Abstract
Although purinergic receptor activity has lately been associated with epilepsy, little is known about the exact role of purines in epileptogenesis. We have used a rat model of temporal lobe epilepsy induced by pilocarpine to study the dynamics of purine metabolism in the hippocampus during different times of status epilepticus (SE) and the chronic phase. Concentrations of adenosine 5′-triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), and adenosine in normal and epileptic rat hippocampus were determined by microdialysis in combination with high-performance liquid chromatography (HPLC). Extracellular ATP concentrations did not vary along 4 h of SE onset. However, AMP concentration was elevated during the second hour, whereas ADP and adenosine concentrations augmented during the third and fourth hour following SE. During chronic phase, extracellular ATP, ADP, AMP, and adenosine concentrations decreased, although these levels again increased significantly during spontaneous seizures. These results suggest that the increased turnover of ATP during the acute period is a compensatory mechanism able to reduce the excitatory role of ATP. Increased adenosine levels following 4 h of SE may contribute to block seizures. On the other hand, the reduction of purine levels in the hippocampus of chronic epileptic rats may result from metabolic changes and be part of the mechanisms involved in the onset of spontaneous seizures. This work provides further insights into purinergic signaling during establishment and chronic phase of epilepsy.
Keywords: ATP metabolism, Adenosine, Microdialysis, Temporal lobe epilepsy, HPLC
Introduction
Temporal lobe epilepsy (TLE) is among the most frequent types of drug-resistant epilepsy [1]. TLE is accompanied by spontaneous recurrent seizures originating from temporal lobe foci, and learning and memory impairments [2, 3]. This disease is preferentially associated with hippocampal sclerosis, consisting by significant neurodegeneration in the dentate hilus, and CA1 and CA3 subregions [4]. However, the etiology of hippocampal sclerosis and the mechanism of genesis of epilepsy are not completely understood.
The model of TLE induced by systemic administration of pilocarpine in rats has been largely used, as it reproduces the main pathologic characteristics observed in humans [5]. In response to pilocarpine application (360 mg/kg, i.p.), rats exhibit uninterrupted seizures in an acute period lasting 15–18 h. Then, animals show normal behavior and electroencephalographic recording patterns for a period of approximately 14 days, also denominated latent period. This period terminates with the appearance of spontaneous epileptic seizures initiating a chronic phase maintaining for the remaining animal’s lifetime [6].
Various studies using experimental models have suggested that variations in intracellular calcium concentration ([Ca2+]i) are of special importance in epileptogenic processes. Elevated [Ca2+]i levels cause neuronal hyperexcitability, thereby triggering various excitotoxic and degenerative cascades in addition to affecting gene expression, neurogenesis, and maintaining the epileptic state [7, 8]. A previous study by our group suggests the participation of adenosine 5′-triphosphate (ATP) in changes of [Ca2+]i in the hippocampus of epileptic rats, probably by activation of P2X7 receptors [9]. An increase in P2X7 receptor expression was observed in the hippocampus of TLE rats, primarily in glial cells and glutamatergic nerve terminals [10], suggesting participation of this receptor in inflammatory response and excitotoxic processes during epileptogenesis.
The functional roles of extracellular ATP released from neurons and non-neuronal cells are regulated by its breakdown to adenosine [11, 12]. Thus, the extracellular concentration of ATP measured in tissue perfusion samples reflects the rate of release and the breakdown of ATP, which are determined by the activity of ecto-nucleotidases like ecto-ATP diphosphohydrolases (e-NTPDases) or ecto-apyrases and ecto-5′-nucleotidase. Members of the apyrase family are surface-located enzymes capable of hydrolyzing nucleotides (ATP, adenosine diphosphate (ADP), and adenosine monophosphate (AMP)), while ecto-5′-nucleotidase hydrolyzes AMP to form adenosine [12, 13]. Ectoenzymes act to regulate synaptic activity, controlling levels of antagonistic-acting ATP and adenosine in both physiological and pathophysiological conditions, including epilepsy [12, 14].
In this study, we used rats subjected to the TLE model induced by pilocarpine to determine extracellular levels of ATP, ADP, AMP, and adenosine in hippocampal perfusates during status epilepticus and in the chronic phase.
Experimental procedures
Pilocarpine model
The rats housed in groups of five had free access to food and water and were kept under a continuous 12-h/12-h light/dark cycle.
Animal protocols were conducted in accordance with national and international legislation (Guidelines of the Brazilian College of Animal Experimentation, COBEA; NIH Guide for Care and Use of Laboratory Animals) and with the approval of the Ethical Committee of the University (Protocol. 0636/04). All efforts were undertaken to minimize the number of animals used and their suffering.
Male Wistar rats weighing about 250 g were injected with N-methyl-scopolamine (1 mg/kg, sc, Sigma) followed 30 min later by pilocarpine (360 mg/kg, ip. Merck, USA). N-methyl-scopolamine was used to prevent peripheral effects of pilocarpine. The pilocarpine-induced SE is characterized by behavior and electrographic changes as previously described by Turski et al. [5] and Cavalheiro [6]. Briefly, behavioral changes began about 10 min after pilocarpine injection, where animals were motionless with salivation, blinking eyes, twitching of vibrissae, orofacial movements, and yawning. Following 30 min of pilocarpine injection, discontinuous seizures were observed lasting up to 90–150 min. Right after, limbic motor seizures with intense salivation, rearing, upper extremity clonus, and falls occurred every 5–15 min, reaching maximal frequency within 1–2 h. SE remitted 5–6 h after pilocarpine administration. After a latent period (seizure-free period) of 14 ± 3 days (ranging from 4–44 days), all animals were placed in transparent acrylic box, one per box, and monitored by video for 24 h/day to register spontaneous and recurrent seizures. Spontaneous seizures were characterized by staring and mouth clonus, automatisms, monolateral forelimb clonus, and bilateral forelimb clonus, followed by rearing and falling and tonic-clonic seizure. The spontaneous seizures lasted for lifetime of the animals [5, 6].
Microdialysis and HPLC assay of 1,N6-etheno-purines
Our microdialysis protocol was adapted based on previous method described by Ballarin et al. (1991) [15]. Briefly, the animals were anesthetized by intraperitoneal injection with a cocktail containing 67 and 13 mg/kg ketamine and xylazine, respectively, and immobilized in a stereotaxic device. A cannula was implanted in the dorsal hippocampus in agreement with the stereotaxic coordinates of the Paxinos’ atlas (anterior-posterior = −5.3 of Bregma; lateral-lateral = 4.0, and depth = 2.0). Twenty-four hours after the surgery, a catheter for microdialysis (CMA11, Stockholm, Sweden; CMA 11 Microdialysis Probe with membrane length 2 mm and cut-off of 6000 Da) was inserted into the cannula and then a polyethylene catheter (∼90 cm of length) was connected to a 2.5-ml syringe (Hamilton, Reno, NV) filled up with sodium lactate Ringer solution. Perfusion was initiated by using a microinfusion pump with a flow rate fixed at 1.5 μl/min following a 180-min interval necessary for establishment of local stabilization of neurotransmission.
Endogenous purines (e.g., ATP, ADP, AMP, and adenosine) in superfusates were quantified after derivatization to 1,N6-etheno-derivatives to increase detection sensitivity as described previously by Levitt et al. (1984) [16]. Samples of 50 μl volume each were fractionated in time intervals of 32 min, immediately derivatized with 2-chloro-acetaldehyde and analyzed by high-performance liquid chromatography (HPLC) [16]. For derivatization, 22.3 μl of 0.1 M citrate phosphate buffer and 2.5 μl of 2-chloro-acetaldehyde were added to each 50 μl of microdialysis sample, and the mixture was heated for 40 min at 80 °C. The obtained 1,N6-etheno-purines were separated by a reversed-phase HPLC system (Shimadzu Scientific Instruments, Columbia, MD) equipped with an analytical Chromolith C18 column (Merck, Darmstadt, Germany). Derivatized samples were injected into the HPLC system followed by isocratic elution of the column for 35 min using 83 % eluent A (0.1 M citrate phosphate buffer, pH 6.0) and 17 % eluent B (eluent A + 25 % methanol).
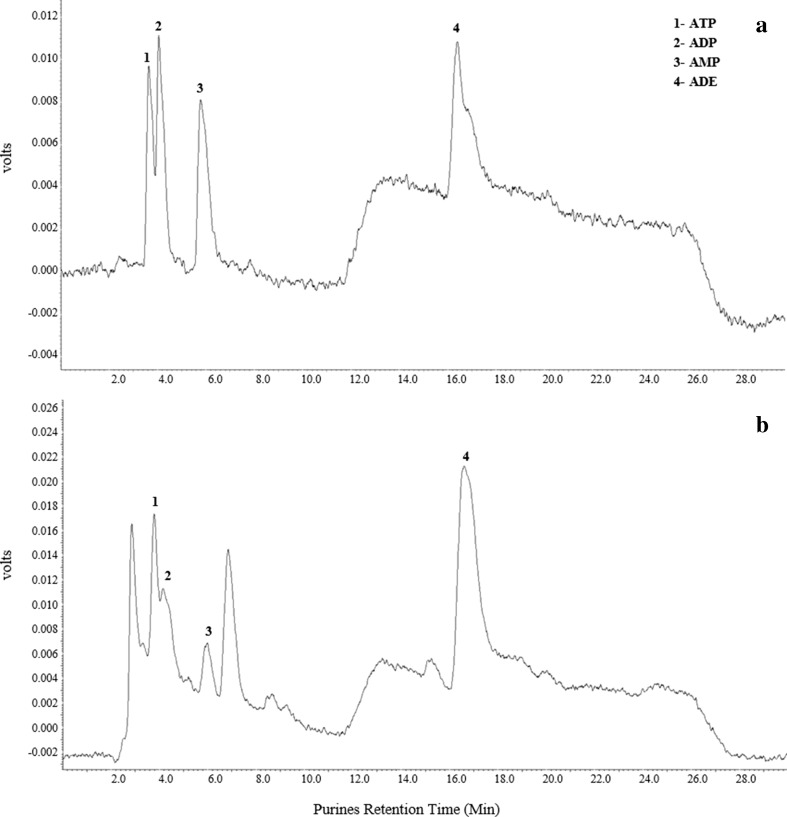
A fluorescence detector (RF-10 AXL, Shimadzu) with excitation and emission wavelengths of 230 and 420 nm, respectively, was used to detect 1,N6-etheno-purine elution from the column. Chromatograms were stored onto a PC and analyzed by using the Software Class VP, version 2.0. For calibration curves, known concentrations of ATP, ADP, AMP, and adenosine were derivatized as described to obtain 1,N6-etheno-purines, injected into the HPLC system, and analyzed (see a standard chromatogram in Fig. 1). Compound peak form samples were identified by the retention times and quantified by comparison of the peak areas of the samples with those of authentic standards in a 50 μl injection volume.
Fig. 1.
HPLC separation of a standard mixture of 1,N 6-etheno-purines (a) and of a hippocampal sample from chronic rat (b). Obtained peaks and their retention times correspond to: (1) ATP at 3.7 min, (2) ADP at 4.1 min, (3) AMP at 5.9 min, and (4) ADE (adenosine) at 16.6 min
Groups of study
After pilocarpine injection, two groups were obtained to study purine metabolism (n = 6/group): status epilepticus group (acute period) and chronic group. Microdialysates of purinergic metabolites were taken at 30 min, 1, 2, 3, and 4 h after pilocarpine administration. Three samples were collected for the determination of basal average concentrations of ε-purines, and another sample was taken 30 min after N-methyl-scopolamine (NMS) injection.
The chronic group consisted of animals (n = 6/group) studied for 90 days after pilocarpine injection. Each animal suffered from at least three severe spontaneous seizures per week. The seizures were considered severe when the animals had tonic-clonic seizures, on the stage 5 of Racine scale [17]. The concentrations of purines were obtained as mean values of the first three microdialysis samples collected from epileptic rats. Animals were subjected to microdialysis for 360 min, following the stabilization period of 180 min. Samples of 50 μl volume each were fractionated in time intervals of 32 min, independently from the onset of the seizure period. During the microdialysis procedure, three rats had one episode of spontaneous seizures with about 15–20-s duration and their samples were also used to quantify purines during the ictal period.
The control group consisted of six animals injected (i.p.) with 0.9 % NaCl solution instead of pilocarpine. Purines were quantified using identical conditions as described for the chronic group.
Histological staining of hippocampal slices
Histological staining was done in order to verify the correct position of microdialysis probes. Animals were sacrificed following each microdialysis experiment. Brains were removed and immediately frozen, and slices of 40 μm each were obtained by using a cryostat. The slices were transferred to gelatinized slides and stained with cresyl violet.
Statistical analyses
One way ANOVA followed by Tukey’s post hoc analysis was employed for comparison of variations in extracellular purine concentrations for the studied time points (basal levels, N-methyl-scopolamine, 30 min, 1, 2, 3, and 4 h after pilocarpine injection) in the hippocampi of rats with SE. The Student t test was employed for comparison of purine concentrations between chronic and control groups. Results were considered significant for p values <0.05.
Results
A rat model of TLE induced by pilocarpine was used to study alterations in the dynamics of extracellular ATP metabolism during acute and chronic phases of the disease. Microdialysis and HPLC were used to quantify 1,N6-etheno-purine (ATP, ADP, AMP, and adenosine) concentrations. The correct position of the microdialysis probe was verified in all animals.
Purine metabolism during acute phase of the epilepsy model
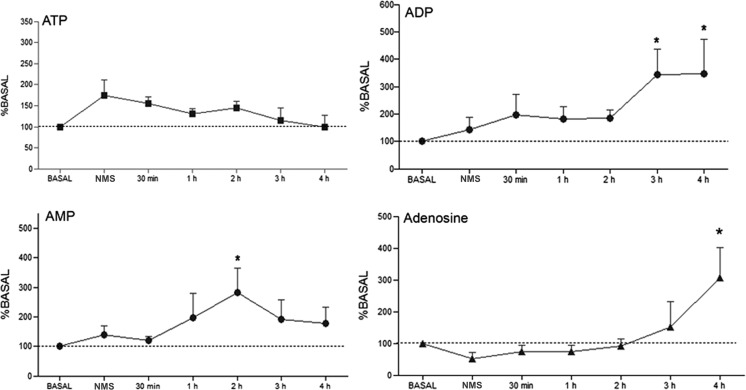
Behavioral manifestations in rats following pilocarpine injection were identical to those previously described [5]. Samples from hippocampal microdialysis collected for 4 h following pilocarpine-induced SE showed significant alterations in concentrations of ATP metabolites (Fig. 2). ADP and AMP concentrations increased to 250 % (p < 0.05 %) and 183 % (p < 0.05 %) following 3 and 4 h of onset of brain insult, whereas adenosine concentration went up to 208 % (p < 0.05 %) when compared to concentrations of purines in basal condition (mean values ± SE: AMP, 8.7 ± 3.6 nM; ADP, 6.0 ± 1.7 nM; and adenosine, 7.0 ± 1.6 nM, respectively). However, no significant difference was observed in extracellular ATP concentrations throughout the experiments (p > 0.05).
Fig. 2.
Extracellular concentrations of 1,N 6-etheno-purines (ATP, ADP, AMP, and adenosine) in hippocampal formation of rats studied at different times: basal, 30 min following N-methyl-scopolamine (NMS) injection, and 30 min, 1, 2, 3, and 4 h after pilocarpine administration. Purine concentrations were determined by HPLC analysis of samples collected by microdialysis. Data are plotted as percentages of basal values. *p < 0.05, determined by ANOVA test, followed by Tukey’s post hoc analysis
Purine metabolism during chronic phase of the epilepsy model
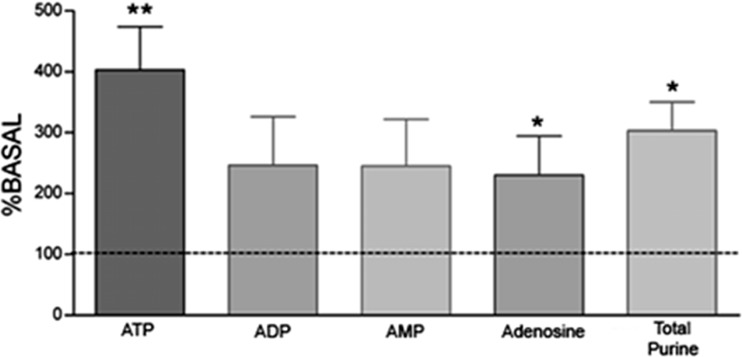
Purine concentrations in the hippocampus from chronic rats were determined by analyzing samples taken 90 days after SE onset. As a general observation, concentrations of ATP and its metabolites ADP, AMP, and adenosine reduced significantly in epileptic rats when compared to control animals (Table 1). However, during microdialysis sample collection, three rats presented spontaneous seizures and purine contents were determined in samples collected at that time. A significant increase in ATP and adenosine concentrations by 300 % (p < 0.009) and 230 % (p < 0.04), respectively, was observed in animals of the chronic group, while elevations of ADP and AMP concentrations were not significant compared to basal levels (Fig. 3) (p > 0.05).
Table 1.
Levels of 1,N 6-etheno-purines in hippocampal dialysates during chronic period compared to basal values of control rats
| Purines | Control (nM) N = 6 |
Chronic (nM) N = 6 |
∆% |
|---|---|---|---|
| ATP | 58.2 ± 11.4 | 22.4 ± 7.0* | −61.5 |
| ADP | 37.0 ± 7.5 | 8.0 ± 0.7** | −58.0 |
| AMP | 19.0 ± 5.7 | 5.2 ± 1.2* | −72.0 |
| ADE | 16.0 ± 4.5 | 2.6 ± 0.3* | −83.0 |
| Purines | 113.0 ± 19 | 39.0 ± 7.7* | −66.0 |
| (ATP + AMP + ADP + ADE) |
Values are means ± SE
Student’s t test, **p < 0.007; *p < 0.03
Fig. 3.
Extracellular concentrations of ATP, ADP, AMP, and adenosine and total purine content in hippocampal formation of epileptic rat during spontaneous seizures. Data are plotted as percentages compared to basal values. The shown values are mean values ± SE of data obtained from three animals each. **p < 0.008; *p < 0.04 as determined by paired Student’s t test
Discussion
This study contributes to a new understanding of the role of ATP metabolism in the TLE. An increase in AMP concentration was detected in the second hour whereas ADP and adenosine concentrations were elevated during the third and fourth hour after pilocarpine administration onset, respectively. In contrast to rising levels of ADP, AMP, and adenosine, ATP concentrations did not significantly change during 4 h following pilocarpine injection when compared to basal values. Increased levels of purine metabolites without changes in ATP concentration suggest that the released ATP is rapidly metabolized in the extracellular space. Augmented expression and/or activity of e-NTPDases preferring ATP over ADP as substrate has been shown [18]. Elevated e-NTPDase 1 and ecto-5′-nucleotidase expression contributes to pathological changes in the hippocampus after transient global cerebral ischemia in rats [19, 20] and after pilocarpine-induced epilepsy [21, 22].
In a previous study using hippocampal synaptosomes of rats, a significant increase in ATP and ADP hydrolysis by ecto-ATP diphosphohydrolase activities occurred 48–52 h after SE induced by pilocarpine [22]. Hydrolysis rates reached maximum values on days 7–9, decreasing gradually, but still remained elevated for 40–50 days when compared to control groups. The profile of 5′-nucleotidase activity was similar, with increased AMP hydrolysis at 48–52 h, 7–9 days, and 45–50 days after SE [22]. Our data, different from those reported by Bonan and co-workers [22], indicate that the hydrolysis of AMP could be detected as early as 2 h after SE onset. ADP levels were increased only 3 h after SE onset suggesting differential activation of ecto-nucleoside triphosphate diphosphohydrolase (e-NTPDase) during SE. Increases in the metabolism of ATP may be part of pathogenic processes manifesting during the initial phase of pilocarpine-induced TLE. This hypothesis is in agreement with previous studies demonstrating liberation of ATP and adenosine following brain insult [23, 24]. Further, it was postulated that ATP and adenosine could mediate antagonistic actions. For instance, ATP could promote depolarization and subsequently neurodegenerative processes, whereas adenosine could exert inhibitory activity [9–11, 21]. The actions exerted by ATP metabolites have not been completely understood, especially those of ADP and AMP. Increased ATP metabolism during SE may indicate increased glutamate release, which can contribute to cell death in this model [25].
ATP released into the extracellular space can be cytotoxic by activating P2X7 receptors whose expression is upregulated in the hippocampus during SE [9, 10]. According to the authors, the increase in P2X7 receptor density was located mainly in glial cells, suggesting that P2X7 receptors participate in inflammatory processes and degenerative effects activated by ATP [10]. This suggests that P2X7 receptor ligands may be useful adjunctive treatments for refractory epilepsy [26].
Increase in extracellular ADP and AMP concentrations observed in the present study can be beneficial for the hippocampus by inhibiting deleterious glutamate neurotransmitter-mediated effects [27–29] and microglial cytokine actions [30, 31]. Recent evidence indicates that AMP also activates A1 receptors and when tested in a model of kainate-induced seizure, AMP prolonged latency of convulsions, but had no effects on seizure severity and mortality. These data are the first to suggest that AMP is an endogenous anticonvulsant acting on A1 receptors [29].
The increase of adenosine concentration, in contrast to the actions of ATP, results in anticonvulsive and neuroprotective actions [21, 32]. These inhibitory effects on excitatory neurotransmission involve A1 adenosine receptor activation and subsequent blockage of excitatory neurotransmitter release including that of glutamate [33]. Direct evidence for adenosine-induced inhibitory effects has been collected in various experimental models in epilepsy [13, 21]. All these data taken together reinforce the hypothesis of neuroprotective roles for adenosine and are in agreement with our results.
In contrast to elevated concentrations of ATP metabolites encountered during acute phase of this model, ATP, ADP, AMP, and adenosine levels decreased during the chronic period when compared to purine concentrations found in the hippocampus of control animals. This reduction in hippocampal extracellular purine concentrations is accompanied by disturbance of cellular metabolism including glucose hypometabolism commonly found in TLE animal models and previously demonstrated by us [34] and also in human patients [35].
Enhanced expression and activity levels of ectonucleotidases including ecto-5′-nucleotidases have been described by us in the hippocampi of chronic rats subjected to pilocarpine as a compensatory inhibitory mechanism [21]. However, in accordance with enhanced ATP breakdown to adenosine as a mechanism of neuroprotection during the interictal period, extracellular concentrations of adenosine are supposed to augment; a postulation that could not be verified in the present study. In agreement with some authors, reduction of extracellular adenosine concentration may result from increased adenosine kinase expression and activity in the epileptic hippocampus facilitating the development of seizures [36].
In view of the fact that extracellular adenosine concentration remains decreased despite the increase in ecto-5′-nucleotidase during the chronic phase, we suppose that nucleotide homeostasis is abnormal in the epileptic hippocampus. In fact, functional neuroimaging studies demonstrated a decrease in glucose utilization (“hypometabolism”) in seizure foci and adjacent brain structures of epilepsy patients [35, 37] and in experimental models [34], which might be explained by the dysfunction of mitochondrial oxidative and/or glycolytic energy metabolism. According to the authors, the hypometabolism in TLE could result from dysfunction in cellular energy metabolism than neuronal cell loss.
During the microdialysis process, some chronic rats exhibited spontaneous seizures and the levels of all purines assayed at that time were increased. A similar increase in adenosine level was also detected after patients’ seizures [38]. Besides, a significant increased activity of soluble nucleotidases has been shown in blood serum of patients in the postictal phase resulting in high level of adenosine that could be actively transported into the brain [39]. Augmented adenosine concentrations found in our study may be related to transitory metabolic changes that occur during seizures or may result from the transport of adenosine from blood into the brain. However, further studies are needed to clarify this hypothesis.
In summary, results from this study suggest that the increased turnover of ATP during the acute period represents a compensatory mechanism in order to reduce the excitatory role of ATP. The increased adenosine level following 4 h of SE may contribute to block seizures. On the other hand, the reduction of purine levels in the hippocampus of chronic epileptic rats may reflect dysfunction in ATP homeostasis resulting from disturbances of astrocytic glycolysis and/or neuronal-astrocytic metabolic coupling. Further studies may be needed for better understanding of this crucial mechanism in the pathophysiology of TLE and subsequent therapeutic strategies to be developed from such knowledge.
Acknowledgments
This work was supported by the Brazilian funds from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Nº. 2006/06502-4 and 2006/61285-9), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES). We thank Hilda Silva Reis for technical assistance.
Compliance with ethical standards
Animal protocols were conducted in accordance with national and international legislation (Guidelines of the Brazilian College of Animal Experimentation, COBEA; NIH Guide for Care and Use of Laboratory Animals) and with the approval of the Ethical Committee of the University (Protocol. 0636/04).
References
- 1.Engel J., Jr Intractable epilepsy: definition and neurobiology. Epilepsia. 2001;42(Suppl 6):3. doi: 10.1046/j.1528-1157.2001.0420s6003.x. [DOI] [PubMed] [Google Scholar]
- 2.Devinsky O. Diagnosis and treatment of temporal lobe epilepsy. Rev Neurol Dis. 2004;1(1):2–9. [PubMed] [Google Scholar]
- 3.Detour J, Schroeder H, Desor D, Nehlig A. A 5-month period of epilepsy impairs spatial memory, decreases anxiety, but spares object recognition in the lithium-pilocarpine model in adult rats. Epilepsia. 2005;46(4):499–508. doi: 10.1111/j.0013-9580.2005.38704.x. [DOI] [PubMed] [Google Scholar]
- 4.Sloviter RS. The neurobiology of temporal lobe epilepsy: too much information, not enough knowledge. C R Biol. 2005;328(2):143–153. doi: 10.1016/j.crvi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Turski WA, Czuczwar SJ, Kleinrok Z, Turski L. Cholinomimetics produce seizures and brain damage in rats. Experientia. 1983;39(12):1408–1411. doi: 10.1007/BF01990130. [DOI] [PubMed] [Google Scholar]
- 6.Cavalheiro EA. The pilocarpine model of epilepsy. Ital J Neurol Sci. 1995;16(1-2):33–37. doi: 10.1007/BF02229072. [DOI] [PubMed] [Google Scholar]
- 7.Delorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol Ther. 2005;105(3):229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raza M, Blair RE, Sombati S, Carter DS, Deshpande LS, DeLorenzo RJ. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc Natl Acad Sci U S A. 2004;101(50):17522–17527. doi: 10.1073/pnas.0408155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vianna EP, Ferreira AT, Naffah-Mazzacoratti MG, Sanabria ER, Funke M, Cavalheiro EA, Fernandes MJ. Evidence that ATP participates in the pathophysiology of pilocarpine-induced temporal lobe epilepsy: fluorimetric, immunohistochemical, and Western blot studies. Epilepsia. 2002;43(Suppl 5):227–229. doi: 10.1046/j.1528-1157.43.s.5.26.x. [DOI] [PubMed] [Google Scholar]
- 10.Dona F, Ulrich H, Persike DS, Conceicao IM, Blini JP, Cavalheiro EA, Fernandes MJ. Alteration of purinergic P2X4 and P2X7 receptor expression in rats with temporal-lobe epilepsy induced by pilocarpine. Epilepsy Res. 2009;83(2-3):157–167. doi: 10.1016/j.eplepsyres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95(2):229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32(1):19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Bruno AN, Oses JP, Amaral O, Coitinho A, Bonan CD, Battastini AM, Sarkis JJ. Changes in nucleotide hydrolysis in rat blood serum induced by pentylenetetrazol-kindling. Brain Res Mol Brain Res. 2003;114(2):140–145. doi: 10.1016/S0169-328X(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 14.Majumder P, Trujillo CA, Lopes CG, Resende RR, Gomes KN, Yuahasi KK, Britto LR, Ulrich H. New insights into purinergic receptor signaling in neuronal differentiation, neuroprotection, and brain disorders. Purinergic Signal. 2007;3(4):317–331. doi: 10.1007/s11302-007-9074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballarin M, Fredholm BB, Ambrosio S, Mahy N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol Scand. 1991;142(1):97–103. doi: 10.1111/j.1748-1716.1991.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 16.Levitt B, Head RJ, Westfall DP. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal Biochem. 1984;137(1):93–100. doi: 10.1016/0003-2697(84)90352-X. [DOI] [PubMed] [Google Scholar]
- 17.Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 18.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2(2):409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun N, Zhu Y, Krieglstein J, Culmsee C, Zimmermann H. Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J Neurosci. 1998;18(13):4891–4900. doi: 10.1523/JNEUROSCI.18-13-04891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chitolina Schetinger MR, Bonan CD, Schierholt RC, Webber A, Arteni N, Emanuelli T, Dias RD, Freitas Sarkis JJ, Netto CA. Nucleotide hydrolysis in rats submitted to global cerebral ischemia: a possible link between preconditioning and adenosine production. J Stroke Cerebrovasc Dis. 1998;7(5):281–286. doi: 10.1016/S1052-3057(98)80044-X. [DOI] [PubMed] [Google Scholar]
- 21.Vianna EP, Ferreira AT, Dona F, Cavalheiro EA, da Silva Fernandes MJ. Modulation of seizures and synaptic plasticity by adenosinergic receptors in an experimental model of temporal lobe epilepsy induced by pilocarpine in rats. Epilepsia. 2005;46(Suppl 5):166–173. doi: 10.1111/j.1528-1167.2005.01027.x. [DOI] [PubMed] [Google Scholar]
- 22.Bonan CD, Walz R, Pereira GS, Worm PV, Battastini AM, Cavalheiro EA, Izquierdo I, Sarkis JJ. Changes in synaptosomal ectonucleotidase activities in two rat models of temporal lobe epilepsy. Epilepsy Res. 2000;39(3):229–238. doi: 10.1016/S0920-1211(00)00095-4. [DOI] [PubMed] [Google Scholar]
- 23.Berman RF, Fredholm BB, Aden U, O’Connor WT. Evidence for increased dorsal hippocampal adenosine release and metabolism during pharmacologically induced seizures in rats. Brain Res. 2000;872(1-2):44–53. doi: 10.1016/S0006-8993(00)02441-0. [DOI] [PubMed] [Google Scholar]
- 24.Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;47(6):442–448. doi: 10.1016/j.neuint.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Smolders I, Khan GM, Manil J, Ebinger G, Michotte Y. NMDA receptor-mediated pilocarpine-induced seizures: characterization in freely moving rats by microdialysis. Br J Pharmacol. 1997;121(6):1171–1179. doi: 10.1038/sj.bjp.0701231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engel T, Jimenez-Pacheco A, Miras-Portugal MT, Diaz-Hernandez M, Henshall DC. P2X7 receptor in epilepsy; role in pathophysiology and potential targeting for seizure control. Int J Physiol Pathophysiol Pharmacol. 2012;4(4):174–187. [PMC free article] [PubMed] [Google Scholar]
- 27.Luthardt J, Borvendeg SJ, Sperlagh B, Poelchen W, Wirkner K, Illes P. P2Y(1) receptor activation inhibits NMDA receptor-channels in layer V pyramidal neurons of the rat prefrontal and parietal cortex. Neurochem Int. 2003;42(2):161–172. doi: 10.1016/S0197-0186(02)00069-4. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci. 2005;25(27):6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muzzi M, Coppi E, Pugliese AM, Chiarugi A. Anticonvulsant effect of AMP by direct activation of adenosine A1 receptor. Exp Neurol. 2013;250:189–193. doi: 10.1016/j.expneurol.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21(6):1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fields RD, Burnstock G. Purinergic signalling in neuron–glia interactions. Nat Rev Neurosci. 2006;7(6):423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavalheiro EA, Calderazzo Filho LS, Bortolotto ZA, Mello L, Turski L. Anticonvulsant role of adenosine. Pol J Pharmacol Pharm. 1987;39(5):537–543. [PubMed] [Google Scholar]
- 33.Dunwiddie TV, Fredholm BB. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J Pharmacol Exp Ther. 1989;249(1):31–37. [PubMed] [Google Scholar]
- 34.Dube C, Boyet S, Marescaux C, Nehlig A. Relationship between neuronal loss and interictal glucose metabolism during the chronic phase of the lithium-pilocarpine model of epilepsy in the immature and adult rat. Exp Neurol. 2001;167(2):227–241. doi: 10.1006/exnr.2000.7561. [DOI] [PubMed] [Google Scholar]
- 35.Theodore WH. Cerebral blood flow and glucose metabolism in human epilepsy. Adv Neurol. 1999;79:873–881. [PubMed] [Google Scholar]
- 36.Gouder N, Scheurer L, Fritschy JM, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci. 2004;24(3):692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casse R, Rowe CC, Newton M, Berlangieri SU, Scott AM. Positron emission tomography and epilepsy. Mol Imaging Biol. 2002;4(5):338–351. doi: 10.1016/S1536-1632(02)00071-9. [DOI] [PubMed] [Google Scholar]
- 38.During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32(5):618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 39.Grosso S, Rocchi R, Margollicci M, Vatti G, Luddi A, Marchi F, Balestri P. Postictal serum nucleotidases activities in patients with epilepsy. Epilepsy Res. 2009;84(1):15–20. doi: 10.1016/j.eplepsyres.2008.11.020. [DOI] [PubMed] [Google Scholar]