Summary
Recent high‐profile studies report GDF11 to be a key circulating ‘anti‐aging’ factor. However, a screen of extracellular proteins attempting to identify factors with ‘anti‐aging’ phenotypes in aged murine skeletal muscle satellite cells did not identify GDF11 activity. We have been unable to confirm the reported activity of GDF11, similar to other laboratories offering conflicting data and describe our attempts to do so in this short take.
Keywords: Satellite cell, GDF11, aging
Numerous reports have demonstrated the existence of factors present in the circulatory system of young rodents that can promote tissue regeneration in aged rodents (Lunsford et al., 1963; Conboy et al., 2005; Conboy et al., 2013). GDF11 was recently proposed to decline in concentration in old mice and to restore young tissue function phenotypes in the heart, CNS, and skeletal muscle (Loffredo et al., 2013; Sinha et al., 2014). It was proposed that GDF11 promotes skeletal muscle regeneration by restoring genomic integrity of old skeletal muscle satellite cells (SCs) and thereby promoting their outgrowth in response to injury (Sinha et al., 2014). To identify factors that may regulate the growth with aging, we screened a comprehensive expression library of extracellular proteins (Zhang et al., 2014) on aged skeletal muscle SCs (additional screen details to be published elsewhere). Given the reported activity of GDF11, we expected to identify it in our screen. However, this activity was not identified despite a GDF11 clone being present. To better understand the lack of GDF11 activity, we considered the conditions under which the screening assay was performed versus the conditions used by Sinha et al. Screening conditions used F10 basal medium supplemented with 20% heat‐inactivated horse serum (HI‐HS) and 25 ng/mL bFGF to support the outgrowth of SC cultures (Conboy et al., 2005). The studies published by Sinha et al. used a non‐mitogen‐containing medium for their experiments testing GDF11's ability to promote outgrowth of aged SCs (see Figure S19 in Sinha et al.). In addition, the authors used a modified culture system consisting of F10 plus 20% knockout serum replacement (KSR) and bFGF as the control condition and F10 plus 20% KSR and recombinant GDF11 (rGDF11) or recombinant GDF8 (rGDF8) with no bFGF as the test conditions. We therefore reasoned GDF11 was not identified in our screen because of these different culture conditions. We directly tested this hypothesis.
Commercially obtained rGDF11 was confirmed active and potent using a SMAD2/3 luciferase reporter assay (Fig. 1A), where rGDF11 exhibited an EC50 of ~67 pM (or ~4 ng/mL). Similarly, purchased rGDF8 was also active and potent in this assay. Modulation of SC outgrowth from young (3 months) or old mice (24 months) using conditions matching Sinha et al. was tested. rGDF8 reduced young SC numbers (Fig. 1B, P < 0.05) consistent with published findings (Sinha et al., 2014; Egerman et al., 2015). However, we observed rGDF11 also tended to reduce young SC numbers (P < 0.05) in agreement with Egerman et al. but differing from the data reported by Sinha et al. that showed enhanced outgrowth. SC expansion was sustained by bFGF in young cultures (Fig. 1B, P < 0.001) as expected. Cultures of old SCs responded to bFGF, but none of the tested concentrations of rGDF8 or rGDF11 had an effect (Fig. 1B).
Figure 1.
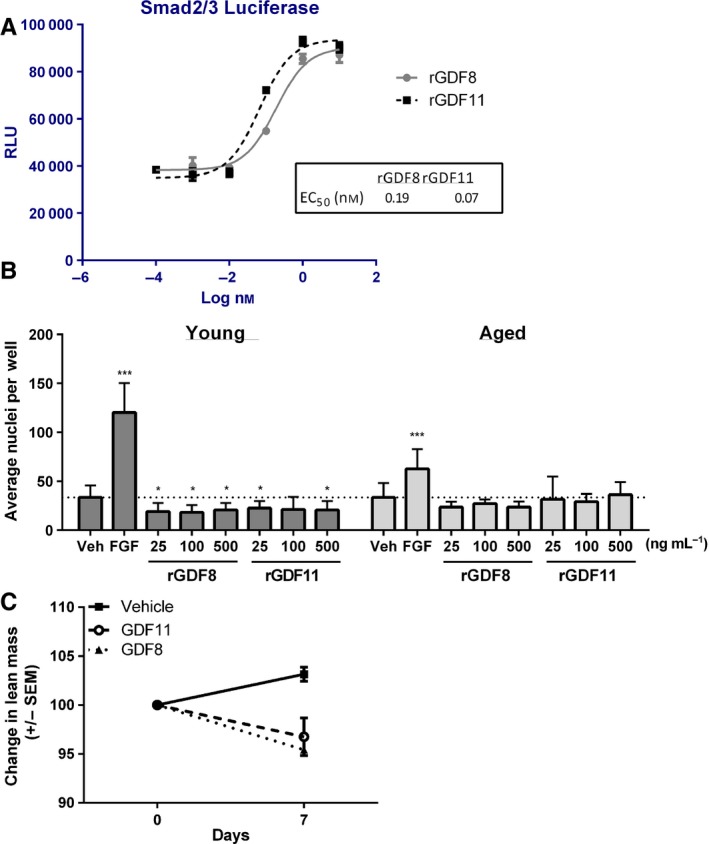
Similar activities of GDF8 and GDF11. (A) Purified rGDF11 and rGDF8 are potent and efficacious in a luciferase reporter gene assay of SMAD2/3 activity in HEK cells. (B) Satellite cells from young or aged animals were treated with rGDF11 or rGDF8 at various concentrations in F10 + 20% KSR. FGF stimulated outgrowth of both young and old cells. rGDF8 and rGDF11 had a modest negative effect on young satellite cell numbers and no effect in cultures of old satellite cells. Average values obtained from two distinct experiments (six replicates for each condition per experiment for a total of 12 wells) are shown (mean ± SD). P ‐values were calculated by Student's t‐test with statistically significant differences indicated (*P < 0.05, ***P < 0.001) matching methods by Sinha et al. (C) Expression of GDF8 or 11 in the liver by injection of expression vectors promotes rapid lean mass loss (P < 0.05).
Interestingly, we observed SC outgrowth in the KSR‐containing media is not as robust as observed in HI‐HS (Bareja et al., 2014). This is different from the data by Sinha et al. in Figure S19 where SC outgrowth with bFGF was similar in KSR to that in HI‐HS media (e.g., 200 seeded cells growing out to 1000–2000 cells in approximately 5 days). We do not understand the basis for this difference between our observations versus those of Sinha et al.; however, our data suggest KSR media do not support survival and growth of the SCs as well as HI‐HS media, although the cells still respond to the mitogenic activity of bFGF.
Recent papers indicate GDF11 is not decreased in the circulation of aged rodents or older humans and that GDF11 is actually deleterious toward muscle repair in mice (Egerman et al., 2015; Rodgers & Eldridge, 2015). The experiments performed in Egerman et al. on isolated SCs demonstrated a small decrease in the number of cells after 3 days of rGDF8 or rGDF11 treatment. However, the conditions used for culture (DMEM with 20% horse serum) were different from those used by Sinha et al. as discussed above (see Table S1 for a comparison). The data we obtained using the culture system of Sinha et al. are most consistent with that presented by Egerman et al., at least with regard to the effects of rGDF11 on young SCs. In addition, we tested for the effects of both GDF8 and GDF11 on lean mass in young mice via expression in the liver by injection of expression plasmids containing GDF8 or GDF11 cDNA. Lean mass in these mice was reduced over a period of 7 days (Fig. 1C). These data are consistent with the fact that GDF11 shares a high degree of sequence identity with GDF8, activates similar signaling pathways (Lach‐Trifilieff et al., 2014), does not promote SC outgrowth, and reduces lean mass in mice. Thus in these findings, GDF11 exhibits activity similar to that of GDF8. Clearly, further investigation is needed to fully clarify whether, and under what, if any, conditions GDF11 is a factor that beneficially regulates muscle activity during aging.
Supplemental materials
Animal Care and Models: For studies involving mice, all procedures performed were in compliance with the Animal Welfare Act and US Department of Agriculture regulations and approved by the GlaxoSmithKline or Five Prime Therapeutics Institutional Animal Care and Use Committee. For satellite cell isolations, mice were maintained on standard laboratory chow and allowed access to food and water ad libitum. Male C57Bl6/J were used for these experiments (Charles River Labs, Wilmington, MA, USA).
Satellite isolation and culture: Isolation and culture of murine SCs was performed as in (Bareja et al., 2014) and references therein. Cells were fixed, stained with Hoechst 33342 to visualize nuclei, and counted as previously described at the end of the culture period (Bareja et al., 2014). Recombinant mouse/rat/human GDF8 and GDF11 was obtained from R and D Systems and reconstituted according the manufacturer's directions.
In vivo studies
Female BalbC mice (Charles River Labs) were randomly assigned into treatment groups (n = 10). On day 0, mice were subjected to lean mass analysis (EchoMRI®, Houston, TX, USA) to establish baseline measurements, then injected with expression vectors encoding GDF8, GDF11 or vehicle control and on day 7 lean mass was measured.
Funding
No funding information provided.
Conflict of interest
All authors are employees and/or stockholders of GlaxoSmithKline or Five Prime Therapeutics, Inc.
Supporting information
Table S1 Comparison of experimental conditions used in Sinha et al (2014) and Egerman et al. (2015).
References
- Bareja A, Holt JA, Luo G, Chang C, Lin J, Hinken AC, Freudenberg JM, Kraus WE, Evans WJ, Billin AN (2014) Human and mouse skeletal muscle stem cells: convergent and divergent mechanisms of myogenesis. PLoS One 9, e90398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764. [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Cerletti M, Wagers AJ, Conboy IM (2005) Immuno‐analysis and FACS sorting of adult muscle fiber‐associated stem/precursor cells. Methods Mol. Biol. 621, 165–173. [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Conboy IM, Rando TA (2013) Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 12, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach‐Trifilieff E, Shavlakadze T, Trendelenburg AU, Brack AS, Glass DJ (2015) GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 22, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lach‐Trifilieff E, Minetti GC, Sheppard K, Ibebunjo C, Feige JN, Hartmann S, Brachat S, Rivet H, Koelbing C, Morvan F, Hatakeyama S, Glass DJ (2014) An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol. Cell. Biol. 34, 606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT (2013) Growth differentiation factor 11 is a circulating factor that reverses age‐related cardiac hypertrophy. Cell 153, 828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsford WR, Mc CC, Lupien PJ, Pope FE, Sperling G (1963) Parabiosis as a method for studying factors which affect aging in rats. Gerontologia 7, 1–8. [DOI] [PubMed] [Google Scholar]
- Rodgers BD, Eldridge JA (2015) Reduced circulating GDF11 is unlikely responsible for age‐dependent changes in mouse heart, muscle, and brain. Endocrinology 156, 3885–3888. [DOI] [PubMed] [Google Scholar]
- Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ (2014) Restoring systemic GDF11 levels reverses age‐related dysfunction in mouse skeletal muscle. Science 344, 649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Pao LI, Zhou A, Brace AD, Halenbeck R, Hsu AW, Bray TL, Hestir K, Bosch E, Lee E, Wang G, Liu H, Wong BR, Kavanaugh WM, Williams LT (2014) Deorphanization of the human leukocyte tyrosine kinase (LTK) receptor by a signaling screen of the extracellular proteome. Proc. Natl. Acad. Sci. U. S. A. 111, 15741–15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Comparison of experimental conditions used in Sinha et al (2014) and Egerman et al. (2015).


