Abstract
Vestibular migraine (VM), defined as vestibular symptoms caused by migraine mechanisms, is very common but poorly understood. Because dizziness is often provoked in VM patients when the semicircular canals and otolith organs are stimulated concurrently (e.g., tilting the head relative to gravity), we measured tilt perception and eye movements in patients with VM and in migraine and normal control subjects during fixed-radius centrifugation, a paradigm that simultaneously modulates afferent signals from the semicircular canals and otoliths organs. Twenty-four patients (8 in each category) were tested with a motion paradigm that generated an inter-aural centrifugal force of 0.36 G, resulting in a 20° tilt of the gravito-inertial force in the roll plane. We found that percepts of roll tilt developed slower in VM patients than in the two control groups, but that eye movement responses, including the shift in the eye’s rotational axis, were equivalent in all three groups. These results demonstrate a change in vestibular perception in VM that is unaccompanied by changes in vestibular-mediated eye movements and suggest that either the brain’s integration of canal and otolith signals or the dynamics of otolith responses are aberrant in patients with VM.
Keywords: vestibular, migraine, perception, centrifugation, eye movements
INTRODUCTION
Vestibular symptoms caused by migraine mechanisms (vestibular migraine, VM) are common and disabling. VM occurs in at least 15 % of migraine patients (Neuhauser et al. 2001), and conversely, VM is one of the most common causes of dizziness in the general population (Neuhauser et al. 2006). Despite its frequency, the pathophysiology of VM remains uncertain. Potential interactions between migraine mechanisms and the vestibular system are numerous and complex (reviewed in Furman et al. 2013), and no vestibulo-ocular or vestibulo-spinal abnormalities have been defined that are specific to VM and not shared by migraine subjects without vestibular symptoms (Casini et al. 2009; Furman et al. 2005).
Vestibular tests focus primarily on reflexive eye movements (the vestibulo-ocular reflex, VOR), which are elicited by angular or linear head motion. There is evidence, however, that vestibular-mediated percepts and eye movements are generated by different mechanisms (Merfeld et al. 2005) and pathways (Cullen 2011) in the brain. Perception appears more dependent on interactions between semicircular canals signals (which encode angular head velocity) and otolith signals (which encode the vector sum of gravity and the inertial force produced by linear acceleration, gravito-inertial force or GIF, Merfeld et al. 2005). Since vestibular symptoms and signs in VM are often positional (provoked or exacerbated by tilting the head relative to gravity, Kayan and Hood 1984; Polensk and Tusa, 2009) and these head movements modulate activity in the canals and otolith organs concurrently, vestibular psychophysics could be a particularly effective way to investigate the pathophysiology of VM (Lewis et al. 2011a, b).
We therefore investigated the hypothesis that percepts of head orientation are abnormal in VM patients during combined stimulation of the canals and otolith organs. We measured percepts of head tilt and VOR responses during fixed-radius centrifugation, a motion paradigm which concurrently activates the canals and otolith organs, in VM patients and normal and migraine control subjects, and found that tilt percepts developed more slowly in VM patients relative to control subjects but eye movements were equivalent in all three groups.
MATERIALS AND METHODS
The study was approved by the hospital’s Institutional Review Board, and informed consent was obtained from each subject. We studied eight VM, migraine, and normal control subjects. In all cases, there was no history of other otologic or neurologic disease, subjects were not on migraine prophylactic medications, and none had a migraine or dizziness episode within 2 weeks of testing. VM subjects met the currently accepted clinical criteria for definite VM (Lempert et al. 2012) and had normal physical exams, audiograms, brain MRIs, and vestibular testing (bi-thermal caloric and sinusoidal rotational testing). Migraine subjects met International Headache Society criteria for migraine without aura (Headache Classification Subcommittee 2004), had no vestibular symptoms, and had normal brain MRIs and vestibular testing. Normal subjects had no history of migraine or vestibular symptoms and had normal vestibular testing. Motion sickness sensitivity was quantified with the revised Golding questionnaire (Golding 1998).
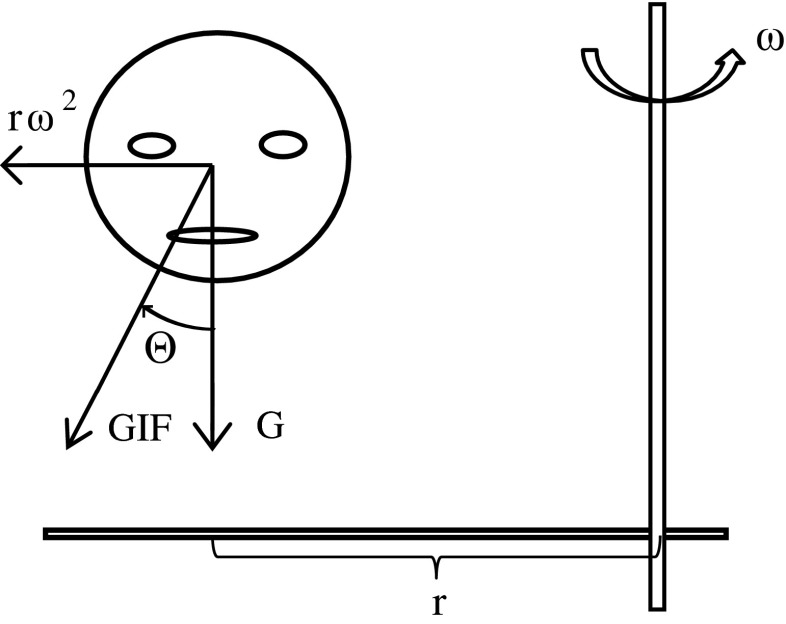
During centrifugation, subjects sat in a padded chair, were restrained with a harness, and their head was immobilized. The head was fixed 0.29 m from the earth-vertical yaw rotation axis with the right ear outmost (Fig. 1). In complete darkness, subjects accelerated about the yaw rotational axis at 13.3 deg/s/s in the facing-motion direction for 15 s, held at a constant angular velocity of 200 deg/s for 2 min, then symmetrically decelerated to a stop. This paradigm generated an inter-aural centrifugal force of 3.5 m/s/s (0.36 G) in a parabolic manner over the 15 s of acceleration, rotating the GIF by 20 deg in roll towards the patient’s right (Fig. 1).
FIG. 1.
Illustration of the centrifugation paradigm and associated vestibular inputs. The head is located a distance “r” from the rotational axis with the right ear outward. The chair rotates in yaw at an angular velocity “w” in the facing-motion direction (counter-clockwise when viewed from above) generating a centrifugal force of rw 2, which sums with the earth-vertical gravitational force (“G”) to yield a net gravito-inertial force (GIF) that is tilted by an angle theta relative to the head’s upright orientation.
Horizontal and vertical VOR responses were recorded with a standard (Neurokinetics, Pittsburgh PA) head-mounted infra-red video system at 100 Hz, smoothed using a nonlinear order statistic filter (predictive FIR-median hybrid), and then smoothed with a fourth-order phase-less Butterworth low pass filter with a corner frequency of 30 Hz, using the Matlab “filtfilt” function (Engelken and Stevens 1990). Quick phases were removed with a semi-automated system based on acceleration criteria, and slow phase velocity was extrapolated across quick phases with a linear fit based on the median of the three slow phase values immediately prior to the quick phase, to the median of the three slow phase values immediately subsequent to the fast phase, yielding a horizontal and vertical slow phase velocity (SPV) time series (Fig. 2A). Positive SPV directions were rightward and upward. The horizontal VOR was characterized by its gain, (peak horizontal SPV)/(200 deg/s), and time constant which was calculated by fitting an exponential to the horizontal SPV, starting at 15 s, when chair velocity reached its peak value. The rotational axis of the VOR in the frontal plane (Fig. 2B, grey line), which is thought to reflect the brain’s estimated orientation of gravity relative to the head (Cohen et al. 1999), was calculated as the tan−1[vertical SPV/horizontal SPV], so an axis of zero degrees is earth-vertical (horizontal eye rotation) and an axis of 90 ° is earth-horizontal (vertical eye rotation). This calculation is valid except when the horizontal SPV (in the denominator of the equation) is near zero (e.g., at rotation onset and after about 30 s) where it is unstable. Since the VOR axis initially shifted very slowly and then rose more rapidly to a plateau (Fig. 2B), these data were fit with a lag (interval from the onset of rotation to the time when the axis shifted at >0.5 deg/s for more than 0.5 s), and the subsequent rise was fit with a single exponential A = Amax(1–e-Tc/t) where Amax is the peak amplitude of the axis shift and Tc is the time constant.
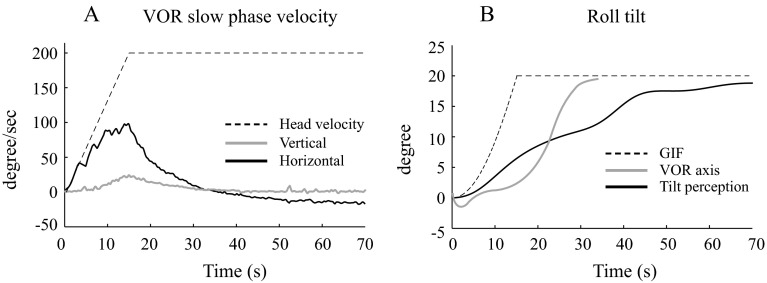
FIG. 2.
Vestibulo-ocular reflex (VOR) and tilt perception in a patient with vestibular migraine. A Shows the horizontal (black line) and vertical (grey line) slow phase eye velocity (SPV), and the dashed line shows the angular velocity of the head. Positive directions are rightward and upward. B Show the axis shift of the VOR (grey line) and the perception of tilt (black line) in the roll plane, with the dashed line showing the orientation of the gravito-inertial force (GIF). The VOR axis trace is clipped at 34 s because it becomes unstable when the horizontal SPV approaches zero, since it is calculated as the tan−1(vertical SPV/horizontal SPV).
A somatosensory bar was used to measure tilt perception (Park et al. 2006). Subjects grasped a bar that rotated in roll in the frontal plane with one hand at either end of the bar and were instructed to maintain the bar parallel to the perceived direction of the ground (e.g., perpendicular to gravity). A potentiometer co-axial with the bar’s rotational axis provided a measure of bar orientation in the frontal roll plane. The dynamic characteristics of the tilt percept (Fig. 2B, black line) were qualitatively similar to the axis shift so they were also fit by a lag followed by a single exponential characterized by its peak amplitude and time constant (exactly as described above for the VOR axis).
Statistical tests were performed using the SigmaStat 3.5 software package. Standard parametric comparisons (t tests, analysis of variance [ANOVA]) were used whenever data passed normality tests; otherwise, the appropriate non-parametric comparisons were used. T tests were paired and assumed equal variance; Bonferroni corrections were used for multiple t tests. ANOVA was performed without repeated measures.
RESULTS
VOR and perceptual responses were qualitatively similar in the three subject groups, and results from a VM patient are shown in Figure 2. The horizontal VOR peaked at about 15 s, when chair velocity reached its maximum, and then rapidly decayed to zero, followed by a small reversal in direction (Fig. 2A); a vertical VOR developed more gradually (Fig. 2A) and shifted the VOR axis towards the orientation of the GIF (Fig. 2B); and the perception of tilt in the roll plane (Fig. 2B) also lagged behind the shift in GIF, but gradually approached it over a time-frame of about 60 s. The horizontal VOR did not differ between the three subject groups, as there was no significant difference in VOR gain between the normal, migraine, and VM subjects (means +/− SE were 0.51+/−0.03, 0.53+/0.02. and 0.54 +/−0.06, respectively; ANOVA F(2) = 1.47, p = 0.25,) or VOR time constant (10.3+/−0.5, 9.5+/−1.3, and 9.6+/−0.7 respectively; ANOVA F(2) = 0.2, p = 0.8). The dynamic features of the VOR rotational axis (Figure 3, grey icons), quantified as the initial delay (lag) and subsequent rate of change (time constant), were equivalent in the normal, migraine, and VM subjects (lag, ANOVA F(2) = 0.7, p = 0.5, time constant ANOVA F(2) = 1.3, p = 0.2), as was the peak amplitude of the axis shift (ANOVA F(2) = 0.2, p = 0.8). No characteristics of the VOR response, therefore, segregated VM patients from the control subjects.
FIG. 3.
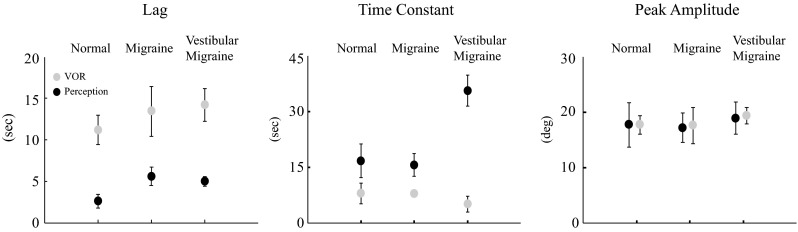
Characteristics of the VOR axis shift and tilt perception in the normal, migraine, and vestibular migraine subjects. Icons are the means of the eight subjects in each group, and error bars are one standard error, and are smaller than the icons in some cases.
Tilt perception (Figure 3, black icons) was characterized by an initial lag which was slightly longer in the VM and migraine subjects compared to normal controls, but peak tilt percepts were equivalent in the three groups (ANOVA F(2) = 0.05, p = 0.9). In contrast, the perceptual time constant was more than twice as long in VM (mean 35.7 s) than in migraine (mean 15.7 s) and normal (mean 16.8 s) subjects. Comparisons of time constant values between VM and migraine were significant (t-test t(14) = 2.2, p = 0.04) as was VM and normal subjects (t test t(14) = 2.1, p = 0.05), and Holm-Sidek test measuring dependence of tilt perception time constant on subject group was significant (F(2) = 7.2, p value of 0.03). Motion sickness susceptibility did not correlate with either the perceptual lag or time constant.
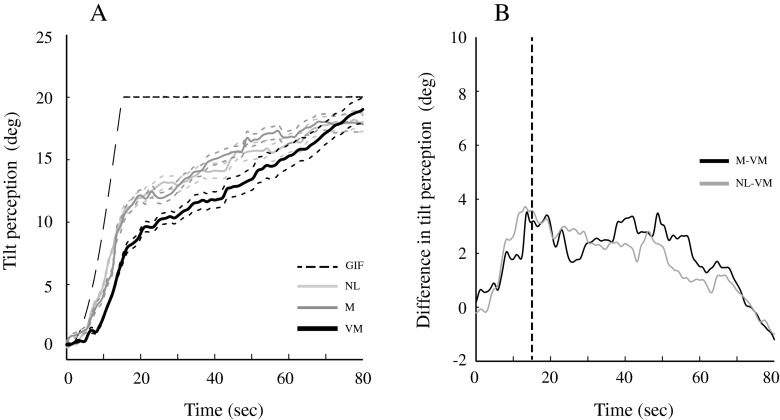
The dynamics of the tilt percept (Fig. 4A) showed little change for each group during the initial 4–5 s of chair acceleration, but over the next 10 s, the VM patients diverged from the control groups, with the tilt percept developing slower in VM subjects. This is most readily observed in Figure 4B, as the difference in tilt perception between the VM patients and control groups increased rapidly from 5 to 15 s, the time where the head’s angular velocity reached its steady-state value (dashed vertical line). The difference in perceived tilt between the VM and control subjects peaked at 3.5 deg (migraine—VM) and 3.7 deg (normal—VM). After 15 s, tilt percepts in all three groups increased in parallel (Fig. 4A), evidenced by the constant value of the difference between subject groups (Fig. 4B). After about 50 s, tilt percepts in the two control groups began to plateau while tilt percepts in VM patients increased relative to the control subjects and then converged to nearly the same final values as the control groups at about 80 s.
FIG. 4.
Tilt perception in the normal (NL), migraine (M), and vestibular migraine (VM) groups. A plots the means (solid lines) +/− one SE (dashed lines) for the three groups and the tilt of the GIF. B Plots the difference in tilt perception between the VM patients and the two control groups, with the vertical line indicating the time that the chair reaches its peak angular velocity.
Although the peak tilt percepts were equivalent to the peak VOR axis shifts in the three groups (t test t(46) = 0.2 p = 0.9), the dynamic characteristics differed between the VOR and perceptual measures (Fig. 3) as the VOR axis shift lags were longer than the perceptual lags (Mann-Whitney T(24) = 876, p < 0.001) while the VOR axis shift time constants were shorter than perceptual time constants (Mann-Whitney T(24) = 765, p < 0.001). The VM and control groups did not differ significantly in age (means VM 34.6, migraine 31.8, normal 31.6, ANOVA F(2) = 0.04, p = 0.9), motion sickness susceptibility (Golding 1998; means VM 12.7, migraine 11.6, normal 7.3, ANOVA on ranks H(2) = 3.2, p = 0.26) or gender (male female for VM 1:7, Migraine 2:6, normal 1:7, Fisher exact test p = 0.25).
DISCUSSION
Our principal finding is that during centrifugation, percepts of head tilt developed slower in VM patients than in migraine or normal control subjects, while VOR responses were equivalent in the three groups. Although prior studies of peripheral (e.g., Cousins et al. 2013) and central (e.g., Shaikh et al. 2013) vestibular disorders have described quantitative differences between VOR and perceptual abnormalities, our study is the first to demonstrate an abnormality that is evident in perceptual responses but is absent from VOR responses.
To interpret these results, it is useful to review the vestibular signals generated by centrifugation. The yaw rotational (canal) and roll tilt of the GIF (otolith) cues both increase during the first 15 s as the chair accelerates to a constant angular velocity, but then the rotational cue rapidly decays (Fig. 2A) while the GIF tilt remains static (Fig. 2B). Either head tilt or linear acceleration can produce shifts in GIF orientation (Angelaki et al. 1999), but if the brain processes the GIF shift as tilt then the vestibular inputs indicate the head is rotating in yaw about an axis tilted away from upright. In this situation, however, otolith signals continuously modulate due to re-orientation of gravity as the head rotates about a tilted axis. In normal subjects, the sensory conflict caused by the absence of these otolith modulations is considered the reason why the tilt estimate (e.g., perceived tilt, shift in the VOR axis) develops gradually during centrifugation—the brain is believed to suppress the estimate of tilt until the rotational cue subsides, thereby minimizing the sensory conflict (Merfeld et al. 2001).
Given these factors, slowed tilt percepts in VM patients could result from stronger yaw rotational cues, weaker dynamic GIF tilt cues, or abnormal central canal-otolith interactions. The angular VOR responses do not suggest that the yaw rotational cue differed between VM and other subjects, as the VOR amplitude and its rate of decay were equivalent in all three groups. In contrast, either dynamic abnormalities in otolith responses or aberrant central integration of canal and otolith cues could be responsible for the slowed development of tilt percepts we observed in VM patients. Both of these putative mechanisms could result in VM responses diverging from the control subjects during the period of angular acceleration, since the otolith signal is modulating dynamically and the canal signal rises to a peak during this time. After the period of angular acceleration, however, the otolith signal becomes time-invariant (e.g., static), and the angular velocity signal in the brain declines rapidly (evidenced by rapid slowing of the horizontal VOR), so both potential mechanisms abate after the angular velocity of the head reaches its peak value.
Data that support an otolith mechanism in VM consist of numerous reports that both cervical (testing the saccule) and ocular (testing primarily the utricle) vestibular evoked myogenic potentials are weaker than normal in VM subjects (c.f. Zaleski et al., 2015). If dynamic otolith responses were weak in VM, however, one would expect that motions that dynamically modulate otolith function in isolation (e.g., linear translations) would be perceived abnormally, but a recent report indicates that perceptual thresholds for linear motion along the three cardinal axes were normal in VM patients (Bremova et al. 2016). Further, since roll tilt about an earth-horizontal axis also dynamically modulates otolith activity, perceptual thresholds in VM patients during roll tilt should be abnormally high if these patients have impaired dynamic otolith responses, but instead were found to be abnormally low (Lewis et al. 2011a, b). These thresholds studies, therefore, do not support an otolith mechanism for our current centrifugation results.
Since central canal-otolith interactions are essential to the dynamics but not the final amplitude of the tilt estimate during centrifugation (Lewis et al. 2008), aberrant canal-otolith integration in VM patients is an alternate explanation for slowed tilt perception during centrifugation. Motions that activate otolith signals in isolation would not be affected by abnormal canal-otolith integration, so this putative mechanism predicts that translational thresholds would be normal in VM, as was recently reported (Bremova et al. 2016). Furthermore, if the interaction between canal and otolith signals in the brain is abnormally sensitized in VM, then behavioral responses during coplanar activation of canal and otolith cues (e.g., roll tilt, where both indicate roll) should be enhanced but responses during orthogonal activation (e.g., centrifugation, where canals indicate yaw rotation while otoliths indicate roll tilt) show be inhibited, consistent with our prior (Lewis et al., 2011a; b) and current observations.
In sum, while our centrifugation results do not allow us to determine if VM perceptual responses are slowed because of abnormalities in the dynamic response of the otolith organs or aberrant canal-otolith integration, when these findings are interpreted in the context of other VM psychophysical studies, the preponderance of evidence is consistent with latter rather than the former. Potential anatomic loci that could be responsible for aberrant canal-otolith integration include the caudal cerebellar vermis (lobules IX and X, where canal and otolith signals are first synthesized, Angelki et al., 2010) or neurons that receive these integrated signals from the cerebellum such as the vestibular nuclei, thalamus, or vestibular cerebral cortex. Recent fMRI findings that localized abnormalities in inter-ictal VM patients to the thalamus (Russo et al. 2014) are consistent with this proposed mechanism, as most thalamic neurons that receive vestibular inputs carry integrated canal-otolith signals (Meng et al. 2007), and a thalamic locus would predict perceptual but not VOR abnormalities, as we observed.
It is also notable that the dynamics of the tilt percept and the VOR axis shift differed in all subject groups. Since the tilt percept and VOR axis are both believed to reflect the brain’s estimated orientation of gravity relative to the head, differences in dynamics between these two behaviors (longer lag and shorter time constant for the VOR, Fig. 3) imply that eye movements and tilt percepts reflect different gravity estimates. As previously suggested (Merfeld et al. 2005), percepts may be more dependent on canal-otolith interactions while VOR responses may reflect the frequency characteristics of the motion, a concept that aligns with our hypothesis that aberrant canal-otolith integration occurs in VM. In the vestibular nuclei, neurons are segregated into populations that project rostrally to the thalamus or to oculomotor pathways (Cullen 2011), and it may be that the former receive integrated signals from the cerebellum while the latter do not.
Acknowledgments
We thank Dan Merfeld for the comments on the study and Dave Balkwill for technical assistance. This study is supported by the National Institutes of Health grant DC012528 to RFL.
References
- Angelaki DE, McHenry MQ, Dickman JD, Newlands SD, Hess BJ. Computation of inertial motion: neural strategies to resolve ambiguous otolith information. J Neurosci. 1999;19:316–327. doi: 10.1523/JNEUROSCI.19-01-00316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremova T, Caushaj A, Ertl M, Strobl R, Bottcher N, Strupp M, MacNeilage PR (2016) Comparison of linear motion perception thresholds in vestibular migraine and Meniere’s disease. Eur Arch Otorhinolaryngol epub ahead of print [DOI] [PMC free article] [PubMed]
- Casini AP, Sellari-Franceschini S, Napolitano A, Muscatello L, Dallan I. Otoneurologic dysfunctions in migraine patients with and without vertigo. Otol Neurotol. 2009;30:961–967. doi: 10.1097/MAO.0b013e3181b4e780. [DOI] [PubMed] [Google Scholar]
- Cohen B, Wearne S, Dai M, Raphan T. Spatial orientation of the angular vestibulo-ocular reflex. J Vestib Res. 1999;9:163–172. [PubMed] [Google Scholar]
- Cousins S, Kaski D, Cutfield N, Seemungal B, Golding JF, Gresty M, Glasauer S, Bronstein AM. Vestibular perception following acute unilateral vestibular lesions. PLOSone. 2013;8 doi: 10.1371/journal.pone.0061862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE. The neural encoding of self-motion. Curr Opin Neurobiol. 2011;21:587–595. doi: 10.1016/j.conb.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Engelken EJ, Stevens KW. A new approach to the analysis of nystagmus: an application for order-statistic filters. Aviat Space Environ Med. 1990;61:859–864. [PubMed] [Google Scholar]
- Furman JM, Sparto PJ, Soso M, Marcus D. Vestibular function in migraine-related dizziness: a pilot study. J Vestib Res. 2005;5:327–332. [PubMed] [Google Scholar]
- Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706–715. doi: 10.1016/S1474-4422(13)70107-8. [DOI] [PubMed] [Google Scholar]
- Golding JF. Motion sickness susceptibility questionnaire revised and its relationship to other forms of sickness. Brain Res Bull. 1998;47:507–516. doi: 10.1016/S0361-9230(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders. Cephalalgia. 2004;24:60–63. doi: 10.1111/j.1468-2982.2004.00641.x. [DOI] [PubMed] [Google Scholar]
- Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain. 1984;107:1123–1142. doi: 10.1093/brain/107.4.1123. [DOI] [PubMed] [Google Scholar]
- Lempert T, Olesen J, Furman J, Waterson J, Seemungal B, Carey J, Biddorff A, Versino M, Evers S, Newman-Toker D. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22:167–172. doi: 10.3233/VES-2012-0453. [DOI] [PubMed] [Google Scholar]
- Lewis RF, Haburcakova C, Merfeld DM. Roll tilt psychophysics in rhesus monkeys during vestibular and visual stimulation. J Neurophysiol. 2008;100:140–153. doi: 10.1152/jn.01012.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF, Priesol A, Nicoucar K, Lim K, Merfeld DM. Abnormal motion perception in vestibular migraine. Laryngoscope. 2011;121:1124–1125. doi: 10.1002/lary.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF, Priesol A, Nicoucar K, Lim K, Merfeld DM. Dynamic tilt thresholds are reduced in vestibular migraine. J Vestib Res. 2011;21:323–330. doi: 10.3233/VES-2011-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, May PJ, Dickman JD, Angelaki DE. Vestibular signals in primate thalamus: properties and origins. J Neurosci. 2007;27:13590–13602. doi: 10.1523/JNEUROSCI.3931-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld DM, Zupan LH, Gifford CA. Neural processing of gravito-inertial cues in humans. II. Influence of semicircular canals during eccentric rotation. J Neurophysiol. 2001;85:1648–1660. doi: 10.1152/jn.2001.85.4.1648. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during translation and tilt. J Neurophysiol. 2005;94:186–198. doi: 10.1152/jn.00904.2004. [DOI] [PubMed] [Google Scholar]
- Neuhauser H, Leopold D, vonBrevern M, Arnold G, Lempert T. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56:436–441. doi: 10.1212/WNL.56.4.436. [DOI] [PubMed] [Google Scholar]
- Neuhauser HK, Radtke A, von Breven M, Feldmann M, Lezius F, Ziese LT. Migrainous vertigo: prevelance and impact on quality of life. Neurology. 2006;67:1028–1033. doi: 10.1212/01.wnl.0000237539.09942.06. [DOI] [PubMed] [Google Scholar]
- Park S, Gianna-Poulin C, Black FO, Woods S, Merfeld DM. Roll rotation cues influence roll tilt perception assayed using a somatosensory technique. J Neurophysiol. 2006;96:486–489. doi: 10.1152/jn.01163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Marcelli V, Esposito F, Corvino V, Marcuccio L, Giannone A, Conforti R, Marciano E, Tedeschi G, Tessitore A. Abnormal thalamic function in patients with vestibular migraine. Neurology. 2014;82:2120–2126. doi: 10.1212/WNL.0000000000000496. [DOI] [PubMed] [Google Scholar]
- Shaikh AC, Palla A, Marti S, Olasagasti I, Optican LM, Zee DS, Straumann D. Role of cerebellum in motion perception and vestibulo-ocular reflex—similarities and disparities. Cerebellum. 2013;12:97–107. doi: 10.1007/s12311-012-0401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleski A, Bogle J, Starling A, Zapala DA, Davis L, Wester M, Cevette M. Vestibular evoked myogenic potentials in patients with vestibular migraine. Otol Neurotol. 2015;36:295–302. doi: 10.1097/MAO.0000000000000665. [DOI] [PubMed] [Google Scholar]