Abstract
The roles of androgens on cardiovascular physiology and pathophysiology are controversial as both beneficial and detrimental effects have been reported. Although the reasons for this discrepancy are unclear, multiple factors such as genetic and epigenetic variation, sex-specificity, hormone interactions, drug preparation and route of administration may contribute. Recently, growing evidence suggests that androgens exhibit beneficial effects on cardiovascular function though the mechanism remains to be elucidated. Endothelial cells (ECs) which line the interior surface of blood vessels are distributed throughout the circulatory system, and play a crucial role in cardiovascular function. Endothelial progenitor cells (EPCs) are considered an indispensable element for the reconstitution and maintenance of an intact endothelial layer. Endothelial dysfunction is regarded as an initiating step in development of atherosclerosis and cardiovascular diseases. The modulation of endothelial functions by androgens through either genomic or nongenomic signal pathways is one possible mechanism by which androgens act on the cardiovascular system. Obtaining insight into the mechanisms by which androgens affect EC and EPC functions will allow us to determine whether androgens possess beneficial effects on the cardiovascular system. This in turn may be critical in the prevention and therapy of cardiovascular diseases. This article seeks to review recent progress in androgen regulation of endothelial function, the sex-specificity of androgen actions, and its clinical applications in the cardiovascular system.
Keywords: Androgen, Cardiovascular diseases, Endothelial cells, Endothelium, Estrogen
1. Introduction
Epidemiological studies suggest that males are at greater risk for cardiovascular disease when compared to age-matched females during their reproductive years.[1]–[3] In the Framingham cohort, men developed cardiovascular diseases (CVD) at more than twice the rate of women in ages younger than 60-years old.[4] This gender difference has been attributed to an estrogen protective effect in women, and/or an androgen detrimental effect in men. However, this hypothesis has not been confirmed in large prospective studies.[5]–[7] More significantly, a growing body of evidence is suggesting the opposite, that androgens exhibit a protective action on the cardiovascular system.[3] Multiple prospective clinical trials and a recent meta-analysis have shown that endogenous androgen level is correlated with mortality and risk factors of CVD.[3],[8]–[10] A reduction in plasma testosterone may contribute to increased arterial stiffness, a risk factor for CVD.[11],[12] Two recent retrospective studies revealed that testosterone therapy is associated with reduced obesity, fat mass, and waist circumference, while improving glycemic control, and reducing mortality. Several retrospective clinical trials in men with coronary artery disease or heart failure have reported improved cardiovascular function in men who received testosterone compared to those treated with placebo.[13]
Androgen withdrawal in men has been associated with decreased central arterial compliance,[14],[15] and increased risk for CVD and mortality.[3],[16] Androgens have been shown to inhibit atherosclerosis through inhibiting carotid intima-media thickness,[17] atheroma formation,[17],[18] and immunomodulation of plaque development and stability.[19] Administration of testosterone has been demonstrated to produce coronary vasodilation, increase the angina threshold in men with coronary artery disease,[20] and induce both endothelium-dependent and independent vasorelaxation.[21] English, et al.[22] discovered that, in men with stable angina, treatment with a testosterone patch increased the time to ST-segment depression on exercise stress testing when compared to placebo. In studying symptomatic hypogonadal men, Malkin, et al.[23] found that, compared with placebo, intramuscular injection of testosterone decreased proinflammatory cytokines (TNFα, IL-1β) and total cholesterol, while it increased anti-inflammatory cytokine IL-10, indicating a less atherogenic state. Moreover, a subjective and objective improvement in hypogonadal men with ischemic heart disease was observed when they were treated with testosterone, as compared to placebo treatment.[24] Taken together, this data suggests that the beneficial effects of androgens are mediated through favorable actions on lipid profile,[25],[26] glycometabolism,[27] inflammation,[28] and haemostatic parameters.[14],[26] This in turn translates into beneficial effects, including anti-angina,[22]–[24] anti-atherosclerosis, vasorelaxation,[21],[29] and increased coronary blood flow.[30]
The understanding of cellular and molecular mechanisms of these beneficial androgen actions on the cardiovascular system will be critical for translation of androgen beneficial effects to the bedside. Since endothelial cells (ECs) and endothelial progenitor cells (EPCs) play a vital role in cardiovascular functions, this review is therefore focused on the current knowledge of androgen effects on ECs and EPCs along with the relevant intracellular mechanisms.
2. Biosynthesis and biological actions of androgens
In the mammalian system, there are two natural potent androgens: testosterone and dihydrotestosterone (DHT).[31] Both testosterone and DHT bind to the same androgen receptor (AR) in the cytoplasm of target cells, and DHT exerts higher affinity to the AR and stronger androgenic activity than testosterone.[32],[33] Testosterone is the primary androgen and is mainly synthesized and secreted by the testes (> 95%) in males. The biosynthesis of testosterone initiates from cholesterol, involving five enzymatic reactions in the process: cholesterol 20,22-desmolase, 3β-hydroxysteroid dehydrogenases, 17α-hydroxylase, 17,20-desmolase, and 17β-hydroxysteroid dehydrogenase.[31] Testosterone can be converted to DHT under the actions of 5alpha-reductase (5αRD) isozymes, mainly in peripheral tissues. Testosterone can also be converted to estrogen by aromatase in the cytoplasm.[34] Testosterone plays an essential role in the anabolic process and androgenic effects in the male,[35],[36] while DHT is critical in the formation of male external genitalia, prostate development and growth, and hair growth.[37] Interestingly, endothelium of the cerebral blood vessels also expresses sex steroid receptors and enzymes that metabolize sex steroids. Gonzales, et al.[38] found that estrogen receptor (ER) alpha, AR, 5αRD-2 and aromatase were expressed in the endothelium of cerebral arteries, suggesting that local production of 17β-estradiol and DHT can occur within the cerebral arteries. The expression of aromatase that converts testosterone to 17β-estradiol alters the local balance of androgenic and estrogenic influences. Thus, the activity of cerebral vessels is affected by circulating sex hormones as well as locally produced sex steroids. The differential expression of hormone effectors in the vascular system may have important implications in the gender differences of cerebrovascular physiology and pathophysiology.[39]
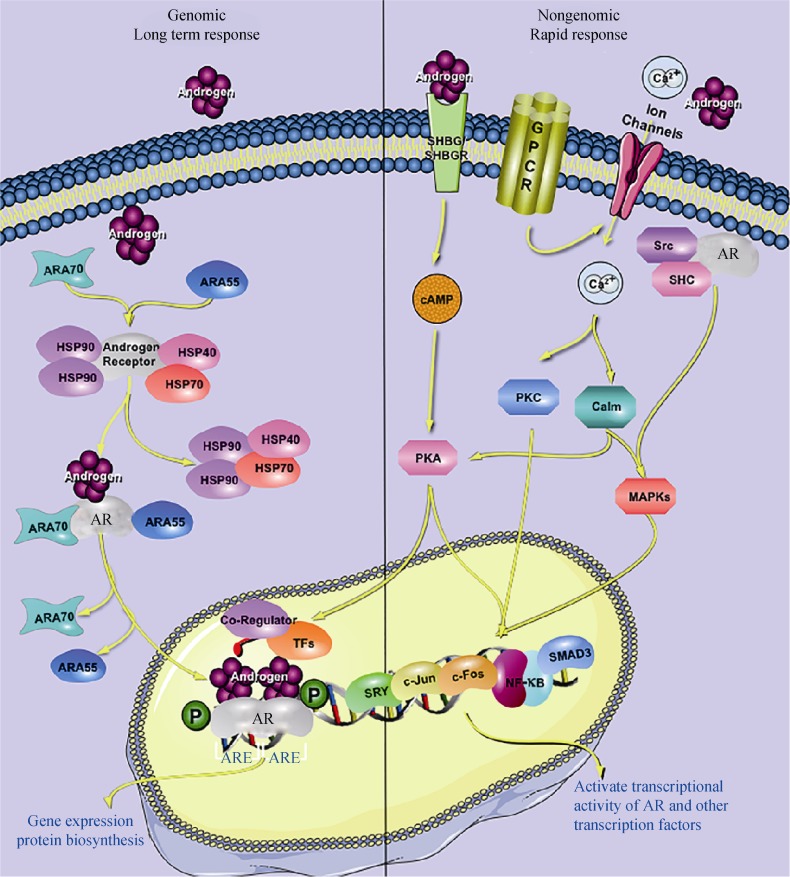
The biological actions of androgens are mainly mediated through binding to the AR, the classical genomic pathway (see Figure 1). Androgen binding to the AR results in an AR conformational change that promotes the dissociation of chaperone proteins and facilitates receptor dimerization, nuclear transportation, phosphorylation, and DNA binding.[40] Upon the recruitment of co-regulators and general transcription factors, the transcription of target-genes is either induced or inhibited, leading ultimately to changes in androgen-target gene expression, and cellular or biological structures and functions.[32],[41] This process usually takes hours before resulting in biological changes in target cells.
Figure 1. The androgen signal pathways.
The steroidal androgens, testosterone and DHT, mediate their biological effects predominantly through genomic pathway by binding to the AR and translocation into the nucleus, thereby facilitating the ability of AR to bind to its cognate response element, and recruiting coregulators to regulate the expression of target genes (left side). The nongenomic stimulation of second messenger cascades by androgens exerts biological effects through modulation of the transcriptional activity of AR or other transcription factors (right side). AR: androgen receptor; ARA: androgen receptor activator; ARE: androgen response element; cAMP: cyclic adenosine monophosphate; DHT: dihydrotestosterone; GPCR: G-protein coupled receptor; HSP: heat shock protein; MAPKs: mitogen-activated protein kinases; PKA: protein kinase A; PKC: protein kinase C; SHBG: steroid hormone binding globulin; SHBGR: SHBG receptor; Src: tyrosine kinase C; SHC: Src homology 2 domain-containing.
In addition to this classical genomic pathway, a rapid nongenomic pathway that is independent of DNA transcription has been demonstrated for androgen actions.[42]–[44] This nongenomic pathway of androgen actions may occur through multiple receptors. Androgens may activate cyclic adenosine monophosphate (cAMP) and protein kinase-A (PKA) through the sex hormone binding globulin (SHBG)/SHBG receptor complex,[45] or increase intracellular Ca2+ levels through a G-protein coupled receptor non-voltage-gated Ca2+ channel couple mechanism.[46]–[48] The elevation of intracellular calcium activates multiple signal transduction cascades, including PKA,[46],[49],[50] Protein kinase C,[51]–[53] and Mitogen-Activated Protein Kinase (MAPKs),[53]–[55] leading to a modification of AR activity and other transcription factors. AR may also interact with the intracellular tyrosine kinase C (Src), triggering cSrc activation and subsequent biological actions. The rapid stimulation of second messenger cascades by androgens via the nongenomic pathway may ultimately result in biological functions through posttranslational modulation of AR or other transcription factors. Since these posttranslational modifications do not involve gene transcription and new protein biosynthesis, it may take only seconds or minutes to occur. AR can also be activated by various growth factors, such as epithelial growth factor (EGF) and insulin-like growth factor-1(IGF-1), in the absence of its cognate ligands, which may also alter AR activity via posttranslational modification.[32] Whether the biological activity of androgens is mediated through the genomic or nongenomic pathway depends on cell type and AR-ligand.[56] The identification of molecules related to androgen-AR functions may facilitate the understanding of the cellular and molecular mechanisms of androgen actions.
3. Effects and mechanisms of androgens in the endothelium
Endothelium damage is thought to be an initiating step in formation of blood clotting, plaques, and atherosclerosis. It causes an imbalance between vasodilating and vasoconstricting substances produced by or acting on the vascular wall, resulting in increased arterial stiffness.[57]–[59] With mounting knowledge in vascular homoeostasis, EPCs, especially circulating EPCs, are considered an indispensable element for the reconstitution and maintenance of an intact endothelial layer.[60] The number and function of EPCs are correlated inversely with cardiovascular risk factors, and an increased level of EPCs is associated with a reduced risk of death from cardiovascular causes.[61] The deficiency of EC and EPCs indicates an inadequate restoration of endothelium in pathological conditions that are underlying the development and progression of CVD. Since the endothelium plays a critical role in the cardiovascular system, the modulation of endothelial functions by androgens may be a promising mechanism for mediating androgen actions on cardiovascular physiology and pathophysiology.
3.1. Androgen regulation of EC proliferation and its modification by estrogens
Both DHT and testosterone have been shown to regulate cell proliferation and function via either a classical genomic pathway or nongenomic pathway in endothelial cells from a variety of origins.[62] Somjen, et al.[63],[64] demonstrated that DHT at doses ranging from 3 to 3000 nmol/L produced a dose-dependent stimulation of [3H] thymidine incorporation and an activation of creatine kinase and MAPKs in a human endothelial cell line, ECV304. These androgen effects were blocked by the addition of flutamide, an AR-antagonist, indicating an AR-related mechanism. We have recently shown that both DHT and testosterone at doses within physiological range stimulated cell growth via an AR-VEGF (vascular endothelial growth factor)/cyclins mediated pathway in human primary aortic endothelial cells (HAECs).[65] Both DHT and testosterone produced a time- and dose-dependent increase in viable cell number and DNA biosynthesis in HAECs. This effect was blocked by the concomitant administration of casodex, a specific AR-antagonist, and by knockdown of AR using specific small interfering RNAs. This androgen action is mediated through upregulation of VEGF expression (Figure 2), which then upregulates cyclin expression in an autocrine fashion, resulting in the stimulation of cell proliferation.[65] Although 5α-reductase isozymes are expressed in HAECs, the effect of testosterone on HAEC proliferation is independent of DHT and estradiol since the testosterone effect was not blocked by dutarsteride, a specific 5α-reductase inhibitor that blocks the conversion of testosterone to DHT (Figure 3). In addition, 17β-estradiol does not stimulate cell proliferation in HAECs originated from males (Figure 4).[65] More recently, Campelo, et al.[66] reported that testosterone via a membrane-AR dependent mechanism enhanced cell growth through direct action on endothelial nitric oxide (NO) production in female rat ECs. Regardless of the exact mechanism by which androgen enhances EC growth, the stimulation of EC proliferation by direct androgen actions in the vascular system should assist in repair of endothelial injury and prevent endothelium impairments and dysfunction – a primary risk factor of vascular stiffness, hypertension, and atherosclerosis.
Figure 2. Illustration of androgen regulation of ECs via autocrine and paracrine mechanisms and its modification by estrogens.
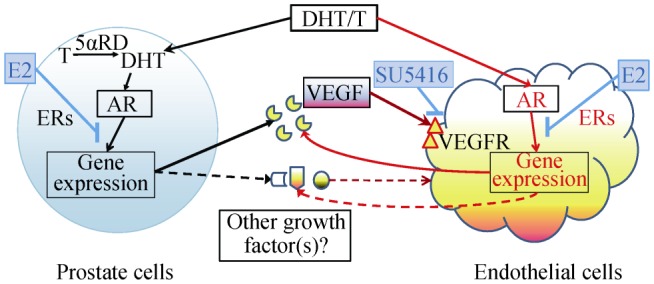
Androgens, T and DHT, regulate EC growth and functions through the regulation of gene expression of VEGF and other growth factors either directly in ECs (autocrine) or indirectly in other androgen-target cells such as prostate epithelial cells (paracrine). AR: androgen receptor; DHT: dihydrotestosterone; ECs: endothelial cells; ERs: estrogen receptors; E2: estrogens; SU5416: a specific VEGFR antagonist; T: testosterone; VEGF: vascular endothelial growth factor; VEGFR: VEGF receptor; 5αRD: 5alpha-reductases.
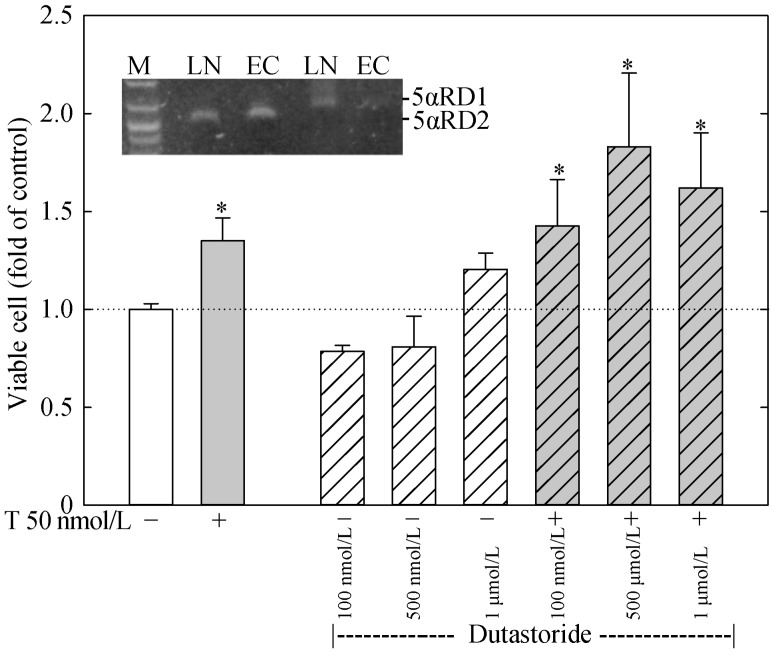
Figure 3. Dutarsteride fails to affect T stimulation of ECs growth.
HAECs were plated in 96-wells and treated with or without T (50 nmol/L) and various doses of dutarsteride, a specific 5alpha-reductase inhibitor, for 48 h. The number of viable cells was determined. The insert shows a RT-PCR analysis of 5αRD1 and 5αRD2 expression in ECs and LN. *P < 0.05 compared to untreated control. ECs: human aortic endothelial cells; LN: LNCaP prostate cancer cells; M: makers; T: testosterone; 5αRD1/2: 5alpha-reductase type 1 or type 2.
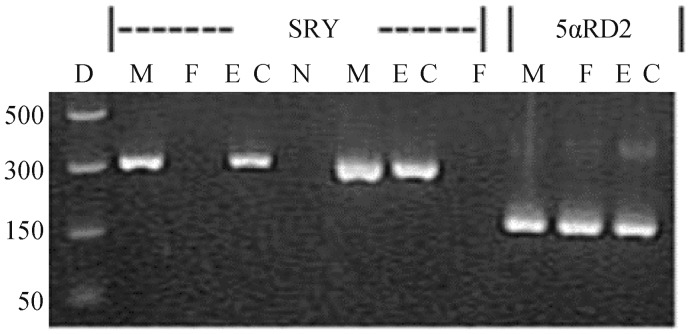
Figure 4. The determination of male originality of ECs by PCR analysis.
Genomic DNA was isolated from M, F, or ECs and subjected to PCR amplification of the SRY gene (two specific primer pairs), a specific Y chromosome gene, and the 5alpha-reductase-2 gene (one specific primer pair). PCR products were analyzed by agarose gel electrophoresis. ECs: human aortic endothelial cells; F: females; Lane D: DNA size markers, M: males; N: negative control; 5αRD2: 5alpha-reductase type 2.
It is well known that ECs possess significant heterogeneity in the vascular system. Furthermore, the endothelium represents a group of small enterprises of cells located within blood vessels of different tissues where each enterprise is uniquely adapted to meet the demands of the underlying tissue.[67] It has been shown that AR is not expressed in the ECs from microvessels of some organs such as the prostate gland and skin, and androgen is therefore unable to stimulate EC growth directly via AR.[68]–[70] However, androgen is able to affect EC proliferation indirectly through a paracrine fashion (Figure 2). By using prostate cancer cells and ECs from mouse skin, we have demonstrated that androgen was able to upregulate VEGF expression in prostate cancer cells, which was secreted from these cancer cells and stimulated EC proliferation.[70],[71] This paracrine action may be an important mechanism in synchronizing angiogenesis and tumor growth in tumor progression.
It is worthwhile to note that both direct and indirect androgen actions on EC proliferation (see Figure 2) may be modulated by other hormones such as estrogens.[70],[71] We have shown that estrogens via ERs produce a ligand, receptor-isoform and cell specific modulation of androgen actions in multiple systems.[72]–[75] In ECs that express both AR and ERs, estrogens produce an ER-dependent modification of androgen actions on gene expression and cell proliferation in an ER-ligand specific manner.[71] On the other hand, in ECs that do not express AR, estrogens were able to modulate androgen-induced paracrine stimulation of cell growth via modification of androgen actions in paracrine cells.[70] Although VEGF as either an autocrine or paracrine hormone is the major factor in stimulating EC proliferation, other factors that remain to be elucidated may be involved in the androgen-estrogen regulation of gene expression and cell functions in ECs (see Figure 2).[70],[76] The concept of ER-ligand, ER-isoform, and cell specific modulation of androgen actions by estrogens may have significant application for anti-androgen therapy of prostate cancer by estrogen analogs to maximize anti-tumor activity and to minimize cardiovascular side effects,[72],[77],[78] as well as for understanding the biology of sex steroid interaction in cardiovascular physiology and pathophysiology.[79],[80]
3.2. Androgen regulation of EPC proliferation
The role of androgens in the cardiovascular system may also be displayed through regulation of EPC growth and functions (Figure 5). EPCs derived from bone marrow play an important role in vascular repair, angiogenesis, and replacement of damaged endothelial cells of blood vessels.[57]–[59] The circulating levels of EPCs correlate inversely with cardiovascular risk factors, and an increased level of EPCs is associated with a reduced risk of death from cardiovascular causes.[61],[81],[82] Moreover, EPC infusions may improve the outcome of the patients with cardiovascular diseases.[61],[83],[84] It is interesting to note that circulating EPC levels are correlated with plasma androgen concentrations.
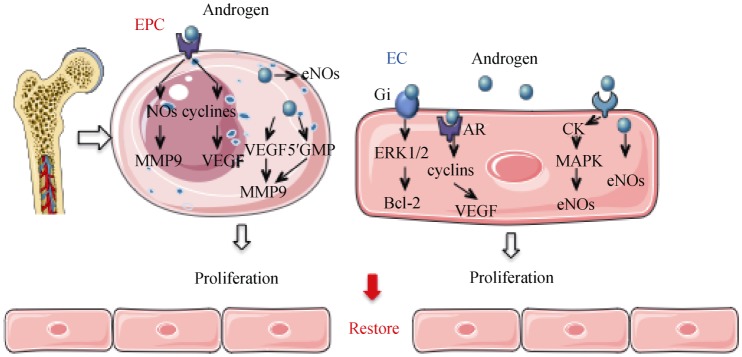
Figure 5. Potential mechanisms of androgens in regulating EC and EPC proliferation.
In ECs, androgen diffuses into the cell directly activating a cascade of signaling creatine kinase and MAPK. The AR ligand may upregulate VEGF and cyclins through the genomic pathway. Androgen may also induce eNOS synthesis. In EPC, androgens may either upregulate VEGF and mitotic cyclins or increase the expression of MMP-9 and NO in the cytoplasm. Androgen activates a cascade of signaling resulting in increased NO and VEGF. AR: androgen receptor; CK: creatine kinase; EC: endothelial cells; EPC: endothelial projenitor cells; ERK1/2: extracellular signal-regulated kinase 1/2; eNOs: endothelial nitric oxide synthase; Gi: inhibitory regulative G-protein; MAPK: mitogen-activated protein kinases; MMP9: matrix metallopeptidase 9; NOs: nitric oxide synthase; VEGF: vascular endothelial growth factor.
A few of studies have demonstrated that, compared to normal controls, the circulating levels of EPCs were lower in patients with hypogonadotrophic hypogonadism, a condition with low plasma androgen levels, which was restored by testosterone replacement therapy (TRT).[85],[86] Furthermore, AR is detected in human EPCs, and androgens via AR are able to stimulate EPC proliferation, migration, and colony formation.[85],[87] Recently, it has been suggested that androgens regulate EPC proliferation and adhesion through the phosphatidylinositol 3-OH kinase (PI3K)/Akt pathway.[88] The effects of androgens on EC and EPC proliferation and function may be a significant factor mediating the beneficial actions of androgens in the cardiovascular system in males, and may explain findings that low levels of circulating androgens are associated with increased CVD morbidity and mortality in males.[88]
3.3. Androgens on endothelium-related vasodilatation and vasoconstriction
Endothelial tissue also plays a role in the regulation of vasoconstriction and vasodilatation, and hence the control of blood pressure. Although many studies have reported that androgens attenuate resistance of arteries predominantly by activation of K+ channels and/or blockade of Ca2+ channels in vascular muscle cells, a growing number of studies have revealed that androgens induce vasodilation in resistance vessels mediated through endothelium-derived NO (see Figure 6).[89],[90] Studies on in vitro ECs have found that dehydroepiandrosterone (DHEA) rapidly increased the expression of endothelial nitric oxide synthase (eNOS) and the activity of extracellular-signal-regulated kinases 1/2 (ERK1/2) via nongenomic pathway, which may increase NO secretion, leading to an increased flow-mediated dilation in vivo.[91] Moreover, Simoncini, et al.[92] revealed that DHEA triggered a G protein coupled receptors-ERK1/2-MAPK cascade to regulate eNOS protein synthesis in human umbilical venous endothelial cells (HUVECs); DHEA also initiated a prolonged genomic action to increase eNOS protein and NO synthesis. Goglia, et al.[93] revealed that both testosterone and DHT when administered at physiological concentrations, produced dose-dependent genomic and nongenomic actions in HUVECs. Androgens rapidly increased endothelial synthesis of NO in HUVECs, which is mediated through a rapid recruitment of ERK1/2 and PI3K/Akt cascades.[93] It is worthy to note that both the genomic and nongenomic actions of testosterone and DHT were mediated via AR as evidenced by the fact that they were blocked by flutamide, a specific AR-antagonist, and the fact that testosterone effects were also mediated through conversion to estradiol.[93] Similar androgen effects have been reported by Yu, et al.[94] in HAECs.
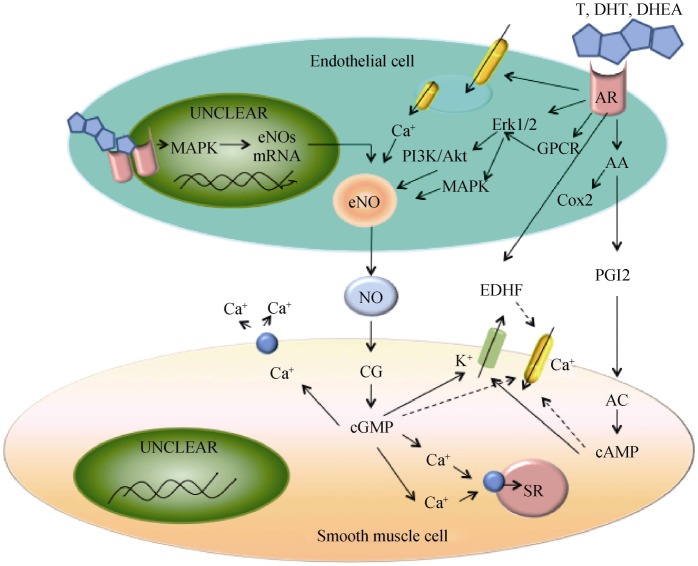
Figure 6. The mechanisms of androgen-induced endothelium-dependent VSM relaxation.
T binds to endothelial cytosolic AR, leading to an activation of MAPK, gene transcription, endothelial cell proliferation, and eNOS production. Androgen also binds to endothelial surface membrane ARs, which is coupled to an increased Ca2+ release from the endoplasmic reticulum and a stimulation of the MAPK/Akt pathway, leading to an activation of eNOS and NO production. NO diffuses into VSM cells, binds to CG, and increases cGMP production. cGMP causes VSM relaxation by decreasing [Ca2+]i and myofilament sensitivity to Ca2+. Endothelial AR activation may also activate COX2 and increase PGI2 production. PGI2 activates prostacyclin receptors in VSM, resulting in AC activation and cAMP production. cAMP causes VSM relaxation by mechanisms similar to cGMP. AR activation may also increase the production of EDHF, which activates K+ channels and causes hyperpolarization and inhibition of Ca2+ influx via Ca2+ channels, leading to a VSM relaxation. Solid lines depict stimulatory effects and dashed lines indicate inhibitory effects. AA: arachidonic acid; AC: adenylate cyclase; Akt: protein kinase B; APK: mitogen-activated protein kinases; AR: androgen receptor; CG: guanylate cyclase; cGMP: cyclic guanosine monophosphate; COX2: cyclooxygenases; DHEA: dehydroepiandrosterone; DHT: dihydrotestosterone; EDHF: endothelium-derived hyperpolarizing factor; eNO: endothelial nitric oxide; ERK1/2: extracellular signal-regulated kinase 1/2; GPCR: G-protein coupled receptor; NO: nitric oxide; PGI2: prostacyclin; PI3K: phosphatidylinositol 3-OH kinase; SR: sarcoplasmic reticulum; T: testosterone; VSM: vascular smooth muscle.
Intracellular free calcium concentration is considered an important physiological regulator of NO production and vasodilatation. Testosterone binds to specific membrane receptor (s) in endothelial cells and stimulates Ca2+ release from the endoplasmic reticulum, eNOS activity, and NO production. NO activates guanylate cyclase and increases cyclic guanosine monophosphate (cGMP) production, causing vascular smooth muscle (VSM) relaxation by inhibiting Ca2+ influx and stimulating Ca2+ extrusion. Testosterone may also increase the release of endothelium-derived hyperpolarizing factor (EDHF), open large-conductance K+ channels, cause VSM hyperpolarization and inhibit Ca2+ influx via Ca2+ channels.[95] This testosterone effect may be mediated, at least in part, via its aromatization to 17β-estradiol.[96],[97]
It has been reported that the androgen effect on vasodilation may be mediated through modulation of arachidonic acid metabolism although the contribution of arachidonic acid metabolites to cardiovascular function is still controversial.[98]–[100] Marrachelli, et al.[98] found that testosterone induced an acute vasorelaxation that is dependent on endothelium-derived eNOS, iNOS, and prostacyclin. It has been shown that androgen induction of cyclooxygenase-2 was accompanied by a preferential increase in prostacyclin synthesis in regulation of vascular homeostasis without an impact on thromboxane A2 (TXA2) production,[101],[102] which would favor vasodilation. Most recently, androgens have been demonstrated to induce vasodilation via induction of hydrogen sulphide, a gaseous mediator involved in cardiovascular homeostasis, mediated through an AR-hsp90-cystathionine-γ lyase pathway.[103] Moreover, androgens have also been shown to increase arachidonic acid metabolites such as 20-hydroxyeicosatetraenoic acid and TXA2, leading to an endothelium-dependent vasoconstriction,[99],[100],[104] which results in a detrimental effect on the cardiovascular system (Figure 6).
Androgens may also modulate the production and secretion of endothelium-derived contracting factors such as endothelin-1 (ET-1) and TXA2 to regulate vascular functions. A cross-sectional study revealed a positive association between plasma ET-1, T, and blood pressure in postmenopausal women.[105] Meanwhile, studies in female-to-male transsexual subjects who received large doses of testosterone have shown high plasma levels of ET-1 in these subjects.[106] However, the association between androgens and ET-1 production is still obscure. It has been reported that plasma endothelin level was increased in males with hypogonadism,[107] and seemed to decrease after testosterone administration,[108] suggesting that androgens may decrease ET-1 production.
3.4. Androgens on endothelium-related inflammation
It has been documented that inflammation is involved in the process of endothelial dysfunction and impairment, and that androgens play a role in both pro-inflammation and anti-inflammation in the cardiovascular system.[90],[109] Clinical studies have suggested that the elevation of inflammatory biomarkers is associated with endothelial cell dysfunction and CVD, and that there is a positive association between circulating androgens and inflammatory biomarker levels in humans.[1],[2],[105] Androgens have been shown to produce a dose-dependent induction of vascular cell adhesion molecule-1 (VCAM-1) expression and monocyte cell adhesion and to potentiate tumor necrosis factors (TNF)-induced VCAM-1 and cell adhesion molecule E-selectin expression and monocyte cell adhesion in ECs, which are presumably mediated through the activation of the transcriptional nuclear factor-kappa B (NF-κB).[110]–[114] In contrast, androgens have also been reported to produce anti-inflammatory effects by promoting endothelial cell survival, reducing endothelial expression of pro-inflammatory markers, and inhibiting proliferation and intimal migration of vascular smooth muscle cells. Norata, et al.[115] reported that incubation of human ECs with DHT or testosterone reduced the inflammatory response induced by TNF-α and lipopolysaccharide, which was mediated by AR and resulted in a decreased expression of VCAM-1, intercellular adhesion molecule-1 (ICAM-1), IL-6, monocyte chemoattractant protein-1 and proteases. These androgen effects were probably mediated through inhibition of the NF-κB signaling pathway. Furthermore, androgens have been reported to inhibit the expression of caspase-3, caspase-9, and phosphor-p38 MAPK induced by oxidative stress and attenuate cell apoptosis in HUVECs.[111],[112],[116] Although the reasons for these apparent discrepant findings are unclear, differences in the origins of ECs, the gender of donors, and the relevant variations of intrinsic molecules such as hormone receptors and hormone metabolic enzymes may account, at least in part, for the underlying mechanisms.
3.5. Androgens on endothelium-related anticoagulant
Tissue plasminogen activator (tPA), plasminogen activator inhibitor type 1 (PAI-1), and tissue factor pathway inhibitor (TFPI) are critical molecules released from ECs in the regulation of anticoagulant activity. Studies in humans have revealed that total and free testosterone levels are independent predictors of plasma TFPI concentrations and a low testosterone level in elderly men is associated with a low free TFPI antigen and a shortened initiation phase of TF-induced coagulation.[117] Moreover, human serum testosterone levels are positively correlated with tPA levels and negatively correlated with PAI-1 and clotting factor VII concentrations.[118]–[120] These findings are further supported by in vitro cell culture studies in which testosterone has been shown to increase TFPI and tPA expression while inhibiting PAI-1 secretion in the endothelium.[121] This data supports the concept that androgens possess anticoagulant activity, resulting in a protective action on the cardiovascular system.
4. Gender specificity of androgen actions in endothelium
Gender disparity in the incidence and progression of CVD indicates an intrinsic sexual dimorphism in the cardiovascular system, which is determined not only by gender-related differences in sex steroid levels, but also by gender-specific tissue, cellular, and molecular differences that mediate gender-specific responses. The effect of sex steroids on the cardiovascular system are complex via either direct or indirect influences on a multitude of cardiovascular biological processes, often in a gender-specific manner.[79] Accumulating evidence has demonstrated that both androgens and estrogens regulate various endothelium biological processes in a sex-dependent fashion.[79],[113],[122],[123] Regarding the actions of androgens, both DHT and testosterone have been shown to induce gene expression and cell proliferation via multiple molecular mechanisms in the ECs of males but not females.[65],[123],[124] Maddox, et al.[125] found that the vascular rings from female rat aorta were much less sensitive and less contractile to prostaglandin F2 alpha (a vasoconstrictor) stimulation than those from male rats, which was endothelium-dependent and affected by pretreatment with testosterone and estradiol. McCrohon, et al.[113] reported that DHT increased human monocyte adhesion to vascular endothelium, mediated at least in part through an AR-dependent upregulation of VCAM-1 expression in ECs originating from males but not females. This sex-specific androgen effect may be related to an approximately two to five fold higher AR expression in ECs from males compared to those from females.[112],[126] However, a recent study by Annibalini, et al.[114] failed to demonstrate any gender-specific androgen action on TNF-α stimulated inflammatory responses. They showed that the inflammatory effect of TNF-α on VCAM-1, ICAM-1, and E-selectin gene expression and on cell adhesion was amplified by co-administration of testosterone or DHT while 17β-estradiol had no effect in HUVECs from both males and females under identical culture conditions. In sum, accumulating evidence supports the concept that androgens produce direct biological functions in ECs in a gender-specific manner though there is some inconsistent data. Further investigations are warranted to delineate the underlying reasons for these conflicting reports and to elucidate the clinical application of sex-specific endothelial actions of androgens.
5. Summary and prospective
The recent flourish of research into androgens in the cardiovascular system has made significant progress in revealing androgen effects and mechanisms. Findings suggest that androgens via genomic and/or nongenomic pathways possess significant effects on cell proliferation and migration, vessel contractility, and pathogenic processes such as inflammation, atherosclerosis, and clotting in the endothelium. Although a number of studies indicate that androgens drive beneficial effects in the endothelium, reports espousing the opposed conclusion are equally presented. The reasons for these apparent discrepancies are unclear although sex specificity of androgen actions and heterogeneity of ECs or endothelium are major factors to be considered. To further cloud the issue, the mechanisms of direct androgen actions on ECs or endothelium are poorly understood. Although it is clearly documented that androgens are able to interact with AR to modulate EC function, proliferation, and gene expression via the classical genomic pathway, there is also evidence for nongenomic androgen actions via various second messenger pathways or cascades without involvement of AR. Furthermore, significant species and sex differences, in addition to temporal and spatial variations of AR expression in ECs, EPCs, or endothelium have been documented. These may account, at least in part, for the conflicting androgen actions observed in the endothelial system.[62],[70] Further studies with particular attention to experimental conditions that consider intrinsic EC differences such as the temporal and spatial origin of endothelium, sex and species specificity, and genetic variation in molecules along androgen signal pathways are necessary to clarify these discrepancies.
The role of androgens in cardiovascular system in humans is equally controversial. Although TRT of male hypogonadism has been available since 1939, the benefits and safety of such therapy have become a major debate recently. Numerous meta-analyses suggest that most data supports the concept that endogenous normal testosterone and TRT, when used in physiological concentrations, displays favorable cardiovascular impact without an increased risk of adverse cardiovascular events or mortality, in both men and women.[13],[127]–[130] However, two recent studies have raised new concerns about cardiovascular risk with TRT. The TOM trial, designed to investigate the TRT effect on frailty in elderly men, was prematurely terminated due to an increased incidence of cardiovascular events in the treatment arm.[131] A retrospective study, which was designed to assess the association between TRT and all-cause mortality, myocardial infarction, or stroke among male veterans, and to determine whether this association is modified by underlying coronary artery disease, has shown an increased risk of adverse outcomes in men treated with testosterone.[132] A most recent meta-analysis including data from 1940 to 2014 have found only four articles, including the two mentioned above, that indicated increased cardiovascular risks with TRT. On the other hand, dozens of reports, including randomized clinical trials and meta-analyses, suggest beneficial effects of endogenous normal androgens and TRT. While the reasons for this discrepancy are not clear, multiple factors should be considered such as sex difference, hormone interactions, routes of drug administration, androgen preparation, and individual genetic variations related to androgen pharmacokinetics and pharmacodynamics. The influence of sex and hormone milieu on androgen actions has been well documented as discussed above. The preparation and route of testosterone administration have also shown significant impact on androgen actions. Oral testosterone administration results in increased cardiovascular risks when compared to intramuscular and transdermal administration.[130] One explanation for discrepancies in these data is the individual genetic polymorphisms of participants in these trials, which may affect the disposition and efficacy of androgens. Individuals treated with the same regimen of TRT have shown large intra- and inter-individual variations in serum total testosterone and free testosterone levels, partially due to polymorphisms of genes related to testosterone disposition such as SHBG, phosphodiesterase (PDE7B) and uridine 5′-diphospho-glucuronosyltransferase.[133]–[135] Moreover, genetic polymorphisms along androgen signal pathways have been found to have a significant impact on androgen efficacy.[136] Genetic polymorphisms of AR gene have been demonstrated to alter the efficacy of androgens,[137],[138] and may serve as a useful genetic marker(s) for the assessment of cardiovascular risk.[139] With advances in molecular genetic technology and the emphasis of personalized/precision medicine, more genetic polymorphisms related to androgen disposition and efficacy will be revealed. Long-term, well-designed prospective trials with large cohorts and careful consideration of individual genetic polymorphisms are required to verify the cardiovascular efficacy/safety of androgens in both men and women. Until then, current data strongly suggests a beneficial relationship between normal androgens and cardiovascular health, a finding that remains to be widely appreciated.
Acknowledgments
The researches described in this article were partially supported by grants from the National Natural Science Foundation of China (No. 81570271 and 81400357) and NIH (UL1 RR024996). We are very grateful to John R Lee (Assistant Professor of Medicine, Weill Cornell Medical College, New York), and Jeff J Zhu (Research Manager, Weill Cornell Medical College, New York) for critical review of the article. The authors have nothing to disclosure.
References
- 1.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 2.Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24:183–217. doi: 10.1210/er.2001-0025. [DOI] [PubMed] [Google Scholar]
- 3.Nettleship JE, Jones RD, Channer KS, et al. Testosterone and coronary artery disease. Front Horm Res. 2009;37:91–107. doi: 10.1159/000176047. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Hjortland MC, McNamara PM, et al. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 5.Bernini GP, Moretti A, Sgro M, et al. Influence of endogenous androgens on carotid wall in postmenopausal women. Menopause. 2001;8:43–50. doi: 10.1097/00042192-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Hendrix SL, Wassertheil-Smoller S, Johnson KC, et al. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Tivesten A, Vandenput L, Labrie F, et al. Low serum testosterone and estradiol predict mortality in elderly men. J Clin Endocrinol Metab. 2009;94:2482–2488. doi: 10.1210/jc.2008-2650. [DOI] [PubMed] [Google Scholar]
- 9.Kintzel PE, Chase SL, Schultz LM, et al. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy. 2008;28:1511–1522. doi: 10.1592/phco.28.12.1511. [DOI] [PubMed] [Google Scholar]
- 10.Smith MR. Androgen deprivation therapy for prostate cancer: new concepts and concerns. Curr Opin Endocrinol Diabetes Obes. 2007;14:247–254. doi: 10.1097/MED.0b013e32814db88c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dockery F, Bulpitt CJ, Donaldson M, et al. The relationship between androgens and arterial stiffness in older men. J Am Geriatr Soc. 2003;51:1627–1632. doi: 10.1046/j.1532-5415.2003.51515.x. [DOI] [PubMed] [Google Scholar]
- 12.Hougaku H, Fleg JL, Najjar SS, et al. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Metab. 2006;290:E234–E242. doi: 10.1152/ajpendo.00059.2005. [DOI] [PubMed] [Google Scholar]
- 13.Morgentaler A, Miner MM, Caliber M, et al. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90:224–251. doi: 10.1016/j.mayocp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Dockery F, Bulpitt CJ, Agarwal S, et al. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 2003;104:195–201. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 15.Dockery F, Bulpitt CJ, Agarwal S, et al. Effect of androgen suppression compared with androgen receptor blockade on arterial stiffness in men with prostate cancer. J Androl. 2009;30:410–415. doi: 10.2164/jandrol.108.006924. [DOI] [PubMed] [Google Scholar]
- 16.Treatment and survival of patients with cancer of the prostate. The Veterans Administration Co-operative Urological Research Group. Surg Gynecol Obstet. 1967;124:1011–1017. [PubMed] [Google Scholar]
- 17.Chan YX, Knuiman MW, Hung J, et al. Testosterone, dihydrotestosterone and estradiol are differentially associated with carotid intima-media thickness and the presence of carotid plaque in men with and without coronary artery disease. Endocr J. 2015;62:777–786. doi: 10.1507/endocrj.EJ15-0196. [DOI] [PubMed] [Google Scholar]
- 18.Alexandersen P, Haarbo J, Byrjalsen I, et al. Natural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbits. Circ Res. 1999;84:813–819. doi: 10.1161/01.res.84.7.813. [DOI] [PubMed] [Google Scholar]
- 19.Malkin CJ, Pugh PJ, Jones RD, et al. Testosterone as a protective factor against atherosclerosis--immunomodulation and influence upon plaque development and stability. J Endocrinol. 2003;178:373–380. doi: 10.1677/joe.0.1780373. [DOI] [PubMed] [Google Scholar]
- 20.Rosano GM, Leonardo F, Pagnotta P, et al. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–1670. doi: 10.1161/01.cir.99.13.1666. [DOI] [PubMed] [Google Scholar]
- 21.Kang SM, Jang Y, Kim JY, et al. Effect of oral administration of testosterone on brachial arterial vasoreactivity in men with coronary artery disease. Am J Cardiol. 2002;89:862–864. doi: 10.1016/s0002-9149(02)02202-6. [DOI] [PubMed] [Google Scholar]
- 22.English KM, Steeds RP, Jones TH, et al. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–1911. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 23.Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 24.Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90:871–876. doi: 10.1136/hrt.2003.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gyllenborg J, Rasmussen SL, Borch-Johnsen K, et al. Cardiovascular risk factors in men: The role of gonadal steroids and sex hormone-binding globulin. Metabolism. 2001;50:882–888. doi: 10.1053/meta.2001.24916. [DOI] [PubMed] [Google Scholar]
- 26.Svartberg J, von Muhlen D, Mathiesen E, et al. Low testosterone levels are associated with carotid atherosclerosis in men. J Intern Med. 2006;259:576–582. doi: 10.1111/j.1365-2796.2006.01637.x. [DOI] [PubMed] [Google Scholar]
- 27.Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–3639. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 28.Ng MK, Liu PY, Williams AJ, et al. Prospective study of effect of androgens on serum inflammatory markers in men. Arterioscler Thromb Vasc Biol. 2002;22:1136–1141. doi: 10.1161/01.atv.0000022167.80130.a6. [DOI] [PubMed] [Google Scholar]
- 29.Bernini G, Versari D, Moretti A, et al. Vascular reactivity in congenital hypogonadal men before and after testosterone replacement therapy. J Clin Endocrinol Metab. 2006;91:1691–1697. doi: 10.1210/jc.2005-1398. [DOI] [PubMed] [Google Scholar]
- 30.Qiao X, McConnell KR, Khalil RA. Sex steroids and vascular responses in hypertension and aging. Gend Med. 2008;5(Suppl. A):S46–S64. doi: 10.1016/j.genm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Zhu YS, Katz MD, Imperato-McGinley J. Natural potent androgens: lessons from human genetic models. Baillieres Clin Endocrinol Metab. 1998;12:83–113. doi: 10.1016/s0950-351x(98)80478-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhu YS. Molecular basis of steroid action in the prostate. Cellscience. 2005;1:27–55. doi: 10.1901/jaba.2005.1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breiner M, Romalo G, Schweikert HU. Inhibition of androgen receptor binding by natural and synthetic steroids in cultured human genital skin fibroblasts. Klin Wochenschr. 1986;64:732–737. doi: 10.1007/BF01734339. [DOI] [PubMed] [Google Scholar]
- 34.Meinhardt U, Mullis PE. The aromatase cytochrome P-450 and its clinical impact. Horm Res. 2002;57:145–152. doi: 10.1159/000058374. [DOI] [PubMed] [Google Scholar]
- 35.Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 36.Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009;5:427–448. doi: 10.2147/tcrm.s3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amory JK, Anawalt BD, Matsumoto AM, et al. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on bone mineral density, serum lipoproteins, hemoglobin, prostate specific antigen and sexual function in healthy young men. J Urol. 2008;179:2333–2338. doi: 10.1016/j.juro.2008.01.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzales RJ, Ansar S, Duckles SP, et al. Androgenic/estrogenic balance in the male rat cerebral circulation: metabolic enzymes and sex steroid receptors. J Cereb Blood Flow Metab. 2007;27:1841–1852. doi: 10.1038/sj.jcbfm.9600483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krause DN, Duckles SP, Gonzales RJ. Local oestrogenic/androgenic balance in the cerebral vasculature. Acta Physiol (Oxf) 2011;203:181–186. doi: 10.1111/j.1748-1716.2011.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 41.Quigley CA, De Bellis A, Marschke KB, et al. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- 42.Boonyaratanakornkit V, Scott MP, Ribon V, et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 43.Kousteni S, Bellido T, Plotkin LI, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 44.Migliaccio A, Castoria G, Di Domenico M, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahn SM, Hryb DJ, Nakhla AM, et al. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002;175:113–120. doi: 10.1677/joe.0.1750113. [DOI] [PubMed] [Google Scholar]
- 46.Bagchi G, Wu J, French J, et al. Androgens transduce the G alphas-mediated activation of protein kinase A in prostate cells. Cancer res. 2008;68:3225–3231. doi: 10.1158/0008-5472.CAN-07-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyng FM, Jones GR, Rommerts FF. Rapid androgen actions on calcium signaling in rat sertoli cells and two human prostatic cell lines: similar biphasic responses between 1 picomolar and 100 nanomolar concentrations. Biol Reprod. 2000;63:736–747. doi: 10.1095/biolreprod63.3.736. [DOI] [PubMed] [Google Scholar]
- 48.Mellstrom B, Naranjo JR. Mechanisms of Ca(2+)-dependent transcription. Curr Opin Neurobiol. 2001;11:312–319. doi: 10.1016/s0959-4388(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 49.Kline LW, Karpinski E. Testosterone and dihydrotestosterone inhibit gallbladder motility through multiple signalling pathways. Steroids. 2008;73:1174–1180. doi: 10.1016/j.steroids.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Sadar MD. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem. 1999;274:7777–7783. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 51.Christian HC, Rolls NJ, Morris JF. Nongenomic actions of testosterone on a subset of lactotrophs in the male rat pituitary. Endocrinology. 2000;141:3111–3119. doi: 10.1210/endo.141.9.7662. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Montelongo MC, Marin R, Gomez T, et al. Androgens induce nongenomic stimulation of colonic contractile activity through induction of calcium sensitization and phosphorylation of LC20 and CPI-17. Mol Endocrinol. 2010;24:1007–1023. doi: 10.1210/me.2009-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sen A, O'Malley K, Wang Z, et al. Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J Biol Chem. 285:28787–28795. doi: 10.1074/jbc.M110.134064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Estrada M, Espinosa A, Muller M, et al. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- 55.Gatson JW, Kaur P, Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology. 2006;147:2028–2034. doi: 10.1210/en.2005-1395. [DOI] [PubMed] [Google Scholar]
- 56.Kim SB, Kanno A, Ozawa T, et al. Nongenomic activity of ligands in the association of androgen receptor with SRC. ACS Chem Biol. 2007;2:484–492. doi: 10.1021/cb7000439. [DOI] [PubMed] [Google Scholar]
- 57.Gates PE, Strain WD, Shore AC. Human endothelial function and microvascular ageing. Exp Physiol. 2009;94:311–316. doi: 10.1113/expphysiol.2008.043349. [DOI] [PubMed] [Google Scholar]
- 58.Balakumar P, Kaur T, Singh M. Potential target sites to modulate vascular endothelial dysfunction: current perspectives and future directions. Toxicology. 2008;245:49–64. doi: 10.1016/j.tox.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 60.Fadini GP, Agostini C, Sartore S, et al. Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis. 2007;194:46–54. doi: 10.1016/j.atherosclerosis.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 61.Werner L, Deutsch V, Barshack I, et al. Transfer of endothelial progenitor cells improves myocardial performance in rats with dilated cardiomyopathy induced following experimental myocarditis. J Mol Cell Cardiol. 2005;39:691–697. doi: 10.1016/j.yjmcc.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 62.Torres-Estay V, Carreno DV, San Francisco IF, et al. Androgen receptor in human endothelial cells. J Endocrinol. 2015;224:R131–R137. doi: 10.1530/JOE-14-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Somjen D, Kohen F, Gayer B, et al. Role of putative membrane receptors in the effect of androgens on human vascular cell growth. J Endocrinol. 2004;180:97–106. doi: 10.1677/joe.0.1800097. [DOI] [PubMed] [Google Scholar]
- 64.Somjen D, Kohen F, Jaffe A, et al. Effects of gonadal steroids and their antagonists on DNA synthesis in human vascular cells. Hypertension. 1998;32:39–45. doi: 10.1161/01.hyp.32.1.39. [DOI] [PubMed] [Google Scholar]
- 65.Cai J, Hong Y, Weng C, et al. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am J Physiol Heart Circ Physiol. 2011;300:H1210–H1221. doi: 10.1152/ajpheart.01210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campelo AE, Cutini PH, Massheimer V. Testosterone Modulates Platelet Aggregation and Endothelial Cell Growth through Nitric Oxide Pathway. J Endocrinol. 2012;213:77–87. doi: 10.1530/JOE-11-0441. [DOI] [PubMed] [Google Scholar]
- 67.Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31:S221–S230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- 68.Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- 69.Gao J, Arnold JT, Isaacs JT. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Res. 2001;61:5038–5044. [PubMed] [Google Scholar]
- 70.Wen J, Zhao Y, Li J, et al. Suppression of DHT-induced paracrine stimulation of endothelial cell growth by estrogens via prostate cancer cells. Prostate. 2013;73:1069–1081. doi: 10.1002/pros.22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weng C, Cai J, Wen J, et al. Differential effects of estrogen receptor ligands on regulation of dihydrotestosterone-induced cell proliferation in endothelial and prostate cancer cells. Int J Oncol. 2013;42:327–337. doi: 10.3892/ijo.2012.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu YS, Cai LQ, Huang Y, et al. Receptor isoform and ligand-specific modulation of dihydrotestosterone-induced prostate specific antigen gene expression and prostate tumor cell growth by estrogens. J Androl. 2005;26:500–508. doi: 10.2164/jandrol.05002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qiao Y, Zhang ZK, Cai LQ, et al. 17alpha-estradiol inhibits LAPC-4 prostatic tumor cell proliferation in cell cultures and tumor growth in xenograft animals. Prostate. 2007;67:1719–1728. doi: 10.1002/pros.20656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu YS, Imperato-McGinley JL. 5alpha-reductase isozymes and androgen actions in the prostate. Ann N Y Acad Sci. 2009;1155:43–56. doi: 10.1111/j.1749-6632.2009.04115.x. [DOI] [PubMed] [Google Scholar]
- 75.Qiao Y, Wang L, Cai LQ, et al. Inhibition of aberrant androgen receptor induction of prostate specific antigen gene expression, cell proliferation and tumor growth by 17alpha-estradiol in prostate cancer. J Urol. 2011;185:305–314. doi: 10.1016/j.juro.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai J, Hong Y, Tan C, et al. Androgens stimulate human aortic endothelial cell proliferation through an androgen receptor-VEGF/cyclin A mediated mechanism. Circulation. 2010;122:e66. [Google Scholar]
- 77.Zhu YS, Cai LQ, Huang Y, et al. ER-isoform and ligand specific modulation of androgen actions is mediated via differential mechanisms. Program and Abstracts of The Endocrine Society 86th Annual Meeting, New Orleans, LA, USA, June 16-19, 2004. 92 [Google Scholar]
- 78.Dehm SM, Tindall DJ. Editorial Commentary. J Androl. 2005;26:509–510. [Google Scholar]
- 79.Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6:532–542. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 80.Zheng HY, Li Y, Dai W, et al. Imbalance of testosterone/estradiol promotes male CHD development. Biomed Mater Eng. 2012;22:179–185. doi: 10.3233/BME-2012-0705. [DOI] [PubMed] [Google Scholar]
- 81.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 82.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 83.Flores-Ramirez R, Uribe-Longoria A, Rangel-Fuentes MM, et al. Intracoronary infusion of CD133+ endothelial progenitor cells improves heart function and quality of life in patients with chronic post-infarct heart insufficiency. Cardiovasc Revasc Med. 2010;11:72–78. doi: 10.1016/j.carrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Wang XX, Zhang FR, Shang YP, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 85.Foresta C, Caretta N, Lana A, et al. Reduced number of circulating endothelial progenitor cells in hypogonadal men. J Clin Endocrinol Metab. 2006;91:4599–4602. doi: 10.1210/jc.2006-0763. [DOI] [PubMed] [Google Scholar]
- 86.Liao CH, Wu YN, Lin FY, et al. Testosterone replacement therapy can increase circulating endothelial progenitor cell number in men with late onset hypogonadism. Andrology. 2013;1:563–569. doi: 10.1111/j.2047-2927.2013.00086.x. [DOI] [PubMed] [Google Scholar]
- 87.Foresta C, Zuccarello D, De Toni L, et al. Androgens stimulate endothelial progenitor cells through an androgen receptor-mediated pathway. Clin Endocrinol (Oxf) 2008;68:284–289. doi: 10.1111/j.1365-2265.2007.03036.x. [DOI] [PubMed] [Google Scholar]
- 88.Liu R, Ding L, Yu MH, et al. Effects of dihydrotestosterone on adhesion and proliferation via PI3-K/Akt signaling in endothelial progenitor cells. Endocrine. 2014;46:634–643. doi: 10.1007/s12020-013-0081-1. [DOI] [PubMed] [Google Scholar]
- 89.Chou TM, Sudhir K, Hutchison SJ, et al. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996;94:2614–2619. doi: 10.1161/01.cir.94.10.2614. [DOI] [PubMed] [Google Scholar]
- 90.Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217:R47–R71. doi: 10.1530/JOE-12-0582. [DOI] [PubMed] [Google Scholar]
- 91.Williams MR, Dawood T, Ling S, et al. Dehydroepiandrosterone increases endothelial cell proliferation in vitro and improves endothelial function in vivo by mechanisms independent of androgen and estrogen receptors. J Clin Endocrinol Metab. 2004;89:4708–4715. doi: 10.1210/jc.2003-031560. [DOI] [PubMed] [Google Scholar]
- 92.Simoncini T, Mannella P, Fornari L, et al. Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinology. 2003;144:3449–3455. doi: 10.1210/en.2003-0044. [DOI] [PubMed] [Google Scholar]
- 93.Goglia L, Tosi V, Sanchez AM, et al. Endothelial regulation of eNOS, PAI-1 and t-PA by testosterone and dihydrotestosterone in vitro and in vivo. Mol Hum Reprod. 2010;16:761–769. doi: 10.1093/molehr/gaq049. [DOI] [PubMed] [Google Scholar]
- 94.Yu J, Akishita M, Eto M, et al. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology. 2010;151:1822–1828. doi: 10.1210/en.2009-1048. [DOI] [PubMed] [Google Scholar]
- 95.Wynne FL, Khalil RA. Testosterone and coronary vascular tone: implications in coronary artery disease. J Endocrinol Invest. 2003;26:181–186. doi: 10.1007/BF03345150. [DOI] [PubMed] [Google Scholar]
- 96.Sun D, Yan C, Jacobson A, et al. Contribution of epoxyeicosatrienoic acids to flow-induced dilation in arteries of male ERalpha knockout mice: role of aromatase. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1239–R1246. doi: 10.1152/ajpregu.00185.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sierra-Ramirez A, Morato T, Campos R, et al. Acute effects of testosterone on intracellular Ca2+ kinetics in rat coronary endothelial cells are exerted via aromatization to estrogens. Am J Physiol Heart Circ Physiol. 2004;287:H63–H71. doi: 10.1152/ajpheart.00784.2003. [DOI] [PubMed] [Google Scholar]
- 98.Marrachelli VG, Miranda FJ, Centeno JM, et al. Role of NO-synthases and cyclooxygenases in the hyperreactivity of male rabbit carotid artery to testosterone under experimental diabetes. Pharmacol Res. 61:62–70. doi: 10.1016/j.phrs.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 99.Wong SL, Leung FP, Lau CW, et al. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ Res. 2009;104:228–235. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]
- 100.Wu CC, Schwartzman ML. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011;96:45–53. doi: 10.1016/j.prostaglandins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bishop-Bailey D, Pepper JR, Haddad EB, et al. Induction of cyclooxygenase-2 in human saphenous vein and internal mammary artery. Arterioscler Thromb Vasc Biol. 1997;17:1644–1648. doi: 10.1161/01.atv.17.9.1644. [DOI] [PubMed] [Google Scholar]
- 102.Caughey GE, Cleland LG, Penglis PS, et al. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of prostacyclin synthesis by COX-2. J Immunol. 2001;167:2831–2838. doi: 10.4049/jimmunol.167.5.2831. [DOI] [PubMed] [Google Scholar]
- 103.Brancaleone V, Vellecco V, Matassa DS, et al. Crucial role of androgen receptor in vascular H2S biosynthesis induced by testosterone. Br J Pharmacol. 2015;172:1505–1515. doi: 10.1111/bph.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gonzales RJ, Ghaffari AA, Duckles SP, et al. Testosterone treatment increases thromboxane function in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;289:H578–H585. doi: 10.1152/ajpheart.00958.2004. [DOI] [PubMed] [Google Scholar]
- 105.Maturana MA, Breda V, Lhullier F, et al. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism. 2008;57:961–965. doi: 10.1016/j.metabol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 106.Polderman KH, Stehouwer CD, van Kamp GJ, et al. Influence of sex hormones on plasma endothelin levels. Ann Intern Med. 1993;118:429–432. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- 107.Kumanov P, Tomova A, Kirilov G, et al. Increased plasma endothelin levels in patients with male hypogonadism. Andrologia. 2002;34:29–33. doi: 10.1046/j.1439-0272.2002.00468.x. [DOI] [PubMed] [Google Scholar]
- 108.Kumanov P, Tomova A, Kirilov G. Testosterone replacement therapy in male hypogonadism is not associated with increase of endothelin-1 levels. Int J Androl. 2007;30:41–47. doi: 10.1111/j.1365-2605.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- 109.Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65:968–978. doi: 10.1016/j.eururo.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 110.Zhang X, Wang LY, Jiang TY, et al. Effects of testosterone and 17-beta-estradiol on TNF-alpha-induced E-selectin and VCAM-1 expression in endothelial cells. Analysis of the underlying receptor pathways. Life Sci. 2002;71:15–29. doi: 10.1016/s0024-3205(02)01567-9. [DOI] [PubMed] [Google Scholar]
- 111.Hatakeyama H, Nishizawa M, Nakagawa A, et al. Testosterone inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cells. FEBS letters. 2002;530:129–132. doi: 10.1016/s0014-5793(02)03440-3. [DOI] [PubMed] [Google Scholar]
- 112.Death AK, McGrath KC, Sader MA, et al. Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-kappaB-dependent pathway. Endocrinology. 2004;145:1889–1897. doi: 10.1210/en.2003-0789. [DOI] [PubMed] [Google Scholar]
- 113.McCrohon JA, Jessup W, Handelsman DJ, et al. Androgen exposure increases human monocyte adhesion to vascular endothelium and endothelial cell expression of vascular cell adhesion molecule-1. Circulation. 1999;99:2317–2322. doi: 10.1161/01.cir.99.17.2317. [DOI] [PubMed] [Google Scholar]
- 114.Annibalini G, Agostini D, Calcabrini C, et al. Effects of sex hormones on inflammatory response in male and female vascular endothelial cells. J Endocrinol Invest. 2014;37:861–869. doi: 10.1007/s40618-014-0118-1. [DOI] [PubMed] [Google Scholar]
- 115.Norata GD, Tibolla G, Seccomandi PM, et al. Dihydrotestosterone decreases tumor necrosis factor-alpha and lipopolysaccharide-induced inflammatory response in human endothelial cells. J Clin Endocrinol Metab. 2006;91:546–554. doi: 10.1210/jc.2005-1664. [DOI] [PubMed] [Google Scholar]
- 116.Xu ZR, Hu L, Cheng LF, et al. Dihydrotestosterone protects human vascular endothelial cells from H(2)O(2)-induced apoptosis through inhibition of caspase-3, caspase-9 and p38 MAPK. Eur J Pharmacol. 643:254–259. doi: 10.1016/j.ejphar.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 117.Agledahl I, Brodin E, Svartberg J, et al. Plasma free tissue factor pathway inhibitor (TFPI) levels and TF-induced thrombin generation ex vivo in men with low testosterone levels. Thromb Haemost. 2009;101:471–477. [PubMed] [Google Scholar]
- 118.Glueck CJ, Glueck HI, Stroop D, et al. Endogenous testosterone, fibrinolysis, and coronary heart disease risk in hyperlipidemic men. J Lab Clin Med. 1993;122:412–420. [PubMed] [Google Scholar]
- 119.Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb. 1994;14:701–706. doi: 10.1161/01.atv.14.5.701. [DOI] [PubMed] [Google Scholar]
- 120.Pugh PJ, Channer KS, Parry H, et al. Bio-available testosterone levels fall acutely following myocardial infarction in men: association with fibrinolytic factors. Endocr Res. 2002;28:161–173. doi: 10.1081/erc-120015055. [DOI] [PubMed] [Google Scholar]
- 121.Jin H, Lin J, Fu L, et al. Physiological testosterone stimulates tissue plasminogen activator and tissue factor pathway inhibitor and inhibits plasminogen activator inhibitor type 1 release in endothelial cells. Biochem Cell Biol. 2007;85:246–251. doi: 10.1139/O07-011. [DOI] [PubMed] [Google Scholar]
- 122.Ng MK, Jessup W, Celermajer DS. Sex-related differences in the regulation of macrophage cholesterol metabolism. Curr Opin Lipidol. 2001;12:505–510. doi: 10.1097/00041433-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 123.Sieveking DP, Lim P, Chow RW, et al. A sex-specific role for androgens in angiogenesis. J Exp Med. 2010;207:345–352. doi: 10.1084/jem.20091924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu J, Wu S, Wei H, et al. Effects of sex hormones and their balance on the proliferation of rat vascular endothelial cells. Horm Res. 2002;58:16–20. doi: 10.1159/000063211. [DOI] [PubMed] [Google Scholar]
- 125.Maddox YT, Falcon JG, Ridinger M, et al. Endothelium-dependent gender differences in the response of the rat aorta. J Pharmacol Exp Ther. 1987;240:392–395. [PubMed] [Google Scholar]
- 126.McCrohon JA, Death AK, Nakhla S, et al. Androgen receptor expression is greater in macrophages from male than from female donors. A sex difference with implications for atherogenesis. Circulation. 2000;101:224–226. doi: 10.1161/01.cir.101.3.224. [DOI] [PubMed] [Google Scholar]
- 127.Fernandez-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 128.Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165:687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 129.Carson CC, 3rd, Rosano G. Exogenous testosterone, cardiovascular events, and cardiovascular risk factors in elderly men: a review of trial data. J Sex Med. 2012;9:54–67. doi: 10.1111/j.1743-6109.2011.02337.x. [DOI] [PubMed] [Google Scholar]
- 130.Borst SE, Shuster JJ, Zou B, et al. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC Med. 2014;12:211. doi: 10.1186/s12916-014-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vigen R, O'Donnell CI, Baron AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 133.Ohlsson C, Wallaschofski H, Lunetta KL, et al. Genetic determinants of serum testosterone concentrations in men. PLoS Genet. 2011;7:e1002313. doi: 10.1371/journal.pgen.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ekstrom L, Schulze JJ, Guillemette C, et al. Bioavailability of testosterone enanthate dependent on genetic variation in the phosphodiesterase 7B but not on the uridine 5′-diphospho-glucuronosyltransferase (UGT2B17) gene. Pharmacogenet Genomics. 2011;21:325–332. doi: 10.1097/FPC.0b013e328344c5c6. [DOI] [PubMed] [Google Scholar]
- 135.Bang AK, Jorgensen N, Rajpert-De Meyts E, et al. UGT2B17 genotype and the pharmacokinetic serum profile of testosterone during substitution therapy with testosterone undecanoate. A retrospective experience from 207 men with hypogonadism. Front Endocrinol (Lausanne) 2013;4:94. doi: 10.3389/fendo.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zitzmann M. Pharmacogenetics of testosterone replacement therapy. Pharmacogenomics. 2009;10:1341–1349. doi: 10.2217/pgs.09.58. [DOI] [PubMed] [Google Scholar]
- 137.Tirabassi G, Delli Muti N, Corona G, et al. Androgen receptor gene CAG repeat polymorphism regulates the metabolic effects of testosterone replacement therapy in male postsurgical hypogonadotropic hypogonadism. Int J Endocrinol. 2013:816740. doi: 10.1155/2013/816740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Francomano D, Fattorini G, Gianfrilli D, et al. Acute endothelial response to testosterone gel administration in men with severe hypogonadism and its relationship to androgen receptor polymorphism: a pilot study. J Endocrinol Invest. doi: 10.1007/s40618-015-0325-4. Published Online First: 11 July 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Agiannitopoulos K, Bakalgianni A, Marouli E, et al. Gender specificity of a genetic variant of androgen receptor and risk of coronary artery disease. J Clin Lab Anal. doi: 10.1002/jcla.21837. Published Online First: 25 February 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]