Abstract
Background and Objective
Stroke is a leading cause of long-term disability. Currently, there are no consistently effective rehabilitative treatments for chronic stroke patients. Our recent studies demonstrate that VNS paired with rehabilitative training improves recovery of function in multiple models of stroke. Here, we evaluated the ability of VNS paired with rehabilitative training to improve recovery of forelimb strength when initiated many weeks after a cortical and subcortical ischemic lesion in subjects with stable, chronic motor deficits.
Methods
Rats were trained to perform an automated, quantitative measure of voluntary forelimb strength. Once proficient, rats received injections of endothelin-1 to cause a unilateral cortical and subcortical ischemic lesion. Six weeks after lesion, rats underwent rehabilitative training paired with VNS (Paired VNS; n = 10), rehabilitative training with equivalent VNS delivered two hours after daily rehabilitative training (Delayed VNS; n = 10), or rehabilitative training without VNS (Rehab, n = 9).
Results
VNS paired with rehabilitative training significantly improved recovery of forelimb function compared to control groups. The Paired VNS group displayed an 86% recovery of strength, the Rehab group exhibited 47% recovery, and the Delayed VNS group exhibited 42% recovery. Improvement in forelimb function was sustained in the Paired VNS group after the cessation of stimulation, potentially indicating lasting benefits. No differences in intensity of rehabilitative training, lesion size, or MAP-2 expression were observed between groups.
Conclusion
VNS paired with rehabilitative training confers significantly greater recovery of forelimb function after chronic ischemic stroke in rats.
Keywords: Vagus nerve, vagal stimulation, ischemic stroke, recovery, rehabilitation
INTRODUCTION
Ischemic stroke affects approximately 800,000 people in the United States each year and is one of the leading causes of disability1. Rehabilitative interventions aimed at improving motor function are effective in some patients; however, most patients are left with some degree of disability2. Rehabilitative strategies have the highest potential to improve functional recovery when delivered early, and efficacy diminishes substantially with increasing time after stroke in animal models and patients3-8. There are as many as 4 million stroke survivors living with permanent neurological disability1,9. Therefore, the development of strategies that improve functional recovery even when initiated long after stroke would provide benefits for many suffering from chronic post-stroke disability.
Recently, vagus nerve stimulation (VNS) paired with rehabilitative training has emerged as a potential therapeutic strategy to improve recovery of motor function after stroke10-13. VNS is believed to support recovery by promoting neuroplasticity to enhance the benefits of rehabilitation14. VNS paired with rehabilitative training significantly improves forelimb strength and movement speed compared to equivalent rehabilitative training without VNS in models of ischemic stroke10-12. Additionally, VNS paired with rehabilitative training improves recovery of forelimb function in a severe model of subcortical hemorrhagic stroke affecting gray and white matter13. In these studies, VNS therapy was initiated approximately one week after stroke. It remains to be determined whether VNS can enhance recovery when initiated during the chronic phase. It is possible that VNS must be delivered shortly after stroke to capitalize on the pro- plasticity period in order to effectively promote recovery. Alternatively, VNS may drive plasticity and recovery independent of the post-stroke timing and would therefore be effective even when delivered during the chronic phase. Determining the optimal window for the efficacy of VNS therapy is critical to its development as a post-stroke intervention.
The purpose of the current study is to determine whether VNS paired rehabilitative training enhances recovery of forelimb function when the therapy is initiated during the chronic phase after a combined cortical and subcortical ischemic stroke. We find that VNS paired with rehabilitative training significantly improves recovery of forelimb function compared to equivalent rehabilitative training without VNS when therapy is initiated on the seventh week after stroke.
METHODS
Subjects
All procedures were approved by the University of Texas Institutional Animal Care and Use Committee. Sixty-five four month old female Sprague-Dawley rats (Charles River), weighing approximately 250 grams at the beginning of the experiment, were used. The rats were housed in a 12:12 hr reversed light cycle environment and were food deprived to no less than 85% of their normal body weight during training.
Isometric Force Task
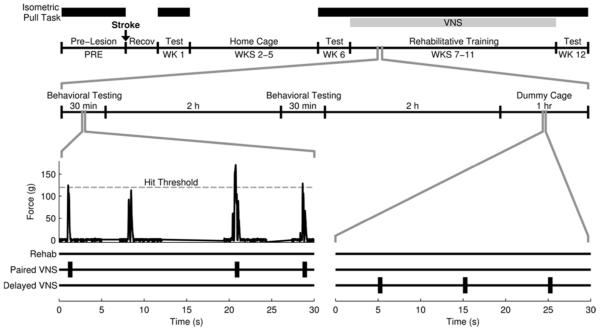
The isometric force task was used to measure volitional forelimb strength as previously described10,12,15,16. The behavioral training chamber consisted of an acrylic box (10 × 12 × 4.75 in) with a slot in the front right corner through which rats could access a manipulandum. Rats were trained to pull a handle attached to a force transducer (Motor Pull Device and Motor Controller, Vulintus LLC, Sachse, TX). If pull force exceeded 120 g within 2 s, the trial was recorded as a success and a reward pellet (45 mg dustless precision pellet, BioServ, Frenchtown, NJ) and VNS, when appropriate, was delivered. If the force did not exceed 120 g within 2 s, the trial was recorded as a failure and no reward was delivered. Rats underwent training and testing according to the timeline shown in Fig. 1. Behavioral training sessions lasted 30 min and were conducted twice daily, five days per week, with daily sessions separated by at least 2 hr.
Fig. 1. Experimental Timeline.
Illustration of the experimental timeline. Example data from the isometric force task is pictured. VNS treatment began in the appropriate groups on week 7 and continued through week 11. VNS was delivered coincident with successful trials in the Paired VNS group. The Delayed VNS group received a matched number of stimulations delivered 2 hours after daily rehabilitative training.
Unilateral ischemic lesion
Unilateral cortical/subcortical ischemic lesions were performed similar to previous descriptions with modifications11,12. Rats were anesthetized with ketamine hydrochloride (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and given supplemental doses as needed. Rats were placed in a stereotaxic frame and a craniotomy exposed primary motor cortex contralateral to the trained forelimb. Endothelin-1 (ET-1, Bachem, Torrance, CA, 1 mg/mL in saline) was injected into nine different locations using a 26-gauge Hamilton syringe. The first eight injections were within the forelimb area of motor cortex: 2.5, 1.5 0.5, and −0.5 AP and 2.5 and 3.5 ML from bregma, at a depth of 1.8 from the cortical surface. The ninth injection was within the dorsolateral striatum: 0 AP, 3.0 ML to bregma at a depth of 6.0 ventral to the skull surface. At all sites, 1.0 µL of ET-1 was injected over 2 minutes, and the syringe was left in place for 3 additional minutes. KwikCast silicone polymer (World Precision Instruments, Sarasota, FL) was placed in the craniotomy and sealed with a thin layer of acrylic, and the skin was sutured.
Vagus Nerve Stimulating Cuff Implantation
Four weeks after stroke, all rats were implanted with a skull-mounted two channel connector and a bipolar stimulating nerve cuff with platinum-iridium leads (5 kΩ impedance), as described in previous studies10-13,17. Blunt dissection isolated the left cervical vagus nerve, which was placed inside the stimulating cuff. Cuff leads were tunneled subcutaneously and attached to the connector atop the skull. All incisions were sutured and treated with antibiotic ointment.
Treatment Group Assignment and Exclusion Criteria
Rats were assigned to balanced treatment groups based on post-lesion performance at week 6 (Online Supplement). The Rehab group underwent rehabilitative training for 6 weeks, which consisted of freely performing the isometric force task during training sessions (Fig. 1). The Paired VNS group underwent identical rehabilitative training, but received stimulation of the vagus nerve paired with each successful trial. The Delayed VNS group underwent identical rehabilitative training and received a matched amount of VNS (every 12 s for 1 hr) delivered at least 2 hrs after the last rehabilitative training session each day10. VNS was delivered using identical parameters to previous studies10-13,17. Each stimulation consisted of a 500 ms train of 15 biphasic 0.8 mA pulses of 100 µs phase duration at 30 Hz. In the Paired VNS group, stimulation was triggered immediately (~70 msec) after the 120 g pull threshold was exceeded. No VNS was delivered on week 12 in any group to allow assessment of persistent effects of VNS pairing. Automated data analysis eliminated any bias.
Thirty-six rats were excluded from the study (Online Supplement) based on the following criteria: 1) did not survive surgery (n = 16), 2) did not display a statistically significant reduction in hit rate compared to pre-lesion at week 1 or 6 after stroke (n = 14), 3) were too impaired to perform trials on week 6 (n = 2), 4) device failure of either headcap connector or vagus nerve cuff (n = 4). Data presented in the main text excludes these subjects, but data for all subjects is included in the supplementary data. Exclusion had no effects on any statistical comparisons (Online Supplement).
Histological Processing
At the end of behavioral testing, brains were fixed with 4% paraformaldehyde. Tissue was cut in 40µm sections and processed with Nissl and myelin stains for white and gray matter analysis. MAP-2 immunohistochemistry was performed similar to previous studies18 (see Online Supplement). Area fraction analysis was performed on regions of interest from the perilesional cortex, contralesional cortex, and insular cortex as a control.
Statistics
All data and error bars are reported as mean ± standard error of the mean. Significant differences were determined using one-way ANOVA, two-way ANOVA, and two-tailed t-tests where appropriate. Paired t-tests were used to compare repeated measures over time within subjects. Statistical tests for each comparison are noted in the text. Alpha level was set at 0.05 for single comparisons, and Bonferroni-corrected for multiple comparisons where applicable.
RESULTS
Stroke chronically impairs forelimb strength
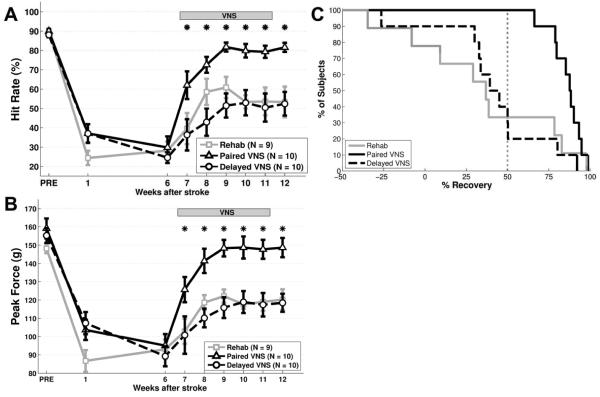
Prior to lesion, all rats were highly proficient on the isometric pull task (Fig. 2A, PRE; Hit Rate, Rehab: 89.8 ± 1.6%; Paired VNS: 89.9 ± 1.1%, Delayed VNS: 88.1 ± 1.2%). Maximal force generated during a trial was similar between groups, indicating comparable forelimb strengths (Fig. 2B, PRE; Maximal Force, Rehab: 148.2 ± 2.6 g; Paired VNS: 159.2 ± 5.5 g, Delayed VNS: 155.3 ± 4.0 g). No differences in performance measures were observed between groups prior to lesion (One-way ANOVA; Hit rate: F[2,28] = 0.71, p = 0.50; Maximal Force: F[2,28] = 1.81, p = 0.18).
Fig. 2. VNS paired with rehabilitative training improves forelimb function after chronic stroke.
(A) Paired VNS improves recovery of hit rate performance on the isometric pull task compared to Rehab alone and Unpaired VNS. (B) Paired VNS similarly improves forelimb strength compared to control groups. (C) All subjects that receive Paired VNS demonstrate >50% recovery of hit rate at the end of therapy. Only a subset of subjects in the control groups demonstrate >50% recovery. * denotes p < 0.05 between Rehab and Paired VNS at each time point. Error bars indicate mean ± SEM.
Ischemic lesion of the motor cortex significantly impaired measures of performance in all groups. One week after lesion, hit rate was significantly reduced compared to pre-lesion levels (Fig. 2A, Wk 1; Rehab: 24.2 ± 3.7%, paired t-test v. PRE, p = 1.5 × 10−7; Paired VNS: 37.1 ± 4.8%, p = 6.1 × 10−6; Delayed VNS: 37.0 ± 4.8%, p = 5.1 × 10−7). Maximal pull force was similarly impaired, consistent with a deficit in forelimb strength (Fig. 2B, Wk 1; Rehab: 86.7 ± 5.9 g, paired t-test v. PRE, p = 7.1 × 10−6; Paired VNS: 103.7 ± 5.6 g, p = 5.0 × 10−5; Delayed VNS: 107.4 ± 6.0 g, p = 4.8 × 10−5). After the post-lesion assessment at week one, rats remained in their home cage for 5 weeks, and returned for testing on week six. Hit rate was not significantly different on the sixth week after stroke compared to the first week after stroke in any group (Fig. 2A, Week 6; Rehab, paired t-test v. Week 1, p = 0.49; Paired VNS, p = 0.27; Delayed VNS, p = 0.11). All groups displayed comparable hit rate performance (One-way ANOVA; F[2,28] = 0.31, p = 0.73). A similar sustained reduction was observed for maximal force, with no difference between groups (Fig. 2B, Week 6; Rehab: paired t-test v. Week 1, p = 0.14; Paired VNS: p = 0.54; Delayed VNS: p = 0.11; One-way ANOVA comparing groups; F[2,28] = 0.18, p = 0.83). These findings indicate that forelimb function is chronically impaired six weeks after lesion.
VNS paired with rehabilitative training improves recovery of motor function
We evaluated the effects of rehabilitative training without VNS initiated on the seventh week after stroke. ANOVA on the Rehab group during the therapy period (weeks 7 – 12) revealed a significant effect of time (One-way ANOVA; F[6,62] = 5.76 , p = 1.0 × 10−4). Post hoc examination indicated a small, but significant, improvement in performance compared to post-lesion levels on weeks 9 and 10 (Rehab, paired t-test, Week 6 v. Weeks 7 – 12, p < 0.01 for weeks 9 – 10, statistical table in Online Supplement). On the last week of rehabilitative training (Week 12), subjects in the Rehab group displayed a 47.2 ± 13.4% recovery of forelimb strength. 3 of 9 subjects demonstrated a >50% recovery of hit rate (Fig. 2C). These findings indicate that rehabilitative training is still effective when initiated several weeks after stroke, but the improvements are modest.
Next, we investigated whether VNS paired with rehabilitative training would improve recovery of forelimb function when initiated on the seventh week after stroke. ANOVA on the Paired VNS group during the therapy period revealed a significant effect of time (One-way ANOVA; F[6,69] = 22.38, p = 6.21 × 10−14). Post hoc comparisons indicated that the Paired VNS group exhibits significantly better performance beginning on the first week of therapy (week 7) compared to post-lesion levels (Paired VNS, paired t-test, Week 6 v. Weeks 7 – 12, p < 0.01 for weeks 7 – 12). VNS was not delivered on week 12 to assess whether the benefits of VNS persist after the end of stimulation. Improved forelimb performance was observed even after the cessation of VNS (Paired VNS, Week 11 v. Week 12, paired t-test, p = 0.29), suggesting that VNS may yield long-lasting benefits. The persistence of increased maximal pull force that continues at least one week after the cessation of VNS indicates that stimulation is not directly influencing forelimb strength (Fig. 2B, Weeks 11 and 12). Subjects in the paired VNS group displayed a 85.9 ± 6.1% recovery of forelimb strength on the last week of rehabilitative training, significantly more than the Rehab group (Unpaired t-test, p = 0.0028). All 10 subjects demonstrated a >50% recovery of hit rate (Fig. 2C). These findings demonstrate that VNS paired with rehabilitative training results in a robust improvement in forelimb function in subjects with chronic deficits.
Previous studies report that VNS delivered two hours after behavioral training is less effective than VNS paired with rehabilitative training10,11. ANOVA on the Delayed VNS group revealed a significant effect of time (One-way ANOVA; F[6,69] = 3.00, p = 0.012), with post hoc tests indicating significantly better performance compared to post-lesion levels by week 9 (Delayed VNS, paired t-test, Week 6 v. Weeks 7 – 12, p < 0.01 for weeks 9 – 12). The trajectory of recovery is similar to that observed in the Rehab group, suggesting that recovery of function is a result of rehabilitative training and not of stimulation. Subjects in the Delayed VNS group exhibited a 42.1 ± 8.0% recovery of forelimb strength at the end of rehabilitative training, demonstrating significantly less recovery than the Paired VNS group (Unpaired t-test, p = 5.91 × 10−4). 4 of 10 subjects demonstrated a >50% recovery of hit rate (Fig. 2C). Therefore, despite equivalent training and amount of stimulation, Delayed VNS resulted in substantially less recovery of forelimb function after chronic stroke compared to Paired VNS.
We next sought to determine if VNS paired with rehabilitative training resulted in enhanced recovery compared to rehabilitative training without VNS or Delayed VNS. ANOVA on hit rate revealed a significant effect of group (Two-way ANOVA, F[2,202] = 41.48, p = 1.43 × 10−15). Post hoc tests indicated that Paired VNS results in significantly better performance compared to Rehab during weeks 9 – 12 (Paired VNS v. Rehab, Weeks 7 – 12, unpaired t-test, p < 0.01 for weeks 9 – 12). Additionally, Paired VNS resulted in significantly better performance than Delayed VNS beginning on week 8 (Paired VNS v. Delayed VNS, Weeks 8 – 12, unpaired t-test, p < 0.01 for all weeks). This demonstrates that VNS must be temporally paired with rehabilitative training in order to confer beneficial effects, corroborating previous studies10,11. Rehab and Delayed VNS displayed comparable performance (Rehab v. Delayed VNS, week 7 – 12, unpaired t-test, p > 0.10 for all weeks), indicating that VNS does not yield discernable benefit for recovery unless it is delivered during rehabilitative training. Paired VNS also results in significantly enhanced recovery of forelimb strength. ANOVA on maximal force reveals a significant group effect (Two-way ANOVA, F[2,202] = 47.74, p = 2.15 × 10−17). Post hoc tests demonstrated that Paired VNS results in significantly improved forelimb strength on most weeks during the therapy period compared to Rehab (Paired VNS v. Rehab at each week, unpaired t- test, p < 0.01 for weeks 8 - 12) and Delayed VNS (Paired VNS v. Delayed VNS at each week, unpaired t-test, p < 0.01 for weeks 8 - 12). Together, these findings indicate that VNS paired with rehabilitative training initiated 7 weeks after stroke results in significantly greater recovery of forelimb function than Delayed VNS or rehabilitative training without VNS.
VNS does not change the intensity of training
The intensity of rehabilitative training is associated with functional outcome after stroke19. We tested whether the intensity of rehabilitative training differed between groups and could account for the degree of forelimb recovery. There was no difference in the total number of task-directed pull attempts performed during the therapy period (weeks 7-12) between groups (Rehab: 67,061 ± 5,892 attempts, Paired VNS: 71,713 ± 6,162 attempts, Delayed VNS: 53,203 ± 9,027 attempts; One-way ANOVA; F[2,28] = 1.81, p = 0.18). This indicates that the intensity of rehabilitative training was similar between groups and therefore cannot account for the observed differences in forelimb recovery.
VNS does not affect lesion size
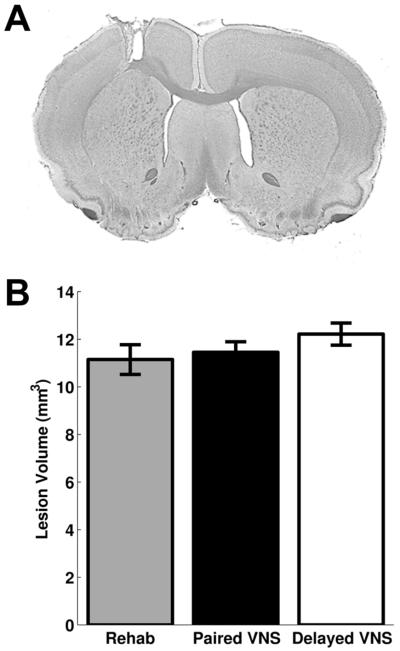
We tested whether VNS affected lesion size and could account for differences in recovery. No difference was observed in white matter lesion size (Rehab: 1.1 ± 0.2 mm3, n = 6, Paired VNS: 1.5 ± 0.2 mm3, n = 9, Delayed VNS: 1.4 ± 0.2 mm3, n = 9; One-way ANOVA; F[2,23] = 0.53, p = 0.60) or total lesion size (Rehab: 11.1 ± 0.6 mm3, Paired VNS: 11.5 ± 0.4 mm3, Delayed VNS: 12.2 ± 0.5 mm3; One-way ANOVA; F[2,23] = 1.20, p = 0.32). These findings corroborate previous studies using similar amounts of VNS and indicate that VNS does not improve recovery by conferring a neuroprotective effect11-13.
Neuroplasticity in motor circuitry in both the peri-infarct region and the contralesional homotopic cortex is believed to contribute to recovery after stroke20-22. We evaluated whether expression of MAP-2, a structural protein associated with dendritic plasticity, was increased in response to VNS paired with rehabilitative training in these regions. No differences in MAP-2 area fraction were observed between groups in the peri-lesional hemisphere (L2/3: Rehab: 0.39 ± 0.09, n = 4; Paired VNS, 0.36 ± 0.04, n = 7; Delayed VNS: 0.30 ± 0.03, n = 7; One-way ANOVA; F[2,17] = 0.96, p = 0.43; L5: Rehab: 0.23 ± 0.04, Paired VNS, 0.31 ± 0.04, Delayed VNS: 0.36 ± 0.04, F[2,17] = 2.07, p = 0.16) or the contralesional hemisphere (L2/3: Rehab: 0.33 ± 0.06, Paired VNS, 0.36 ± 0.05, Delayed VNS: 0.31 ± 0.04, F[2,17] = 0.25, p = 0.78; L5: Rehab: 0.34 ± 0.07, Paired VNS, 0.29 ± 0.02, Delayed VNS: 0.32 ± 0.03, F[2,17] = 0.30, p = 0.75). Consistent with previous studies, this finding suggests that long after stroke, sustained increases in MAP-2 expression are not required for functional improvement23.
DISCUSSION
This study demonstrates that VNS paired with rehabilitative training significantly improves recovery of forelimb function compared to equivalent rehabilitative training without VNS when initiated many weeks after stroke. Delayed VNS delivered two hours after daily rehabilitative training failed to improve recovery, indicating that VNS must be temporally coupled with rehabilitation to be effective. VNS did not change intensity of rehabilitative training, lesion size, or MAP-2 expression.
Previous studies have indicated that VNS paired with rehabilitative training improves recovery of motor function in multiple models of brain injury when initiated approximately one week after injury10-13. Here, we extend these findings and demonstrate that VNS paired with rehabilitative training enhances recovery of forelimb strength even when initiated long after stroke in subjects with chronic, stable deficits in motor function. Consistent with previous studies, the benefits of VNS therapy persist even after the cessation of stimulation, which may suggest that functional improvements are long-lasting12,13. The trajectory of recovery after chronic stroke looks similar to that observed in previous studies after acute stroke, potentially suggesting that the efficacy of VNS paired with rehabilitative training does not substantially decline with time after stroke10-12. These findings provide additional support for VNS paired with rehabilitation as a post-stroke intervention to improve recovery of motor function.
Intensive rehabilitative training is largely recognized as one of the most effective post- stroke interventions. All subjects in the study underwent intensive rehabilitative training, performing greater than 60,000 task-directed forelimb movements on average during the therapy period. Intensive rehabilitative training without VNS does result in a modest improvement in forelimb function. This indicates that rehabilitative interventions can yield benefits after chronic stroke, consistent with previous studies24,25. The addition of VNS provides significantly greater benefits than intensive rehabilitative training alone, demonstrating that VNS therapy may yield improvements beyond current effective interventions.
A matched amount of VNS delivered two hours after daily rehabilitative training fails to improve recovery compared to rehabilitative training paired with VNS, consistent with previous studies10,11. The inability for delayed VNS to improve motor function indicates that precise timing of VNS with concurrent rehabilitation is required for benefits. Neuroprotection and neurogenesis have been associated with VNS and stroke recovery26-30. These processes are unlikely to depend on precise temporal coupling of rehabilitation and stimulation and therefore would be expected to be engaged equally in the Paired VNS and Delayed VNS groups. Because Paired VNS improves recovery and Delayed VNS does not, it is unlikely that neuroprotection or neurogenesis contribute to VNS-dependent benefits observed in this study. Rather, Paired VNS likely improves recovery by enhancing neuroplasticity, a timing-dependent phenomenon. Neuroplasticity is strongly influenced by the relative timing of stimuli and neuromodulator release. Therefore, temporal dissociation of VNS-dependent neuromodulator release and rehabilitation-dependent neural activity likely prevents the beneficial plasticity associated with enhanced recovery14. The requirement for precise temporal coupling differentiates VNS from other pro-plasticity therapies that are effective in the chronic phase, such as anti-Nogo-A immunotherapy. While anti-Nogo-A therapy is believed to counter anti-plasticity processes and support an environment permissive for plasticity, VNS likely acts by specifically labeling ongoing neural activity to support plasticity and recovery.
In the current study, VNS delivery began during a time when the majority of pro- plasticity factors induced by stroke have returned to baseline levels22. The observed efficacy of paired VNS at this time suggests that VNS does not act by piggybacking on the pro-plasticity cascades induced by the lesion. Consistent with this, VNS paired with motor training drives robust cortical plasticity in the absence of brain damage31. This lesion-independent induction of plasticity may account for the ability of VNS to improve recovery when initiated long after stroke.
Previous studies have reported a neuroprotective effect of VNS when stimulation is delivered during or shortly (<2 hrs) after brain injury26-29. In the present study, VNS was initiated long after any neuroprotective effects would be expected. Consistent with previous reports using similar stimulation paradigms, VNS not reduce lesion size11-13. These findings suggest that VNS therapy, when initiated at least a week after stroke, acts through a mechanism independent of neuroprotection to support recovery, most likely by promoting neuroplasticity.
Stimulation of the vagus nerve engages multiple neuromodulatory pathways associated with neuroplasticity14. More than 80% of the vagus nerve consists of afferent projections to the central nervous system, and VNS drives neural activity in the noradrenergic locus coeruleus and cholinergic basal forebrain32-35. Moreover, VNS increases levels of these neuromodulators and brain-derived neurotrophic factor (BDNF) throughout the brain36-38. These neuromodulatory systems have clear links to both neuroplasticity and recovery after brain lesion39-42. A reduction of either noradrenergic or cholinergic signaling prevents VNS-dependent effects in the central nervous system, further suggesting that VNS engages these systems43,44. While these lines of evidence provide a potential rationale, future studies are needed to conclusively define the mechanisms of VNS-dependent enhancement of stroke recovery.
Neuroplasticity in multiple areas, including the peri-infarct region and the contralesional homotopic cortex, is associated with recovery after stroke22. Increased expression of MAP-2, a somatodendritically enriched protein associated with neuroplasticity, has been reported21,45. In this study, no differences in MAP-2 expression were observed between groups in any region examined. While no MAP-2 changes were evident, the enhanced recovery observed with Paired VNS suggests that neuroplasticity has taken place. It is possible that the gross changes in dendritic plasticity that can be observed with MAP-2 expression are insufficient to identify relevant neural changes, such as changes in synapse number or strength, this long after lesion. Other studies have also failed to observe changes in MAP-2 expression following rehabilitative training after stroke despite increases in recovery, suggesting that lasting MAP-2 changes are not obligatory for functional gains23. To identify the mechanisms that support recovery, future studies should examine the time course of finer-scale neuronal changes that accompany VNS- dependent recovery of function.
Delivery of VNS during rehabilitation has clear translation potential. VNS is FDA approved, and over 60,000 patients receive semi-continuous VNS for epilepsy and depression46,47. Plasticity-based VNS therapies, such as that employed in this study, use brief bursts of stimulation paired with specific rehabilitative events, reducing the amount of total daily charge delivered by 100-fold compared to FDA-approved standards14. This will likely further increase safety and tolerability. This study provides additional preclinical evidence in support of VNS therapy as a post-stroke intervention. VNS paired with rehabilitative training improves motor function in a variety of mechanistically distinct models of brain injury and when initiated at various times post-injury10-13. Future studies should evaluate VNS therapy in other preclinical models incorporating clinically-relevant complications present in the target population, including advanced age. Based on the safety record of VNS and the preclinical efficacy across multiple models, clinical trials evaluating VNS paired with rehabilitation in stroke patients are underway48,49.
Supplementary Material
Fig. 3. VNS does not affect lesion size.
(A) Example of typical lesion size. (B) No differences were observed in lesion size between groups.
ACKNOWLEDGEMENTS
We would like to thank Dr. Ann Stowe and Katie Poinsatte for technical assistance with histological imaging and Dr. Andrew Sloan for engineering support.
SOURCES OF FUNDING
This work was supported by grants from the US National Institute for Neurological Disorders and Stroke, US National Institute for Deafness and Other Communicative Disorders, and the Texas Biomedical Device Center.
Footnotes
DISCLOSURES
Dr. Khodaparast is a consultant for, Dr. Kilgard is a consultant and has a financial interest in, and Dr. Casavant is an employee of MicroTransponder, Inc. Dr. Rennaker owns Vulintus, Inc. The other authors report no conflicts.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wade DT, Langton-Hewer R, Wood VA, Skilbeck CE, Ismail HM. The hemiplegic arm after stroke: Measurement and recovery. J Neurol Neurosurg Psychiatry. 1983;46(6):521–524. doi: 10.1136/jnnp.46.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horn SD, DeJong G, Smout RJ, Gassaway J, James R, Conroy B. Stroke rehabilitation patients, practice, and outcomes: Is earlier and more aggressive therapy better? Arch Phys Med Rehabil. 2005;86(12):101–114. doi: 10.1016/j.apmr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Katherine Salter B, Mark Hartley B, Norine Foley B. Impact of early vs delayed admission to rehabilitation on functional outcomes in persons with stroke. J Rehabil Med. 2006;38:113–117. doi: 10.1080/16501970500314350. [DOI] [PubMed] [Google Scholar]
- 5.Hayes SH, Carroll SR. Early intervention care in the acute stroke patient. Arch Phys Med Rehabil. 1986;67(5):319–321. [PubMed] [Google Scholar]
- 6.Turton A, Pomeroy V. When should upper limb function be trained after stroke? evidence for and against early intervention. NeuroRehabilitation. 2002;17(3):215–224. [PubMed] [Google Scholar]
- 7.Kwakkel G, Kollen BJ, van der Grond J, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34(9):2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- 8.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. The Journal of Neuroscience. 2004;24(5):1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai S, Papadopoulos CM, Schwab ME, Kartje GL. Delayed anti-nogo-a therapy improves function after chronic stroke in adult rats. Stroke. 2011;42(1):186–190. doi: 10.1161/STROKEAHA.110.590083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hays SA, Khodaparast N, Ruiz A, et al. The timing and amount of vagus nerve stimulation during rehabilitative training affect post-stroke recovery of forelimb strength. NeuroReport. 2014;25(9):676–682. doi: 10.1097/WNR.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khodaparast N, Hays SA, Sloan AM, et al. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil Neural Repair. 2014;28:698–706. doi: 10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khodaparast N, Hays SA, Sloan AM, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis. 2013;60(0):80–88. doi: 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Hays SA, Khodaparast N, Hulsey DR, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke. 2014;45:10–3097. doi: 10.1161/STROKEAHA.114.006654. 3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hays SA, Rennaker IIRL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. 2013;207:275–299. doi: 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hays SA, Khodaparast N, Sloan AM, et al. The isometric pull task: A novel automated method for quantifying forelimb force generation in rats. J Neurosci Methods. 2012;212(2):329–37. doi: 10.1016/j.jneumeth.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Pruitt D, Hays S, Schmid A, et al. Controlled-cortical impact reduces volitional forelimb strength in rats. Brain Res. 2014 doi: 10.1016/j.brainres.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Engineer ND, Riley JR, Seale JD, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470(7332):101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the "unaffected" forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22(19):8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veerbeek JM, van Wegen E, van Peppen R, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PloS one. 2014;9(2):e87987. doi: 10.1371/journal.pone.0087987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. The Journal of Neuroscience. 2001;21(14):5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29(9):1972–80. doi: 10.1161/01.str.29.9.1972. discussion 1980-1. [DOI] [PubMed] [Google Scholar]
- 22.Murphy TH, Corbett D. Plasticity during stroke recovery: From synapse to behaviour. Nature Reviews Neuroscience. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 23.Maldonado M, Allred R, Felthauser E, Jones T. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabilitation and Neural Repair. 2008;22(3):250–261. doi: 10.1177/1545968307308551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Deville WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: Results from a single-blind randomized clinical trial. Stroke. 1999;30(11):2369–2375. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- 25.Volpe BT, Lynch D, Rykman-Berland A, et al. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22(3):305–310. doi: 10.1177/1545968307311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ay I, Lu J, Ay H, Gregory Sorensen A. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci Lett. 2009;459(3):147–151. doi: 10.1016/j.neulet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Hiraki T, Baker W, Greenberg JH. Effect of vagus nerve stimulation during transient focal cerebral ischemia on chronic outcome in rats. J Neurosci Res. 2012 doi: 10.1002/jnr.22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Z, Baker W, Hiraki T, Greenberg JH. The effect of right vagus nerve stimulation on focal cerebral ischemia: An experimental study in the rat. Brain stimulation. 2012;5(1):1–10. doi: 10.1016/j.brs.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ay I, Ay H. Ablation of the sphenopalatine ganglion does not attenuate the infarct reducing effect of vagus nerve stimulation. Autonomic Neuroscience. 2013;174(1):31–35. doi: 10.1016/j.autneu.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Revesz D, Tjernstrom M, Ben-Menachem E, Thorlin T. Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol. 2008;214(2):259–265. doi: 10.1016/j.expneurol.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Porter BA, Khodaparast N, Fayyaz T, et al. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cerebral Cortex. 2011;22:2365–2374. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- 32.Foley JO, DuBois FS. Quantitative studies of the vagus nerve in the cat. I. the ratio of sensory to motor fibers. J Comp Neurol. 1937;67(1):49–67. [Google Scholar]
- 33.Detari L, Juhasz G, Kukorelli T. Effect of stimulation of vagal and radial nerves on neuronal activity in the basal forebrain area of anaesthetized cats. Acta Physiol Hung. 1983;61(3):147–154. [PubMed] [Google Scholar]
- 34.Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006;318(2):890–898. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- 35.Groves DA, Bowman EM, Brown VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci Lett. 2005;379(3):174–179. doi: 10.1016/j.neulet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 36.Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006;1119(1):124–132. doi: 10.1016/j.brainres.2006.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Follesa P, Biggio F, Gorini G, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 38.Furmaga H, Carreno FR, Frazer A. Vagal nerve stimulation rapidly activates brain-derived neurotrophic factor receptor TrkB in rat brain. PloS one. 2012;7(5):e34844. doi: 10.1371/journal.pone.0034844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldstein LB, Coviello A, Miller GD, Davis JN. Norepinephrine depletion impairs motor recovery following sensorimotor cortex injury in the rat. Restorative Neurol Neurosci. 1991;3(1):41–47. doi: 10.3233/RNN-1991-3105. [DOI] [PubMed] [Google Scholar]
- 40.Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111(4):815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- 41.Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38(5):819–829. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- 42.Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46(2):173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 44.Nichols J, Nichols A, Smirnakis S, Engineer N, Kilgard M, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011;189:207–214. doi: 10.1016/j.neuroscience.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 45.Johnson G, Jope R. The role of microtubule-associated protein 2 (MAP-2) in neuronal growth, plasticity, and degeneration. J Neurosci Res. 1992;33(4):505–512. doi: 10.1002/jnr.490330402. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. The Lancet Neurology. 2002;1(8):477–482. doi: 10.1016/s1474-4422(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 47.Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS™) for treatment-resistant depression: Efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713–728. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 48.Microtransponder. Paired vagus nerve stimulation (VNS) with rehabilitation for upper limb function improvement after stroke. 2014 ClinicalTrials gov Bethesda (MD): National Library of Medicine (US) NCT01669161: http://clinicaltrials.gov/ct2/show/NCT01669161.
- 49.Microtransponder. VNS during rehabilitation for improved upper limb motor function after stroke. 2014 ClinicalTrials gov Bethesda (MD): National Library of Medicine (US) NCT02243020: http://clinicaltrials.gov/ct2/show/study/NCT02243020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.