Abstract
Background
Although particulate matter, with diameters < 2.5 µm (PM2.5) and < 10 µm (PM10), and other pollutants have been associated with cardiovascular morbidity and mortality, the effect of pollutants on acute myocardial infarctions (AMIs) has rarely been investigated in Asia, especially in Shanghai, China.
Methods
Between 1 November 2013 and 27 April 2014, 972 patients from the Pudong District, Shanghai City, were assessed by the Emergency Medical Service. A case-crossover design was used to analyze exposure to air pollution and the AMI risk. Exposures to PM2.5, PM10, nitrogen dioxide (NO2), sulphurdioxide (SO2), and carbon monoxide (CO) were based on the mean urban background levels. The associations among AMI admissions, the included pollutants, temperature, and relative humidity were analyzed using correlation and logistic regression.
Results
The urban background levels of PM2.5, PM10 and CO were associated with an increased risk of AMI, unlike NO2 and SO2 levels. The OR (95% CI) for AMI were 1.16 (1.03–1.29), 1.05 (1.01–1.16), 0.82 (0.75–1.02), 0.87 (0.63–1.95), and 1.08 (1.02-1.21) for PM2.5, PM10, NO2, SO2, and CO, respectively. Increases in the air quality index (AQI) were associated with more AMI occurrences. There was no correlation between fluctuations in temperature and relative humidity with AMI hospital admissions.
Conclusions
Short-term exposure to moderate-serious pollution levels is associated with increased risk of AMI. Increased PM2.5, PM10 and CO levels are related to increased AMI admissions.
Keywords: Air pollution, Myocardial infarction, Particulate matter
1. Introduction
Air pollution has deleterious effects on human health and is a major problem for the global community. Several studies have demonstrated that there are associations between exposure to common environmental pollutants and both overall and cardiovascular mortality,[1]–[7] as well as the incidence of acute myocardial infarctions (AMIs). Most of these studies primarily examined long-term pollutant exposure. Initially, a recent systematic review suggested that the association between long-term air pollution exposure and myocardial infarction, specifically, is less clear, with less than half of the identified studies finding evidence of the detrimental effects of particles with diameters < 2.5 µm (PM2.5) or < 10 µm (PM10), ozone, carbon monoxide (CO), nitrogen dioxide (NO2), or sulfur dioxide (SO2).[8] Although some studies focused on the relationship between air pollution and AMIs,[9],[10] the data were extracted from high income regions in Western countries. At present, there remains a scarcity of evidence regarding the impact of air pollutants on AMI risk in middle-income nations, especially in Asia.
In the present study, we investigated the relationship between short-term exposure to air pollution and the AMI risk in Shanghai, China.
2. Methods
2.1. Shanghai City
This study examined the daily variations in hospital admissions, due to AMIs, relative to air pollutant levels in Pudong District, Shanghai City between 1 November 2013 and 27 April 2014. Shanghai is the largest metropolitan area in China, with a population of approximately 24.15 million and is located along the country's southeast coast. Pudong District is the largest area within the metropolitan area, having a population of 5,500,000. Historically, the monsoons have prevented the southeast coast of China from experiencing serious air pollution concerns. At the end of 2013, fog and haze swept south from northern China and was associated with a sharp increase in the rate of cardiovascular emergency admissions, including those for AMIs, in Shanghai City.
2.2. Hospital admissions
The inclusion criteria were AMIs which was defined according to the universal definition of myocardial infarction.[11] Daily hospital admission counts, due to AMIs [International Classification of Diseases, 9th revision (ICD-9)], were extracted from the Emergency Medical Service documents covering the 2013–2014 period. Computerized records of daily clinic visitor admissions in Pudong District were available for each contracted medical institution. All medical institutions must submit standard claim documents for medical expenses on a computerized form that includes the dates of admission and discharge, identification number, sex, birthday, and the diagnostic code for each admission. Therefore, the information from the register database is sufficiently complete and accurate for use in epidemiological studies. At recruitment, the location of each AMI occurrence was recorded, and was dichotomized as occurring indoors or outdoors. Home, indoor workplace, public transportation and taxi, car, airport, and shopping mall or store were defined as indoor places, whereas streets and outdoor workplaces were regarded as outdoor places. The location information was verified by the patient, witness, or emergency medical service staff.
All patients were stratified according to circadian rhythm, and the time, one hour before hospital admission, was recorded as falling within one of two windows: 6: 00–18: 00 or 18: 00–6: 00. The study was approved by the Ethical Committee of Shanghai East Hospital, Tongji University. Informed consent was obtained from each patient or an immediate family member.
2.3. Air pollution and meteorological data
Six air quality monitoring stations in Pudong District have been established by the Shanghai Environmental Monitoring Center, a central government agency. The monitoring stations are fully automated and provide daily PM2.5, PM10, SO2, NO2 and CO levels. For each day, hourly air pollution data were obtained from each monitoring station. After calculating the hourly mean for each pollutant from the 6 stations, the average 24-h levels of these pollutants were computed. Additional daily data for the mean temperature and mean humidity were provided by the Shanghai Meteorological Service. Pollution severity was divided into quartiles, according to the air quality index (AQI), which was calculated using the concentrations of the five indicated pollutants: an AQI of 0–100 indicated no pollution, an AQI of 101–150 indicated mild pollution, AQI 151–200 represented moderate pollution, and an AQI > 201 indicated serious pollution.[12]
2.4. Statistical analysis
Data were analyzed using the case-crossover technique.[13]–[15] This design is an alternative to Poisson time-series regression models for studying the short-term effects attributed to air pollution.[16] The exposure effect was analyzed using conditional logistic regression, after constructing 24-h means for each pollutant. The period immediately preceding the time of the event was the case period, and all other comparable time intervals within the same time stratum were control periods. The reported time of the event was rounded to the full hour preceding the event.
The associations among hospital admissions, air contaminant levels and the temperature and relative humidity were estimated using the OR and 95% CI, which were produced using conditional logistic regression with weights equal to the number of hospital admissions on that day. The estimates are expressed as ORs and 95% CIs per 50 µg/m3 for PM2.5, PM10, NO2, SO2 and 50 mg/m3 for CO. A P-value < 0.05 was considered significant for an interaction. Data management, descriptive statistics, and analysis were performed using Prism, version 5.0 (GraphPad Software, San Diego, CA, USA). Air pollutant exposure levels were entered into the models as continuous variables. Meteorological variables (daily average temperature and humidity), for the same days, were also included in the model as they were potentially confounding.
3. Results
3.1. Study population and exposure characteristics
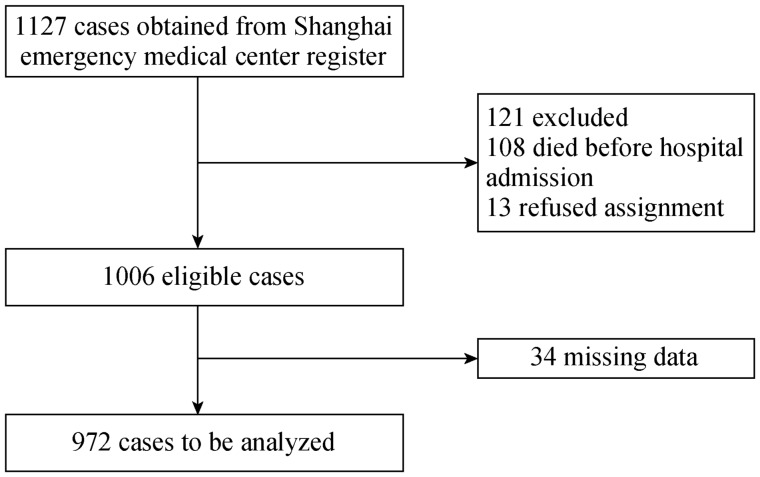
Out of 1,127 Emergency Medical Service-assessed occurring during the study period, 972 met the inclusion criteria (Figure 1). The distribution of AMI occurrences, shown in Table 1, does not demonstrate any sex-related differences in the pollution effect on AMI risk. More than half of the total AMIs occurred in patients aged 61–80 years, and nearly 50% of the AMIs occurred in patients who were outdoors.
Figure 1. Flow chart of inclusion of acute myocardial infarction patients.
Table 1. Study population characteristics.
| Category | Men, n = 515 | Women, n = 457 | P |
| Age | |||
| ≤ 40 yrs | 27 (5.5%) | 21 (4.7%) | 0.28 |
| 41–60 yrs | 132 (25.5%) | 122 (26.6%) | 0.69 |
| 61–80 yrs | 281 (54.5%) | 246 (53.9%) | 0.42 |
| > 80 years | 75 (14.5%) | 68 (14.8%) | 0.51 |
| Current smoker | 115 (22.3%) | 57 (12.5%) | 0.02 |
| COPD | 63 (12.4%) | 48 (10.5%) | 0.39 |
| Platelet count, ×109 | 202 | 183 | 0.74 |
| CRP, mg/L | 7 | 5 | 0.42 |
| LTA | |||
| AA aggregation, % | 38 | 44 | 0.66 |
| ADP aggregation, % | 42 | 51 | 0.23 |
| PT, s | 11.8 | 12.9 | 0.82 |
| APTT, s | 22.5 | 25.8 | 0.59 |
| Place of AMI | |||
| Home | 99 (19.3%) | 98 (21.4%) | 0.36 |
| Street | 230 (44.8%) | 184 (40.2%) | 0.15 |
| Indoor workplace | 16 (3.1%) | 11 (2.4%) | 0.76 |
| Outdoor workplace | 30 (5.8%) | 28 (6.1%) | 0.83 |
| Public transportation | |||
| Taxi | 36 (6.9%) | 28 (6.2%) | 0.79 |
| Car | 28 (5.5%) | 21 (4.7%) | 0.85 |
| Airport | 11 (2.0%) | 14 (3.1%) | 0.47 |
| Shopping mall/store | 19 (3.4%) | 10 (2.3%) | 0.66 |
| Other place not classified | 46 (8.9%) | 63 (14.1%) | 0.09 |
Data are presented as n (%) unless other indicated. AA: arachidonic acid; ADP: adenosine diphosphate; AMI: acute myocardial infarction; APTT: activated partial prothrombin time; COPD: chronic obstructive pulmonary disease; CRP: C-reactive protein; LTA: light turbidity aggregation; PT: prothrombin time.
3.2. Pollution and meteorology trends during the 6-month follow-up period
Air pollutants and meteorological conditions were measured during the study period, and showed that there were moderate or serious pollution days during the follow-up period; all of the pollutant levels were higher than those that occurred on mild or no pollution days. During the follow-up, the highest pollution levels recorded were 167.0 µg/m3 (6-month follow-up period PM2.5), 205.5 µg/m3 (PM10), 88.8 µg/m3 (NO2), 55.4 µg/m3 (SO2), and 1740 mg/m3 (CO). The PM2.5, PM10 and CO trends correlated well with each other every month. The pollution levels were particularly high and there were more consecutive days of high pollution levels during November, December and January than in other months. When the winter ended, on about March 21, the levels of the pollutants decreased (data was not shown).
3.3. Relationships among air pollutants
The urban background levels of PM2.5, PM10, NO2, SO2 and CO correlated with each other. The Pearson's correlation coefficients for the pollutants are shown in Table 2. There were correlations among the pollutant levels, including between PM2.5 and PM10 (r = 0.55), PM2.5 and CO (r = 0.71), PM2.5 and SO2 (r = 0.63) and between SO2 and NO2 (r = 0.54).
Table 2. Correlation coefficients among air pollutants.
| Variable | PM2.5 | PM10 | NO2 | SO2 | CO |
| PM2.5 | 1.00 | 0.55 (0.38−0.62)* | 0.38 (0.29−0.41)* | 0.63 (0.44−0.72)* | 0.71 (0.65−0.97)* |
| PM10 | 1.00 | 0.26 (0.18−0.34)* | 0.41 (0.32−0.68)* | 0.19 (0.06−0.35)* | |
| NO2 | 1.00 | 0.54 (0.48−0.76)* | 0.26 (0.11−0.49)* | ||
| SO2 | 1.00 | 0.33 (0.18−0.66)* | |||
| CO | 1.00 |
Data are presented as Spearman correlation coefficients. *P < 0.01. CO: carbon monoxide; NO2: nitrogen dioxide; PM: particulate matter; SO2: sulphur dioxide.
3.4. Relationship among air pollutants, temperature and relative humidity
During the study, there was no relationship between any of the pollutants and temperature, nor was there a relationship with relative humidity (data was not shown).
3.5. Associations between air pollution and AMIs
The associations between the various monthly air pollutant levels and the number of hospital admissions due to AMIs were analyzed. In the single pollutant model, AMI admissions were significantly associated with the presence of each pollutant. The correlation coefficients for AMI admissions ranged 1.03–1.29 for PM2.5, 1.01–1.16 for PM10, 0.75–1.02 for NO2, 0.63–1.95 for SO2 and 1.02–1.21 for CO (Table 3).
Table 3. Odds ratios (ORs) and 95% CI for acute myocardial infarction admissions for each interquartile range increase, in the single-pollutant model.
| Pollutant | OR (95% CI) | |
| PM2.5 | 1.16 (1.03−1.29)* | |
| PM10 | 1.05 (1.01−1.16)* | |
| NO2 | 0.82 (0.75−1.02) | |
| SO2 | 0.87 (0.63−1.95) | |
| CO | 1.08 (1.02−1.21)* |
*P < 0.05. CO: carbon monoxide; NO2: nitrogen dioxide; PM: particulate matter; SO2: sulphur dioxide.
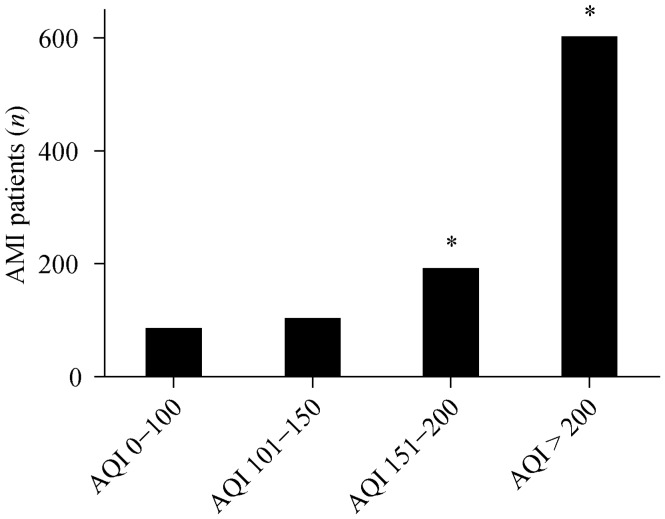
3.6. AMI patient distribution according to AQI quartiles
During the six months of monitoring, increases in the AQI were associated with more frequent AMI occurrences. Compared to the AQI 0–100 and the AQI 101–150 quartiles, the occurrence of AMIs was much higher (P < 0.01, Figure 2) when the AQI was in the 151–200 and > 200 quartiles (data was not shown).
Figure 2. Distribution of AMI patients according to AQI quartile.
*P < 0.01 compared to AQI 0–100 and AQI 101–150. AMI: acute myocardial infarction; AQI: air quality index.
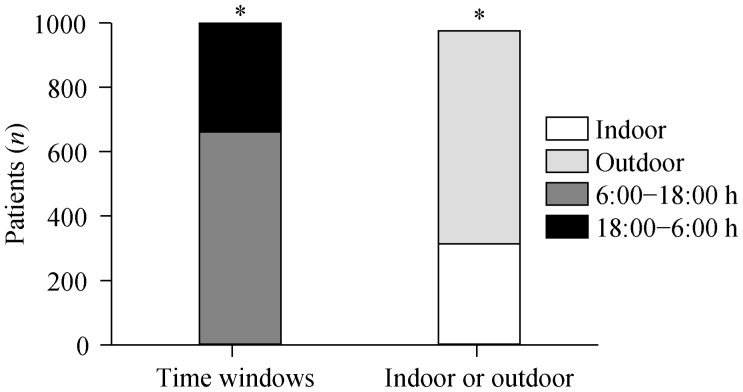
3.7. AMI patient distribution according to time of day and location
Figure 3 shows that AMIs occurred more frequently between 6:00 and 18:00 than between18:00 and 6:00. Consistently, if patients were categorized according to their being indoors or outdoors preceding of their AMIs, more AMIs were observed to occur following spending time outdoors. There were no differences in symptoms and laboratory findings between patients having indoor or outdoor onset (data not shown).
Figure 3. Distribution of acute myocardial infarctions according to time windows and indoor/outdoor occurrence.
*P < 0.05 for 6:00–18:00/18:00–6:00 and indoor/outdoor occurrence.
4. Discussion
This study describes the short-term effects of ambient air pollutant exposure on hospital admissions due to AMIs in Shanghai, China. Data show that PM2.5, PM10 and CO levels were positively associated with increases in the daily numbers of AMI hospitalizations on polluted days; NO2 and SO2 levels did not show a relationship with AMI admissions and there was no correlation with ambient temperature or humidity.
Certain populations seem to be more vulnerable to the deleterious effects of air pollution. Nuvolone, et al.[7] found that elderly persons (age ≥ 75 years) were at greater risk for AMIs following short-term exposure to air pollutants than younger patients. These results were in accordance with our study wherein more than half of the population was 61–80 years-old. Patients with preexisting coronary artery disease were also found to have an increased risk for acute ischemic events (unstable angina or myocardial infarctions), compared with healthy subjects, during periods of increased PM2.5 concentrations,[17] as in the present study.
The most common and consistent associations between air pollutants and hospital admissions due to AMIs were found for particulate matter levels,[18]–[21] which are now of great health and regulatory concern. Various biological mechanisms have been proposed to associate particulate air pollution with adverse cardiac endpoints.[21],[22] Mustafic, et al,[23] demonstrated that PM2.5 and PM10 levels are significantly associated with increased AMI risk. One reviewed study, by Peters, et al.,[18] involved a case-crossover study of 772 patients presenting to Boston area hospitals with strictly defined myocardial infarctions. The authors reported that increased concentrations of ambient particulates (PM2.5 and PM10) were strongly associated with higher myocardial infarction risk. In industrialized Australia, the 24-h PM2.5 was reported to be 8.1–11.0 µg/m3, whereas in developing Brazil,[24] the PM10 concentration was 48.34 µg/m3; both were much lower than reported in this study. In Asia, Taiwan has an average PM10 concentration of 77.93 µg/m3,[25] also much lower than the concentration in the present study. Even in these conditions, positive relationships between particulate matter levels and AMIs have been reported. Therefore, there is no doubt that particulate matter is an independent risk factor for AMI admissions.
In our study, a significant association was also demonstrated between elevated CO levels and hospital admissions for AMIs. CO is a well-recognized cardiovascular toxin. Allred, et al.[26] reported that even low levels of carboxyhemoglobin exacerbated myocardial ischemia in subjects with coronary artery disease. Several studies have suggested an association between ischemic heart disease mortality and CO exposure.[19],[20],[27],[28] The effect of CO on cardiovascular disease is considered to be related to CO replacing oxygen in the blood stream.
Our study failed to indicate an association between increased daily AMI admissions and higher NO2 levels, unlike in previous studies.[19],[20] The reason for this difference is unclear, but may be related to ambient air differences between Shanghai and Western cities.
An association between SO2 exposure and AMI admissions has been previously reported in Brazil.[24] In that study, SO2 showed a significant association with AMI admissions, particularly when the average concentration of SO2 was 14.75 ± 8.16 µg/m3. In the present study, the concentration of SO2 was higher, but did not demonstrate a relationship to AMI occurrences. This discrepancy between SO2 increases and AMI hospitalizations may also be related to ethnicity and differences between countries and the amount of vehicular traffic. The weak relationship between AMI admissions and temperature and relative humidity strongly suggested that the association between air pollutants and AMIs is not confounded by meteorological factors.
More AMIs occurred during the 6:00–18:00 time frame, when people were more likely to be outdoors, than during the 18:00–6:00 period. Additionally, during a 3-month of the study period (November, December and January), the outdoor pollution level was particularly high (data was not shown). These observations suggest that more outdoor activities result in greater whole body exposures to air pollutants and subsequently resulted in increased AMI hospital admissions.
Experimental evidence has revealed plausible biological mechanisms underlying the relationship between air pollution and the occurrence of cardiovascular disease.[22] One suggested pathway involves the initiation of pulmonary and systemic oxidative stress and inflammation by pollutants. Subsequently, a cascade of physiological responses occurs that triggers cardiovascular events. These responses include high coagulability, high platelet activity, cardiac arrhythmias, acute vascular dysfunction and plaque instability.
Our study was conducted in a subtropical city and the population was racially homogeneous compared with other Chinese populations. Therefore, the study conclusions can be generalized to the whole of southern China because of the similar racial and economic characteristics of the populations and the similar meteorological conditions within the area.
In conclusion, this study provides evidence of the association between short-term exposure to air pollutants, including PM2.5, PM10, CO and hospital admissions due to AMIs. There is good reason to believe that lowering air pollution levels will lead to fewer hospital admissions due to AMIs.
Acknowledgments
The authors extend sincere thanks to the patients for their willingness to participate in the study. This study was supported by Shanghai Pudong New Area Health and Family Planning Commission (PW2014A-16).
References
- 1.Bhaskaran K, Hajat S, Armstrong B, et al. The effects of hourly differences in air pollution on the risk of myocardial infarction: case crossover analysis of the MINAP database. BMJ. 2011;343:d5531. doi: 10.1136/bmj.d5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonne C, Wilkinson P. Long-term exposure to air pollution is associated with survival following acute coronary syndrome. Eur Heart J. 2013;34:1306–1311. doi: 10.1093/eurheartj/ehs480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson RW, Carey IM, Kent AJ, et al. Long-term exposure to outdoor air pollution and incidence of cardiovascular disease. Epidemiology. 2013;24:44–53. doi: 10.1097/EDE.0b013e318276ccb8. [DOI] [PubMed] [Google Scholar]
- 4.Madrigano J, Kloog I, Goldberg R, et al. Long-term exposure to PM2.5 and incidence of acute myocardial infarction. Environ Health Perspect. 2013;121:192–196. doi: 10.1289/ehp.1205284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufman JD, Adar SD, Allen RW, et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Am J Epidemiol. 2012;176:825–837. doi: 10.1093/aje/kws169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wichmann J, Folke F, Torp-Pedersen C, et al. Out-of-hospital cardiac arrests and outdoor air pollution exposure in Copenhagen, Denmark. PLoS One. 2013;8:e53684. doi: 10.1371/journal.pone.0053684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuvolone D, Balzi D, Chini M, et al. Short-term association between ambient air pollution and risk of hospitalization for acute myocardial infarction: results of the cardiovascular risk and air pollution in Tuscany (RISCAT) study. Am J Epidemiol. 2011;174:63–71. doi: 10.1093/aje/kwr046. [DOI] [PubMed] [Google Scholar]
- 8.Beelen R, Stafoggia M, Raaschou-Nielsen O, et al. Long-term exposure to air pollution and cardiovascular mortality: an analysis of 22 European cohorts. Epidemiology. 2014;25:368–378. doi: 10.1097/EDE.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh YL, Yang YH, Wu TN, et al. Air pollution and hospital admissions for myocardial infarction in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health A. 2010;73:757–765. doi: 10.1080/15287391003684789. [DOI] [PubMed] [Google Scholar]
- 10.Goggins WB, Chan EY, Yang CY. Weather, pollution, and acute myocardial infarction in Hong Kong and Taiwan. Int J Cardiol. 2013;168:243–249. doi: 10.1016/j.ijcard.2012.09.087. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF task force for the redefinition of myocardial infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 12. USEPA Uniform air quality index (AQI) and daily reporting 2015. US Environmental Protection Agency Homepage. http://www.Gpo.gov/fdsys/pkg/CFR-2013-title40-vol6/xml/CFR-2013-title40-vol6-part58-appG.xml (accessed June 24, 2015) [Google Scholar]
- 13.Maclure M. The case-crossover design: A method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 14.Marshall RJ, Jackson RT. Analysis of case-crossover designs. Stat Med. 1993;12:2333–2341. doi: 10.1002/sim.4780122409. [DOI] [PubMed] [Google Scholar]
- 15.Mittleman MA, Maclure M, Robins JM. Control sampling strategies for case-crossover studies: An assessment of relative efficiency. Am J Epidemiol. 1995;142:91–98. doi: 10.1093/oxfordjournals.aje.a117550. [DOI] [PubMed] [Google Scholar]
- 16.Levy D, Lumley T, Sheppard L, et al. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology. 2001;12:186–192. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Pope CA, 3rd, Muhlestein JB, May HT, et al. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 18.Peters A, Dockery DW, Muller JE, et al. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 19.Zanobetti A, Schwartz J. Air pollution and emergency admissions in Boston, MA. J Epidemiol Community Health. 2006;60:890–895. doi: 10.1136/jech.2005.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Ippoliti D, Forastiere F, Ancona C, et al. Air pollution and myocardial infarction in Rome: a case-crossover analysis. Epidemiology. 2003;14:528–535. doi: 10.1097/01.ede.0000082046.22919.72. [DOI] [PubMed] [Google Scholar]
- 21.Kodavanti UP, Schladweiler MC, Ledbetter AD, et al. Temporal association between pulmonary and systemic effects of particulate matter in healthy and cardiovascular compromised rats. J Toxicol Environ Health A. 2002;65:1545–1569. doi: 10.1080/00984100290071667. [DOI] [PubMed] [Google Scholar]
- 22.Brook RD, Franklin B, Cascio W, et al. Expert Panel on Population and Prevention Science of the American Heart Association. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 23.Mustafic H, Jabre P, Caussin C, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307:713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 24.Cendon S, Pereira LA, Braga AL, et al. Air pollution effects on myocardial infarction. Rev Saude Publica. 2006;40:414–419. doi: 10.1590/s0034-89102006000300008. [DOI] [PubMed] [Google Scholar]
- 25.Cheng MF, Tsai SS, Yang CY. Air pollution and hospital admissions for myocardial infarction in a tropical city: Kaohsiung, Taiwan. J Toxicol Environ Health A. 2009;72:1135–1140. doi: 10.1080/15287390903091756. [DOI] [PubMed] [Google Scholar]
- 26.Allred EN, Bleecker ER, Chaitman BR, et al. Short-term effects of carbon monoxide exposure on the exercise performance of subjects with coronary artery disease. N Engl J Med. 1989;321:1426–1432. doi: 10.1056/NEJM198911233212102. [DOI] [PubMed] [Google Scholar]
- 27.Koskela RS, Mutanen P, Sorsa JA, et al. Factors predictive of ischemic heart disease mortality in foundry workers exposed to carbon monoxide. Am J Epidemiol. 2000;152:628–632. doi: 10.1093/aje/152.7.628. [DOI] [PubMed] [Google Scholar]
- 28.Yang W, Jennison BL, Omaye ST. Cardiovascular disease hospitalization and ambient levels of carbon monoxide. J Toxicol Environ Health A. 1998;55:185–196. doi: 10.1080/009841098158485. [DOI] [PubMed] [Google Scholar]