Abstract
Brain-derived neurotrophic factor (BDNF) and adenosine are widely recognized as neuromodulators of glutamatergic transmission in the adult brain. Most BDNF actions upon excitatory plasticity phenomena are under control of adenosine A2A receptors (A2ARs). Concerning gamma-aminobutyric acid (GABA)-mediated transmission, the available information refers to the control of GABA transporters. We now focused on the influence of BDNF and the interplay with adenosine on phasic GABAergic transmission. To assess this, we evaluated evoked and spontaneous synaptic currents recorded from CA1 pyramidal cells in acute hippocampal slices from adult rat brains (6 to 10 weeks old). BDNF (10–100 ng/mL) increased miniature inhibitory postsynaptic current (mIPSC) frequency, but not amplitude, as well as increased the amplitude of inhibitory postsynaptic currents (IPSCs) evoked by afferent stimulation. The facilitatory action of BDNF upon GABAergic transmission was lost in the presence of a Trk inhibitor (K252a, 200 nM), but not upon p75NTR blockade (anti-p75NTR IgG, 50 μg/mL). Moreover, the facilitatory action of BDNF onto GABAergic transmission was also prevented upon A2AR antagonism (SCH 58261, 50 nM). We conclude that BDNF facilitates GABAergic signaling at the adult hippocampus via a presynaptic mechanism that depends on TrkB and adenosine A2AR activation.
Keywords: BDNF, GABAergic transmission, Adenosine A2A receptors, Hippocampus
Introduction
Adenosine and brain-derived neurotrophic factor (BDNF) are two well-known modulators of synaptic maturation, plasticity, and signaling [1, 2]. Most of the present knowledge about BDNF actions upon synaptic signaling at the hippocampus, as well as its modulation by adenosine, relies on its influence upon glutamatergic synaptic transmission. BDNF postsynaptically enhances NMDA receptor activity in cultured neurons [3, 4] and presynaptically enhances glutamate release in developing neurons [4–8] and isolated hippocampal nerve endings from young [9, 10] and adult [11] rats. Furthermore, BDNF is also known to facilitate long-term plasticity of excitatory synapses, such as long-term potentiation (LTP) [12, 13] and long-term depression (LTD) [14] in an A2A receptor (A2AR)-dependent manner [15, 16]. Information on the ability of A2AR to modulate actions of BDNF on GABAergic transmission is restricted to the actions of the neurotrophin on GAT-1-mediated GABA transport in astrocytes [17] or nerve endings, either when transport is in the inward [18] or outward [19] direction.
The action of BDNF upon synaptic GABAergic transmission at the hippocampus is itself poorly known; the information so far available refers mostly to developing neurons in culture, where BDNF has been shown to cause a postsynaptically mediated fast facilitation of GABAergic currents followed by a prolonged depression [20] or to cause a presynaptic facilitation of GABAergic inputs to glutamatergic neurons [21]. Prolonged applications of BDNF (>24 h) have been shown to facilitate maturation of GABAergic synapses and presynaptically increase GABAergic transmission [22] or to prevent down scaling of GABAergic responses caused by activity deprivation [23]. Changes in modulation of GABAergic currents by BDNF are expected to occur during maturation since BDNF affects K+/Cl− transport in hippocampal neurons [21]. Indeed, in neurons acutely isolated at the postnatal day 6 (P6), BDNF has been shown to cause a postsynaptically mediated facilitation of GABAergic currents, while causing a long-lasting inhibition of GABAergic currents at P12–P18 neurons [24, 25]. On the other hand, highly variable actions of BDNF on GABAergic transmission have been reported for hippocampal slices acutely prepared from 2 to 8 weeks old rats, but data were not discriminated as a function of age [26].
We now evaluated the action of BDNF on synaptic GABAergic transmission at the adult hippocampus as well as its dependence on adenosinergic tuning. We tested the influence of BDNF on evoked and spontaneous inhibitory synaptic currents (inhibitory postsynaptic currents (IPSCs) and miniature inhibitory postsynaptic currents (mIPSCs), respectively) recorded from adult hippocampal pyramidal neurons in acutely isolated slices and assessed the ability of an A2AR antagonist to modify those actions. We report that BDNF facilitates GABAergic signaling at the adult hippocampus via a presynaptic mechanism that depends on TrkB and adenosine A2AR activation.
Materials and methods
Animals
Adult (6 to 10 weeks old) male Wistar rats were used in this study. The animals were kept under standardized conditions of light, temperature, and humidity and had access to food and water ad libitum. All animal procedures were carried out in strict accordance with the ethical recommendations by EU (Directive 210/63/EU), the Portuguese (DL 113/2013) legislation for the protection of animals used for scientific purposes, and the Ethics Committee of the Instituto de Medicina Molecular and of the Faculty of Medicine, University of Lisbon, Lisbon, Portugal, who approved this study. Animals were deeply anesthetized under isoflurane atmosphere before being sacrificed, and all efforts were made to minimize suffering before anesthesia. Deep anesthesia was confirmed by the absence of response to paw pinching while the animals were still breathing.
Hippocampal slice preparation
After decapitation, the brain was rapidly removed from the skull cavity and the hippocampi were dissected in ice-cold dissecting solution containing (mM) the following: sucrose 110; KCl 2.5; CaCl2 0.5; MgCl2 7; NaHCO3 25; NaH2PO4 1.25; and glucose 7, pH 7.4, oxygenated with 95 % O2 and 5 % CO2. Each hippocampus was then transversely sliced (300 μm thick) on a vibratome (VT 1000 S; Leica, Nussloch, Germany) under the same ice-cold dissecting solution. The slices were then incubated at 35 °C for 20 min in artificial cerebrospinal fluid (aCSF) (mM: NaCl 124; KCl 3; NaH2PO4 1.25; NaHCO3 26; MgSO4 1; CaCl2 2; and glucose 10, pH 7.4) before being placed at room temperature in the same solution for at least 1 h before use in patch clamp experiments.
Patch clamp recordings
Individual slices were fixed with a grid in a recording chamber of about 1-mL volume and were continuously superfused by a gravitational system at 2–3 mL/min with aCSF at room temperature. Drugs were added to this superfusion solution and reached the recording chamber within approximately 1 min.
Patch pipettes were made from borosilicate glass capillaries (1.5-mm outer diameter, 0.86 inner diameter, Harvard Apparatus, Holliston, MA, USA) and had a resistance of 4–9 MΩ when filled with an internal solution containing (mM) the following: CsCl 125; NaCl 8; CaCl2 1; EGTA 10; HEPES 10; glucose 10; MgATP 5; NaGTP 0.4; and pH 7.2, adjusted with CsOH (50 wt% in H2O), 280–290 Osm.
Whole-cell recordings were obtained from pyramidal cells located at CA1 stratum pyramidale. The cells were visualized with a microscope (Zeiss Axioskop 2FS, Jena, Germany) equipped with infrared video microscopy and differential interference contrast optics. All recordings were performed in voltage clamp mode (VH = −70 mV) at room temperature (22–24 °C) with either an EPC-7 (List Biologic) or an Axopatch 200B (Molecular Devices) amplifier, under the control of pClamp10 software (Molecular Devices). Before the giga-seal formation, the offset potentials were nulled. Immediately after having whole-cell access, the membrane potential of the neurons was measured in current clamp mode (VH approximately −60 mV). Through all the recordings, the holding current was constantly monitored, and if it varied by more than 20 %, the experiment would be rejected. Data were low-pass filtered using a 3- and 10-kHz three-pole Bessel filter, digitized at 5 Hz, and registered by the Clampex Software version 10.2 (Molecular Devices, Sunnyvale, CA, USA).
Afferent-evoked IPSCs were recorded as described elsewhere [27]. Briefly, every 15 s, a stimuli (1–15 μA) was delivered via monopolar stimulation with a patch-type pipette filled with aCSF and positioned in stratum radiatum, 80–120 μm from the recorded cell. Averages of eight consecutive individual recordings were used for analysis. Recordings were performed using the internal solution previously described plus 1 mM QX-314, a voltage-gated Na+ channel blocker. During all recordings, the aCSF was supplemented with kynurenic acid (Kyn, 1 mM), to block glutamate receptors.
mIPSCs were recorded in aCSF supplemented with tetrodotoxin (TTX, 0.5 μM) and Kyn (1 mM). In some experiments and only where specified, K252a (200 nM) and SCH58261 (100 nM) were also added to the superfused aCSF. When testing the dependency on p75NTR activation for the effect of BDNF, the slices were preincubated for 2 h at room temperature with a blocking antibody anti-p75NTR IgG (REX Ab, 50 μg/mL) [28]. Miniature event analysis was performed using the MiniAnalysis software (Synaptosoft, GA, USA), with the amplitude threshold for event detection being set at 5× the average RMS noise. The rise time was calculated by finding the first data point to the left of the peak that shows 0.5 % of the peak amplitude and subtracting the time at this point from the time at the peak. The decay time was calculated by finding the first data point to the right of the peak that shows 10 % of the peak amplitude and taking a difference between the time at this point and the time at the peak. mIPSC frequency and amplitude were analyzed for 40 s every 2 min in order to obtain their time course variances. Statistical differences were assessed between two different periods: (1) the 10 min prior to BDNF superfusion and (2) the last 10 min in its presence (30 to 40 min after BDNF addition to the bath solution).
Drugs
BDNF was generously supplied by Regeneron Pharmaceuticals (Tarrytown, NY), in a 1.0 mg/mL stock solution in 150 mM NaCl, 10 mM sodium phosphate buffer, and 0.004 % Tween 20; aliquots of this sock solution were kept frozen at −80 °C and diluted in aCSF in the day of the experiment (solvent concentration in the perfusion solution 0.003 % v/v). REX Ab (anti-p75NTR IgG) was a generous gift from Louis Reichardt. Tetrodotoxin (TTX, octahydro-12-(hydroxymethyl)-2-imino-5,9:7,10a-dimethano-10aH-[1, 3] dioxocino[6,5-d] pyrimidine-4,7,10,11,12-pentol, a sodium-channel blocker) was obtained from Ascent Scientific (Bristol, UK). K252a (tyrosine kinase inhibitor) and SCH58261 (2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e] [1, 2, 4] triazolo[1,5-c]pyrimidin-5-amine, selective A2A receptor antagonist), QX-314 chloride (N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium chloride, blocker of voltage-activated Na+ channel), and bicuculline ([R-(R*,S*)]-6-(5,6,7,8-tetrahydro-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl)furo[3,4-e]-1,3-benzodioxol-8(6H)-one, selective GABAAR antagonist) were purchased from Tocris Cookson (Ballwin, MO). Kynurenic acid (4-hydroxyquinoline-2-carboxylic acid, glutamate receptor antagonist) was from Abcam. SCH58261 was prepared in a 5 mM, K252a in a 1 mM, and Bicuculline in a 100-mM stock solution, all in dimethyl sulfoxide (DMSO). TTX (1 mM) and kynurenic acid (100 mM) were prepared in water. Stock solutions were aliquoted and stored at −20 °C until use. Dilutions of these solutions to the final concentration were made freshly before each experiment. The percentage of DMSO in each experiment did not exceed 0.001 % and did not affect neither IPSCs nor mIPSCs [27].
Statistical analysis
Results are expressed as the mean ± SEM of n experiments, where n corresponds to the number of tested cell from different slices. Statistical significance was evaluated by two-tailed Student’s t test, when comparing before and after BDNF perfusion or by performing one-way ANOVA followed by Bonferroni’s post hoc test for comparison between multiple experimental groups. Statistical significance was assumed if p value was inferior to 0.05.
Results
BDNF increases IPSCs in CA1 pyramidal cells
IPSCs evoked by afferent stimulation were recorded to evaluate the influence of BDNF upon GABAAR-mediated responses at the hippocampus of adult rats. All the recordings were performed in voltage clamp mode (VH = −70 mV) in the presence of kynurenic acid (Kyn, 1 mM), to block glutamate receptors [29] and isolate GABAergic currents from interference of fast excitatory transmission [30]. Under such conditions, the recorded currents were completely blocked when applying bicuculline (20 μM), a selective GABAAR antagonist, thus confirming their GABAergic nature (Fig. 1a). In addition, QX-314 (1 mM) was added to the intracellular solution avoiding GABA current contamination with action potentials.
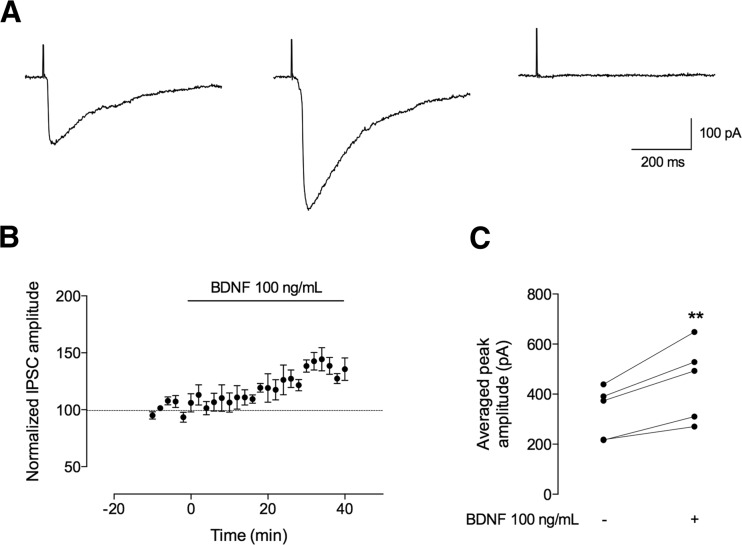
Fig. 1.
BDNF increases the amplitude of evoked IPSCs recorded from CA1 pyramidal neurons. The recordings represented here were performed in CA1 pyramidal cells in whole-cell configuration, and inhibitory postsynaptic currents (IPSCs) were evoked by afferent stimulation in the continuous presence of a glutamate receptor antagonist (kynurenic acid, Kyn, 1 mM). a Representative IPSC recordings in the absence (left panel) and presence (middle panel) of BDNF (100 ng/mL) and in the presence of bicuculline (20 μM) (right panel). b Time course changes, induced by BDNF (100 ng/mL, n = 5), in the peak amplitude of the evoked IPSCs. One hundred percent represents the average peak amplitude of the currents recorded for 10 min prior to BDNF application. BDNF effect was quantified by comparing the peak amplitude from the 10-min period before BDNF application (baseline) to the 30–40 min after BDNF application. c The individual (dots) changes in peak amplitude are shown as individual data obtained in the 10 min in the absence (−) of BDNF and 30–40 min after (+) BDNF administration (n = 5). Values are mean ± SEM. **p < 0.01 (two-tailed paired Student’s t test)
Once achieving a stable baseline of electrically evoked IPSC amplitudes for at least 10 min, BDNF was perfused, remaining in the bath for 40 min. First, we used a moderately high concentration of BDNF (100 ng/mL), since at this concentration, BDNF has been shown to postsynaptically affect GABAergic transmission in acute slices prepared from developing hippocampus taken from preweaning (P12–18) rats (22). As shown in Fig. 1, BDNF (100 ng/mL) induced a progressive increase in the peak amplitude of IPSCs recorded from CA1 pyramidal cells in slices prepared from adult rats. The effect of BDNF on the amplitude of GABAergic currents started 15 min after its perfusion, the maximum increase being observed between the 30 and 40 min of BDNF application (Fig. 1b). The averaged increase in peak amplitude of GABA currents caused by BDNF (100 ng/mL) was 39 ± 4.9 % (n = 5, p < 0.01, as compared with baseline values), being this value calculated from IPSCs recorded 30–40 min after starting perfusion of BDNF (100 ng/mL). These data indicate that BDNF facilitates phasic GABAergic transmission in the adult hippocampal neurons.
BDNF increases frequency, but not amplitude, of mIPSCs
To evaluate whether the facilitatory action of BDNF upon fast GABAergic transmission results from presynaptic or postsynaptic mechanisms, recordings of miniature inhibitory postsynaptic currents (mIPSCs) were performed in the presence of tetrodotoxin (TTX, 0.5 μM) and Kyn (1 mM). Changes in frequency of these events are usually interpreted as changes at presynaptic level, while changes in the amplitude are thought to relate with a postsynaptic modulation.
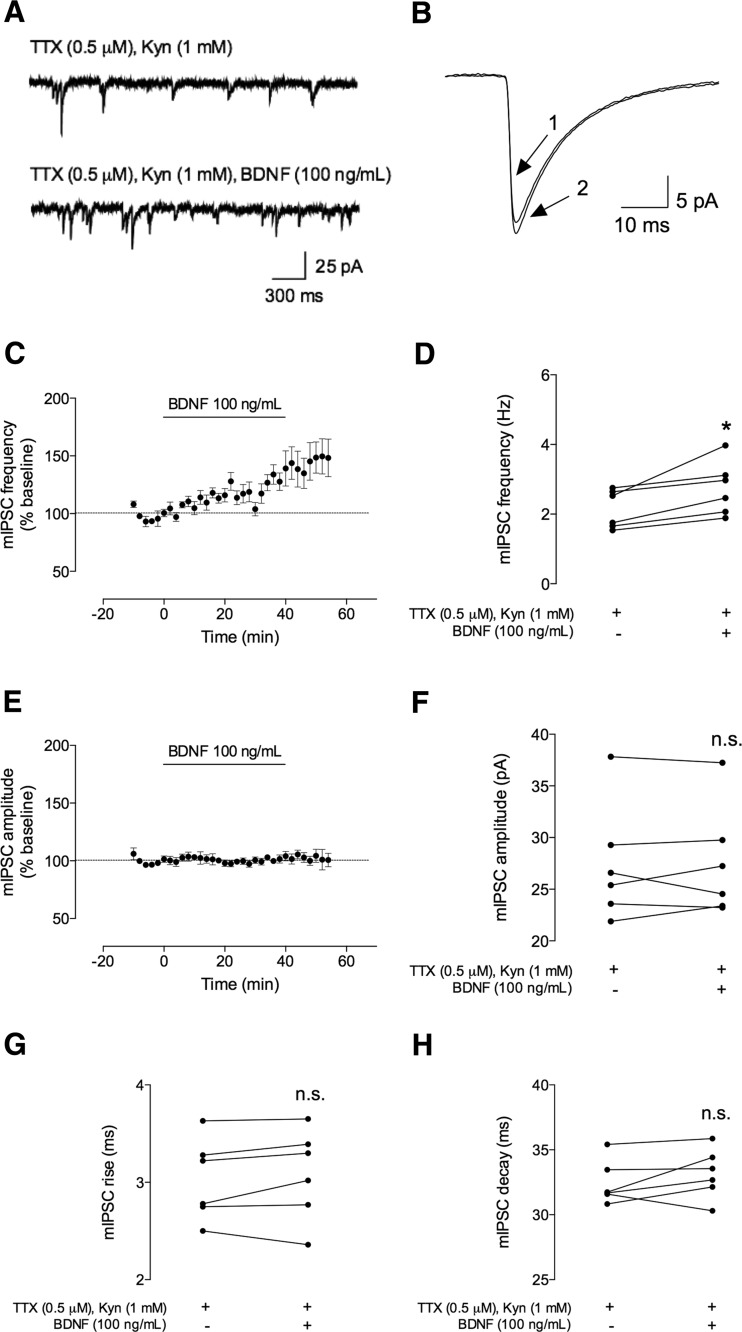
As illustrated in Fig. 2, application of BDNF (100 ng/mL) to the bathing solution consistently increased the frequency of mIPSCs by 28 ± 7.0 % (n = 6, p < 0.05, measured 30–40 min after starting BDNF superfusion, Fig. 2a, c, d). Baseline frequency of mIPSC was 2.1 ± 0.22 Hz and increased to 2.7 ± 0.31 Hz, 30–40 min after BDNF addition. At this concentration of BDNF (100 ng/mL), the increase in mIPSC frequency persisted even after washout. BDNF (100 ng/mL) was virtually devoid of effect in mIPSC amplitude (% change as compared with baseline 1.0 ± 2.3, n = 6, p > 0.05). The averaged absolute amplitude values were 27 ± 2.3 and 27 ± 2.2 pA, before and 30–40 min after BDNF addition, respectively. Additionally, the duration of mIPSCs showed no statistical significance upon BDNF application (Fig. 2g, h). Indeed, mIPSC rising time changed to 1.9 ± 1.9 % of baseline (3.0 ± 0.17 and 3.1 ± 0.19 ms, before and after BDNF application, respectively) and the decay time to 2.2 ± 1.7 % (from 33 ± 0.69 ms before and 33 ± 0.79 ms after BDNF perfusion).
Fig. 2.
BDNF (100 ng/mL) increases the frequency, but not the amplitude, of spontaneous miniature inhibitory postsynaptic currents (mIPSCs). The miniature IPSC (mIPSC) recordings represented here were performed in CA1 pyramidal cells in whole-cell configuration and in the presence of sodium channel blocker (tetrodotoxin (TTX) 0.5 μM) and a glutamate receptor antagonist (Kynurenic acid, Kyn, 1 mM). a Representative tracings of mIPSCs recorded in the absence (upper trace) and presence (lower trace) of BDNF (100 ng/mL). b Representative average tracings of mIPSCs of two superimposed events in the absence (1) and presence (2) of BDNF (100 ng/mL), from the same cell. c, e Time course changes in mIPSC frequency (c) and amplitude (e) induced by application of BDNF (n = 6). One hundred percent represents the average mIPSC frequency or amplitude recorded for 10 min prior to BDNF application. mIPSC frequency and amplitude changes were quantified by comparing the events from the 10-min period before BDNF application (baseline) to the final 10 min in its presence. d, f The averages of absolute values of frequency (d) or amplitude (f) are shown as individual data obtained in the 10 min in the absence (−) of BDNF and 30–40 min after (+) BDNF administration (n = 6). g, h Averages of the absolute values of rise (g) or decay (h) times are shown as individual data obtained in the 10 min in the absence (−) of BDNF and 30–40 min after (+) BDNF administration (n = 6). Values are mean ± SEM. *p < 0.05 and n.s. p > 0.05 (two-tailed paired Student’s t test)
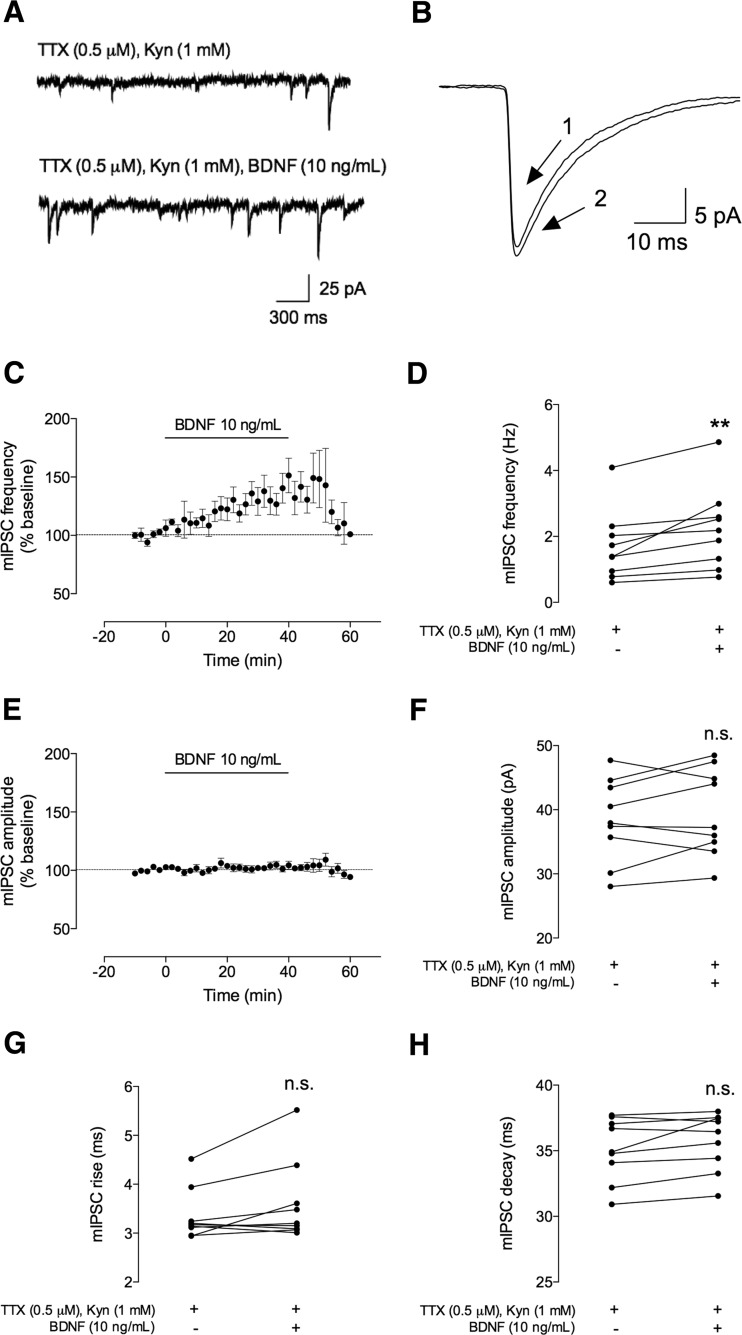
A lower concentration of BDNF (10 ng/mL) also increased mIPSC frequency, without affecting mIPSC amplitude (Fig. 3), the averaged increase in mIPSC frequency being 38 ± 11 % (n = 9, p < 0.01) for the last 10 min of its perfusion. Frequency values increased from 1.7 ± 0.35 Hz (before the perfusion of BDNF) to 2.2 ± 0.41 Hz (30–40 min after BDNF addition). The effect was not statistically different from that obtained with 100 ng/mL BDNF (p > 0.05, one-way ANOVA). However, contrary to what was observed for 100 ng/mL BDNF (Fig. 2c), the effect of 10 ng/mL was fully washed out (Fig. 3c). BDNF (10 ng/mL) had no effect on mIPSC amplitude (% change 3.3 ± 2.7 as compared to the baseline values; 38 ± 2.2 pA before and 40 ± 2.3 pA after BDNF application, Fig. 3e, f) or on mIPSC duration (rising time 6.9 ± 3.4 % of baseline, 3.4 ± 0.17 ms before and 3.6 ± 0.28 ms after BDNF perfusion; decay time 1.8 ± 0.83 % of baseline, 35 ± 0.80 ms before and 36 ± 0.74 ms after BDNF application, Fig. 3g, h).
Fig. 3.
BDNF (10 ng/mL) increases the frequency, but not the amplitude, of spontaneous miniature inhibitory postsynaptic currents (mIPSCs). The mIPSCs were recorded in the same conditions as described for Fig. 2. a The upper panel represents mIPSC tracings recorded in the absence (upper trace) and presence (lower trace) of BDNF (10 ng/mL). b Representative average tracings of mIPSCs of two superimposed events in the absence (1) and presence (2) of BDNF (10 ng/mL), from the same cell. c, e Time course changes in mIPSC frequency (c) and amplitude (e) induced by application of BDNF (n = 9). d, f Averages of the absolute values of frequency (d) or amplitude (f) are shown as individual data points in the absence (−) of BDNF and after (+) BDNF administration (n = 9). g, h Averages of the absolute values of rise (g) or decay (h) times are shown as individual data obtained in the absence (−) of BDNF and after (+) BDNF administration (n = 9). Values are mean ± SEM. **p < 0.01 and n.s. p > 0.05 (two-tailed paired Student’s t test
These data thus suggest that BDNF positively modulates GABAergic transmission via presynaptic changes that lead to increased GABA release.
The BDNF facilitatory effect onto GABAergic transmission is TrkB, but not p75NTR, dependent
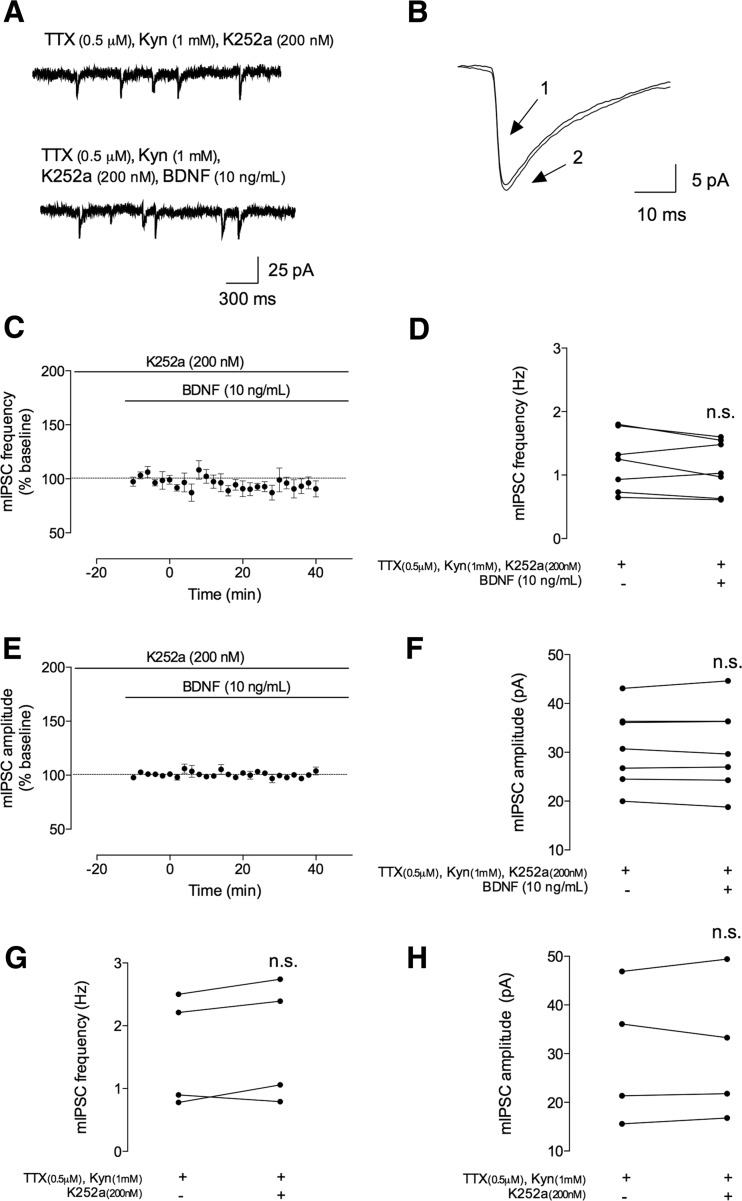
BDNF can activate a high-affinity TrkB receptor and a low-affinity p75NTR receptor. K252a is a tyrosine kinase inhibitor that prevents Trk receptor activation [31]. Thus, to test for the involvement of TrkB receptors on the effect of BDNF, mIPSC recordings in the presence of K252a (200 nM) were performed. To do so, slices were perfused with K252a immediately after going to whole cell and currents allowed to stabilize before applying BDNF in the presence of K252a for at least 20 min. In the presence of K252a, 40 min of incubation of BDNF did not affect either mIPSC frequency or mIPSC amplitude (Fig. 4). Indeed, in the last 10 min of BDNF application, the percentage of change in mIPSC frequency was −7.3 ± 5.3 % as compared to baseline (n = 7, p > 0.05), the absolute values being 1.2 ± 0.18 Hz before and 1.1 ± 0.16 Hz after BDNF application. Similarly, for mIPSC amplitude, the percentage of change was −1.0 ± 1.4 % (n = 7, p > 0.05), with absolute values of 31 ± 3.0 pA before and 31 ± 3.3 pA after BDNF application. Perfusion of K252a (200 nM) alone did not alter neither frequency nor amplitude of mIPSCs (frequency 1.6 ± 0.44 to 1.7 ± 0.48 Hz; amplitude 30 ± 7.1 to 30 ± 7.2 pA, n = 4, p > 0.05, Fig. 4g, h).
Fig. 4.
The facilitatory effect of BDNF on mIPSCs is dependent on TrkB receptor activation. The mIPSCs were recorded in the same conditions as described for Fig. 2. During all the recordings, a tyrosine kinase inhibitor (K252a, 200 nM) was further added to the perfusion. a In the upper panel, a representation of mIPSC tracings recorded in the absence (upper trace) and presence (lower trace) of BDNF (10 ng/mL). b Representative average tracings of mIPSCs of two superimposed events in the absence (1) and presence (2) of BDNF (10 ng/mL), from the same cell. c, e Time course changes in mIPSC frequency (c) and amplitude (e) induced by application of BDNF (n = 7). mIPSC frequency and amplitude changes were quantified by comparing the events before (baseline) and after BDNF application. d, f Averages of the absolute values of frequency (d) or amplitude (f) are shown as individual data in the absence (−) and presence (+) of BDNF (n = 7). g, h Averages of the absolute values of frequency (g) or amplitude (h) are shown as individual data obtained in the absence (−) or presence (+) of K252a (n = 4). Values are mean ± SEM. n.s. p > 0.05 (two-tailed paired Student’s t test
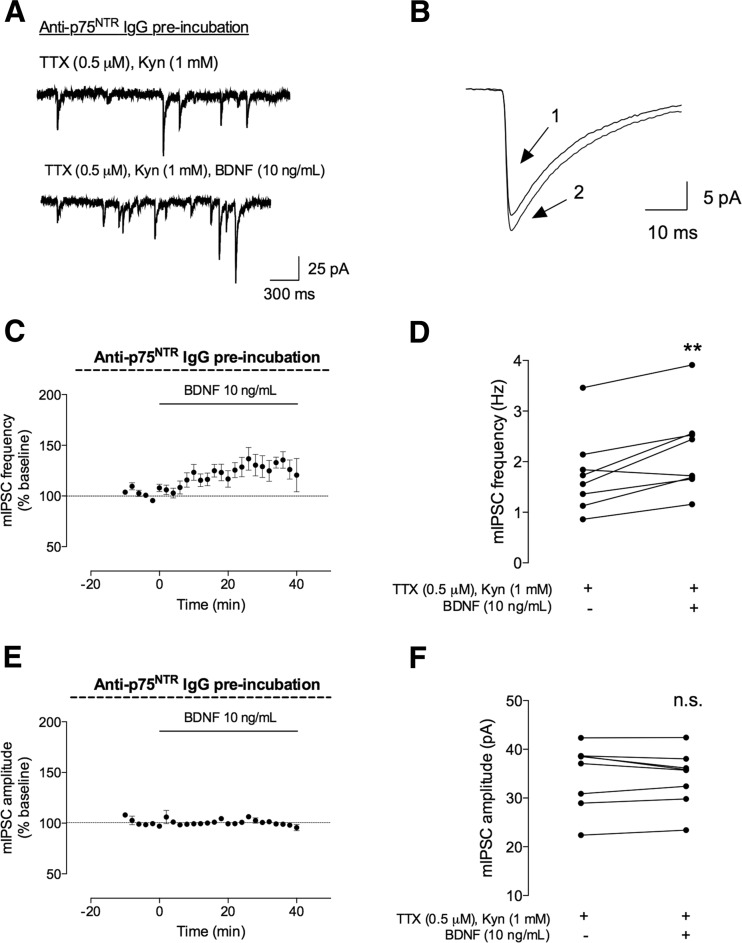
To evaluate the involvement of p75NTR on the facilitatory action of BDNF on GABAergic currents, the hippocampal slices were preincubated for 2 h with an anti-p75NTR IgG (50 μg/mL), prior to whole-cell recordings. This antibody binds to the p75NTR receptor and blocks its activation by preventing BDNF binding to the receptor. As shown in Fig. 5, the excitatory action of BDNF on GABAergic transmission was not affected in slices preincubated with the p75NTR antibody. Thus, after BDNF application, there was a significant increase in mIPSC frequency (29 ± 7.8 %, n = 8, p < 0.01; 1.8 ± 0.28 Hz before and 2.2 ± 0.30 Hz after BDNF application), without changes in mIPSC amplitude (−0.84 ± 1.5 %, n = 8, p > 0.05; 35 ± 2.3 pA before and 34 ± 2.0 pA after BDNF application). The effect of BDNF in the presence of p75NTR antibody was not significantly different from to the effect caused by BDNF (either 10 or 100 ng/mL) alone (p > 0.05, one-way ANOVA).
Fig. 5.
The facilitatory effect of BDNF on mIPSC frequency is not dependent on p75NTR receptor activation. The mIPSCs were recorded in the same conditions as described for Fig. 2. The slices were preincubated with an anti-p75NTR IgG (50 mg/mL) for 2 h. a In the upper panel, a representation of mIPSC tracings recorded in the absence (upper trace) and presence (lower trace) of BDNF (10 ng/mL). b Representative average tracings of mIPSCs of two superimposed events in the absence (1) and presence (2) of BDNF (10 ng/mL), from the same cell. c, e Time course changes in mIPSC frequency (c) and amplitude (e) induced by application of BDNF (n = 8). mIPSC frequency changes were quantified by comparing the events before (baseline) and after BDNF application. d, f Averages of the absolute values of frequency (d) or amplitude (f) are shown as individual data in the absence (−) of BDNF and after (+) BDNF administration (n = 8). Values are mean ± SEM. **p < 0.01 and n.s. p > 0.05 (two-tailed paired Student’s t test)
The above data show that the excitatory effect of BDNF in mIPSC frequency is prevented by the presence of K252a, but not by the anti-p75NTR IgG, suggesting that the presynaptic enhancement of GABAergic transmission caused by BDNF is mediated by Trk receptor activation, but not by p75NTR.
Adenosine A2A receptor blockade prevents BDNF effect onto GABAergic transmission
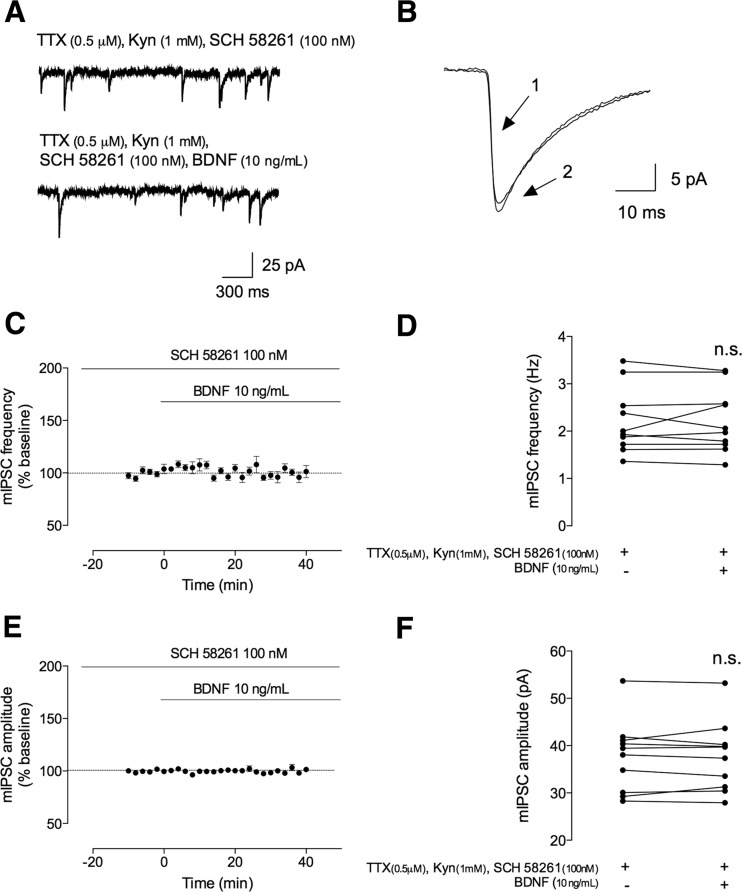
The interplay between TrkB and adenosine A2A receptors has been demonstrated in several contexts, with the activation of A2AR being a requisite for most of the effects of BDNF on excitatory synaptic transmission [15, 16, 18, 32–34]. We thus hypothesized that A2AR, in spite of disynaptically modulating GABAergic transmission to pyramidal cells [35], could directly affect the monosynaptic action of BDNF to principal cells. We thus tested whether endogenous activation of adenosine A2AR could also influence the action of BDNF on GABAergic transmission. To do so, slices were perfused with the selective A2AR antagonist, SCH 58261 (100 nM) [36], immediately after going to whole cell and currents were allowed to stabilize for at least 20 min before applying BDNF and still in the presence of the A2AR antagonist. However, in contrast with what occurred in the absence of SCH 58261 (Fig. 2), after 40 min of BDNF application in the presence of SCH 58261 (100 nM), there was virtually no effect in mIPSC frequency (% change: −0.38 ± 3.5, n = 10, p > 0.05, Fig. 6a, c, d) and amplitude (% change 0.16 ± 1.2 of change n = 10, p > 0.05, Fig. 6b, e, f). When analyzing the period between 30 and 40 min of BDNF application (in the presence of SCH 58261), the averaged absolute values were 2.2 ± 0.22 Hz and 37 ± 2.4 pA, while in the 10 min prior to BDNF application, they were 2.2 ± 0.22 Hz and 38 ± 2.4 pA.
Fig. 6.
BDNF-mediated effect on mIPSC frequency is dependent on adenosine A2A receptor activation. The mIPSCs were recorded in the same conditions as described for Fig. 2. During all the recordings, an adenosine A2A receptor antagonist was further added to the perfusion. a In the upper panel, a representation of mIPSC tracings recorded in the absence (upper trace) and presence (lower trace) of BDNF (10 ng/mL). b Representative average tracings of mIPSCs of two superimposed events in the absence (1) and presence (2) of BDNF (10 ng/mL), from the same cell. c, e Time course changes in mIPSC frequency (c) and amplitude (e) induced by application of BDNF (n = 10). mIPSC frequency and changes were quantified by comparing the events before (baseline) and after BDNF application. d, f Averages of the absolute values of frequency (d) or amplitude (f) are shown as individual data obtained in the absence (−) and presence (+) of BDNF (n = 10). Values are mean ± SEM. n.s. p > 0.05 (two-tailed paired Student’s t test
These data thus suggest that A2AR activation is required for the BDNF facilitatory effect onto inhibitory transmission.
Discussion
We herein show that, through TrkB receptor activation, BDNF positively modulates GABAergic transmission in the adult hippocampus by operating a presynaptic mechanism that requires adenosine A2AR co-activation.
GABA-releasing neurons are crucial for a proper regulation of pyramidal cells, controlling their firing rate, spike timing, and synchronized activity [37]. BDNF is a critical player in the modulation of neuronal networks. It mediates positive fast actions onto glutamatergic transmission [3–6] and synaptic plasticity [12, 13]. The increase in mIPSC frequency caused by BDNF may be interpreted as an increase in number of release sites or increase in number of docked vesicles, resulting in increased probability of release and/or an increase in the number of presynaptic interneuron terminals. Glutamic acid decarboxylase 65 (GAD65) is the key enzyme for GABA synthesis, and chronic administration of BDNF to organotypic hippocampal slices and hippocampal cultures led to increased GAD expression [38–40]. Moreover, enhanced GABA release probability due to a BDNF-mediated redistribution of Ca2+ channels to vesicle release sites was shown in cultured hippocampal neurons [41]. Our data obtained in slices from adult rats is consistent with these presynaptically mediated actions of BDNF, though contrasting with data from slices or acutely isolated neurons from P12 to P18 [24, 25], where BDNF has been reported to cause a postsynaptically mediated inhibition of GABAergic signaling. Interestingly, BDNF affects Cl− transport in hippocampal neurons [21]. The developmental shift from excitatory to inhibitory GABAergic currents observed around P13–16 results from changes in Cl− transport and is regulated by GABA itself, since blockade of GABAARs is able to prevent the switch from excitatory to inhibitory GABA [42]. One may thus speculate that BDNF changes the shifts from excitatory to inhibitory during a critical period of inhibitory GABAergic synapse maturation, eventually delaying it by inhibiting GABAergic transmission, but returns to its facilitatory role upon phasic transmission once synapses mature. Further studies on the role of BDNF in GABAergic synapse maturation are indeed required to directly assess this possibility.
Adenosine is a ubiquitous molecule released by neurons and glia that through the activation of its receptors is able to modulate synaptic transmission [43]. For instance, adenosine A2ARs are up-regulators of TrkB receptor signaling, being able to boost BDNF facilitatory effects on synaptic transmission [32], synaptic plasticity [15], and neuromuscular junction [34]. Moreover, in hippocampal neurons, adenosine A2ARs are more abundantly located in nerve terminals [44] and are required to translocate TrkB receptors to lipid rafts during high-frequency neuronal firing, allowing a BDNF effect upon glutamate release [10]. Interestingly, as we now show, the presynaptic influence of TrkB receptors upon GABA release at GABAergic synapses is also under control of tonic adenosine A2AR activation since the BDNF-induced increase on mIPSC frequency was prevented in the presence of an adenosine A2AR antagonist. Remarkably, A2ARs, though present in a subset of GABAergic nerve terminals, do not directly affect GABAergic inputs to pyramidal neurons [35], but as we now show, they can do so in an indirect way by allowing facilitatory BDNF actions in GABAergic nerve terminals.
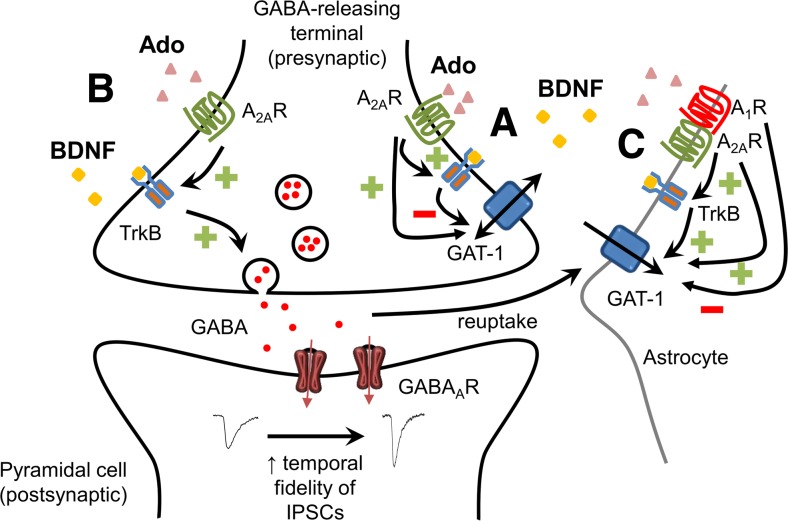
Although BDNF is only expressed in pyramidal cells, but not interneurons [45, 46], it is generally accepted that interneurons do express TrkB receptors [45, 47, 48]. Increase in synaptic activity will favor the release of BDNF [49] and the formation of adenosine from released ATP [50], with consequent activation of TrkB receptors and adenosine A2ARs [2]. BDNF is well known to increase neuronal excitability either directly, by facilitating glutamatergic synapses [1], or indirectly, by increasing GAT-1-mediated GABA uptake into astrocytes [17] and decreasing non-exocytotic GAT-1 reversal-mediated GABA release from synaptosomes [8, 19]. BDNF-mediated inhibition of GAT-1 activity at nerve endings also occurs when the transporter works in the inward direction [18], which may lead to an attenuation of excitation. Concerning exocytotic GABA release, we now show that BDNF enhances the activity of interneurons to release GABA, thus most probably contributing to a negative feedback mechanism to refrain excitability. Moreover, A2AR-dependent BDNF facilitation of GABA release, herein described, together with A2AR-dependent BDNF facilitation of GABA uptake into astrocytes [17] will synergistically contribute to increase temporal fidelity of GABA transmission (the time window available for synaptic integration) and synchronize pyramidal cell firing. In fact, deleting TrkB/BDNF signaling in fast-spiking interneurons induces a reduction of GABAergic inputs to pyramidal cells and the disruption of the typical rhythmic neuronal activity observed in neuronal circuits at gamma oscillation frequency [51]. Altogether, the available information allows to suggest that BDNF and adenosine A2AR synergistically control synaptic communication by increasing neuronal excitability without losing GABAergic temporal fidelity necessary for proper network functioning and the maintenance of hippocampal oscillations (see Fig. 7). Indeed, by increasing GABA release to principal cells (present work) and by facilitating a major mechanism for GABA clearance from the synapses, GABA uptake into astrocytes [17], BDNF might not only be increasing the power of GABAergic signaling but also be shortening the time window of GABA action, so increasing, in this way, the temporal fidelity of GABAergic transmission.
Fig. 7.
Summary of the influence of BDNF over GABAergic transmission and its control by adenosine A2ARs. BDNF inhibits GAT-1-mediated non-exocytotic GABA release [9] and inhibits neuronal GABA reuptake [18] (A); A2ARs facilitate BDNF actions [18, 19]. In addition, BDNF facilitates exocytotic GABA release (B) (present work) and astrocytic GAT-1-mediated uptake of GABA (C) [17]; in both cases, these actions are gated by A2AR. Direct influences of adenosine over hippocampal GABAergic transmission also occur, and some involving A2ARs are depicted in this figure (see [2] for details). Thus, A2ARs also directly facilitate GAT-1-mediated GABA transport into nerve endings (A) [52] as well as facilitate GAT-1- and GAT-3-mediated GABA transport into astrocytes (C), these actions being counteracted by an inhibitory action mediated by A1R which is heteromerized with A2AR at the plasma membrane of the astrocytes (C) [53]. Altogether, these A2AR-dependent actions of BDNF may contribute to increase neuronal excitability without losing temporal fidelity of GABA transmission
In summary, the present work clearly shows that BDNF facilitates GABAergic inputs to pyramidal neurons in the adult hippocampus in an A2AR-dependent manner. How the interplay between A2AR and BDNF at the tripartite GABAergic synapse (Fig. 7) impacts in pathological conditions as epilepsy, where GABA, BDNF, and adenosine play a role [54, 55], deserves further investigation.
Acknowledgments
The authors thank Regeneron for the gift of brain-derived neurotrophic factor and Louis Reichardt (Department of Physiology and Neuroscience Program, University of California, San Francisco) for the gift of anti-p75NTR IgG. Work was supported by a Fundação para a Ciência e Tecnologia (FCT) Research Project (EXPL/BIM-MEC/0009/2013). M.C-O., D.M.R., and R.B.D. were receipt of FCT Fellowships (SFRH/BD/73276/2010, SFRH/BD/60386/2009, and SFRH/BPD/89057/2012).
Compliance with ethical standards
All animal procedures were carried out in strict accordance with the ethical recommendations by EU (Directive 210/63/EU), the Portuguese (DL 113/2013) legislation for the protection of animals used for scientific purposes, and the Ethics Committee of the Instituto de Medicina Molecular and of the Faculty of Medicine, University of Lisbon, Lisbon, Portugal, who approved this study.
References
- 1.Leal G, Afonso PM, Salazar IL, Duarte CB. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015;1621:82–101. doi: 10.1016/j.brainres.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Sebastião AM, Ribeiro JA. Neuromodulation and metamodulation by adenosine: impact and subtleties upon synaptic plasticity regulation. Brain Res. 2015;1621:102–113. doi: 10.1016/j.brainres.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YX, Zhang Y, Lester HA, et al. Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J Neurosci. 1998;18:10231–10240. doi: 10.1523/JNEUROSCI.18-24-10231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lessmann V, Gottmann K, Heumann R. BDNF and NT enhance glut synaptic transmission in cultured hippocampal neurons. Neuroreport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- 6.Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyler WJ, Zhang X, Hartman K, et al. BDNF increases release probability and the size of a rapidly recycling vesicle pool within rat hippocampal excitatory synapses. J Physiol. 2006;574:787–803. doi: 10.1113/jphysiol.2006.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amaral MD, Pozzo-Miller L. Intracellular Ca 2+ stores and Ca 2+ influx are both required for BDNF to rapidly increase quantal vesicular transmitter release. Neural Plast. 2012 doi: 10.1155/2012/203536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canas N, Pereira IT, Ribeiro JA, Sebastião AM. Brain-derived neurotrophic factor facilitates glutamate and inhibits GABA release from hippocampal synaptosomes through different mechanisms. Brain Res. 2004;1016:72–78. doi: 10.1016/j.brainres.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 10.Assaife-Lopes N, Sousa VC, Pereira DB, et al. Regulation of TrkB receptor translocation to lipid rafts by adenosine A2A receptors and its functional implications for BDNF-induced regulation of synaptic plasticity. Purinergic Signal. 2014;10:251–267. doi: 10.1007/s11302-013-9383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerónimo-Santos A, Vaz SH, Parreira S, et al. Dysregulation of TrkB receptors and BDNF function by amyloid-β peptide is mediated by calpain. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu105. [DOI] [PubMed] [Google Scholar]
- 12.Figurov A, Pozzo-Miller LD, Olafsson P, et al. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Kolbeck R, Barde YA, et al. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J Neurosci. 1999;19:7983–7990. doi: 10.1523/JNEUROSCI.19-18-07983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikegaya Y, Ishizaka Y, Matsuki N. BDNF attenuates hippocampal LTD via activation of phospholipase C: implications for a vertical shift in the frequency-response curve of synaptic plasticity. Eur J Neurosci. 2002;16:145–148. doi: 10.1046/j.1460-9568.2002.02051.x. [DOI] [PubMed] [Google Scholar]
- 15.Fontinha BM, Diógenes MJ, Ribeiro JA, Sebastião AM. Enhancement of long-term potentiation by brain-derived neurotrophic factor requires adenosine A2A receptor activation by endogenous adenosine. Neuropharmacology. 2008;54:924–933. doi: 10.1016/j.neuropharm.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues TM, Jerónimo-Santos A, Sebastião AM, Diógenes MJ. Adenosine A2A Receptors as novel upstream regulators of BDNF-mediated attenuation of hippocampal Long-Term Depression (LTD) Neuropharmacology. 2014;79:389–398. doi: 10.1016/j.neuropharm.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Vaz SH, Jørgensen TN, Cristóvão-Ferreira S, et al. Brain-derived neurotrophic factor (BDNF) enhances GABA transport by modulating the trafficking of GABA transporter-1 (GAT-1) from the plasma membrane of rat cortical astrocytes. J Biol Chem. 2011;286:40464–40476. doi: 10.1074/jbc.M111.232009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaz SH, Cristóvão-Ferreira S, Ribeiro JA, Sebastião AM. Brain-derived neurotrophic factor inhibits GABA uptake by the rat hippocampal nerve terminals. Brain Res. 2008;1219:19–25. doi: 10.1016/j.brainres.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Vaz SH, Lérias SR, Parreira S, et al. Adenosine A2A receptor activation is determinant for BDNF actions upon GABA and glutamate release from rat hippocampal synaptosomes. Purinergic Signal. 2015 doi: 10.1007/s11302-015-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jovanovic JN, Thomas P, Kittler JT, et al. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardle RA, Poo M-M. Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci. 2003;23:8722–8732. doi: 10.1523/JNEUROSCI.23-25-08722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolton MM, Pittman AJ, Lo DC. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 2000;20:3221–3232. doi: 10.1523/JNEUROSCI.20-09-03221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanwick CC, Murthy NR, Kapur J. Activity-dependent scaling of GABAergic synapse strength is regulated by brain-derived neurotrophic factor. Mol Cell Neurosci. 2006;31:481–492. doi: 10.1016/j.mcn.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizoguchi Y, Ishibashi H, Nabekura J. The action of BDNF on GABA(A) currents changes from potentiating to suppressing during maturation of rat hippocampal CA1 pyramidal neurons. J Physiol. 2003;548:703–709. doi: 10.1113/jphysiol.2003.038935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- 27.Rombo DM, Dias RB, Duarte ST et al (2014) Adenosine A1 receptor suppresses tonic GABAA receptor currents in hippocampal pyramidal cells and in a defined subpopulation of interneurons. Cereb Cortex:1–15. doi:10.1093/cercor/bhu288 [DOI] [PubMed]
- 28.Woo NH, Teng HK, Siao C-J, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 29.Robinson MB, Anderson KD, Koerner JF. Kynurenic acid as an antagonist of hippocampal excitatory transmission. Brain Res. 1984;309:119–126. doi: 10.1016/0006-8993(84)91015-1. [DOI] [PubMed] [Google Scholar]
- 30.Andrásfalvy BK, Mody I. Differences between the scaling of miniature IPSCs and EPSCs recorded in the dendrites of CA1 mouse pyramidal neurons. J Physiol. 2006;576:191–196. doi: 10.1113/jphysiol.2006.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knüsel B, Hefti F. K-252 compounds: modulators of neurotrophin signal transduction. J Neurochem. 1992;59:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- 32.Diógenes MJ, Fernandes CC, Sebastião AM, Ribeiro JA. Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices. J Neurosci. 2004;24:2905–2913. doi: 10.1523/JNEUROSCI.4454-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diógenes MJ, Assaife-Lopes N, Pinto-Duarte A, et al. Influence of age on BDNF modulation of hippocampal synaptic transmission: interplay with adenosine A2A receptors. Hippocampus. 2007;17:577–585. doi: 10.1002/hipo.20294. [DOI] [PubMed] [Google Scholar]
- 34.Pousinha PA, Diogenes MJ, Ribeiro JA, Sebastião AM. Triggering of BDNF facilitatory action on neuromuscular transmission by adenosine A2A receptors. Neurosci Lett. 2006;404:143–147. doi: 10.1016/j.neulet.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 35.Rombo DM, Newton K, Nissen W, et al. Synaptic mechanisms of adenosine A 2A receptor-mediated hyperexcitability in the hippocampus. Hippocampus. 2015;25:566–580. doi: 10.1002/hipo.22392. [DOI] [PubMed] [Google Scholar]
- 36.Zocchi C, Ongini E, Ferrara S, et al. Binding of the radioligand [3H]-SCH 58261, a new non-xanthine A2A adenosine receptor antagonist, to rat striatal membranes. Br J Pharmacol. 1996;117:1381–1386. doi: 10.1111/j.1476-5381.1996.tb15296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klausberger T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci. 2009;30:947–957. doi: 10.1111/j.1460-9568.2009.06913.x. [DOI] [PubMed] [Google Scholar]
- 38.Marty S, Wehrlé R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohba S, Ikeda T, Ikegaya Y, et al. BDNF locally potentiates GABAergic presynaptic machineries: target-selective circuit inhibition. Cereb Cortex. 2005;15:291–298. doi: 10.1093/cercor/bhh130. [DOI] [PubMed] [Google Scholar]
- 40.Sánchez-Huertas C, Rico B. CREB-dependent regulation of gad65 transcription by BDNF/TrκB in cortical interneurons. Cereb Cortex. 2011;21:777–788. doi: 10.1093/cercor/bhq150. [DOI] [PubMed] [Google Scholar]
- 41.Baldelli P, Hernandez-Guijo J-M, Carabelli V, Carbone E. Brain-derived neurotrophic factor enhances GABA release probability and nonuniform distribution of N- and P/Q-type channels on release sites of hippocampal inhibitory synapses. J Neurosci. 2005;25:3358–3368. doi: 10.1523/JNEUROSCI.4227-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganguly K, Schinder AF, Wong ST, Poo MM. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/S0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro JA, Sebastião AM. Modulation and metamodulation of synapses by adenosine. Acta Physiol. 2010;199:161–169. doi: 10.1111/j.1748-1716.2010.02115.x. [DOI] [PubMed] [Google Scholar]
- 44.Rebola N, Canas PM, Oliveira CR, Cunha RA. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience. 2005;132:893–903. doi: 10.1016/j.neuroscience.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Gorba T, Wahle P. Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. Eur J Neurosci. 1999;11:1179–1190. doi: 10.1046/j.1460-9568.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 46.Swanwick CC, Harrison MB, Kapur J. Synaptic and extrasynaptic localization of brain-derived neurotrophic factor and the tyrosine kinase B receptor in cultured hippocampal neurons. J Comp Neurol. 2004;478:405–417. doi: 10.1002/cne.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cellerino A, Maffei L, Domenici L. The distribution of brain-derived neurotrophic factor and its receptor trkB in parvalbumin-containing neurons of the rat visual cortex. Eur J Neurosci. 1996;8:1190–1197. doi: 10.1111/j.1460-9568.1996.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 48.Fernandes CC, Pinto-Duarte A, Ribeiro JA, Sebastião AM. Postsynaptic action of brain-derived neurotrophic factor attenuates alpha7 nicotinic acetylcholine receptor-mediated responses in hippocampal interneurons. J Neurosci. 2008;28:5611–5618. doi: 10.1523/JNEUROSCI.5378-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodman LJ, Valverde J, Lim F, et al. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 50.Wieraszko A, Goldsmith G, Seyfried TN. Stimulation-dependent release of adenosine triphosphate from hippocampal slices. Brain Res. 1989;485:244–250. doi: 10.1016/0006-8993(89)90567-2. [DOI] [PubMed] [Google Scholar]
- 51.Zheng K, An JJ, Yang F, et al. TrkB signaling in parvalbumin-positive interneurons is critical for gamma-band network synchronization in hippocampus. Proc Natl Acad Sci. 2011;108:17201–17206. doi: 10.1073/pnas.1114241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cristóvão-Ferreira S, Vaz SH, Ribeiro JA, Sebastião AM. Adenosine A2A receptors enhance GABA transport into nerve terminals by restraining PKC inhibition of GAT-1. J Neurochem. 2009;109:336–347. doi: 10.1111/j.1471-4159.2009.05963.x. [DOI] [PubMed] [Google Scholar]
- 53.Cristóvão-Ferreira S, Navarro G, Brugarolas M, et al. A1R-A2AR heteromers coupled to Gs and Gi/0 proteins modulate GABA transport into astrocytes. Purinergic Signal. 2013;9:433–449. doi: 10.1007/s11302-013-9364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simonato M. Gene therapy for epilepsy. Metab Brain Dis. 2014;38:125–130. doi: 10.1016/j.yebeh.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 55.Boison D (2015) Adenosinergic signaling in epilepsy. Neuropharmacology 1–9. doi:10.1016/j.neuropharm.2015.08.046 [DOI] [PMC free article] [PubMed]