Abstract
Glioblastoma multiforme (GBM) is a deadly cancer characterized by a pro-tumoral immune response. T-regulatory (Treg) lymphocytes suppress effector immune cells through cytokine secretion and the adenosinergic system. Ecto-5′-nucleotidase/CD73 plays a crucial role in Treg-mediated immunosuppression in the GBM microenvironment (GME). Methotrexate (MTX) is an immunosuppressive drug that can increase the extracellular concentration of adenosine. In this manuscript, C6 GBM cells were treated with 1.0 μM MTX, and ecto-5′-nucleotidase/CD73 expression and extracellular AMP metabolism were analyzed in vitro. For in vivo studies, rats with implanted GBM were treated for 10 days with MTX-loaded lipid-core nanocapsules (MTX-LNCs, 1 mg/kg/day). The activity of ectonucleotidase and the expression of NTPDase1/CD39 and ecto-5′-nucleotidase/CD73 were measured. The frequencies of T lymphocytes (CD3+CD4+, CD3+CD8+, and CD4+CD25highCD39+) were quantified. In vitro, treatment with MTX increased CD73 expression and activity in C6 cells, which is in agreement with higher levels of extracellular adenosine. In vivo, MTX-LNC treatment increased CD39 expression on CD3+CD8+ lymphocytes. In addition, MTX-LNC treatment up-regulated CD73 expression in tissue isolated from GBM, a finding that is in agreement with the higher activity of this enzyme. More specifically, the treatment increased CD73 expression on CD3+CD4+ and CD3+CD8+ lymphocytes. Treatment with MTX-LNCs decreased the frequencies of T-cytotoxic, T-helper, and Treg lymphocytes in the GME. Although more studies are necessary to better understand the complex cross-talk mediated by supra-physiological concentrations of adenosine in the GME, these studies demonstrate that MTX treatment increases CD73 enzyme expression and AMP hydrolysis, leading to an increase in adenosine production and immunosuppressive capability.
Keywords: Ecto-5′-nucleotidase/CD73, Adenosine, NTPDase1/CD39, Regulatory T lymphocytes, Glioblastoma, Methotrexate
Introduction
Glioblastoma multiforme (GBM) is the most aggressive brain tumor and has an awful prognosis. The average survival of a patient with GBM who is undergoing the current standard chemotherapy is only 14 months [1, 2]. Some reasons for treatment failure and GBM recurrence are multidrug resistance, intrinsic limitations due to the blood brain barrier (BBB), the inherent resistance of glioblastoma cancer stem cells (GSCs) to chemotherapy, and the ability of the cancer cells to escape from a compromised immune system [3, 4]. In fact, although GBM has an extensive inflammatory infiltrate, the immune cells are functionally impaired, resulting in a pro-tumoral immune response [1]. The escape from the immunosurveillance is partially mediated by T-regulatory (Treg) lymphocytes that are present at high levels in the GBM microenvironment (GME). The quantity of Treg cells is associated with reduced survival and increased recurrence in GBM patients [4].
Treg cells may suppress anti-tumoral immune cells in a process that is mediated, at least in part, by the supra-physiological concentrations of adenosine that are present in the GME [5]. The metabolism of adenine nucleotides in the extracellular compartment in cancer patients may contribute to the pro-tumoral response, leading to the initiation and development of cancer [6, 7]. The cancer cells and the surrounding cells work in a synchronized way to metabolize ATP to adenosine in the extracellular milieu. For example, ATP and ADP may be hydrolyzed following the overexpression of NTPdase1/CD39 (hereinafter referred to as CD39) in regulatory immune cells, and AMP may be hydrolyzed following the overexpression of ecto-5′-nucleotidase/CD73 (hereinafter referred to as CD73) in cancer cells [5]. Adenosine may in turn modulate the immune cells to form a more suppressive microenvironment in the tumor in a process that is mediated by P1 adenosine receptors (A1, A2A, A2B, and A3), specifically the A2A receptor [8].
Adenosine is an anti-inflammatory nucleoside that suppresses T-effector (Teff) lymphocytes either by directly binding to the A2A receptor on Teff cells or by modulating cytokine secretion by Treg and Teff cells. Treg cells produce suppressive cytokines such as IL-10 and TGF-β that may decrease the proliferation and differentiation of Teff cells and/or lead to Teff cell apoptosis [9–11]. Therefore, the depletion of Treg cells has been evaluated as a means of restoring the anti-cancer immune response. For example, the immunosuppressant cyclophosphamide depletes peripheral Treg cells in cancer patients, restoring the function of T and natural killer (NK) effector cells [12]. The supra-physiological concentrations of adenosine that are present in the cancer microenvironment may lead to cancer cell death by a mechanism that is mediated by the A3 adenosine receptor [13]. In accordance with this observation, the overexpression of CD73 on breast cancer cells has been associated with a good prognosis [14].
Methotrexate (MTX) is a cytostatic and cytotoxic drug that inhibits dihydrofolate reductase and consequently reduces purine and pyrimidine biosynthesis [15]. Furthermore, MTX is an immunosuppressive drug that is extensively used for the treatment of cancer and inflammatory processes [16]. In addition to producing a classical antimetabolite effect, MTX is thought to directly suppress effector immune cells by increasing the adenosine concentration in the extracellular milieu. However, the direct modulation of CD73 expression by MTX has not been reported [17, 18]. MTX has also been shown to increase the intracellular concentration of adenine nucleotides. This in turn would raise the extracellular concentration to maintain the concentration gradient, and thus increase the extracellular production of adenosine by the CD73 enzyme [19]. MTX has been tested in clinical trials for GBM. However, a high dose of this drug is necessary to traverse the BBB and increase event-free survival rates [20]. Therefore, drug delivery systems have been studied to increase the bioavailability of MTX in the brain and consequently enhance its anti-cancer activity [16, 21].
Recently, we have shown that MTX-loaded lipid-core nanocapsules (MTX-LNCs) substantially decreased glioblastoma growth to a greater extent than free MTX in vitro and in vivo. MTX-LNCs induce the apoptosis of GBM cells in a rat glioma model without altering the biochemical markers of toxicity. However, this effect is accompanied by a decrease in peripheral leukocytes [21]. To expand upon this previous study, the present work sought to evaluate the role of MTX in the modulation of the lymphocyte microenvironment in a rat glioblastoma model by interfering with the adenosinergic system by using the nanocapsules to deliver MTX to the GME.
Materials and methods
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), Fungizone®, penicillin/streptomycin, and 0.5 % trypsin/EDTA were obtained from Gibco (USA). Dimethyl sulfoxide (DMSO) was obtained from Sigma-Aldrich (USA). Poly(ε-caprolactone) (PCL; MW = 14,000 g/mol; α,ω-hydroxylated) was supplied by Sigma-Aldrich (Japan). Caprylic/capric triglyceride (CCT) and polysorbate 80 were obtained from Delaware (Brazil). Span 60® (sorbitan monostearate), N,N-dicyclohexylcarbodiimide (DCC) and 4-(N,N-dimethyl)aminopyridine (DMAP) were obtained from Sigma-Aldrich (USA). MTX was supplied by Pharma Nostra (Brazil). Unloaded lipid-core nanocapsules (LNCs) and MTX-loaded LNCs (MTX-LNCs) were prepared and characterized as described previously [21].
Maintenance of the glioblastoma cell line
The C6 rat glioblastoma cell line was obtained from the American Type Culture Collection (ATCC, USA). The cells were grown up to passage 25 and maintained in DMEM containing antibiotics (0.5 U/mL penicillin/streptomycin) and supplemented with 5 % FBS. The cells were kept at a temperature of 37 °C, a minimum relative humidity of 95 %, and an atmosphere of 5 % CO2.
In vitro activity and expression of ecto-5′-nucleotidase/CD73 in the C6 glioblastoma cell line
Ecto-5′-nucleotidase/CD73 activity was assayed as described by Wink et al. [22]. Twenty-four-well plates containing C6 glioblastoma cells that were exposed to 1.0 μM MTX for 24 h were washed three times with phosphate-free incubation medium. The enzymatic reaction was initiated by the addition of 200 μL of incubation medium (2 mM MgCl2, 120 mM NaCl, 5 mM KCl, 10 mM glucose, and 20 mM Hepes buffer, pH 7.4) containing 2 mM AMP at 37 °C. After a 10-min incubation, the reaction was stopped by collecting 150 μL of the incubation medium that was in contact with the cells and transferring it to Eppendorf tubes containing ice-cold trichloroacetic acid (at a final concentration of 5 % w/v). The release of inorganic phosphate was measured using the malachite green method [23]. Control samples were used to determine the non-enzymatic release of Pi. The protein concentration was quantified by the Coomassie blue method using bovine serum albumin as the standard [24]. The specific activity was expressed as nanomole Pi released per minute per milligram of protein. The C6 glioblastoma cells were also analyzed to determine CD73 expression after MTX treatment. The cells were stained with an anti-CD73 antibody using a method that is similar to the one described below for the analysis of lymphocytes, and the stained cells were analyzed by flow cytometry using a FACSCalibur (BD Biosciences, USA). The data were analyzed using the FlowJo software®.
Analysis of AMP metabolism in vitro by high-pressure liquid chromatography
For the determination of AMP hydrolysis by high-pressure liquid chromatography (HPLC), C6 GBM cells were seeded in 24-well plates, grown for 24 h and treated with 1.0 μM MTX for 24 h. After treatment, the cells were washed three times with the AMP incubation medium described above. The reaction was initiated by adding 50 μM AMP in a volume of 300 μL at 37 °C. After a 30- or 60-min incubation, the reaction was stopped by placing 200 μL of the supernatant on ice. The collected supernatants were centrifuged for 30 min at 16,000×g at 4 °C, and 20-μL aliquots were analyzed by reverse-phase HPLC (Shimadzu, Japan) using a C18 column (Ultra C18, 25 cm × 4.6 mm × 5 μm; Restek, USA). The elution was carried out applying a linear gradient from 100 % solvent A (60 mM KH2PO4 and 5 mM tetrabutylammonium chloride, pH 6.0) to 100 % solvent B (solvent A plus 30 % methanol) over a 30-min period (at a flow rate of 1.4 mL/min) according to a method described previously [25]. The amount of purines was measured by measuring the absorption at 254 nm. The retention time of the standards was used as a parameter for identification and quantification. Purine concentrations are expressed as nanomole per milligram of protein.
In vivo glioblastoma model
Glioblastoma implantation was performed as described previously [21, 26]. Briefly, C6 rat glioblastoma cells at approximately 80 % confluence were trypsinized, washed once with DMEM, centrifuged, and resuspended in the same medium. Approximately 106 cells in a volume of 3 μL were injected at a depth of 6.0 mm into the right striatum, using a Hamilton microsyringe coupled with an infusion pump (1 μL/min) (from Bregma coordinates, 0.5 mm posterior and 3.0 mm lateral) of anesthetized adult male Wistar rats (8–9 weeks old, 220–300 g) by intraperitoneal (i.p.) administration of ketamine/xylazine [27].
Animal treatments and tumor resection
Ten days after C6 glioblastoma cell implantation, the animals were randomly divided into three groups: (1) untreated animals (GBM), (2) animals treated with non-drug-loaded LNCs (LNC group), and (3) animals treated with 1 mg/kg/day MTX-LNCs (MTX-LNC group). The formulations were intraperitoneally administered to the animals once a day for 10 consecutive days. After treatment, the rats were decapitated. The entire brain was then removed and the tumor dissociated by collagenase tissue digestion. To assay the purines in the tumor microenvironment, the tumor tissue was collected with a scalpel, transferred to 1.5 mL of collagenase IV and dissociated with the aid of a Pasteur pipette at 5-min intervals at 37 °C until the tissue was homogenized. After two to three rounds of dissociation, the collagenase was inactivated with EDTA in saline (0.05 M, pH 7.4), and the cells were harvested twice by centrifugation in saline at 400×g for 6 min. After the cells were counted, they were immediately used in the experiments.
All of the procedures used in the present study followed the “Principles of Laboratory Animal Care” from the National Institutes of Health (NIH) and were approved by the Ethical Committee of the Universidade Federal do Rio Grande do Sul (Protocol # 26389).
Ex vivo ectonucleotidase activity
The ATPase, ADPase, and AMPase activities in the GBM tissue were measured using a modified version of the method described by Fillippini et al. The reaction medium contained 2 mM CaCl2 (for ATP and ADP) or 2 mM MgCl2 (for AMP), 120 mM NaCl, 5 mM KCl, 60 mM glucose, 1 mM sodium azide, 0.1 % mM albumin, and 20 mM Hepes buffer, pH 7.6 in a final volume of 200 μL. One million of the dissociated cells from the glioblastoma tissue were added to the reaction medium, and the enzymatic reaction was initiated by the addition of ATP, ADP, or AMP at a final concentration of 2 mM. After a 30-min incubation at 37 °C, the reaction was stopped by the addition of 200 μl of 10 % TCA. The samples were chilled on ice, and the amount of Pi that was released was measured using the malachite green method [23]. In order to correct for non-enzymatic hydrolysis, we performed control reactions by adding the cells after the reaction was stopped with TCA. All of the samples were assayed in triplicate.
Surface protein staining
The surface proteins of tumoral lymphocytes were stained with specific antibodies for analysis by flow cytometry. The cells were phenotyped by incubation with staining buffer (1 % FBS plus 0.09 % sodium azide in PBS) containing anti-rat CD73 and CD39 primary antibodies (both from http://ectonucleotidases-ab.com) and a FITC-conjugated anti-rabbit secondary antibody (Invitrogen, USA). The conjugated antibodies were CD3-PE, CD4-APC, CD8-PerCP, and CD25-PE (all from BD Biosciences, USA). Briefly, the cells from the tumor resection were incubated with primary antibodies against CD39 and CD73 for 30 min on ice and then washed twice. After the last wash, the cells were incubated with a secondary antibody specific to the CD39 and CD73 primary antibodies as well as antibodies against the lymphocyte markers for 30 min in the dark. There were four different staining sets: CD39-FITC/CD3-PE/CD8-PerCP/CD4-APC, CD73-FITC/CD3-PE/CD8-PerCP/CD4-APC, CD39-FITC/CD25-PE/CD4-APC, and CD73-FITC/CD25-PE/CD4-APC. A secondary antibody or isotype control was used as a non-specific binding control. At the end of the incubation period, the cells were washed twice and immediately analyzed using the BD Accuri™ flow cytometer and the C6 software (BD Biosciences, USA).
Immunohistochemical staining
The tumor sections were embedded in paraffin and analyzed by immunohistochemical (IHC) staining. To perform the IHC analysis, the sample sections were processed by antigenic Tris/EDTA recuperation at a high temperature and a pH of 9.0, blocked against peroxidase with 3 % methanol and blocked against non-specific antibody binding with 5 % FBS. The samples were then incubated with the rabbit anti-rat CD73 antibody (1:1500; Santa Cruz Biotechnology™) overnight at 4 °C. Next, the tissue sections were incubated with the REVEAL Complement secondary antibody (Spring Bioscience). All of the IHC evaluations were carried out on ten randomly chosen fields per tumor (Nikon Eclipse e800 microscope, ×400).
Statistical analysis
The data were analyzed for statistical significance by one-way analysis of variance (ANOVA) followed by a post hoc test for multiple comparisons (Tukey test) or by Student’s t test using the GraphPad Prism software®. The data are expressed as the mean ± SEM. Differences were considered significant at p < 0.05.
Results
MTX treatment increases the expression and activity of ecto-5′-nucleotidase/CD73 and the production of adenosine in the C6 glioblastoma cell line
In order to evaluate the effect of MTX on ecto-5′-nucleotidase/CD73, we measured the expression and activity of CD73 as well as AMP metabolism after C6 glioblastoma cells were treated with 1.0 μM MTX for 24 h. MTX treatment increased CD73 expression (mean fluorescence intensity, MFI) after 24 h (from 209.9 ± 7.9 to 298.8 ± 24.9, Fig. 1a) when compared to the DMSO control group. In agreement with this finding, MTX treatment increased AMP hydrolysis and the release of inorganic phosphate (from 136.9 ± 3.9 to 166.8 ± 3.9 nmol Pi/min/mg of protein, Fig. 1b) in comparison to the DMSO-treated cells. To investigate whether the increase in CD73 expression and activity is related to adenosine accumulation, AMP metabolism was analyzed by HPLC. MTX treatment increased the amount of adenosine after 30 and 60 min of AMP incubation (from 808.5 ± 14.57 to 1146.0 ± 110.6 nmol/mg of protein, Fig. 1c, and from 715.7 ± 44.8 to 1077.0 ± 88.4 nmol/mg of protein, Fig. 1d, respectively). In accordance with the literature and despite the MTX-induced increase in adenosine production, there was an accumulation of AMP in the extracellular milieu compared to DMSO-treated cells after 30 min of AMP incubation (from 547.5 ± 160.0 to 1302.0 ± 280.0 nmol/mg of protein, Fig. 1c) and 60 min of incubation (from 135.2 ± 50.5 to 474.0 ± 3.3 nmol/mg of protein, Fig. 1d). In regard to the inosine measurements, there was no difference between the groups studied for 30 min, but there was a slight increase after a 60-min incubation period (Fig. 1).
Fig. 1.
MTX treatment increases the expression and activity of CD73 and the production of adenosine in C6 glioblastoma cells in vitro. C6 glioblastoma cells were seeded into plates and treated with 1.0 μM MTX for 24 h. After treatment, CD73 expression (n = 3) was analyzed (a) and AMP hydrolysis was measured by the malachite green method (n = 3) (b) or by HPLC after a 30- (c) or 60-min (n = 2) (d) incubation as described in the “Materials and Methods” section. The values represent the mean ± SEM. Asterisk indicates a significant difference from the DMSO vehicle group (p < 0.05) as determined by Student’s t test
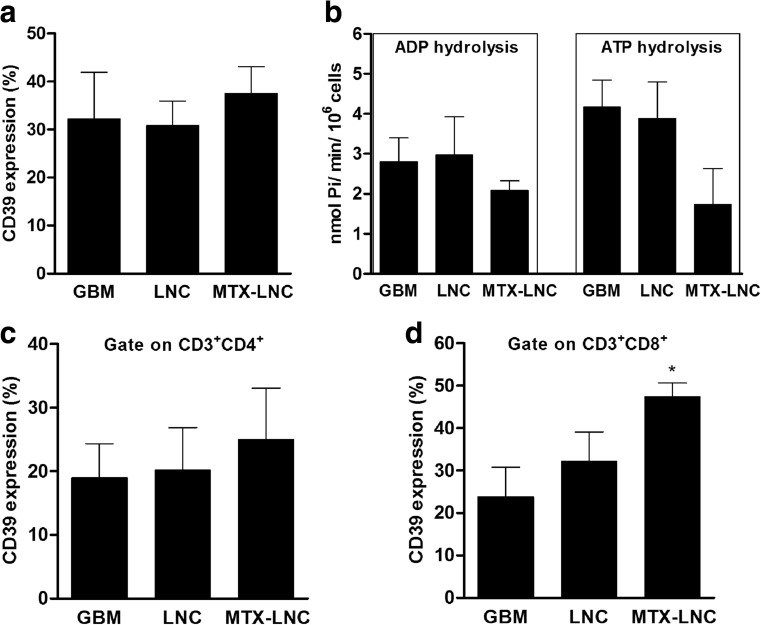
MTX-LNC treatment increases NTPDase1/CD39 expression on CD3+CD8+ lymphocytes in the glioblastoma microenvironment
Rat C6 cells were implanted into the rat brain. Twenty days later (after 10 days of tumor growth and 10 days of treatment with MTX-LNCs), the tumor cells were isolated and analyzed with an anti-CD39-specific antibody. Figure 2a shows that MTX-LNC treatment did not alter the number of cells expressing CD39 protein in the dissociated tumor tissue. In agreement with this finding, MTX treatment did not alter the hydrolysis of ADP or ATP when these cells were incubated with ADP and ATP to measure nucleotidase activity (Fig. 2b). Specifically, MTX treatment did not change CD39 expression on CD3+CD4+ cells (Fig. 2c), but it significantly increased the percentage of CD39 protein on CD3+CD8+ subset of T lymphocytes compared to the GBM control group (from 23.8 ± 7.0 to 47.4 ± 3.3 %, Fig. 2d). In accordance with this data, the MFI of the CD39-stained cells increased in parallel with the percentage of CD39+ cells (data not shown).
Fig. 2.
MTX-LNC treatment increases CD39 expression on the CD3+CD8+ cells of the glioblastoma microenvironment. Rats with implanted glioblastoma were treated with 1.0 mg/kg/day MTX-loaded lipid-core nanocapsules (MTX-LNCs) for 10 days. After tumor dissociation, CD39 expression was determined by immunostaining followed by flow cytometry. CD39 expression was analyzed using a gating strategy that identified the cells of the glioblastoma microenvironment (a). The determination of ADP and ATP hydrolysis in the cells of the glioblastoma microenvironment was performed using the malachite green method (b). CD39 expression was also analyzed in CD3+CD4+ (c) and CD3+CD8+ lymphocytes (d). The values represent the mean ± SEM from at least four animals. Asterisk indicates a significant difference from the GBM group (p < 0.05) as determined by one-way ANOVA followed by Tukey’s test
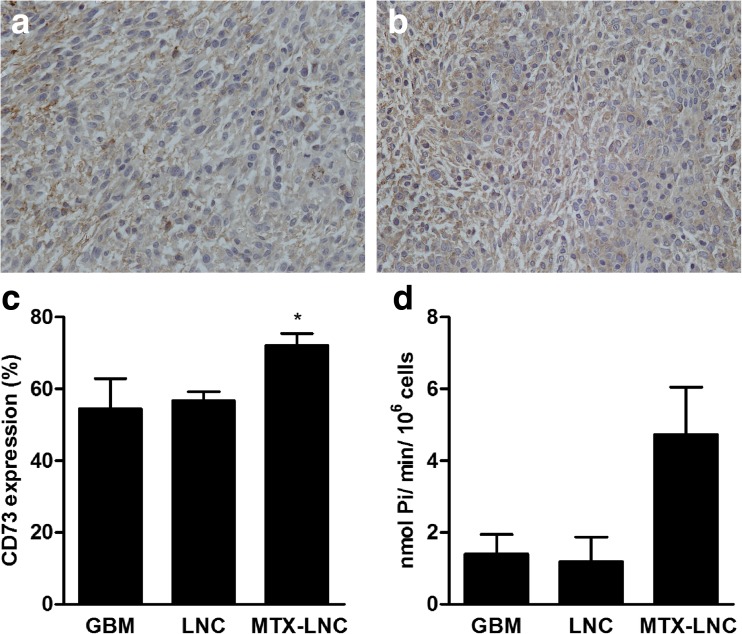
MTX-LNC treatment up-regulates ecto-5′-nucleotidase/CD73 in glioblastoma tissue
Twenty days following glioblastoma implantation (after 10 days of tumor growth and 10 days of MTX-LNC treatment), brain tissues were isolated for IHC. GBM tissue from both untreated animals and animals treated with MTX-LNC had a high level of CD73 expression (Fig. 3a and b, respectively). In order to quantify CD73 expression, we isolated tumor tissue, measured CD73 protein by flow cytometry and measured AMP hydrolysis by enzymatic activity as described in the “Materials and Methods” section. Treatment with MTX-LNCs significantly increased the percentage of cells expressing the CD73 protein (from 56.7 ± 2.5 to 72.1 ± 3.3 %, Fig. 3c) as well as the MFI of the CD73 enzyme in the cells in the tumor microenvironment (data not shown) when compared to vehicle treatment. In agreement with the enzyme expression, MTX-LNC treatment increased CD73 enzymatic activity as determined by the increase in AMP hydrolysis in the dissociated cells of the GME (from 1.2 ± 0.7 to 4.7 ± 1.3, Fig. 3d).
Fig. 3.
MTX-LNC treatment increases the expression and activity of CD73 in the cells of the glioblastoma microenvironment. Rats with implanted glioblastoma were treated with 1.0 mg/kg/day MTX-LNCs for 10 days. After tumor isolation, immunohistochemistry was carried out using a Nikon Eclipse e800 microscope (×400) to visualize CD73 expression in the control (a) and MTX-LNC-treated group (b). Additionally, CD73 expression was determined by immunostaining followed by flow cytometry (c). AMP hydrolysis in the cells of the GBM microenvironment was measured using the malachite green method (d). The values represent the mean ± SEM from at least four animals. Asterisk indicates a significant difference from the LNC vehicle group (p < 0.05) as determined by one-way ANOVA followed by Tukey’s test
MTX-LNC treatment increases ecto-5′-nucleotidase/CD73 expression on CD3+CD4+ and CD3+CD8+ T lymphocytes from the glioblastoma microenvironment
After tumor implantation and treatment, the cells of the tumor tissue were isolated, and the lymphocytes were analyzed for CD73 expression by flow cytometry. CD73 was evaluated on both CD3+CD4+ (Fig. 4a) and CD3+CD8+ (Fig. 4b) subsets. In accordance with the results generated from the whole tissue, the treatment of rats with MTX-LNCs significantly increased the percentage of CD3+CD4+ lymphocytes expressing CD73 on their membrane in comparison to treatment with the control (from 34.8 ± 3.5 to 69.7 ± 10.6 %, Fig. 4a). Likewise, MTX-LNC treatment significantly increased the percentage of CD73-positive CD3+CD8+ cells in comparison to the GBM group (from 36.0 ± 3.0 to 51.9 ± 2.4 %, Fig. 4b). The MFI of the CD73-stained cells followed the same profile for both the CD3+CD4+ and CD3+CD8+ subsets (data not shown).
Fig. 4.
MTX-LNC treatment increases CD73 expression on CD3+CD4+ and CD3+CD8+ lymphocytes. Rats with implanted glioblastoma were treated with 1.0 mg/kg/day MTX-LNCs for 10 days. After tumor dissociation, CD73 expression on CD3+CD4+ lymphocytes (a) and CD3+CD8+ lymphocytes (b) was determined by immunostaining followed by flow cytometry. The values represent the mean ± SEM from at least four animals. Asterisk indicates a significant difference from the control groups (p < 0.05) as determined by one-way ANOVA followed by Tukey’s test
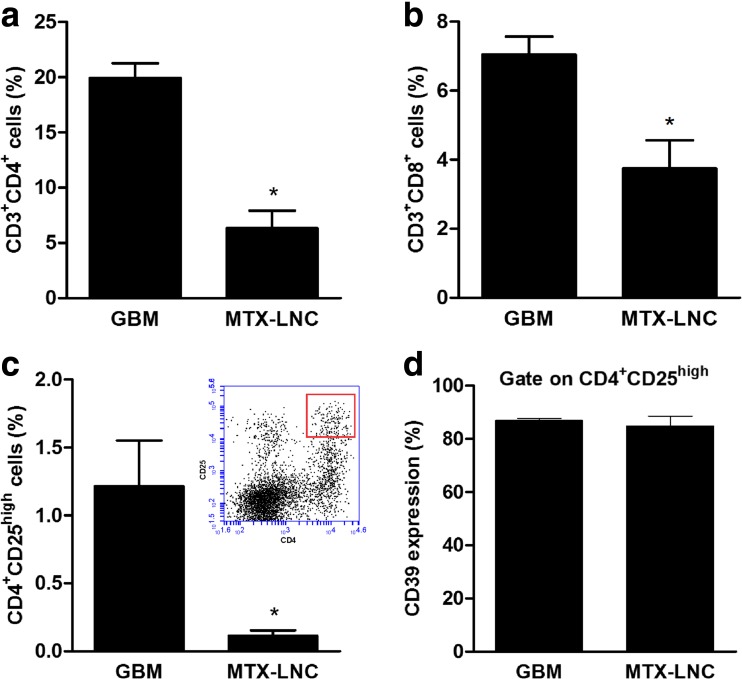
MTX-LNC treatment decreases the T lymphocyte population in the glioblastoma microenvironment
The treatment of rats with MTX-LNCs for 10 days decreased the percentage of both CD3+CD4+ lymphocytes (from 19.9 ± 1.3 to 6.3 ± 1.6 %, Fig. 5a) and CD3+CD8+ lymphocytes (from 7.0 ± 0.5 to 3.7 ± 0.8 %, Fig. 5b) in the GME. The percentage of Treg (CD4+CD25high) cells in the GME was practically abolished in rats subjected to MTX-LNC treatment in comparison to the GBM group (from 1.21 ± 0.33 to 0.11 ± 0.04 %, Fig. 5c). The Treg phenotype was also confirmed by the expression of CD39, a previously described Treg marker [28]. Figure 5d shows that most of the CD4+CD25high lymphocytes were also positive for CD39 protein in both the control group (86.9 ± 0.8 %) and MTX-treated group (84.8 ± 3.7 %).
Fig. 5.
MTX-LNC treatment decreases the percentage of T lymphocytes in the glioblastoma microenvironment. Rats with implanted glioblastoma were treated with 1.0 mg/kg/day MTX-LNCs for 10 days. After tumor dissociation, the frequency of T lymphocytes was determined by immunostaining followed by flow cytometry. The percentages of T-helper (CD3+CD4+) (a), T-cytotoxic (CD3+CD8+) (b), and T-regulatory (CD4+CD25high, represented in the upper picture) (c) lymphocytes were determined. CD39 expression was also analyzed as a third marker of Treg lymphocytes (d). The values represent the mean ± SEM from at least four animals. Asterisk indicates a significant difference from the control group (p < 0.05) as determined by Student’s t test
Discussion
GBM has an infiltrative capability that leads to incomplete tumor resection and subsequent high recurrence levels and poor survival rates [29]. GBM is also characterized by an impaired immune response that results in tumor progression [30]. The purinergic system, which is known to be involved in cancer development, has a direct influence on cancer cells and mediates the modulation of immune cells through P1 adenosine receptors [9, 31]. Although the supra-physiological concentration of adenosine in the tumor microenvironment has been studied extensively, there is no final consensus regarding the pro- or anti-tumoral role of the adenosinergic system. Most studies have shown the immunosuppressive effects of adenosine (pro-tumoral) [7], whereas other studies have shown that adenosine may lead to tumor cell death (anti-tumoral) [13, 31]. In fact, targeting the CD73 enzyme, the main extracellular factor that produces adenosine, has been discussed as an alternative treatment for solid tumors. However, case-by-case studies are necessary to better understand the adenosinergic system in the intricate tumor microenvironment [32].
Previously, we have shown that MTX-loaded lipid-core nanocapsules (MTX-LNCs) reduced glioblastoma tumor size in vivo by increasing apoptosis to a greater extent than free MTX [21]. In the current study, we applied the same MTX-LNC nanotechnology, which presented adequate particle size, zeta potential, and drug content (data not shown), to evaluate the influence of MTX treatment on adenosine formation resulting from the hydrolysis of ATP, ADP, and AMP in the glioblastoma microenvironment (GME). The results showed that with the exception of the CD3+CD8+ subset, none of the studied cells in the GME displayed a change in ADP/ATP hydrolysis or CD39 expression following treatment with MTX. Furthermore, MTX significantly increased CD73 expression in both cancer cells and T lymphocytes. The up-regulation of CD73 raises the adenosine concentration, and thus may modulate the effector and regulatory T lymphocytes in the GME.
MTX has been shown to interfere with the intracellular concentration of adenine nucleotides, which enforces the output and subsequent production of adenosine in the extracellular milieu. MTX increases the release of adenine nucleotides from cells, and this in turn would lead to AMP hydrolysis by the membrane-anchored CD73 enzyme [18, 19]. Here, we showed that CD73 expression and activity is up-regulated in GBM cells following MTX treatment. As shown previously, MTX increases the intracellular concentration of AMP and in doing so may also maintain high extracellular levels of this nucleotide [19]. The increase in AMP combined with the increase in CD73 expression and activity leads to a positive feedback system that controls the accumulation of AMP and adenosine. Prior to this study, there was no evidence to suggest that MTX up-regulates CD73 enzyme expression in GBM cells to produce even more extracellular adenosine. Therefore, the increase in extracellular adenosine could explain, at least in part, the immunosuppressive effect of MTX.
In contrast to CD73, the CD39 enzyme is poorly expressed on GBM cells but is highly expressed on regulatory immune cells [5]. In this manuscript, we showed that MTX did not alter ADP/ATP hydrolysis by measuring enzymatic activity, and we showed that MTX did not affect CD39 expression by measuring the protein content of cells dissociated from the GME. Specifically, MTX treatment did not change CD39 expression on the CD3+CD4+ subset of T lymphocytes, but it did increase the expression of the enzyme on the CD3+CD8+ subset of T lymphocytes. The precise role of the CD39 enzyme in CD3+CD8+ cells is not completely understood. However, previous work has shown that CD8+CD39+ cells have a greater cytotoxic capability than CD8+CD39− cells [33]. More recently, CD39 expression has been described as a phenotypic and functional marker for CD8+ Treg cells [34] as well as classical CD4+ Treg lymphocytes, which also highly express the CD39 enzyme [35].
In the in vivo GBM model, MTX also up-regulated the expression of the CD73 enzyme. Consistently, an increase was detected in whole tissues and on CD3+CD4+ and CD3+CD8+ lymphocytes when rats were treated with MTX-LNCs for 10 days. This treatment reduced the percentage of CD3+CD4+ and CD3+CD8+ subpopulations in the GME. MTX also suppressed the Treg lymphocytes (CD4+CD25highCD39+) that are extremely important in the pro-tumoral immunosuppressive response in the tumor microenvironment. Indeed, the percentage of Treg cells in the GME was almost abolished by MTX-LNC treatment. The immunosuppressive effect of adenosine on Teff cells (CD4+ or CD8+) is mainly mediated by binding to the A2A receptor on the membrane of these cells, which increases cAMP [5, 36]. The immunosuppression triggered by adenosine binding to A2A has been extensively studied in Teff cells [10, 37]. However, the long-term effect of supra-physiological concentrations of adenosine on Treg cells is still unclear.
The reduction in the number and function of Treg cells in cancer patients has been the challenge of immunotherapy [38]. Treg hyporesponsiveness has been described following different chemotherapic treatments and is manly mediated by the differential modulation of cytokines. For example, low doses of sorafenib reduced the function of Treg lymphocytes, allowing for the activation of Teffs in hepatocellular carcinoma [39]. Similarly, curcumin has been shown to reduce Treg function by suppressing IL-2 production and modulating other pericellular cytokines [40]. Moreover, it has been shown that cyclophosphamide, a chemotherapic drug with immunosuppressive properties, induced an extensive reduction in Treg, thus restoring the functions of Teff and NK cells [12].
Although the mechanism by which MTX inhibits purine and pyrimidine synthesis is well established, the immunosuppressive effect of MTX is not fully understood. The long-term supra-physiological concentrations of adenosine that are induced by MTX in the GME do not only explain the reduction in Teff cells but can also account for the decrease in Treg cells. For instance, it is thought that adenosine suppresses NF-kB activation via the A2A receptor since AMPCP (a CD73 inhibitor) and DMPX (an A2A receptor antagonist) reversed the MTX-mediated suppression of NK-kB activation, and adenosine showed a similar effect to that induced by MTX in the suppression of NF-kB activation [41]. The binding of the A2A receptor by adenosine has also been described to reduce NF-kB nuclear translocation, which may suppress T lymphocytes including A2A-expressing Treg cells [42].
In summary, we describe an increase in CD73 expression on GBM cells and T lymphocytes in the GME. The increase in CD39 expression on CD8+ cells and the increase in CD73 expression on glioblastoma cells and CD4+ and CD8+ T lymphocytes that is induced by MTX may increase the adenosine concentration as seen in the in vitro experiments. High levels of adenosine might contribute to the inhibition of the CD3+CD4+ and CD3+CD8+ subsets of T lymphocytes by acting on the A2A receptor. Moreover, treatment with MTX for 10 days also decreased the percentage of Treg cells, which are critical for cancer progression. Previously, we have shown a reduction in tumor size and an induction of apoptosis in in vivo glioblastoma models subjected to the same treatment [21]. Taken together, the results presented here show an extensive up-regulation of CD73 expression and MTX immunosuppressive capability in tumor tissue. However, future studies are necessary to better understand the long-term effect of high levels of adenosine on the modulation of Treg lymphocytes and its implications in cancer progression.
Acknowledgments
This study was supported by the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Proc. 472577/2013-1, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
Compliance with ethical standards
All of the procedures used in the present study followed the “Principles of Laboratory Animal Care” from the National Institutes of Health (NIH) and were approved by the Ethical Committee of the Universidade Federal do Rio Grande do Sul (Protocol # 26389).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Nieto-Sampedro M, Valle-Argos B, Gomez-Nicola D, Fernandez-Mayoralas A, Nieto-Diaz M. Inhibitors of glioma growth that reveal the tumour to the immune system. Clin Med Insights Oncol. 2011;5:265–314. doi: 10.4137/CMO.S7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pointer KB, Clark PA, Zorniak M, Alrfaei BM, Kuo JS. Glioblastoma cancer stem cells: biomarker and therapeutic advances. Neurochem Int. 2014;71:1–7. doi: 10.1016/j.neuint.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sayour EJ, McLendon P, McLendon R, De Leon G, Reynolds R, Kresak J, Sampson JH, Mitchell DA. Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother. 2015;64(4):419–427. doi: 10.1007/s00262-014-1651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu S, Shao QQ, Sun JT, Yang N, Xie Q, Wang DH, Huang QB, Huang B, Wang XY, Li XG, Qu X. Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human malignant gliomas. Neuro Oncol. 2013;15(9):1160–1172. doi: 10.1093/neuonc/not067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar V. Adenosine as an endogenous immunoregulator in cancer pathogenesis: where to go? Purinergic Signal. 2013;9(2):145–165. doi: 10.1007/s11302-012-9349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29(39):5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 8.Cekic C, Linden J. Adenosine A2A receptors intrinsically regulate CD8+ T cells in the tumor microenvironment. Cancer Res. 2014;74(24):7239–7249. doi: 10.1158/0008-5472.CAN-13-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller-Haegele S, Muller L, Whiteside TL. Immunoregulatory activity of adenosine and its role in human cancer progression. Expert Rev Clin Immunol. 2014;10(7):897–914. doi: 10.1586/1744666X.2014.915739. [DOI] [PubMed] [Google Scholar]
- 10.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 2009;30(3):102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Albesiano E, Han JE, Lim M. Mechanisms of local immunoresistance in glioma. Neurosurg Clin N Am. 2010;21(1):17–29. doi: 10.1016/j.nec.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+ CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardi A, Bavaresco L, Wink MR, Jacques-Silva MC, Delgado-Canedo A, Lenz G, Battastini AM. Indomethacin stimulates activity and expression of ecto-5′-nucleotidase/CD73 in glioma cell lines. Eur J Pharmacol. 2007;569(1–2):8–15. doi: 10.1016/j.ejphar.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 14.Supernat A, Markiewicz A, Welnicka-Jaskiewicz M, Seroczynska B, Skokowski J, Sejda A, Szade J, Czapiewski P, Biernat W, Zaczek A. CD73 expression as a potential marker of good prognosis in breast carcinoma. Appl Immunohistochem Mol Morphol. 2012;20(2):103–107. doi: 10.1097/PAI.0b013e3182311d82. [DOI] [PubMed] [Google Scholar]
- 15.McGuire JJ. Anticancer antifolates: current status and future directions. Curr Pharm Des. 2003;9(31):2593–2613. doi: 10.2174/1381612033453712. [DOI] [PubMed] [Google Scholar]
- 16.Abolmaali SS, Tamaddon AM, Dinarvand R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother Pharmacol. 2013;71(5):1115–1130. doi: 10.1007/s00280-012-2062-0. [DOI] [PubMed] [Google Scholar]
- 17.Tohyama N, Tanaka S, Onda K, Sugiyama K, Hirano T. Influence of anticancer agents on cell survival, proliferation, and CD4 + CD25 + Foxp3+ regulatory T cell-frequency in human peripheral-blood mononuclear cells activated by T cell-mitogen. Int Immunopharmacol. 2013;15(1):160–166. doi: 10.1016/j.intimp.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Morabito L, Montesinos MC, Schreibman DM, Balter L, Thompson LF, Resta R, Carlin G, Huie MA, Cronstein BN. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101(2):295–300. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montesinos MC, Takedachi M, Thompson LF, Wilder TF, Fernandez P, Cronstein BN. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: findings in a study of ecto-5′-nucleotidase gene-deficient mice. Arthritis Rheum. 2007;56(5):1440–1445. doi: 10.1002/art.22643. [DOI] [PubMed] [Google Scholar]
- 20.Wolff JE, Kortmann RD, Wolff B, Pietsch T, Peters O, Schmid HJ, Rutkowski S, Warmuth-Metz M, Kramm C. High dose methotrexate for pediatric high grade glioma: results of the HIT-GBM-D pilot study. J Neurooncol. 2011;102(3):433–442. doi: 10.1007/s11060-010-0334-2. [DOI] [PubMed] [Google Scholar]
- 21.Figueiró F, Oliveira CP, Rockenbach L, Mendes FB, Bergamin L, Jandrey EHF, Edelweiss MI, Guterres SS, Pohlmann AR, Battastini AM (2015) Pharmacological improvement and preclinical evaluation of methotrexate-loaded lipid-core nanocapsules in a glioblastoma model. J Biomed Nanotechnol 11(10):1808–1818 [DOI] [PubMed]
- 22.Wink MR, Lenz G, Braganhol E, Tamajusuku AS, Schwartsmann G, Sarkis JJ, Battastini AM. Altered extracellular ATP, ADP and AMP catabolism in glioma cell lines. Cancer Lett. 2003;198(2):211–218. doi: 10.1016/S0304-3835(03)00308-2. [DOI] [PubMed] [Google Scholar]
- 23.Chan KM, Delfert D, Junger KD. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem. 1986;157(2):375–380. doi: 10.1016/0003-2697(86)90640-8. [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Voelter W, Zech K, Arnold P, Ludwig G. Determination of selected pyrimidines, purines and their metabolites in serum and urine by reversed-phase ion-pair chromatography. J Chromatogr. 1980;199:345–354. doi: 10.1016/S0021-9673(01)91386-X. [DOI] [PubMed] [Google Scholar]
- 26.Figueiro F, Bernardi A, Frozza RL, Terroso T, Zanotto-Filho A, Jandrey EH, Moreira JC, Salbego CG, Edelweiss MI, Pohlmann AR, Guterres SS, Battastini AM. Resveratrol-loaded lipid-core nanocapsules treatment reduces in vitro and in vivo glioma growth. J Biomed Nanotechnol. 2013;9(3):516–526. doi: 10.1166/jbn.2013.1547. [DOI] [PubMed] [Google Scholar]
- 27.Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7(9):1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 28.Mandapathil M, Lang S, Gorelik E, Whiteside TL. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods. 2009;346(1–2):55–63. doi: 10.1016/j.jim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prados MD, Byron SA, Tran NL, Phillips JJ, Molinaro AM, Ligon KL, Wen PY, Kuhn JG, Mellinghoff IK, de Groot JF, Colman H, Cloughesy TF, Chang SM, Ryken TC, Tembe WD, Kiefer JA, Berens ME, Craig DW, Carpten JD, Trent JM. Toward precision medicine in glioblastoma: the promise and the challenges. Neuro Oncol. 2015 doi: 10.1093/neuonc/nov031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolle CE, Sengupta S, Lesniak MS. Mechanisms of immune evasion by gliomas. Adv Exp Med Biol. 2012;746:53–76. doi: 10.1007/978-1-4614-3146-6_5. [DOI] [PubMed] [Google Scholar]
- 31.Kim TH, Kim YK, Woo JS. The adenosine A3 receptor agonist Cl-IB-MECA induces cell death through Ca(2)(+)/ROS-dependent down regulation of ERK and Akt in A172 human glioma cells. Neurochem Res. 2012;37(12):2667–2677. doi: 10.1007/s11064-012-0855-5. [DOI] [PubMed] [Google Scholar]
- 32.Corbelini PF, Figueiro F, das Neves GM, Andrade S, Kawano DF, Oliveira Battastini AM, Eifler-Lima VL (2015) Insights into ecto-5′-nucleotidase as a new target for cancer therapy: a medicinal chemistry study. Curr Med Chem [DOI] [PubMed]
- 33.Gouttefangeas C, Mansur I, Schmid M, Dastot H, Gelin C, Mahouy G, Boumsell L, Bensussan A. The CD39 molecule defines distinct cytotoxic subsets within alloactivated human CD8-positive cells. Eur J Immunol. 1992;22(10):2681–2685. doi: 10.1002/eji.1830221031. [DOI] [PubMed] [Google Scholar]
- 34.Parodi A, Battaglia F, Kalli F, Ferrera F, Conteduca G, Tardito S, Stringara S, Ivaldi F, Negrini S, Borgonovo G, Simonato A, Traverso P, Carmignani G, Fenoglio D, Filaci G. CD39 is highly involved in mediating the suppression activity of tumor-infiltrating CD8+ T regulatory lymphocytes. Cancer Immunol Immunother. 2013;62(5):851–862. doi: 10.1007/s00262-013-1392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, Gorelik E, Lang S, Johnson JT, Whiteside TL. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res. 2009;15(20):6348–6357. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su Y, Huang X, Raskovalova T, Zacharia L, Lokshin A, Jackson E, Gorelik E. Cooperation of adenosine and prostaglandin E2 (PGE2) in amplification of cAMP-PKA signaling and immunosuppression. Cancer Immunol Immunother. 2008;57(11):1611–1623. doi: 10.1007/s00262-008-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hausler SF, Montalban del Barrio I, Strohschein J, Anoop Chandran P, Engel JB, Honig A, Ossadnik M, Horn E, Fischer B, Krockenberger M, Heuer S, Seida AA, Junker M, Kneitz H, Kloor D, Klotz KN, Dietl J, Wischhusen J. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother. 2011;60(10):1405–1418. doi: 10.1007/s00262-011-1040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colombo MP, Piconese S. Regulatory T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7(11):880–887. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 39.Cabrera R, Ararat M, Xu Y, Brusko T, Wasserfall C, Atkinson MA, Chang LJ, Liu C, Nelson DR. Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2012;62(4):737–746. doi: 10.1007/s00262-012-1380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao G-J, Lu Z-Q, Tang L-M, Wu Z-S, Wang D-W, Zheng J-Y, Qiu Q-M. Curcumin inhibits suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. Int Immunopharmacol. 2012;14(1):99–106. doi: 10.1016/j.intimp.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 41.Majumdar S, Aggarwal BB. Methotrexate suppresses NF-kappaB activation through inhibition of IkappaBalpha phosphorylation and degradation. J Immunol. 2001;167(5):2911–2920. doi: 10.4049/jimmunol.167.5.2911. [DOI] [PubMed] [Google Scholar]
- 42.Mediero A, Perez-Aso M, Cronstein BN. Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFkappaB nuclear translocation. Br J Pharmacol. 2013;169(6):1372–1388. doi: 10.1111/bph.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]