VO groups, BMMNC administration significantly reduced the number of FJB-positive cells; this effect was enhanced by preconditioning with VEGF. No difference was observed between the 2VO+ SU5416 +BMMNC group and the 2VO+vehicle group. *p<0.05 vs. 2VO+vehicle; †p<0.05 vs. 2VO+ BMMNC. n=8/group. Values are mean±SD
Neuroprotective Effect
Quantification showed that the 2VO procedure significantly increased the number of FJB-positive cells in the ipsilateral cortex (n=8; F=138; p<0.05). ANOVA followed by the LSD test revealed that the 2VO+VEGF+BMMNC group had the fewest FJB-positive cells of all the 2VO groups (31.9±11.8; p<0.05 vs. other three 2VO groups (Fig. 7b, d). 2VO rats that received BMMNC transplantation had fewer FJB-positive cells than did 2VO rats that received vehicle (2VO + BMMNC, 51.2±6.3; 2VO+vehicle, 81.8.3±9.1; p<0.05), but also showed no differences compared with the 2VO + SU5416 +BMMNC group (73.2 ± 11.6; p >0.05 vs. 2VO+ BMMNC group).
Discussion
Our study provides the novel finding that preconditioning with VEGF can improve the therapeutic effects of BMMNC transplantation in a rat model of chronic cerebral hypoperfusion, most likely by enhancing migration of BMMNCs into the brain. Intravenous transplantation of BMMNCs has been shown to reduce neurologic impairments in rodent models of ischemic stroke [9] and in patients with acute ischemic stroke [11]. We chose to use the intravenous route for transplantation because it is easier to perform than icv or intra-arterial injection, it saves time, and it is more applicable for clinical use. However, the underlying mechanism of action for intravenous transplantation of BMMNCs in ischemic diseases is unclear. Some investigators have shown that transplanted cells, such as MSCs [39], neural stem cells [40], endothelial progenitor cells [41], and BMMNCs [7, 19, 28], are able to migrate to the ischemic lesion, remain viable, and function effectively via paracrine effects or cell differentiation. However, others have reported that, after intravenous delivery, most BMMNCs are initially trapped in the lungs or liver [14, 42]. Because only 4–5 % of the injected BMMNCs can pass through the pulmonary system, only a small percentage reach the ischemic lesion [42]. Prockop and colleagues [43] also showed that the biodistribution of MSCs, which are an important component of BMMNCs, was complete within 5 min. They concluded that the effects of MSC transplantation on myocardial infarction in mice arise from the anti-inflammatory protein TSG-6 being secreted by activated MSCs trapped in the lungs, rather than from cell migration to the ischemic heart tissue. Saviz et al. [12] also showed that intravenous BMMNC transplantation significantly altered the profile of inflammatory cytokines in the peripheral blood, suggesting that BMMNCs trapped in the lung or other organs may play a vital role in immune regulation in the MCAO rat model [44]. Therefore, the extent to which BMMNC migration to the brain contributes to the therapeutic effect in brain ischemia remains unclear.
As described above, the use of BMMNCs is limited by their small number and the short period of time during which they are able to penetrate the BBB [26]. Our research and that of others has shown that a permeable BBB is essential for BMMNC biodistribution into the ischemic brain [7, 12, 19]. In this study, we administered rrVEGF by icv injection to permeabilize the BBB 6 h before BMMNC transplantation. We found that at a dose of 0.2 μg/rat, rrVEGF significantly reduced the levels of tight junction proteins ZO-1 and claudin-5 in the ischemic brain of 2VO rats. Quantification of Evans Blue staining, albumin staining, and water content also indicated that rrVEGF increased BBB permeability without causing brain edema. Notably, we found that more BMMNCs had migrated to the brain of 2VO rats that were preconditioned with VEGF than in those that were preconditioned with vehicle. However, the lung BMMNC migration and peripheral blood cytokine levels did not differ significantly between the 2VO+BMMNC and 2VO+VEGF+BMMNC groups, suggesting that icv injection of VEGF may have no influence on the biodistribution or immune regulatory effect of BMMNCs outside the ischemic brain. An important finding was that rats that received VEGF and BMMNC transplantation showed better cognitive functions, greater angiogenesis, and less neuronal apoptosis in the ischemic brain than did rats that received BMMNC transplantation only. These results indicate that increased brain BMMNC migration may enhance the therapeutic effects of BMMNC transplantation in the rat global ischemia model.
To exclude the effect of VEGF on the ischemic brain, we used SU5416 to further confirm our hypothesis. SU5416 is a specific inhibitor of VEGFR2, which mediates most of the downstream angiogenic and neuroprotective effects of VEGF [45]. We found that preconditioning with SU5416 significantly reduced the migration of BMMNCs to the brain but had no effect on lung BMMNC migration or peripheral blood cytokine levels. SU5416 also abolished the therapeutic effects of VEGF+BMMNC therapy on cognitive function, angiogenesis, and neuroprotection. The biodistribution of BMMNCs after intravenous transplantation is complete in a very short time. Our data indicate that facilitating the migration of more BMMNCs to the ischemic lesion in a limited time can improve the efficiency of BMMNC therapy for brain ischemia, suggesting a novel way to improve the efficiency of cell therapies for various brain diseases.
In conclusion, our study suggests that preconditioning with VEGF enhances the therapeutic effects of BMMNC transplantation in a rat model of chronic cerebral hypoperfusion by facilitating the migration of BMMNCs to the chronic ischemic brain.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81271284, 81571137), The American Heart Association (13GRNT15730001), and the National Institutes of Health (R01NS078026, R01AT007317). We thank Yoyo Wang, Jiarui Wang, and Claire Levine for assistance with this manuscript.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare no conflict of interest.
References
- 1.Liu J, Wang LN, Tan JP. Dementia in China: current status. Neurology. 2013;81(12):1077–1078. doi: 10.1212/WNL.0b013e3182a4a3cb. doi:10.1212/WNL.0b013e3182a4a3cb. [DOI] [PubMed] [Google Scholar]
- 2.Jia J, Wang F, Wei C, Zhou A, Jia X, Li F, Tang M, Chu L, et al. The prevalence of dementia in urban and rural areas of China. Alzheimer Dement: J Alzheimer Assoc. 2014;10(1):1–9. doi: 10.1016/j.jalz.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7(9):812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37(1):56–74. doi: 10.1111/j.1365-2990.2010.01139.x. doi:10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baskys A, Cheng JX. Pharmacological prevention and treatment of vascular dementia: approaches and perspectives. Exp Gerontol. 2012;47(11):887–891. doi: 10.1016/j.exger.2012.07.002. doi:10.1016/j.exger.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Giraldi-Guimaraes A, de Freitas HT, Coelho Bde P, Macedo-Ramos H, Mendez-Otero R, Cavalcante LA, Baetas-da-Cruz W. Bone marrow mononuclear cells and mannose receptor expression in focal cortical ischemia. Brain Res. 2012;1452:173–184. doi: 10.1016/j.brainres.2012.03.002. doi:10.1016/j. brainres.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Yu L, Jiang C, Chen M, Ou C. Bone marrow mononuclear cells exert long-term neuroprotection in a rat model of ischemic stroke by promoting arteriogenesis and angiogenesis. Brain Behav Immun. 2013;34:56–66. doi: 10.1016/j.bbi.2013.07.010. doi:10.1016/j.bbi.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Liu X, Lu H, Jiang C, Cui X, Yu L, Fu X, Li Q. CXCR4(+)CD45(−) BMMNC subpopulation is superior to unfractionated BMMNCs for protection after ischemic stroke in mice. Brain Behav Immun. 2015;45:98–108. doi: 10.1016/j.bbi.2014.12.015. doi:10.1016/j.bbi.2014.12. 015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr, Aronowski J, Grotta JC, Savitz SI. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30(1):140–149. doi: 10.1038/jcbfm.2009.198. doi:10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, Yang B, Strong R, Xi X, Brenneman M, Grotta JC, Aronowski J, Savitz SI. Bone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic stroke. J Neurosci Res. 2010;88(13):2869–2876. doi: 10.1002/jnr.22452. doi:10.1002/jnr.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Jr, Alderman S, Aisiku I, Kar S, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70(1):59–69. doi: 10.1002/ana.22458. doi:10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 12.Yang B, Migliati E, Parsha K, Schaar K, Xi X, Aronowski J, Savitz SI. Intra-arterial delivery is not superior to intravenous delivery of autologous bone marrow mononuclear cells in acute ischemic stroke. Stroke. 2013;44(12):3463–3472. doi: 10.1161/STROKEAHA.111.000821. doi:10.1161/STROKEAHA.111. 000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich MA, Martins MP, Araujo MD, Klamt C, Vedolin L, Garicochea B, Raupp EF, Sartori El Ammar J, et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012;21(1):S13–S21. doi: 10.3727/096368912x612512. doi:10.3727/096368911X612512. [DOI] [PubMed] [Google Scholar]
- 14.Rosado-de-Castro PH, Schmidt Fda R, Battistella V, de Souza SAL, Gutfilen B, Goldenberg RC, Kasai-Brunswick TH, Vairo L, et al. Biodistribution of bone marrow mononuclear cells after intra-arterial or intravenous transplantation in subacute stroke patients. Regen Med. 2013;8(2):145–155. doi: 10.2217/rme.13.2. doi:10.2217/rme.13.2. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Hu X, Wang L, Jiang Z, Liu X, Yu H, Zhang Z, Chen H, et al. Preconditioning via angiotensin type 2 receptor activation improves therapeutic efficacy of bone marrow mononuclear cells for cardiac repair. PLoS One. 2013;8(12):e82997. doi: 10.1371/journal.pone.0082997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matoba S, Matsubara H. Therapeutic angiogenesis for peripheral artery diseases by autologous bone marrow cell transplantation. Curr Pharm Des. 2009;15(24):2769–2777. doi: 10.2174/138161209788923840. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Salmeron R, de la Cuesta-Diaz A, Constantino-Bermejo M, Perez-Camacho I, Marcos-Sanchez F, Hmadcha A, Soria B. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant. 2011;20(10):1629–1639. doi: 10.3727/096368910X0177. [DOI] [PubMed] [Google Scholar]
- 18.Jiwa NS, Garrard P, Hainsworth AH. Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. J Neurochem. 2010;115(4):814–828. doi: 10.1111/j.1471-4159.2010.06958.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Fu X, Jiang C, Yu L, Wang M, Han W, Liu L, Wang J. Bone marrow mononuclear cell transplantation promotes therapeutic angiogenesis via upregulation of the VEGF-VEGFR2 signaling pathway in a rat model of vascular dementia. Behav Brain Res. 2014;265:171–180. doi: 10.1016/j.bbr.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miki Y, Nonoguchi N, Ikeda N, Coffin RS, Kuroiwa T, S-i M. Vascular endothelial growth factor gene-transferred bone marrow stromal cells engineered with a herpes simplex virus type 1 vector can improve neurological deficits and reduce infarction volume in rat brain ischemia. Neurosurgery. 2007;61(3):586–594. doi: 10.1227/01.NEU.0000290907.30814.42. discussion 594–585. [DOI] [PubMed] [Google Scholar]
- 21.Chang YS, Ahn SY, Jeon HB, Sung DK, Kim ES, Sung SI, Yoo HS, Choi SJ, et al. Critical role of VEGF secreted by mesenchymal stem cells in hyperoxic lung injury. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2013-0385OC. doi:10.1165/rcmb.2013-0385OC. [DOI] [PubMed] [Google Scholar]
- 22.Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29(2):274–285. doi: 10.1002/stem.584. doi:10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Qu Y, Fei Z. Vascular endothelial growth factor in cerebral ischemia. J Neurosci Res. 2011;89(7):969–978. doi: 10.1002/jnr.22628. doi:10.1002/jnr. 22628. [DOI] [PubMed] [Google Scholar]
- 24.Zechariah A, ElAli A, Doeppner TR, Jin F, Hasan MR, Helfrich I, Mies G, Hermann DM. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke. 2013;44(6):1690–1697. doi: 10.1161/STROKEAHA.111.000240. doi:10. 1161/STROKEAHA.111.000240. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25(2):281–290. doi: 10.1038/sj.jcbfm.9600034. doi:10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleier BS, Kohman RE, Feldman RE, Ramanlal S, Han X. Permeabilization of the blood–brain barrier via mucosal engrafting: implications for drug delivery to the brain. PLoS One. 2013;8(4):e61694. doi: 10.1371/journal.pone.0061694. doi:10.1371/journal.pone.0061694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choy M, Ganesan V, Thomas DL, Thornton JS, Proctor E, King MD, van der Weerd L, Gadian DG, et al. The chronic vascular and haemodynamic response after permanent bilateral common carotid occlusion in newborn and adult rats. J Cereb Blood Flow Metab. 2006;26(8):1066–1075. doi: 10.1038/sj.jcbfm.9600259. doi:10.1038/sj.jcbfm.9600259. [DOI] [PubMed] [Google Scholar]
- 28.Jiang C, Wang J, Yu L, Ou C, Liu X, Zhao X. Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia. Behav Brain Res. 2013;250:222–229. doi: 10.1016/j.bbr.2013.05.011. doi:10.1016/j.bbr.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van Damme P, Rutten B, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8(1):85–92. doi: 10.1038/nn1360. doi:10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 30.Nowacka MM, Obuchowicz E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: a new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides. 2012;46(1):1–10. doi: 10.1016/j.npep.2011.05.005. doi:10.1016/j.npep.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Yang B, Strong R, Sharma S, Brenneman M, Mallikarjunarao K, Xi X, Grotta JC, Aronowski J, et al. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89(6):833–839. doi: 10.1002/jnr.22614. doi:10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang CF, Cho S, Wang J. (−)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann Clin Transl Neurol. 2014;1(4):258–271. doi: 10.1002/acn3.54. doi:10.1002/acn3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54(1):162–180. doi: 10.1016/j.brainresrev.2007.01.003. doi:10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Feng Y, Rhodes PG, Bhatt AJ. Neuroprotective effects of vascular endothelial growth factor following hypoxic ischemic brain injury in neonatal rats. Pediatr Res. 2008;64(4):370–374. doi: 10.1203/PDR.0b013e318180ebe6. doi:10. 1203/PDR.0b013e318180ebe6. [DOI] [PubMed] [Google Scholar]
- 35.Cechetti F, Worm PV, Pereira LO, Siqueira IR. The modified 2VO ischemia protocol causes cognitive impairment similar to that induced by the standard method, but with a better survival rate. Braz J Med Biol Res. 2010;43(12):1178–1183. doi: 10.1590/s0100-879x2010007500124. CAN. [DOI] [PubMed] [Google Scholar]
- 36.Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans blue injection routes: intravenous versus intra-peritoneal, for measurement of blood–brain barrier in a mice hemorrhage model. J Neurosci Methods. 2011;195(2):206–210. doi: 10.1016/j.jneumeth.2010.12.013. doi:10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, Moghaddam SJ, Dong C. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U S A. 2014;111(15):5664–5669. doi: 10.1073/pnas.1319051111. doi:10.1073/pnas.1319051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, et al. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med. 2007;43(3):408–414. doi: 10.1016/j.freeradbiomed.2007.04.020. doi:10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donega V, van Velthoven CT, Nijboer CH, Kavelaars A, Heijnen CJ. The endogenous regenerative capacity of the damaged newborn brain: boosting neurogenesis with mesenchymal stem cell treatment. J Cereb Blood Flow Metab. 2013;33(5):625–634. doi: 10.1038/jcbfm.2013.3. doi:10.1038/jcbfm.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darsalia V, Allison SJ, Cusulin C, Monni E, Kuzdas D, Kallur T, Lindvall O, Kokaia Z. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab. 2011;31(1):235–242. doi: 10.1038/jcbfm.2010.81. doi: 10.1038/jcbfm.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hecht N, Schneider UC, Czabanka M, Vinci M, Hatzopoulos AK, Vajkoczy P, Woitzik J. Endothelial progenitor cells augment collateralization and hemodynamic rescue in a model of chronic cerebral ischemia. J Cereb Blood Flow Metab. 2014;34(8):1297–1305. doi: 10.1038/jcbfm.2014.78. doi:10.1038/jcbfm.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS., Jr Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–692. doi: 10.1089/scd.2008.0253. doi:10.1089/scd. 2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. doi:10.1016/j.stem. 2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Yu L, Jiang C, Fu X, Liu X, Wang M, Ou C, Cui X, et al. Cerebral ischemia increases bone marrow CD4+CD25+ FoxP3+ regulatory T cells in mice via signals from sympathetic nervous system. Brain Behav Immun. 2015;43:172–183. doi: 10.1016/j.bbi.2014.07.022. doi:10.1016/j. bbi.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. doi: 10.1038/380435a0. doi:10.1038/380435a0. [DOI] [PubMed] [Google Scholar]