Abstract
The plants of the genus Phyllanthus (Euphorbiaceae) have been used as traditional medicinal materials for a long time in China, India, Brazil, and the Southeast Asian countries. They can be used for the treatment of digestive disease, jaundice, and renal calculus. This review discusses the ethnopharmacological, phytochemical, and pharmacological studies of Phyllanthus over the past few decades. More than 510 compounds have been isolated, the majority of which are lignins, triterpenoids, flavonoids, and tannins. The researches of their remarkable antiviral, antioxidant, antidiabetic, and anticancer activities have become hot topics. More pharmacological screenings and phytochemical investigations are required to support the traditional uses and develop leading compounds.
1. Introduction
Phyllanthus (Euphorbiaceae) is a large genus and widely distributed in tropical and subtropical zones like tropical Africa, tropical America, Asia, and Oceania. This genus, consisting of more than 700 species, can be classified into 11 subgenuses [1, 2]. The most popular 24 species are chiefly belonging to subgenus Kirganelia, Cicca, and Phyllanthus and they are traditionally used by different nationalities.
Genus Phyllanthus has been employed as herbal drugs for a long time in China, India, Brazil, and Southeast Asian countries. The most abundant species are used in India and have a beneficial role in Ayurveda for the treatment of digestive, genitourinary, respiratory, and skin diseases [3, 4]. In China, herbs and their prescriptions are used to treat hepatitis B, hypertension, dropsy, and sore throat [2]. These herbal drugs are employed by local inhabitants of Thailand, Latin America (especially Brazil), and Africa to cure jaundice, renal calculus, and malaria, respectively [5–7].
By virtue of the wide uses of Phyllanthus as anti-HIV, anticancer, and anti-HBV agents, there has been considerable interest in the investigations of this genus in recent years and the researches about pharmacology and chemistry had been finished in a deep going way. This report reviews the ethnopharmacological, phytochemical, and pharmacological investigations of Phyllanthus over the past few decades. More than three hundred articles were selected from the data taken from SciFinder Scholar database by searching the keyword “Phyllanthus”.
2. Ethnopharmacological Uses
The traditional application experiences of these herbs may have reference value for the treatment of recent diseases. Botanical data, folk name, and medicinal properties of twenty-four Phyllanthus species are depicted in Table 1. In Asia, seventeen plants are considered to have bitter and astringent taste. They are regarded as stomachic, diuretic, febrifuge, deobstruent, and antiseptic agents and effective remedies for hepatopathy, hypertensive, diabetes, and jaundice. In Africa, six herbs are widely employed by many tribes for the treatment of malaria wound and tetanus. Six species are used extensively in Latin America for the treatment of urination disorder and diabetes. The distribution and the main uses of Phyllanthus are pictured in Figure 1.
Table 1.
The traditional use of Phyllanthus.
| Species | Region | Local name | Plant part used | Traditional use | Reference |
|---|---|---|---|---|---|
| P. emblica | Bangladesh | Fruit | Constipation, urinary diseases | [8] | |
| Burma | Juice/bark | Constipation, hemostasis, keratitis | [8] | ||
| Cambodia | Leaves | Muscle pain, fever | [8] | ||
| China | Yuganzi | Fruit | Digestive disease, hypertension, fever, respiratory inflammation | [8] | |
| Fiji | Fruit | Tonic | [8] | ||
| India | Amla, Indian gooseberry | Fruit | Diabetes, chronic diarrhea, inflammation, fever, liver diseases, stomach ulcers, metabolic disorders, skin disorders, beauty care | [3, 4] | |
| Indonesia | Leaves/fruit | Diarrhea, abdominal pain, stomach Disease, gallbladder disease, bleeding | [8] | ||
| Iran | Fruit | Parasitic | [8] | ||
| Iraq | Fruit | Bleeding, gastrointestinal system disorder | [8] | ||
| Nepal | Stem/fruit/seed | Urination disorder, constipation, bleeding, diarrhea, ophthalmopathy, asthma, bronchitis | [8] | ||
| Pakistan | Fruit | Diarrhea, preterm, skin diseases, gonorrhea, ophthalmopathy, anemia, hair care | [8, 9] | ||
| Sri Lanka | Fruit/whole plant | Constipation, indigestion, keratitis | [8] | ||
| Thailand | Makham pom | Juice/bark | Diarrhea, leukorrhagia, cough, parasitosis, gastrointestinal chronic diseases, hair treatment and nourishment, skin care | [8, 10, 11] | |
| Turkey | Fruit | Diarrhea, dysentery, hemostasis, gastroenteritis | [8] | ||
|
| |||||
| P. reticulatus | Bangladesh | Whole plant | Edema, constipation, helminthiasis, dysentery, diarrhea, pain | [12] | |
| China | Huangguo yexiazhu | Inflammation, rheumatism | [13] | ||
| India | Pancoli, karineli | Leaves/bark | Urination disorder, fever, smallpox, colic, constipation, diabetes | [12, 14, 15] | |
| Kenya | Malaria | [7] | |||
| Malaysia | Leaves | Smallpox, syphilis, asthma, diarrhea, bleeding from gums, diabetes, urination disorder, sores, burn, suppuration, chafe, venereal sores | [16, 17] | ||
| Sri Lanka | Bark/fruit | Enteritis, urination disorder | [15] | ||
| Sudan | Urination disorder, fever | [15] | |||
| Tanzania | Whole plant/leaves | Dysmenorrhea, gonorrhea, urination disorder, intestinal hemorrhage and anemia, muscle spasms, diarrhea with anal bleeding, promoting fertility, sores | [12, 15] | ||
| Thailand | Urination disorder, asthma, anemia, fever, thirst, astringent, inflammation | [16] | |||
|
| |||||
| P. niruri | Brazil | Quebra-pedra | Whole plant | Kidney calculi | [18] |
| China | zhuzicao | Whole plant | Hepatitis, dysentery, enteritis, urinary infection | [19] | |
| Congo | Whole plant | Malaria | [20] | ||
| India | Chanka piedra, bhuiamlki | Fruit/whole plant | Bronchitis, anaemia, leprosy, asthma, kidney calculi, ulcer, wound, sore, scabies, ring worm, jaundice, gonorrhea, menstruation, diabetes | [18, 21–23] | |
| Indonesia | Whole plant | Viral infection, hepatitis | [22] | ||
| Latin America | Chanca piedra | Whole plant | Gallstone, kidney calculi, fever, excess uric acid | [6, 18, 24] | |
| Malaysia | Dukong anak | Whole plant | Diarrhoea, kidney disorder, gonorrhea, cough | [22] | |
| Thailand | Aerial parts | Anorexia, malaria | [18] | ||
|
| |||||
| P. muellerianus | Africa | Malaria | [25] | ||
| Cameroon | Mbolongo | Stem bark | Wound, tetanus | [26] | |
| Ghana | Wound | [27] | |||
| Ivory Coast | Leaves | Fever | [26] | ||
| Nigeria | Root | Fever | [26] | ||
| Zambia | leaves | Fever | [26] | ||
|
| |||||
| P. amarus | Africa | Whole plant | Urinary concretions, dysentery, jaundice, diarrhoea | [28] | |
| India | Bhuiamlki | Whole plant | Gastropathy, diarrhoea, dysentery, intermittent fevers, ophthalmopathy, scabies, ulcers, wound, malaria, jaundice, diabetes, asthma, hepatitis, tuberculosis, urinary diseases, bodyache, immunomodulatory | [29–34] | |
| Nigeria | Leaves | Diabetes mellitus, obesity, hyperlipidemia, malaria | [35, 36] | ||
| Peru | Chanca piedra | Leaves | Diabetes, jaundice, kidney diseases, urination disorder, sedative, astringent, tonic | [37] | |
| Thailand | Look tai bai | Gonorrhea, jaundice, diabetes, liver diseases | [5] | ||
|
| |||||
| P. urinaria | China | Yexiazhu | Whole plant | Kidney calculi, painful disorder, jaundice, enteritis, diarrhea, dropsy, inflammation | [38–41] |
| India | Inflammation, diarrheal, kidney calculi, painful disorder | [38, 39] | |||
| Thailand | Look tai bai | Inflammation, diarrheal, gonorrhea, jaundice, diabetes | [5, 38] | ||
|
| |||||
| P. acidus | India | Harfarauri | Fruit/leaves/roots | Jaundice, constipation, vomiting, biliousness, urinary concretions, piles, fever, smallpox, rheumatism, asthma, hepatic disease, diabetes, gonorrhea, ophthalmopathy, amnesia, psoriasis | [42, 43] |
| Thailand | Otaheiti gooseberry, star gooseberry, mayom | Leaves/bark/root | Constipation, alcoholic addicts, hypertension, fever, dermatitis, menstruation fever | [44–46] | |
|
| |||||
| P. debilis | India | Bhuiamlki | Swelling, intestinal worms, fever, wound, inflammation, rheumatism | [34] | |
| Sri Lanka | Diabetes | [47] | |||
|
| |||||
| P. simplex | India | Bhuiaveli, uchchiyusirika | Leaves/whole plant | Ophthalmopathy, gonorrhea, jaundice, mammary abscess, pruritus, diarrhea, hepatitis, urinary infection | [48, 49] |
| China | Huang zhuzicao | Ophthalmopathy, diarrhea, hepatitis, urinary infection | [49] | ||
|
| |||||
| P. discoideus | Cameroon | Insomnia, epilepsy | [50] | ||
|
| |||||
| P. fraternus | India | Bhuiamlki | Whole plant | Constipation, jaundice, hepatic disorder, kidney disorders, bacterial infection | [29, 51, 52] |
|
| |||||
| P. hookeri | India | Diabetes, wound, fever, inflammation, snake bite, bacterial infection | [34] | ||
|
| |||||
| P. kozhikodianus | India | Dysentery, jaundice, ulcer, itching, bacterial infection | [34] | ||
|
| |||||
| P. maderaspatensis | India | Bhuiamlki | Whole plant | Headache, constipation, diarrhea, edematous, dysentery, fever, ulcer, burn, jaundice, bacterial infection, immunomodulatory | [34, 52] |
|
| |||||
| P. nozeranii | India | Spasmodic, piles, headache, boils, indigestion, viral and bacterial infection | [34] | ||
|
| |||||
| P. orbicularis | Cuba | Jaundice, diabetes, kidney calculi, ulcer, rheumatism, fever | [53, 54] | ||
|
| |||||
| P. piscatorum | Venezuela | Aerial parts | Wound, fungal infection | [55] | |
|
| |||||
| P. polyanthus | Kenya | Root | Sexually transmitted diseases | [56] | |
|
| |||||
| P. polyphyllus | India | Sirunelli | Leaves | Liver disease | [57] |
|
| |||||
| P. rheedii | India | Whole plant | Diabetes | [58] | |
|
| |||||
| P. sellowianus | South America | Sarandi blanco | Stems/leaves | Urination disorder, diabetes | [59] |
|
| |||||
| P. taxodiifolius | Thailand | Leaves/twigs | Urination disorder | [60] | |
|
| |||||
| P. tenellus | Brazil | Erva pombinha, quebra-pedra | Leaves | Urination disorder, kidney calculi | [61] |
|
| |||||
| P. virgatus | Thailand | Look tai bai | Gonorrhea, jaundice, diabetes, liver disease | [5] | |
Figure 1.
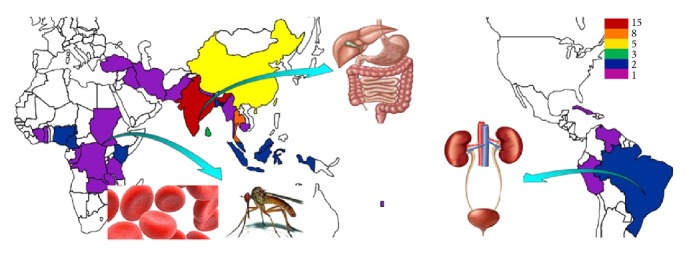
Traditional use of genus Phyllanthus in different countries. Different color represents the number of plants traditionally used in different countries: red, orange, yellow, green, blue, and purple represent fifteen, eight, five, three, two, and one kinds of plants under use, respectively. In Asia, Phyllanthus are used to treat digestive system disease, in south America, Phyllanthus are used to treat urinary system disease, and in Africa, Phyllanthus are used to treat malaria and wound.
2.1. Asia
In Asia, the clinical use of genus Phyllanthus is very prevalent. The fruit of P. emblica has a long history of use in India and is called “amla” or “Indian gooseberry.” As a tonic in Indian Ayurveda, it is often used for liver diseases [3, 4]. This fruit is known as “yuganzi” in China. It has sweet and slightly astringent taste and is used for clearing heat from throat and moistening lung for arresting cough in Traditional Chinese Medicine (TCM). In Tibetan medicine this herb is used to treat blood and bile disease, and its preparations are clinically applicable to hypertension and anuria [2]. In Thailand, it is named “makham pom” and is employed to treat gastrointestinal chronic diseases. P. emblica is commonly used together with Terminalia chebula and T. belerica and called “Triphala.” “Triphala” is used as a clinical treatment protocol of gastropathy in India and as a remedy for pestilence and fatigue in China [62].
In India, fifteen species of genus Phyllanthus are widely used by indigenous medicine. These plants have bitter and astringent taste and are considered as stomachic, diuretic, febrifuge, deobstruent, antiseptic, and effective remedies for hepatopathy. Some herbs such as P. niruri, P. amarus, P. fraternus, P. debilis, and P. maderaspatensis share the same name “bhuiamlki” [29]. The fruits of “bhuiamlki” are employed by Ayurveda to cure jaundice. P. simplex, P. reticulatus, and P. acidus are therapy of urinary disease and have the names of “bhuiaveli,” “pancoli,” and “harfarauri,” respectively. The leaves of P. polyphyllus, called “sirunelli,” are used for liver disease. Additionally, the rest of these herbs can be employed as remedies for diabetes, jaundice, wound, fever, and inflammation.
In China, five herbs are commonly used by TCM, Tibetan medicine, Dai People, and Yi People [2]. They have bitter and sweet taste and are usually used as prescriptions. The whole plant of P. urinaria, known as “yexiazhu,” can clear heat-toxin and remove dampness and is employed to treat jaundice, enteritis, diarrhea, and dropsy. Besides, the TCM prescription, named “yexiazhu capsule,” performs a beneficial role in curing hepatitis B. Other herbs such as P. reticulatus, P. niruri, and P. simplex are beneficial to the treatment of ophthalmopathy, urinary infection, inflammation, and rheumatism.
In Thailand, eight herbs of this genus are widely used by residents. P. amarus, P. urinaria, and P. virgatus share the name “look tai bai,” all of which are used for treating gonorrhea, jaundice, diabetic, and liver disease. P. acidus has three names: “otaheiti gooseberry,” “star gooseberry,” and “mayom,” and it can be used as remedy for hypertensive, constipation, skin disease, and fever. The rest of herbal drugs including P. taxodiifolius, P. niruri, and P. reticulatus are employed for the treatment of urination disorder and malaria.
2.2. Africa
Many African tribes employ six plants of genus Phyllanthus to treat malaria, fever, and wound. P. muellerianus is the most popular herbal drugs of this genus in Africa. It is named “mbolongo” in Cameroon. In Ghana and Cameroon, the stem bark is used for the therapy of wound and tetanus. In Nigeria, Zambia, and Ivory Coast the leaves and root are applied as a fever remedy. In Kenya, the root of P. polyanthus is used to cure sexually transmitted diseases. What is more, the whole plants of P. muellerianus and P. reticulatus can be used for the treatment of malaria.
2.3. Latin America
About six herb species of this genus are used in many countries in Latin America. In Brazil, P. tenellus is popularly known as “quebra-pedras” whose leaves can be used as diuretic. P. amarus is named “chanca piedra” in Peru and the leaves are employed for diabetic and jaundice therapy or as sedative and astringent. P. sellowianus is called “sarandi blanco” in South America and used widely in folk for the treatment of urination disorder and diabetes.
In summary, P. emblica, P. reticulatus, and P. niruri are the top three species widely used around the world. P. niruri is probably the most widespread herb of Phyllanthus, which is named “chanka piedra,” “bhuiamlki,” “zhuzicao,” “dukung anak,” “quebra-pedra,” and “chanca piedra.” Its whole plant can treat inflammation, lithiasis, fever, malaria, hepatitis, and gonorrhea [7, 18, 19, 21, 22].
3. Chemical Constituents
More than 510 compounds have been isolated from Phyllanthus, the majority of which are lignins, triterpenoids, flavonoids, and tannins. The compositions isolated from each species and their biological activities are partially summarized in Table 2. Lignins and tannins exhibit various activities and are considered to be the biological active compounds of this genus. Corilagin, geraniin, and gallic acid are three most prevalent compounds in this genus, and the pharmacological researches mainly focus on phyllanthin, niranthin, and geraniin.
Table 2.
The compounds isolated from the genus Phyllanthus and part of pharmacological effects.
| Number | Compounds | Species | Pharmacological effects | References |
|---|---|---|---|---|
| 1 | (20S)-3α-Acetoxy-24-methylenedammaran-20-ol | P. polyanthus | [56] | |
| 2 | (20S)-3β-Acetoxy-24-methylenedammaran-20-ol | P. polyanthus | [56] | |
| 3 | Ocotillol-II | P. flexuosus | [63] | |
| 4 | Phyllanthenol | P. niruri | [64] | |
| 5 | Phyllanthenone | P. niruri | [64] | |
| 6 | Phyllantheol | P. niruri | [64] | |
| 7 | (+)-Songbodichapetalin | P. songboiensis | [65] | |
| 8 | Acutissimatriterpene A | P. acutissima | [66] | |
| 9 | Acutissimatriterpene B | P. acutissima | [66] | |
| 10 | Acutissimatriterpene C | P. acutissima | [66] | |
| 11 | Acutissimatriterpene D | P. acutissima | [66] | |
| 12 | Acutissimatriterpene E | P. acutissima | [66] | |
| 13 | Flexuosoids A | P. flexuosus | [67] | |
| 14 | Flexuosoids B | P. flexuosus | [67] | |
| 15 | δ-Amyrin acetate | P. polyanthus | [56] | |
| 16 | 12(13)-Dehydro-3α-acetoxyolean-28-oic acid | P. pulcher | [68] | |
| 17 | 3′-O-Acetyl-3-O-α-L-arabinosyl-23-hydroxyolean-12-en-28-oic acid | P. polyphyllus | [69] | |
| 18 | 3α-Acetoxyl-25-hydroxyolean-12-en-28-oic acid | P. pulcher | Antitumor | [68] |
| 19 | 4′-O-Acetyl-3-O-α-L-arabinosyl-23-hydroxyolean-12-en-28-oic acid | P. polyphyllus | [69] | |
| 20 | Olean-12-en-3β,15α,24-triol | P. flexuosus | Antitumor | [70, 71] |
| 21 | Olean-12-en-3β,15α-diol | P. flexuosus | Antitumor | [70, 71] |
| 22 | Olean-12-en-3β,24-diol | P. flexuosus | [70] | |
| 23 | Olean-18-en-3α-ol | P. fraternus | [72] | |
| 24 | Oleana-11:13(18)-dien-3β-ol | P. flexuosus | [70] | |
| 25 | Oleana-11:13(18)-dien-3β,24-diol | P. flexuosus | [70] | |
| 26 | Oleana-9(11):12-dien-3β-ol | P. flexuosus | [70] | |
| 27 | Oleanolic acid | P. urinaria | [73] | |
| 28 | Phyllanoside | P. amarus | [74] | |
| 29 | Phyllenolide A | P. myrtifolius | [75] | |
| 30 | Phyllenolide B | P. myrtifolius | [75] | |
| 31 | Phyllenolide C | P. myrtifolius | [75] | |
| 32 | Taraxerol | P. columnaris | [76] | |
| 33 | Taraxerone | P. reticulatus | [77] | |
| 33 | Taraxerone | P. columnaris | [76] | |
| 34 | Taraxeryl acetate | P. reticulatus | [77] | |
| 35 | α-Amyrin | P. singampattiana | [78] | |
| 36 | β-Amyrin | P. urinaria | [79] | |
| 36 | β-Amyrin | P. flexuosus | [80] | |
| 36 | β-Amyrin | P. acidus | [81] | |
| 37 | 11β-Hydroxy-D:A-friedoolean-1-en-3-one | P. flexuosus | [82] | |
| 38 | 1β,22β-Dihydroxyfriedelin | P. muellerianus | [83] | |
| 39 | 21α-Hydroxyfriedel-4(23)-en-3-one | P. reticulatus | [84] | |
| 40 | 21α-Hydroxyfriedelan-3-one | P. reticulatus | [84] | |
| 41 | 22β-Hydroxyfriedel-1-ene | P. muellerianus | [83] | |
| 42 | 26-Nor-D:A-friedoolean-14-en-3-one | P. watsonii | [85] | |
| 43 | 26-Nor-D:A-friedoolean-14-en-3β-ol | P. watsonii | [85] | |
| 43 | Friedelin | P. columnaris | [86] | |
| 44 | 3,20-Dioxo-dinorfriedelane | P. emblica | [87] | |
| 45 | Epifriedelinol | P. reticulatus | [77] | |
| 45 | Epifriedelinol | P. singampattiana | [78] | |
| 46 | Friedelan-3β-ol | P. reticulatus | [84] | |
| 47 | Friedelin | P. niruri | [88] | |
| 47 | Friedelin | P. reticulatus | [84] | |
| 47 | Friedelin | P. flexuosus | [80] | |
| 47 | Friedelin | P. watsonii | [85] | |
| 47 | Friedelin | P. wightianus | [89] | |
| 47 | Friedelin | P. singampattiana | [78] | |
| 48 | Polpunonic acid | P. oxyphyllus | [90] | |
| 49 | Trichadenic acid B | P. flexuosus | [91] | |
| 50 | 3-Friedelanone | P. muellerianus | [92] | |
| 51 | Betulin | P. reticulatus | [77] | |
| 51 | Betulin | P. flexuosus | Antitumor | [70, 71] |
| 52 | Betulinic acid | P. reticulatus | [84] | |
| 53 | Glochidiol | P. urinaria | [73] | |
| 53 | Glochidiol | P. sellowianus | [93] | |
| 54 | Glochidone | P. virgatus | [94] | |
| 54 | Glochidone | P. sellowianus | [95] | |
| 54 | Glochidone | P. watsonii | [85] | |
| 54 | Glochidone | P. taxodiifolius | Antitumor | [60, 96] |
| 54 | Glochidone | P. pulcher | Antitumor | [68] |
| 54 | Glochidone | P. flexuosus | [80] | |
| 55 | Glochidonol | P. reticulatus | [84] | |
| 55 | Glochidonol | P. sellowianus | [93] | |
| 55 | Glochidonol | P. watsonii | [85] | |
| 55 | Glochidonol | P. pulcher | Antitumor | [68] |
| 56 | Lup-20(29)-en-3β,15α-diol | P. flexuosus | Antitumor | [63, 71] |
| 57 | Lup-20(29)-en-3β,24-diol | P. flexuosus | Antitumor | [70, 71] |
| 58 | Lup-20(29)-en-3β-ol | P. urinaria | [97] | |
| 59 | Lup-20(29)-ene-3β,24-diol | P. flexuosus | [98] | |
| 60 | Lup-20(29)-ene-1β,3β-diol | P. sellowianus | [93] | |
| 60 | Lup-20(29)-ene-1β,3β-diol | P. watsonii | [85] | |
| 61 | Lupanyl acetate | P. urinaria | [99] | |
| 61 | Lupanyl acetate | P. watsonii | [85] | |
| 61 | Lupanyl acetate | P. columnaris | [86] | |
| 61 | Lupanyl acetate | P. pulcher | [68] | |
| 62 | Lupenone | P. polyanthus | [56] | |
| 63 | Lupenyl palmitate | P. watsonii | [85] | |
| 64 | Lupeol | P. emblica | [100] | |
| 64 | Lupeol | P. urinaria | [79] | |
| 64 | Lupeol | P. reticulatus | [17] | |
| 64 | Lupeol | P. flexuosus | Antitumor | [71, 80] |
| 64 | Lupeol | P. oxyphyllus | [90] | |
| 64 | Lupeol | P. watsonii | [85] | |
| 64 | Lupeol | P. taxodiifolius | Antitumor | [60, 96] |
| 64 | Lupeol | P. wightianus | [89] | |
| 64 | Lupeol | P. columnaris | [86] | |
| 65 | Lupeol acetate | P. reticulatus | [17] | |
| 66 | 29-Nor-3,4-seco-friedelan-4(23),20(30)-dien-3-oic acid | P. oxyphyllus | [90] | |
| 67 | 3,7,11,15,19,23-Hexamethyl-2Z,6Z,10Z,14E,18E,22E-tetracosahexen-1-ol | P. niruri | [101] | |
| 68 | Phyllanthol | P. sellowianus | [102] | |
| 68 | Phyllanthol | P. polyanthus | [56] | |
| 68 | Phyllanthol | P. acidus | [81] | |
| 69 | Phyllanthone | P. polyanthus | [56] | |
| 70 | 4′-Hydroxyphyllaemblicin B | P. emblica | [103] | |
| 71 | 5-Hydroxy-6,9-epoxyguaiane | P. oxyphyllus | [90] | |
| 72 | 5-O-Acetyl-6,9-epoxyguaiane | P. oxyphyllus | [90] | |
| 73 | Cloven-2β,9α-diol | P. urinaria | [73] | |
| 74 | Descinnamoylphyllanthocindiol | P. acuminatus | [104] | |
| 75 | Didesacetylphyllanthostatin 3 | P. acuminatus | [104] | |
| 76 | Dihydrophaseic acid-4′-O-β-D-glucopyranoside | P. reticulatus | [105] | |
| 77 | Englerins A | P. engleri | Antitumor | [106] |
| 78 | Englerins B | P. engleri | [106] | |
| 79 | Glochicoccin D | P. emblica | [107] | |
| 80 | Jaslanceoside B | P. cochinchinensis | [108] | |
| 81 | Jasminoside | P. cochinchinensis | [108] | |
| 82 | Phyllaemblic acid | P. emblica | [109] | |
| 83 | Phyllaemblic acid B | P. emblica | [110] | |
| 84 | Phyllaemblic acid C | P. emblica | [110] | |
| 85 | Phyllaemblicin A | P. emblica | [109] | |
| 86 | Phyllaemblicin B | P. emblica | Antiviral and antitumor | [109, 111, 112] |
| 87 | Phyllaemblicin C | P. emblica | Antitumor and antiviral | [109, 111, 113] |
| 88 | Phyllaemblicin D | P. emblica | [110] | |
| 89 | Phyllaemblicin E | P. emblica | [103] | |
| 90 | Phyllaemblicin F | P. emblica | [103] | |
| 91 | Phyllaemblicin G1 | P. emblica | [107] | |
| 92 | Phyllaemblicin G2 | P. emblica | [107] | |
| 93 | Phyllaemblicin G3 | P. emblica | [107] | |
| 94 | Phyllaemblicin G4 | P. emblica | [107] | |
| 95 | Phyllaemblicin G5 | P. emblica | [107] | |
| 96 | Phyllaemblicin G6 | P. emblica | Antiviral | [107] |
| 97 | Phyllaemblicin G7 | P. emblica | [107] | |
| 98 | Phyllaemblicin G8 | P. emblica | [107] | |
| 99 | Phyllaemblinol | P. emblica | [114] | |
| 100 | Phyllanthocin | P. brasiliensis | [115] | |
| 101 | Phyllanthoside | P. acuminatus | Antitumor | [116] |
| 101 | Phyllanthoside | P. veuminatus | Antitumor | [117] |
| 101 | Phyllanthoside | P. brasiliensis | Antitumor | [115] |
| 102 | Phyllanthostatin 1 | P. acuminatus | Antitumor | [116] |
| 102 | Phyllanthostatin 1 | P. veuminatus | Antitumor | [117] |
| 103 | Phyllanthostatin 2 | P. acuminatus | Antitumor | [117] |
| 103 | Phyllanthostatin 2 | P. veuminatus | Antitumor | [117] |
| 104 | Phyllanthostatin 3 | P. acuminatus | Antitumor | [117] |
| 104 | Phyllanthostatin 3 | P. veuminatus | Antitumor | [117] |
| 105 | Phyllanthostatin 6 | P. acuminatus | Antitumor | [104] |
| 106 | Phyllanthusol A | P. acidus | Antitumor | [46] |
| 107 | Phyllanthusol B | P. acidus | Antitumor | [46] |
| 108 | β-Caryophyllene | P. emblica | [113] | |
| 109 | β-Bourbonene | P. emblica | [113] | |
| 110 | 19-Hydroxyspruceanol 19-O-β-D-glucopyranoside | P. reticulatus | [118] | |
| 111 | Cleistanthol | P. urinaria | [73] | |
| 111 | Cleistanthol | P. reticulatus | [13] | |
| 111 | Cleistanthol | P. flexuosus | Antitumor | [119] |
| 111 | Cleistanthol | P. oxyphyllus | [90] | |
| 112 | Ent-3β-Hydroxykaur-l6-ene | P. flexuosus | [80] | |
| 113 | Orthosiphol G | P. niruri | [120] | |
| 114 | Orthosiphol I | P. niruri | [120] | |
| 115 | Phyllanflexoid A | P. flexuosus | Antitumor | [119] |
| 116 | Phyllanflexoid B | P. flexuosus | Antitumor | [119] |
| 117 | Phyllanflexoid C | P. flexuosus | [119] | |
| 118 | Phyllanterpenyl ester | P. fraternus | [121] | |
| 119 | Spruceanol | P. urinaria | [73] | |
| 119 | Spruceanol | P. reticulatus | [13] | |
| 119 | Spruceanol | P. oxyphyllus | [90] | |
| 119 | Spruceanol | P. songboiensis | [65] | |
| 120 | trans-Phytol | P. niruri | [122] | |
| 121 | (3S,5R,6S,9R)-Megastigmane-3,9-diol 3-O-α-L-arabinofuranosyl-(1 → 6)-β-D-glucopyranoside | P. reticulatus | [13] | |
| 122 | (6R)-Menthiafolic acid | P. urinaria | [73] | |
| 123 | 7-Megastigmen-3-ol-9-one 3-O-α-L-arabinofuranosyl-(1 → 6)-β-D-glucopyranoside | P. reticulatus | [13] | |
| 124 | Turpenionoside A | P. reticulatus | [118] | |
| 125 | Turpenionoside B | P. reticulatus | [118] | |
| 126 | 7-O-[(2,3,4-Tri-O-acetyl)-α-L-arabinopyranosyl]diphyllin | P. poilanei | Antitumor | [123] |
| 127 | Arabelline | P. flexuosus | [67] | |
| 128 | Acutissimalignans A | P. songboiensis | [65] | |
| 128 | Acutissimalignans A | P. acutissima | [66] | |
| 129 | Cleistanthin A | P. taxodiifolius | Antitumor | [96, 124] |
| 130 | Cleistanthin A acetate | P. taxodiifolius | Antitumor | [96, 124] |
| 131 | Cleistanthin A Me ether | P. taxodiifolius | Antitumor | [96, 124] |
| 132 | Cleistanthin B | P. poilanei | [123] | |
| 133 | Cleistanthoside A | P. taxodiifolius | [96] | |
| 134 | Cleistanthoside A tetraacetate | P. taxodiifolius | Antitumor | [96, 124] |
| 135 | Dextrobursehernin | P. urinaria | [125] | |
| 136 | Diphyllin | P. poilanei | [123] | |
| 136 | Diphyllin | P. polyphyllus | Anti-inflammatory | [126] |
| 137 | Hypophyllanthin | P. niruri | Hepatoprotection and hypotensive | [127–129] |
| 137 | Hypophyllanthin | P. urinaria | Hypotensive | [125, 130] |
| 137 | Hypophyllanthin | P. virgatus | [131] | |
| 137 | Hypophyllanthin | P. amarus | Antitumor and anti-CYP3A4 | [132–134] |
| 137 | Hypophyllanthin | P. debilis | [135] | |
| 138 | Isolariciresinol | P. emblica | [114] | |
| 139 | Isolintetralin | P. niruri | [136] | |
| 139 | Isolintetralin | P. urinaria | [125] | |
| 139 | Isolintetralin | P. virgatus | [131] | |
| 140 | Justicidin A | P. myrtifolius | [131] | |
| 141 | Iusticidin B | P. myrtifolius | [137] | |
| 141 | Iusticidin B | P. polyphyllus | Anti-inflammatory | [126] |
| 141 | Iusticidin B | P. anisolobus | [138] | |
| 141 | Iusticidin B | P. piscatorum | Antifungal, antitumor, and antiparasitic | [139] |
| 142 | Lintetralin | P. niruri | [128] | |
| 142 | Lintetralin | P. urinaria | [125] | |
| 143 | (+)-Lyoniresinol | P. reticulatus | [13] | |
| 144 | (+)-Lyoniresiol | P. urinaria | [73] | |
| 145 | Mananthoside I | P. reticulatus | [118] | |
| 146 | Neonirtetralin | P. niruri | [140] | |
| 146 | Neonirtetralin | P. urinaria | [141] | |
| 147 | Nirtetralin | P. niruri | Antiviral and hypotensive | [127, 128, 142] |
| 147 | Nirtetralin | P. urinaria | [125] | |
| 147 | Nirtetralin | P. virgatus | Antiviral | [131, 143] |
| 147 | Nirtetralin | P. amarus | Anti-inflammatory and antitumor | [132, 144, 145] |
| 148 | Nirtetralin A | P. niruri | Antiviral | [142] |
| 149 | Nirtetralin B | P. niruri | Antiviral | [142, 146] |
| 150 | Phyllamyricin A | P. myrtifolius | [137] | |
| 151 | Phyllamyricin B | P. myrtifolius | [137] | |
| 152 | Phyllamyricin C | P. myrtifolius | [137] | |
| 152 | Phyllamyricin C | P. polyphyllus | Anti-inflammatory | [126] |
| 153 | Phyllamyricin D | P. myrtifolius | [147] | |
| 154 | Phyllamyricin E | P. myrtifolius | [147] | |
| 155 | Phyllamyricin F | P. myrtifolius | [147] | |
| 156 | Phyllamyricoside A | P. myrtifolius | Anti-HIV | [147] |
| 157 | Phyllamyricoside B | P. myrtifolius | [147] | |
| 158 | Phyllamyricoside C | P. myrtifolius | [147] | |
| 159 | Phyllanthostatin A | P. acuminatus | [148] | |
| 159 | Phyllanthostatin A | P. anisolobus | [138] | |
| 160 | Phyllanthuoside C | P. cochinchinensis | [149] | |
| 161 | Phyllanthusmin A | P. poilanei | [123] | |
| 161 | Phyllanthusmin A | P. oligospermus | Antitumor | [150] |
| 162 | Phyllanthusmin B | P. reticulatus | [13] | |
| 162 | Phyllanthusmin B | P. poilanei | [123] | |
| 162 | Phyllanthusmin B | P. oligospermus | [150] | |
| 163 | Phyllanthusmin C | P. reticulatus | [13] | |
| 163 | Phyllanthusmin C | P. flexuosus | [67] | |
| 163 | Phyllanthusmin C | P. poilanei | Antitumor | [123] |
| 163 | Phyllanthusmin C | P. oligospermus | [150] | |
| 164 | Phyllanthusmin D | P. poilanei | [123] | |
| 165 | Phyllanthusmin E | P. poilanei | [123] | |
| 166 | Phyllanthusmin D′ | P. flexuosus | [67] | |
| 167 | Phyllanthusmin E′ | P. flexuosus | [67] | |
| 168 | Phyllanthusmin F | P. flexuosus | [67] | |
| 169 | Phyltetralin | P. niruri | [128] | |
| 169 | Phyltetralin | P. urinaria | Anti-inflammatory | [125, 151] |
| 169 | Phyltetralin | P. virgatus | [131] | |
| 169 | Phyltetralin | P. amarus | Anti-inflammatory | [145] |
| 170 | Piscatorin | P. piscatorum | Antitumor | [139] |
| 171 | Reticulatuside A | P. reticulatus | [13] | |
| 172 | Reticulatuside B | P. reticulatus | [13] | |
| 173 | Retrojusticidin B | P. myrtifolius | Anti-HIV | [137, 152] |
| 174 | Seco-4-hydroxylintetralin | P. niruri | [153] | |
| 175 | Taxodiifoloside | P. taxodiifolius | Antitumor | [124] |
| 176 | Urinatetralin | P. niruri | [154] | |
| 176 | Urinatetralin | P. urinaria | [125] | |
| 177 | 2,3-Desmethoxy seco-isolintetralin | P. niruri | [155] | |
| 178 | 2,3-Desmethoxy seco-isolintetralin diacetate | P. niruri | [155] | |
| 179 | 4-(3,4-Dimethoxy-phenyl)-1-(7-methoxy-benzo[1,3]dioxol-5-yl)-2,3-bis-methoxymethyl-butan-1-ol | P. amarus | [132] | |
| 180 | 5-Demethoxy niranthin | P. urinaria | [125] | |
| 180 | 5-Demethoxy niranthin | P. amarus | [132] | |
| 181 | 7′-Hydroxy-3′,4′,5,9,9′-pentamethoxy-3,4-methylene dioxy lignan | P. urinaria | Antitumor | [156] |
| 182 | Demethylenedioxyniranthin | P. niruri | [155] | |
| 183 | Dihydrocubebin | P. niruri | [155] | |
| 183 | Dihydrocubebin | P. urinaria | [73] | |
| 184 | Hydroxyniranthin | P. niruri | [153] | |
| 185 | Linnanthin | P. niruri | [155] | |
| 186 | Niranthin | P. niruri | [157] | |
| 186 | Niranthin | P. urinaria | [125] | |
| 186 | Niranthin | P. virgatus | Antiviral | [131, 143] |
| 186 | Niranthin | P. amarus | Anti-inflammatory, antiparasitic, antihyperalgesic, and antitumor | [132, 144, 158, 159] |
| 187 | Nirphyllin | P. niruri | [160] | |
| 188 | Phyllanthin | P. niruri | Hepatoprotection, hypotensive, and antihyperuricemic | [127, 157, 161, 162] |
| 188 | Phyllanthin | P. urinaria | Immunomodulatory and hypotensive | [125, 130, 163] |
| 188 | Phyllanthin | P. amarus | Cell-protection, hepatoprotection, antitumor, and anti-CYP3A4 | [134, 144, 164, 165] |
| 188 | Phyllanthin | P. fraternus | [72] | |
| 188 | Phyllanthin | P. debilis | [135] | |
| 189 | Seco-isolariciresinol | P. oxyphyllus | [90] | |
| 190 | Seco-isolariciresinol trimethyl ether | P. niruri | [153] | |
| 191 | (+)-8-(3,4-(Methylenedioxy)benzyl)-8′-(3′,4′-dimethoxybenzyl)-butyrolactone | P. virgatus | [131] | |
| 192 | (+)-Secoisolariciresinol | P. songboiensis | [65] | |
| 193 | (+)-Songbosin | P. songboiensis | [65] | |
| 194 | 2S,3S-Bursehernin | P. urinaria | [166] | |
| 195 | 3-(3,4-Dimethoxy-benzyl)-4-(7-methoxy-benzo[1,3]dioxol-5-yl-methyl)-dihydrofuran-2-one | P. amarus | [132] | |
| 196 | Acutissimalignans B | P. acutissima | [66] | |
| 197 | Bursehernin | P. amarus | [132] | |
| 198 | Cubebin dimethyl ether | P. niruri | [154] | |
| 199 | Dibenzylbutyrolactone | P. niruri | [153] | |
| 200 | Heliobuphthalmin lactone | P. urinaria | [125] | |
| 200 | Heliobuphthalmin lactone | P. amarus | [132] | |
| 201 | Hinokinin | P. niruri | [136] | |
| 201 | Hinokinin | P. virgatus | Antiviral | [131, 143] |
| 202 | (7 R,7′R,8S,8′S)-Icariol A2 | P. urinaria | [73] | |
| 203 | Phyllnirurin | P. niruri | [160] | |
| 204 | Urinaligran | P. urinaria | [125] | |
| 205 | Virgatusin | P. urinaria | [125] | |
| 205 | Virgatusin | P. virgatus | [131] | |
| 205 | Virgatusin | P. amarus | [132] | |
| 206 | (+)-Diasyringaresinol | P. flexuosus | [67] | |
| 207 | (−)-Episyringaresinol | P. urinaria | [73] | |
| 207 | (−)-Episyringaresinol | P. songboiensis | [65] | |
| 208 | (−)-Lirioresinol-B | P. virgatus | [94] | |
| 209 | 4-Ketopinoresinol | P. emblica | [114] | |
| 210 | 4-Oxopinoresinol | P. urinaria | [73] | |
| 211 | Lirioresinol A | P. emblica | [114] | |
| 212 | Medioresinol | P. emblica | [114] | |
| 213 | Pinoresinol | P. oxyphyllus | [90] | |
| 213 | Pinoresinol | P. songboiensis | [65] | |
| 214 | Syringaresinol | P. emblica | [114] | |
| 214 | Syringaresinol | P. urinaria | [73] | |
| 214 | Syringaresinol | P. reticulatus | [13] | |
| 215 | Virgatyne | P. virgatus | [94] | |
| 216 | 4,9,9′-Trihydroxy-3,4′-dimethoxy-8-O-3′-neolignan | P. emblica | [114] | |
| 217 | Caffeic acid | P. urinaria | [167] | |
| 217 | Caffeic acid | P. sellowianus | [168] | |
| 217 | Caffeic acid | P. muellerianus | [169] | |
| 217 | Caffeic acid | P. simplex | [170] | |
| 218 | Cinnamic acid | P. emblica | Antioxidant | [171] |
| 219 | Coniferyl aldehyde | P. emblica | [114] | |
| 220 | Evofolin B | P. urinaria | [73] | |
| 221 | Ferulic acid | P. urinaria | [172] | |
| 221 | Ferulic acid | P. simplex | [170] | |
| 222 | Methyl caffeate | P. emblica | [114] | |
| 223 | Phyllanthuoside A | P. cochinchinensis | Antitumor | [149] |
| 224 | Phyllanthuoside B | P. cochinchinensis | [149] | |
| 225 | Debelalactone | P. debilis | Hepatoprotection | [173] |
| 226 | Isofraxidin | P. sellowianus | [174] | |
| 227 | Scopoletin | P. sellowianus | [174] | |
| 228 | 1,2,4,6-Tetra-O-galloyl-β-D-glucose | P. emblica | Antiviral | [175] |
| 228 | 1,2,4,6-Tetra-O-galloyl-β-D-glucose | P. niruri | Antiviral | [176, 177] |
| 229 | 1,3,4,6-Tetra-O-galloyl-β-D-glucose | P. virgatus | [94] | |
| 230 | 1,4,6-Tri-O-galloyl-β-D-glucose | P. virgatus | [94] | |
| 231 | 1,6-Di-O-galloyl-β-D-glucose | P. virgatus | [94] | |
| 232 | 1,2-Di-O-galloyl-3,6-(R)-hexa-hydroxydiphenoyl-β-D-glucose | P. niruri | [176] | |
| 233 | Amariin | P. amarus | Hepatoprotection, radioprotective, and antioxidant | [178–181] |
| 234 | Amariinic acid | P. amarus | [182] | |
| 235 | Amarulone | P. amarus | [183] | |
| 236 | Carpinusnin | P. emblica | [184] | |
| 237 | Chebulagic acid | P. emblica | Antioxidant and antitumor | [111, 184, 185] |
| 237 | Chebulagic acid | P. myrtifolius | [186] | |
| 238 | Chebulanin | P. emblica | Antioxidant | [184, 185] |
| 239 | Corilagin | P. emblica | Antioxidant and antitumor | [111, 184, 187] |
| 239 | Corilagin | P. niruri | Antihyperalgesic and anti-inflammatory | [6, 176, 188] |
| 239 | Corilagin | P. urinaria | Antiviral and antiplatelet | [189–191] |
| 239 | Corilagin | P. reticulatus | [192] | |
| 239 | Corilagin | P. virgatus | [94] | |
| 239 | Corilagin | P. amarus | Antidiabetic, radioprotective, and anti-HIV | [179, 181, 193, 194] |
| 239 | Corilagin | P. myrtifolius | [186] | |
| 239 | Corilagin | P. muellerianus | [169] | |
| 239 | Corilagin | P. debilis | Antioxidant | [195] |
| 239 | Corilagin | P. matsumurae | [196] | |
| 239 | Corilagin | P. wightianus | [89] | |
| 239 | Corilagin | P. ussuriensis | Antioxidant | [197, 198] |
| 240 | Excoecarianin | P. urinaria | Antiviral | [199] |
| 241 | Furosin | P. emblica | Antioxidant | [184, 187] |
| 241 | Furosin | P. virgatus | [94] | |
| 241 | Furosin | P. sellowianus | Antihyperalgesic | [200] |
| 241 | Furosin | P. muellerianus | Wound healing | [169] |
| 241 | Furosin | P. debilis | Antioxidant | [195] |
| 242 | Geraniin | P. emblica | Antioxidant and antitumor | [111, 185, 201] |
| 242 | Geraniin | P. niruri | Antiviral | [177] |
| 242 | Geraniin | P. urinaria | Immunomodulatory, antioxidant, and hypotensive | [41, 163] |
| 242 | Geraniin | P. virgatus | Antiviral | [94, 143] |
| 242 | Geraniin | P. amarus | Hepatoprotection, radioprotective, and anti-HIV | [179–181, 194] |
| 242 | Geraniin | P. myrtifolius | [186] | |
| 242 | Geraniin | P. sellowianus | Antihyperalgesic | [200] |
| 242 | Geraniin | P. muellerianus | Wound healing and antimalarial | [169, 202] |
| 242 | Geraniin | P. debilis | Antioxidant | [195] |
| 242 | Geraniin | P. matsumurae | [196] | |
| 242 | Geraniin | P. wightianus | [89] | |
| 242 | Geraniin | P. ussuriensis | [197] | |
| 242 | Geraniin | P. caroliniensis | [203] | |
| 243 | Geraniinic acid B | P. amarus | [182] | |
| 244 | Hippomanin A | P. urinaria | Antiviral | [204] |
| 245 | Isocorilagin | P. emblica | Antioxidant and antitumor | [185, 201, 205] |
| 245 | Isocorilagin | P. niruri | Cholinesterase inhibition | [206, 207] |
| 246 | Isomallotusinin | P. emblica | Antioxidant | [185] |
| 247 | Isostrictinin | P. emblica | [208] | |
| 247 | Isostrictinin | P. urinaria | [209] | |
| 248 | Mallonin | P. emblica | [184] | |
| 249 | Mallotusinin | P. emblica | Antioxidant | [210] |
| 249 | Mallotusinin | P. myrtifolius | [186] | |
| 250 | Neochebulagic acid | P. emblica | [184] | |
| 251 | Phyllanemblinin A | P. emblica | [184] | |
| 251 | Phyllanemblinin A | P. flexuosus | [211] | |
| 252 | Phyllanemblinin B | P. emblica | [184] | |
| 252 | Phyllanemblinin B | P. flexuosus | [211] | |
| 253 | Phyllanemblinin C | P. emblica | [184] | |
| 253 | Phyllanemblinin C | P. flexuosus | [211] | |
| 254 | Phyllanemblinin D | P. emblica | [184] | |
| 254 | Phyllanemblinin D | P. flexuosus | [211] | |
| 255 | Phyllanemblinin E | P. emblica | [184] | |
| 255 | Phyllanemblinin E | P. flexuosus | [211] | |
| 256 | Phyllanemblinin F | P. emblica | [184] | |
| 257 | Phyllanthunin | P. emblica | [212] | |
| 258 | PhyllanthusiinC | P. myrtifolius | [186] | |
| 259 | PhyllanthusiinD | P. niruri | [176] | |
| 259 | PhyllanthusiinD | P. amarus | Radioprotective and antioxidant | [178, 181] |
| 260 | Phyllanthusiin G | P. urinaria | [213] | |
| 261 | Phyllanthusiin U | P. urinaria | [167] | |
| 262 | Pinocembrin-7-O-[3′′-O-galloyl-4′′,6′′-(S)-hexahydroxydiphenoyl]-β-D-glucose | P. tenellus | [214] | |
| 263 | Pinocembrin-7-O-[4′′,6′′-(S)-hexahydroxydiphenoyl]-β-D-glucose | P. tenellus | [214] | |
| 264 | Punicafolin | P. emblica | [184] | |
| 265 | Putranjivain A | P. emblica | [184] | |
| 266 | Putranjivain B | P. emblica | [185] | |
| 267 | Repandusinic acid | P. amarus | Antioxidant | [178, 182] |
| 268 | Terchebin | P. niruri | [176] | |
| 269 | Tercatain | P. emblica | [184] | |
| 270 | Virganin | P. virgatus | [94] | |
| 271 | Dimeric procyanidins mono-gallates | P. orbicularis | Antiviral | [53] |
| 272 | Dimeric procyanidins-3,3′-di-O-gallates | P. orbicularis | Antiviral | [53] |
| 273 | Epicatechin-(4β → 8)-epigallocatechin | P. emblica | [184] | |
| 274 | Oligomeric procyanidins | P. orbicularis | Antiviral | [53] |
| 275 | Oligomeric procyanidins mono-gallates | P. orbicularis | Antiviral | [53] |
| 276 | Phyllemtannin | P. emblica | Antitumor | [111] |
| 277 | Prodelphinidin B1 | P. emblica | [184] | |
| 277 | Prodelphinidin B1 | P. niruri | [215] | |
| 277 | Prodelphinidin B1 | P. sellowianus | [216] | |
| 277 | Prodelphinidin B1 | P. orbicularis | [215] | |
| 277 | Prodelphinidin B1 | P. matsumurae | [217] | |
| 278 | Prodelphinidin B2 | P. emblica | [184] | |
| 278 | Prodelphinidin B2 | P. orbicularis | Antioxidant | [53, 54] |
| 278 | Prodelphinidin B2 | P. simplex | [170] | |
| 278 | Prodelphinidin B2 | P. matsumurae | [218] | |
| 279 | Prodelphinidin B-2,3′-O-gallate | P. emblica | [184] | |
| 280 | 5,7-Dihydroxy-4′-methoxyflavonol | P. virgatus | [94] | |
| 281 | 5,3′-Dihydroxy-6,7,4′-trimethoxyflavone | P. niruri | [207] | |
| 282 | Astragalin | P. urinaria | [141] | |
| 282 | Astragalin | P. virgatus | [94] | |
| 282 | Astragalin | P. muellerianus | [169] | |
| 283 | Avicularin | P. emblica | [219] | |
| 284 | Galangin 3-O-β-D-glucoside 8-sulfonate | P. virgatus | [94] | |
| 285 | Isoquercitrin | P. emblica | [201] | |
| 285 | Isoquercitrin | P. urinaria | [220] | |
| 285 | Isoquercitrin | P. reticulatus | [192] | |
| 285 | Isoquercitrin | P. virgatus | [94] | |
| 285 | Isoquercitrin | P. muellerianus | [169] | |
| 286 | Kaempferol | P. emblica | Antioxidant | [201] |
| 286 | Kaempferol | P. niruri | [79] | |
| 286 | Kaempferol | P. virgatus | [94] | |
| 286 | Kaempferol | P. cochinchinensis | [149] | |
| 287 | Kaempferol-3-O-α-L-(6′′-ethyl)-rhamnopyranoside | P. emblica | [221] | |
| 288 | Kaempferol-3-O-α-L-(6′′-methyl)-rhamnopyranoside | P. emblica | [221] | |
| 289 | Kaempferol-3-O-β-D-glucopyranoside | P. emblica | Antioxidant | [201] |
| 290 | Kaempferol 8-sulfonate | P. virgatus | [94] | |
| 291 | Myricitrin | P. virgatus | [94] | |
| 292 | Quercetin | P. emblica | Antioxidant | [171] |
| 292 | Quercetin | P. urinaria | [215] | |
| 292 | Quercetin | P. virgatus | [94] | |
| 292 | Quercetin | P. caroliniensis | Anti-inflammatory | [203] |
| 293 | Quercetin 3-O-α-L-(2,4-di-O-acetyl) rhamnopyranoside-7-O-α-L-rhamnopyranoside | P. urinaria | [222] | |
| 294 | Quercetin 3-O-α-L-(3,4-di-O-acetyl) rhamnopyranoside-7-O-α-L-rhamnopyranoside | P. urinaria | [222] | |
| 295 | Quercetin 3-O-α-L-rhamnopyranoside | P. urinaria | [222] | |
| 296 | Quercetin-3-O-β-D-glucopyranoside | P. emblica | Antioxidant | [201] |
| 297 | Quercetin-3-O-β-D-glucopyranosyl(1 → 4)-α-rhamnopyranoside | P. niruri | [79] | |
| 298 | Quercetin-3-O-β-D-glucosyl-(1 → 6)-β-D-glucoside | P. virgatus | [94] | |
| 299 | Quercetin 3-O-β-D-glucopyranosyl-(2 → 1)-O-β-D-xylopyranoside | P. niruri | [223] | |
| 300 | Quercetin pentaacetate | P. orbicularis | [54] | |
| 301 | Quercitrin | P. niruri | Antinociceptive | [215, 224] |
| 301 | Quercitrin | P. urinaria | Anti-inflammatory | [151, 215] |
| 301 | Quercitrin | P. virgatus | [94] | |
| 301 | Quercitrin | P. sellowianus | [95] | |
| 301 | Quercitrin | P. muellerianus | [169] | |
| 301 | Quercitrin | P. orbicularis | [54] | |
| 301 | Quercitrin | P. ussuriensis | [225] | |
| 302 | Rhamnocitrin | P. urinaria | Anti-inflammatory | [151] |
| 302 | Rhamnocitrin | P. amarus | [179] | |
| 302 | Rhamnocitrin | P. cochinchinensis | [149] | |
| 302 | Rhamnocitrin | P. simplex | [170] | |
| 303 | Rutin | P. niruri | Anti-inflammatory | [224] |
| 303 | Rutin | P. urinaria | Anti-inflammatory | [151, 215] |
| 303 | Rutin | P. reticulatus | [192] | |
| 303 | Rutin | P. virgatus | [94] | |
| 303 | Rutin | P. amarus | Radioprotective and antioxidant | [178, 181] |
| 303 | Rutin | P. debilis | Antioxidant | [195] |
| 304 | Rutin decaacetate | P. orbicularis | [54] | |
| 305 | Schaftoside | P. cochinchinensis | [149] | |
| 306 | Sodium galangin-8-sulfonate | P. virgatus | [94] | |
| 307 | Sodium galangin-3-O-β-glucoside-8-sulfonate | P. virgatus | [94] | |
| 308 | Sodium kaempferol-8-sulfonate | P. virgatus | [94] | |
| 309 | Vicenin-2 | P. cochinchinensis | [149] | |
| 310 | 4′-Methoxyscutellarein | P. urinaria | [226] | |
| 311 | Apigenin | P. amarus | [74] | |
| 311 | Apigenin | P. orbicularis | Antioxidant | [54] |
| 312 | Apigenin-7-O-(6′′-butyryl-β-glucopyranoside) | P. emblica | [227] | |
| 312 | Apigenin-7-O-(6′′-butyryl-β-glucopyranoside) | P. niruri | [215] | |
| 312 | Apigenin-7-O-(6′′-butyryl-β-glucopyranoside) | P. urinaria | [215] | |
| 313 | Demethoxysudachitin (4′,5,7-trihydroxy-6,8-dimethoxyflavone) | P. atropurpureus | [228] | |
| 314 | Galangin 8-sulfonate | P. virgatus | [94] | |
| 315 | Luteolin | P. amarus | [74] | |
| 315 | Luteolin | P. singampattiana | [78] | |
| 316 | Niruriflavone | P. niruri | Antioxidant | [206] |
| 317 | Urinariaflavone | P. urinaria | [141] | |
| 318 | 2-(4-Hydroxyphenyl)-8-(3-methylbut-2-enyl)-chroman-4-one | P. niruri | [23] | |
| 319 | 7-Hydroxyflavanone | P. sellowianus | [168] | |
| 320 | 8-(3-Methyl-but-2-enyl)-2-phenyl chroman-4-one | P. niruri | Antiparasitic | [23] |
| 321 | Nirurin | P. niruri | [229] | |
| 322 | Nirurinetin | P. niruri | [229] | |
| 323 | (S)-Eriodictyol 7-O-(6′′-O-(E)-β-coumaroyl)-β-D-glucopyranoside | P. emblica | [230] | |
| 324 | (S)-Eriodictyol 7-O-(6′′-O-galloyl)-β-D-glucopyranoside | P. emblica | [230] | |
| 325 | (+)-Catechin | P. niruri | [176] | |
| 325 | (+)-Catechin | P. orbicularis | [53] | |
| 326 | (−)-Epiafzelechin | P. emblica | [184] | |
| 327 | (−)-Epicatechin | P. emblica | [184] | |
| 327 | (−)-Epicatechin | P. niruri | [176] | |
| 327 | (−)-Epicatechin | P. cochinchinensis | [149] | |
| 327 | (−)-Epicatechin | P. orbicularis | [53] | |
| 328 | (−)-Epigallocatechin | P. emblica | [184] | |
| 328 | (−)-Epigallocatechin | P. niruri | [176] | |
| 328 | (−)-Epigallocatechin | P. reticulatus | [118] | |
| 329 | (+)-Gallocatechin | P. emblica | [184] | |
| 329 | (+)-Gallocatechin | P. niruri | [176] | |
| 330 | 8-(2-Pyrrolidinone-5-yl)-(−)-epicatechin | P. cochinchinensis | [149] | |
| 331 | 5,7-Dimethoxy-3,4′-dihydroxy-3′,8-di-C-prenylflavanone | P. niruri | [231] | |
| 332 | 5,6,8,4′-Tetrahydroxy isoflavone | P. atropurpureus | [228] | |
| 333 | 6-Hydroxy-7,8,2′,3′,4′-pentamethoxyisoflavone | P. niruri | [207] | |
| 334 | (−)-β-Sitosterol-3-O-β-D-(6-O-palmitoyl) glucopyranoside | P. songboiensis | [65] | |
| 335 | (3β,22E)-Stigmasta-5,22-diene-3,25-diol | P. urinaria | [73] | |
| 336 | 24-Isopropylcholesterol | P. niruri | [157] | |
| 337 | 5α,6β-Dihydroxysitosterol | P. emblica | [232] | |
| 338 | 5α,6β,7α-Trihydroxysitosterol | P. emblica | [232] | |
| 339 | 6′-(Stigmast-5-en-3-O-β-D-glucopyranosidyl) hexadecanoate | P. emblica | [232] | |
| 340 | 6′-(Stigmast-5-en-7-one-3-O-β-D-glucopyranosidyl) hexadecanoate | P. emblica | [232] | |
| 341 | 7-Ketositosterol | P. emblica | [232] | |
| 342 | 7α-Hydroxysitosterol | P. emblica | [232] | |
| 343 | 7α-Acetoxysitosterol | P. emblica | [232] | |
| 344 | 7β-Ethoxysiterol | P. emblica | [232] | |
| 345 | Amarosterol A | P. amarus | [233] | |
| 346 | Amarosterol B | P. amarus | [233] | |
| 347 | Campesterol | P. sellowianus | [216] | |
| 348 | Daucosterol | P. emblica | [232] | |
| 348 | Daucosterol | P. urinaria | [220] | |
| 348 | Daucosterol | P. amarus | [74] | |
| 349 | Fraternusterol | P. fraternus | [234] | |
| 350 | Phyllanthosecosteryl ester | P. fraternus | [234] | |
| 351 | Phyllanthosterol | P. fraternus | [234] | |
| 352 | Phyllanthostigmasterol | P. fraternus | [234] | |
| 353 | Stigmast-4-en-3-one | P. emblica | [232] | |
| 354 | Stigmast-4-en-3,6-dione | P. emblica | [232] | |
| 355 | Stigmast-4-en-6β-ol-3-one | P. emblica | [232] | |
| 356 | Stigmast-4-ene-3β,6α-diol | P. emblica | [232] | |
| 357 | Stigmast-4,5-en-3-one | P. oxyphyllus | [90] | |
| 358 | Stigmast-5-en-3-ol, oleate | P. amarus | [74] | |
| 359 | Stigmasterol | P. urinaria | [97] | |
| 359 | Stigmasterol | P. sellowianus | [216] | |
| 359 | Stigmasterol | P. columnaris | [76] | |
| 360 | Stigmasterol 3-O-β-D-glucoside | P. urinaria | [97] | |
| 361 | β-Daucosterol | P. emblica | Antioxidant | [171, 212] |
| 362 | β-Sitosterol | P. emblica | [100] | |
| 362 | β-Sitosterol | P. niruri | [157] | |
| 362 | β-Sitosterol | P. urinaria | [220] | |
| 362 | β-Sitosterol | P. reticulatus | [77] | |
| 362 | β-Sitosterol | P. sellowianus | [216] | |
| 362 | β-Sitosterol | P. muellerianus | [92] | |
| 362 | β-Sitosterol | P. oxyphyllus | [90] | |
| 362 | β-Sitosterol | P. fraternus | [72] | |
| 362 | β-Sitosterol | P. debilis | [135] | |
| 362 | β-Sitosterol | P. singampattiana | [78] | |
| 363 | β-Sitosterol-3-O-β-D-glucopyranoside | P. urinaria | [151] | |
| 364 | 14,15-Dihydroallosecurinin-15β-ol | P. discoideus | [148] | |
| 365 | 4-Hydroxysecurinine | P. niruri | [235] | |
| 366 | 4-Methoxydihydronorsecurinine | P. niruri | [235] | |
| 367 | β-Sitosterol-3-β-D-glucopyranoside | P. singampattiana | [78] | |
| 368 | 4-Methoxynorsecurinine | P. niruri | [236] | |
| 369 | 4-Methoxytetrahydrosecurinine | P. niruri | [235] | |
| 370 | Allosecurinine | P. niruri | [235] | |
| 370 | Allosecurinine | P. glaucus | [237] | |
| 371 | Dihydrosecurinine | P. niruri | [235] | |
| 372 | Ent-norsecurinine | P. niruri | [238] | |
| 373 | Epibubbialine | P. niruri | [239] | |
| 373 | Epibubbialine | P. amarus | [240] | |
| 374 | Isobubbialine | P. niruri | [215] | |
| 374 | Isobubbialine | P. urinaria | [215] | |
| 374 | Isobubbialine | P. amarus | [240] | |
| 375 | Methyl (2S)-1-[2-(furan-2-yl)-2-oxoethyl]-5-oxopyrrolidine-2-carboxylate | P. emblica | [114] | |
| 376 | Nirurine | P. niruri | [241] | |
| 377 | Niruroidine | P. niruroides | [242] | |
| 378 | Nitidine | P. sellowianus | [243] | |
| 379 | Norsecurinine | P. niruri | [235] | |
| 379 | Norsecurinine | P. amarus | Antifungal | [240, 244] |
| 379 | Norsecurinine | P. simplex | [245] | |
| 379 | Norsecurinine | P. discoides | [246] | |
| 380 | Phyllanthine | P. niruri | [236] | |
| 380 | Phyllanthine | P. amarus | [240] | |
| 381 | Securinine | P. niruri | [235] | |
| 381 | Securinine | P. amarus | [240] | |
| 381 | Securinine | P. glaucus | [237] | |
| 382 | Securinol A | P. niruri | [235] | |
| 383 | Securinol B | P. niruri | [235] | |
| 384 | Simplexine | P. simplex | [245] | |
| 385 | Tetrahydrosecurinine | P. niruri | [235] | |
| 386 | Virosecurinine | P. discoides | [247] | |
| 387 | 1,12-Diazacyclodocosane-2,11-dione | P. niruri | [248] | |
| 388 | 3-(3-Methylbut-2-en-1-yl) isoguanine | P. reticulatus | [118] | |
| 389 | 5-Hydroxy-isoquinoline | P. emblica | [249] | |
| 390 | E,E-2,4-Octadienamide | P. fraternus | Antimalarial | [250] |
| 391 | E,Z-2,4-Decadienamide | P. fraternus | Antimalarial | [250] |
| 392 | Indole-3-carboxaldehyde | P. virgatus | [94] | |
| 393 | Indole-3-carboxylic acid | P. virgatus | [131] | |
| 394 | Phyllanthimide | P. sellowianus | [251] | |
| 395 | Phyllurine | P. urinaria | [252] | |
| 396 | (−)-Epicatechin 3-O-gallate | P. niruri | [176] | |
| 396 | (−)-Epicatechin 3-O-gallate | P. orbicularis | Antiviral | [53] |
| 397 | (−)-Epigallocatechin 3-O-gallate | P. emblica | [111] | |
| 397 | (−)-Epigallocatechin 3-O-gallate | P. niruri | [176] | |
| 398 | (5R∗R∗)-4,6-Dimethoxycarbonyl-5-[2′,3′,4′-trihydroxy-6′-(methoxycarbonyl) phenyl]-5,6-dihydro-2H-pyran-2-one | P. reticulatus | [16] | |
| 399 | 1-O-Galloyl-6-O-luteoyl-α-D-glucose | P. niruri | Antimalarial | [223] |
| 400 | 1-O-Galloyl-β-D-glucose | P. emblica | Antidiabetic and antitumor | [111, 253, 254] |
| 400 | 1-O-Galloyl-β-D-glucose | P. virgatus | [94] | |
| 401 | 2-(2-Methylbutyryl)phloroglucinol 1-O-(6′′-O-β-D-apiofuranosyl)-β-D-glucopyranoside | P. emblica | [230] | |
| 402 | 2,3,4,5,6-Pentahydroxybenzoic acid | P. urinaria | [255] | |
| 403 | 2,3,5,6-Tetrahydroxybenzyl acetate | P. niruri | [256] | |
| 404 | 2,6-Dimethoxy-4-(2-hydroxyethyl)phenol 1-O-β-D-glucopyranoside | P. emblica | [110] | |
| 405 | 2-Carboxylmethylphenol 1-O-β-D-glucopyranoside | P. emblica | [110] | |
| 406 | 3′′-Hydroxy robustaside A (6′-(3′′,4′′-dihydroxy cinnamoyl) arbutin) | P. atropurpureus | [228] | |
| 407 | 3,3′-Di-O-methylellagic acid | P. reticulatus | [105] | |
| 408 | 3,4,3′-Tri-O-methylellagic acid | P. urinaria | [172] | |
| 408 | 3,4,3′-Tri-O-methylellagic acid | P. reticulatus | [16] | |
| 409 | 3,4,8,9,10-Pentahydroxy-dibenzo[b,d] pyran-6-one | P. emblica | [114] | |
| 410 | 3,4-di-O-Methylellagic acid | P. reticulatus | [105] | |
| 411 | 3,5-Dicaffeoylquinic acid | P. muellerianus | [169] | |
| 412 | 3,5-Dihydroxy-4-methoxybenzoic acid | P. urinaria | [73] | |
| 413 | 3-Ethylgallic acid | P. emblica | [208] | |
| 414 | 3-O-Methylellagic acid 4′-O-α-L-rhamnopyranoside | P. reticulatus | [105] | |
| 415 | 4,4′-Di-O-methylellagic acid | P. reticulatus | [105] | |
| 416 | 4-Hydroxy-3-methoxybenzaldehyde | P. emblica | [114] | |
| 417 | 4-Hydroxy-3-methoxy-benzoic acid | P. amarus | [74] | |
| 418 | 4-O-Caffeoylquinic acid | P. niruri | [257] | |
| 419 | 4-O-Methylellagic acid-3′-α-rhamnoside | P. emblica | [87] | |
| 420 | 4-O-Methylgallic acid | P. polyphyllus | Anti-inflammatory | [126] |
| 421 | 8,9-Epoxy brevifolin | P. simplex | Hepatoprotective | [258] |
| 422 | Bergenin | P. flexuosus | [80] | |
| 422 | Bergenin | P. wightianus | [89] | |
| 423 | Brevifolin | P. urinaria | [259] | |
| 423 | Brevifolin | P. virgatus | [94] | |
| 423 | Brevifolin | P. simplex | Hepatoprotective | [260] |
| 424 | Brevifolin carboxylic acid | P. niruri | [261] | |
| 424 | Brevifolin carboxylic acid | P. urinaria | [209] | |
| 424 | Brevifolin carboxylic acid | P. amarus | Antidiabetic | [193] |
| 424 | Brevifolin carboxylic acid | P. matsumurae | [196] | |
| 425 | Caffeoylmalic acid | P. muellerianus | [169] | |
| 426 | Chebulic acid | P. emblica | [253] | |
| 427 | Chlorogenic acid | P. sellowianus | [168] | |
| 427 | Chlorogenic acid | P. muellerianus | [169] | |
| 428 | Dehydrochebulic acid trimethyl ester | P. urinaria | [73] | |
| 429 | Di [3,4,5-trihydroxy-phenyl] ether | P. atropurpureus | [228] | |
| 430 | Ellagic acid | P. emblica | Antioxidant | [100, 210] |
| 430 | Ellagic acid | P. niruri | Antidiabetic | [202, 261] |
| 430 | Ellagic acid | P. urinaria | Antitumor | [220, 262] |
| 430 | Ellagic acid | P. reticulatus | [192] | |
| 430 | Ellagic acid | P. matsumurae | [196] | |
| 430 | Ellagic acid | P. wightianus | [89] | |
| 431 | Ethyl brevifolin carboxylate | P. niruri | [261] | |
| 431 | Ethyl brevifolin carboxylate | P. urinaria | [189] | |
| 432 | Ethyl gallate | P. emblica | Antitussive | [212, 263] |
| 432 | Ethyl gallate | P. myrtifolius | [186] | |
| 433 | Flavogallonic acid bislactone | P. emblica | [184] | |
| 434 | Gallic acid | P. emblica | Antiulcer and antioxidant | [210, 264] |
| 434 | Gallic acid | P. niruri | Anti-inflammatory | [202, 224] |
| 434 | Gallic acid | P. urinaria | [220] | |
| 434 | Gallic acid | P. virgatus | [94] | |
| 434 | Gallic acid | P. amarus | Antijaundice | [265] |
| 434 | Gallic acid | P. myrtifolius | [186] | |
| 434 | Gallic acid | P. muellerianus | [169] | |
| 434 | Gallic acid | P. debilis | Antioxidant | [195] |
| 434 | Gallic acid | P. simplex | [170] | |
| 434 | Gallic acid | P. matsumurae | [196] | |
| 434 | Gallic acid | P. wightianus | [89] | |
| 434 | Gallic acid | P. ussuriensis | [225] | |
| 435 | Gallic acid 3-O-(6′-O-galloyl)-β-D-glucoside | P. emblica | [184] | |
| 436 | Gallic acid 3-O-β-D-glucoside | P. emblica | [184] | |
| 437 | Gallic acid 4-methyl ether | P. cochinchinensis | [149] | |
| 438 | Gallic acid ethyl ester | P. urinaria | Antihyperalgesic | [266] |
| 438 | Gallic acid ethyl ester | P. sellowianus | [95] | |
| 438 | Gallic acid ethyl ester | P. caroliniensis | Anti-inflammatory | [203] |
| 439 | Koaburaside | P. cochinchinensis | [149] | |
| 440 | L-Malic acid 2-O-gallate | P. emblica | Antitumor | [111, 253] |
| 441 | Methyl-4-hydroxybenzoate | P. emblica | [114] | |
| 442 | Methyl brevifolin carboxylate | P. niruri | Hypotensive and antiplatelet | [206, 267, 268] |
| 442 | Methyl brevifolin carboxylate | P. urinaria | Antioxidant and anti-inflammatory | [151, 269] |
| 442 | Methyl brevifolin carboxylate | P. reticulatus | [192] | |
| 442 | Methyl brevifolin carboxylate | P. virgatus | [94] | |
| 443 | Methyl ester dehydrochebulic acid | P. urinaria | [269] | |
| 444 | Methyl gallate | P. emblica | Antioxidant and antitussive | [187, 263] |
| 444 | Methyl gallate | P. urinaria | Antioxidant and anti-inflammatory | [151] |
| 444 | Methyl gallate | P. reticulatus | [192] | |
| 444 | Methyl gallate | P. virgatus | [94] | |
| 444 | Methyl gallate | P. myrtifolius | [186] | |
| 444 | Methyl gallate | P. muellerianus | [169] | |
| 444 | Methyl gallate | P. ussuriensis | [197] | |
| 445 | Mucic acid 1,4-lactone 2-O-gallate | P. emblica | [253] | |
| 446 | Mucic acid 1,4-lactone 3,5-di-O-gallate | P. emblica | [253] | |
| 447 | Mucic acid 1,4-lactone 3-O-gallate | P. emblica | Antioxidant | [185, 253] |
| 448 | Mucic acid 1,4-lactone 5-O-gallate | P. emblica | [253] | |
| 449 | Mucic acid 1,4-lactone 6-methyl ester 2-O-gallate | P. emblica | [253] | |
| 450 | Mucic acid 1,4-lactone 6-methyl ester 5-O-gallate | P. emblica | [253] | |
| 451 | Mucic acid 1-methyl ester 2-O-gallate | P. emblica | [253] | |
| 452 | Mucic acid 2-O-gallate | P. emblica | Antitumor | [111, 253] |
| 453 | Mucic acid 3-O-gallate | P. emblica | [270] | |
| 454 | Mucic acid 6-methyl ester 2-O-gallate | P. emblica | [253] | |
| 455 | Mucic acid di-methyl ester 2-O-gallate | P. emblica | [253] | |
| 456 | p-Hydroxybenzaldehyde | P. urinaria | [73] | |
| 457 | Phloroglucinol | P. ussuriensis | [225] | |
| 458 | Phyllangin | P. niruri | [256] | |
| 459 | Phyllanthusin F | P. urinaria | [271] | |
| 460 | Potassium brevifolin carboxylate | P. virgatus | [94] | |
| 461 | Protocatechuic acid | P. urinaria | [189] | |
| 461 | Protocatechuic acid | P. matsumurae | [196] | |
| 462 | Pyrogallol | P. emblica | Antitumor and anti-inflammatory | [249, 272] |
| 462 | Pyrogallol | P. urinaria | [167] | |
| 463 | Robustaside A | P. atropurpureus | Antitumor | [228] |
| 464 | Shikimic acid | P. myrtifolius | [186] | |
| 465 | Syringaldehyde | P. emblica | [114] | |
| 466 | Tri-Me dehydrochebulic acid | P. urinaria | [220] | |
| 467 | Trimethyl-3,4-dehydrochebulate | P. urinaria | Antioxidant and anti-inflammatory | [151] |
| 468 | Vanillic acid | P. emblica | [114] | |
| 469 | (−)-7′-Hydroxydivanillyltetrahydrofuran | P. songboiensis | [65] | |
| 470 | (+)-Cucurbic acid | P. urinaria | [73] | |
| 471 | (+)-Methyl cucurbate | P. urinaria | [73] | |
| 472 | (E)-3-(5′-Hydroperoxy-2,2′-dihydroxy[1,1′-biphenyl]-4-yl)-2-propenoic acid | P. urinaria | [255] | |
| 473 | 1′S-11-Dehydroxy penicillide | P. emblica | [114] | |
| 474 | 2R-Diethyl malate | P. emblica | [114] | |
| 475 | 3,6′-Di-O-benzoyl-2′-O-acetylsucrose | P. cochinchinensis | [108] | |
| 476 | 3,6′-Di-O-benzoyl-3′-O-acetylsucrose | P. cochinchinensis | [108] | |
| 477 | 3,6′-Di-O-benzoyl-4′-O-acetylsucrose | P. cochinchinensis | [108] | |
| 478 | 3,6′-Di-O-benzoylsucrose | P. cochinchinensis | [108] | |
| 479 | 3,4-Dimethoxyphenyl-β-D-glucopyranoside | P. cochinchinensis | [149] | |
| 480 | 3,4-Dihydroxyphenylpropanol 3-O-β-D-glucopyranoside | P. reticulatus | [118] | |
| 481 | 3,4,5-Trimethoxy-phenyl-β-D-glucopyranoside | P. cochinchinensis | [149] | |
| 482 | 3-O-Benzoyl-6′-O-(E)-cinnamoylsucrose | P. cochinchinensis | [108] | |
| 483 | 4,4,8-Trimethoxy chroman | P. amarus | [273] | |
| 484 | 5-Hydroxymethyl-2-furaldehyde | P. urinaria | [73] | |
| 485 | 4-Hydroxysesamin | P. niruri | [274] | |
| 486 | 5-Hydroxymethylfurfural | P. emblica | Antioxidant | [171] |
| 487 | Aquilegiolide | P. anisolobus | [138] | |
| 487 | Aquilegiolide | P. klotzschianus | [275] | |
| 488 | Bis(2-ethylicosyl)phthalate | P. muellerianus | [92] | |
| 489 | Bis(2-ethyloctyl)phthalate | P. muellerianus | [92] | |
| 490 | Di-O-methylcrenatin | P. cochinchinensis | [149] | |
| 491 | Byzantionoside B | P. multiflorus | [276] | |
| 492 | Carthamoside B5 | P. reticulatus | [118] | |
| 493 | Dendranthemoside B | P. urinaria | [141] | |
| 494 | Hovetrichoside A | P. reticulatus | [118] | |
| 495 | Isotachioside | P. reticulatus | [118] | |
| 496 | Menisdaurilide | P. anisolobus | [138] | |
| 496 | Menisdaurilide | P. klotzschianus | [275] | |
| 497 | Methyl (1 R,2R,2′Z)-2-(5′-hydroxy-pent-2′-enyl)-3-oxocyclopentaneacetate | P. urinaria | [73] | |
| 498 | Mucic acid | P. emblica | [277] | |
| 499 | Mucic acid 1-methyl ester-6-ethyl ester | P. emblica | [114] | |
| 500 | Penicillide | P. emblica | [114] | |
| 501 | Phthalic acid bis(2,5-dimethylhexyl) ester | P. urinaria | [99] | |
| 502 | Phyllanthoid A | P. cochinchinensis | Antitumor | [278] |
| 503 | Phyllanthoid B | P. cochinchinensis | [278] | |
| 504 | Phyllanthurinolactone | P. urinaria | [279] | |
| 505 | Phyllanthusone | P. fraternus | [121] | |
| 506 | Phyllester | P. niruri | [157] | |
| 507 | Purpactin A | P. emblica | [114] | |
| 508 | Roseoside | P. multiflorus | [276] | |
| 509 | Succinic acid | P. niruri | [280] | |
| 510 | Terephthalic acid mono-[2-(4-carboxy-phenoxycarbonyl)-vinyl] ester | P. urinaria | [255] | |
| 511 | Vanilloloside | P. cochinchinensis | [149] | |
| 512 | Xanthoxyline | P. sellowianus | [281] |
3.1. Terpenoids
Terpenoids are the most prevalent chemical class of the genus. About 125 compounds including 69 triterpenoids (1–69), 40 sesquiterpenes (70–109), 11 diterpenoids (110–120), and 5 monoterpenes (121–125) are mainly identified from P. flexuosus, P. reticulatus, P. watsonii, P. emblica, P. acuminatus, and P. veuminatus. Compounds 1–14 are tetracyclic triterpenoids, and compounds 15–69 are pentacyclic triterpenoids. In pentacyclic triterpenoids, compounds 15–36, compounds 37–49, and compounds 50–65 are oleanane type, friedelane type, and lupine type, respectively. Glochidone and lupeol are representatives of lupine type triterpenoids, which were suggested to have antitumor activities and mainly isolated from Phyllanthus species [68, 80, 96].
3.2. Phenylpropanoids
Phenylpropanoids (126–227) have typical C6–C3 constituents, which chiefly involve three groups including lignins, simple phenylpropanoids, and coumarins. 90 lignins (126–215) have been isolated from genus Phyllanthus since 1944. Compounds 126–176 are arylnaphthalene type lignins with a ring caused by the link of C-6 and C-7′. Compounds 177–190 are dibenzylbutane type lignins with two simple phenylpropanoids bounded by C-8 and C-8′. Phyllanthin, which had been studied to the most extent, was considered to be correlated with anti-inflammatory, immunomodulatory, antitumor, and hypotensive activities [127, 144, 163]. Pharmacokinetic studies of retrojusticidin B, a potential anti-HIV compound, had been done. The oral bioavailabilities dissolved in Tween 80 and in corn oil were found to be 22.1 and 33.1%, respectively [152].
3.3. Tannins
Tannins were progressively reported from the genus Phyllanthus since 1992. Hydrolyzable tannins (228–270) are characterized by the presence of one or more galloyl, hexahydroxydiphenoyl (HHDP), and HHDP metabolites attached to a glucopyranose core, which are mainly isolated from P. emblica, P. amarus, P. niruri, and P. urinaria. Compounds 271–279 are condensed tannins, which are the condensation of flavan-3-ols and linked by C-C. A great many condensed tannins were proved to have antiviral activity [53]. Ellagitannins (232–270) are the largest group of hydrolyzable tannins. Corilagin and geraniin are most extensively obtained from this genus and are characteristic compounds of ellagitannins, which exhibited multiple activities such as antioxidant, anti-HIV, antitumor, and antihyperalgesic activities [6, 111, 188, 195, 196, 199, 201, 202].
3.4. Flavonoids
Compounds 281–334 are flavonoids, which mainly contain flavonols (280–309), flavones (310–317), flavonones (318–324), flavan-3-ols (325–330), flavanonols (331), and isoflavone (332-333). Flavan-3-ols are the basic constitution of condensed tannins. Flavonols such as quercetin, quercitrin, and rutin demonstrated anti-inflammatory and antioxidant activities [151, 171, 178, 195, 203, 224].
3.5. Sterols
Until now, thirty sterols (334–363) from Phyllanthus have been reported. All the sterols are phytosterols with a side chain (C8–C10) substitution at C-17, and half of which were isolated from P. emblica.
3.6. Alkaloids
Thirty-two alkaloids (364–395) have been found in genus Phyllanthus, most of which are securinine and securinine-related compounds and mainly distributed in P. niruri. Compounds 390-391 isolated from P. fraternus are amide type alkaloids and exhibited antimalarial potential [250].
3.7. Phenols and Others
Compounds 396–468 belong to phenols, which have one and several phenolic hydroxyl groups. Thirty other constitutions (469–512) have been isolated. Mucic acid (compounds 445–455) and its derivatives (compounds 498-499) can only be found in P. emblica among this genus.
4. Biological Activity
The remarkable traditional uses of genus Phyllanthus lead to the various researches of biological activities, such as antiviral, antioxidant, antidiabetic, anticancer, and immunomodulatory activities. In this section, biological activity researches of the extracts of the plants are highlighted.
4.1. Antiviral Activity
Various Phyllanthus plants were reported to have strong antiviral potential such as anti-HIV, anti-HCV, anti-HSV, and anti-HCMV. The aqueous extract of P. emblica reduced viral load of HIV significantly at the dose of 400 μg/mL [282]. DNA-polymerase and ribonuclease H (RNase H) activities of HIV-1 reverse transcriptase were inhibited by aqueous extract of P. sellowianus with IC50 values of 2.4 ± 0.8 μg/mL and 5.9 ± 1.4 μg/mL, respectively [283]. Moreover, methanol extract of P. reticulatus strongly inhibited the activity of RNase H by 99% at the dose of 50 μg/mL [284].
HCV-infected HuH7 cells were used to test the anti-HCV activities of methanolic fraction of P. amarus. The fraction was proved to suppress the replication of HCV monocistronic replicon RNA and HCV H77S viral RNA without toxic effect in host cells. Inhibiting HCV-NS3 protease enzyme and NS5B enzyme may be the main mechanism [285]. Aqueous extract of P. orbicularis revealed inhibition activity against the replication of HCMV, HSV-1, and HSV-2 as well as BHV-1 with EC50 values of 57.7, 28.8, 25.7, and 21.27 μg/mL, respectively. The selectivity indexes (SI) were ranged from 8.7 to 37.6 [286, 287].
Friend murine leukemia virus (FMuLv) induced erythroleukemia in BALB/c mice was relieved by metabolic extract of P. amarus. The extract inhibited leukemic cells from infiltrating into the sinusoidal space, decreased the morbidity of anemia, and improved survival rate of leukemia animals. Besides, the extract induced the upregulation of p53 and p45NFE2 and downregulation of Bcl-2 in the spleen [288].
4.2. Antioxidant Activity
Methanolic and aqueous parts of this genus have remarkable antioxidant activity, which may be correlated with the hydroxyl rich compositions. P. acidus, P. polyphyllus, and P. fraternus showed remarkable hepatoprotective activity against liver toxicity which was induced by acetaminophen, carbon tetrachloride, bromobenzene, and thioacetamide [42, 289–291]. The biochemical parameters as well as antioxidants levels were restored by these parts at the dose of 300 mg/kg. What is more, mitochondrial dysfunction in liver, induced by bromobenzene, was relieved by prior oral administration of aqueous part of P. fraternus at the dose of 100 mg/kg [51, 291].
Antimycin A governed mitochondrial protein degeneration, lipid peroxidation and mitochondrial DNA damage, and H2O2 induced membrane damage of Hep3B cells were considerably mitigated by aqueous fraction of P. amarus [164]. Mutagenesis induced by PhIP and 4-ABP and DNA damage induced by γ-ray and UVB were protected by aqueous fraction of P. orbicularis [292–294].
Methanol extract of P. debilis showed strong antioxidant activity when tested by various antioxidant assays including total antioxidant, free radical scavenging, superoxide anion radical scavenging, hydrogen peroxide scavenging, and nitric oxide scavenging assays. Besides, further study demonstrated that total phenolic was correlated with antioxidant activity [52]. In addition, hydromethanolic extract of P. virgatus exhibited substantially antioxidant capacity in both DPPH scavenging (IC50 = 30.4 μg/mL) and linoleic acid oxidation inhibiting (84%) method [5].
4.3. Antidiabetic Activity
Twelve herb drugs such as P. emblica, P. reticulatus, P. niruri, P. amarus, P. urinaria, P. acidus, P. debilis, P. virgatus, P. sellowianus, P. rheedii, P. orbicularis, and P. hookeri are traditionally employed for diabetes in many countries. Recent researches about the hypoglycemic effect of Phyllanthus plants were abundant. Streptozotocin- and alloxan-induced diabetic rats were employed for the evaluation of antidiabetic potential of P. emblica, P. niruri, P. reticulatus, P. sellowianus, P. virgatus, and P. simplex [4, 295–299]. After oral administration of these (aqueous, methanol, and ethanol) extracts for 21–45 days, the concentration of blood glucose was significantly reduced, and the effects of P. sellowianus and P. simplex were similar to the glibenclamide group (10 mg/kg). In addition, methanol fraction of P. virgatus considerably inhibited the activity of α-amylase in the noncompetitive pattern with IC50 of 33.20 ± 0.556 μg/mL [300].
After oral aqueous extract of P. niruri for 28 days, the levels of LPO and MDA were decreased while the concentrations of SOD, CAT, and GPx were increased. After being pretreated with the aqueous fraction of P. sellowianus, hemorheological parameters were ameliorated and red blood cells (RBCs) showed large globular aggregates and agglutination [301].
4.4. Anticancer Activity
Different extracts of the plants have been assessed for anticancer effects and the related mechanisms. Cancer cell lines such as NCI-H1703, MDA-MB-231, HeLa, 143B, PC-3, MCF-7, HepG2, A549, SKOV3, and HT-29 were considerably inhibited by P. emblica, P. urinaria, P. polyphyllus, P. watsonii, and P. pulcher [57, 68, 302–309]. In addition, P. emblica showed no toxicity to normal cells (MRC5). The extracts inhibited growth of cells through fragmentation of DNA and dysfunction of mitochondrial including upregulated mitochondrial fission 1 protein and downregulated optic atrophy type 1 and mitofusin 1 [304]. Moreover, the extracts suppressed the ability of cell invasion, migration, and adhesion. Further researches demonstrated that the fractions induced apoptosis, invasion, and migration through increasing the expression of caspase-3, caspase-7, caspase-8, and p-JNK and decreasing the expression of ERK, p-ERK1/2, JNK, MMP-2, MMP-9, Wnt, NF-κB, Myc/Max, and hypoxia [302, 303, 307].
Ehrlich ascites carcinoma tumor model was used to evaluate the antitumor activity of P. polyphyllus. Oral administration of methanol fraction at the dose of 200 mg/kg could significantly reduce the solid tumor volume. Hematological parameters, protein, packed cellular volume (PCV), and antioxidant enzymes such as LPO, GPx, GST, SOD, and CAT were greatly regulated [57].
4.5. Immunomodulatory Activity
Ethanol extracts of P. urinaria and P. amarus were demonstrated to have inhibitory effects on the chemotaxis of neutrophils and monocytes with IC50 lower than 2.92 μg/mL. In addition, phagocytic activity and CD18 expression of neutrophils and monocytes were downregulated [163].
Oral administration of P. reticulatus extract at the dose of 100 mg/kg demonstrated a significant increase in phagocytic activity, the percentage of neutrophil adhesion, and white blood cell in albino mice [310].
4.6. Analgesic Activity
The extracts of P. corcovadensis, P. niruri, and P. tenellus showed significant reduction in writhing response induced by acetic acid, with ID50 values of 30, 19, and >30 mg/kg, respectively. The late phase of formalin-induced pain could be relieved by P. tenellus with ID50 of 100 mg/kg and both phases of formalin-induced pain could be reduced by P. corcovadensis and P. niruri with ID50 values of 100 and 52 mg/kg, respectively. The analgesic effects could not be antagonized by naloxone [311]. In addition, intraperitoneally given hydroalcoholic extracts of P. amarus, P. orbicularis, and P. fraternus produced a marked analgesic activity by inhibiting acetic acid-induced abdominal constriction, capsaicin-induced neurogenic pain, and late phase of formalin-induced paw licking [312]. The ethanol and aqueous extracts of P. emblica succeeded in inhibiting acetic acid-induced writhing response but failed in the tail-immersion test [313].
4.7. Anti-Inflammatory Activity
In recent years, different inflammatory models such as Freund's complete adjuvant induced arthritis, carrageenin induced paw edema, and cotton pellet induced granuloma were employed to evaluate the anti-inflammatory effect of Phyllanthus. After receiving the aqueous extract of P. amarus, indexes of arthritis, joint diameter, and paw volume were decreased and thresholds of mechanical hyperalgesia and nociceptive were increased [314]. The ethanol fraction of P. simplex ameliorated the parameters of paw edema and granuloma and substantially inhibited nitric oxide (NO) production [315].
4.8. Antispasmodic Activity
Isolated rabbit jejunum and guinea-pig ileum were employed for the in vitro tests for the antispasmodic effects of P. emblica. Carbachol and K+ induced contractions of rabbit jejunum were released by the extract with IC50 values of 0.09 mg/mL and 1.38 mg/mL. The pretreatment of guinea-pig ileum with the extract at 0.3 mg/mL caused a rightward parallel shift in the concentration-response curves of acetylcholine without suppression of the maximum contractile response. Dual blockade of muscarinic receptors and Ca2+ channels can explain its antispasmodic activity [316].
4.9. Hypotensive and Hypolipidemic Activity
Aqueous extract of the leaves of P. amarus was found to restrain both force and rate of myocardial contraction and to inhibit the intrinsic myogenic contraction of isolated rat portal vein [317]. Aqueous part of P. reticulatus was effective in releasing total cholesterol, lipid profile, and oxidative stress in hypercholesterolemic albino rats after oral administrated for 45 days at 250 mg/kg [14].
4.10. Wound Healing
Extracts of P. emblica and P. niruri were demonstrated to have wound healing effect. Topical application with P. emblica could promote the proliferation of cells and cross-link of collagen in the full thickness excision wound [318]. Oral administration of P. emblica at the dose of 60 mg/kg showed healing effect against NSAID-induced gastric ulcer through upregulating the concentration of IL-10 and downregulating the levels of TNF-α and IL-1β [319]. After treatment with P. niruri at the dose of 200 mg/kg, 98.8% of wound could be recovered in the excision and incision wound models on the 16th day [320].
4.11. Antimalarial Activity
Malaria is a prevalent disease in many tropical and subtropical countries and folks of these places especially African people employed Phyllanthus as antimalarial agency. Plasmodium falciparum was suppressed by ethyl acetate fraction of P. acidus with IC50 of 9.37 μg/mL, and the SI equals 4.88 for HEp-2 cells and 11.75 for Vero cells [321]. What is more, chloroquine-resistant P. falciparum could be exhibited by P. amarus and P. muellerianus with IC50 values of 11.7 and 9.4 μg/mL, respectively. P. amarus presented protection effect on human RBCs damage caused by the virus [322]. The SI of P. muellerianus was higher than 5.3 for L-6 and MRC-5 cell lines [25, 202].
4.12. Antidepressant Activity
The aqueous extract of P. emblica (200 mg/kg) significantly decreased immobility period in both tail suspension test and forced swim test by decreasing the levels of MAO-A and GABA [323]. In the plus-maze, Hebb-Williams maze, and passive avoidance apparatus test, preparation of P. emblica produced a dose-dependent upgrade in scores. The preparation was also proved to reverse the amnesia induced by diazepam and scopolamine and to reduce the cholinesterase activity and total cholesterol level in brain [324, 325].
4.13. Others
The essential oil fraction of P. muellerianus exhibited strong antibacterial activity against Clostridium sporogenes, Streptococcus mutans, and S. pyogenes with MIC values ranging from 13.5 to 126 μg/mL [326]. Methanol extract of P. acuminatus (100 mg/mL) showed stronger antifungal than Dithane M-45 (10 000-ppm solution) against Pythium ultimum [327].
Aqueous extract of P. acidus was proved to regulate electrolyte transport in cystic fibrosis airways by increasing the intracellular levels of cAMP and Ca2+, stimulating basolateral K+ channels, and activating and redistributing cellular localization of cystic fibrosis transmembrane conductance regulator [328].
Eight hours after being treated with the aqueous extract of P. sellowianus at a dose of 400 mg/kg, urine output of test animals was decreased from 2.59 to 3.69 mL/100 g [329].
5. Clinical Studies
The extracts of P. niruri were proved to have immunomodulatory effect and played a crucial role in treating pulmonary tuberculosis and vaginal candidiasis as well as varicella. In patients with pulmonary tuberculosis, after oral administration of P. niruri 50 mg/mL for 2–6 months, the level of IL-10 was decreased and the levels of plasma IFN-γ and TNF-α were significantly increased. After 1-month treatment, the increase of the ratio of CD4+/CD8+ was observed. In the vaginal candidiasis patients, after receiving P. niruri 100 mg/mL for 1–3 months, the levels of IFN-γ and IL-12 were elevated. As for varicella patients, the number of papules and the number crusts were decreased after treatment with the extract at the dose of 5 mg/mL [330].
Clinical studies of P. niruri in Brazil had been finished, from which the P. niruri showed beneficial effects on the treatment of urolithiasis. After 3-month treatment, calculi elimination was increased. Furthermore, urinary calcium excretion and residual stone fragments after lithotripsy were decreased. Toxic effects on kidney, cardiovascular, and nervous systems were not found [331].
In China, the clinical study of P. urinaria in treating chronic hepatitis B with 140 patients was well established. The results indicated that, after treatment with P. urinaria capsule for 3 months or 2 years, especially in the long term, the recovery rate in the index of HBV-DNA and HBeAg was 88.2% and 52.5%, respectively. Once the treatment stopped, the recurrence rate was 10.4% to 13.4% [332].
6. Toxicity Studies
After given aqueous leaf extract of P. niruri at the dose of 2000 mg/mL, no acute toxicity was observed at the levels of bilirubin, ALT, AST, total protein, albumin, globulin, ALP, GGT, urea, creatinine, full blood count, and hemoglobin [333]. After being treated with ethanol extract of P. niruri over a period of 90 days at doses of 30 and 300 mg/kg, the rats showed no genotoxic effect at the test of PCE/NCE ratio [334]. Reproductive toxicity of P. niruri was tested using estrogen values, progesterone values, and testosterone levels. The estrogen and progesterone levels increased more than 1.5-fold above the control group after receiving 50 and 500 mg/kg aqueous leaf extract for 90 days, which reminded us of the cytotoxic of male antifertility properties [335].
Nephrotoxicity including interstitial oedema and tubular necrosis were detected after receiving 400 and 800 mg/kg of aqueous extract from P. amarus for 30 days [336]. The test animals were given 800 and 1600 mg/kg of the aqueous extract of P. amarus for 10 days, and significant pathological changes were found in the liver, kidney, and testis. The frequency of MNPCE, sperm abnormalities, total white blood cell, and lymphocyte counts were significantly increased, which suggested the genetic and systemic toxicity of P. amarus [337]. In addition, aqueous, methanolic, and hydromethanolic extracts of P. amarus (400 mg/kg) reduced locomotor activity and showed CNS depressant effect [338].
The LD50 of ethanolic extract from P. fraternus was 1125 mg/kg in the toxicity test. When the rats received the extract at doses of 400 mg/kg for 7 days, no toxicity was detected in liver and kidney [339]. Hydroethanolic extract P. fraternus showed the quick onset and long duration of reduction of locomotor activity at the dose of 400 mg/kg [338].
7. Conclusion
514 compounds have been isolated from different species of Phyllanthus, including 126 terpenoids, 102 phenylpropanoids, 73 phenols, 54 flavonoids, 53 tannins, 33 sterols, 31 alkaloids, and a number of other compositions. Their wide range of biological activities such as antiviral, antioxidant, antidiabetic, anticancer, anti-inflammatory, hypolipidemic, immunomodulatory, and antidepressant activities are tested using polar solvents (water, methanol, and ethanol) extracts. These extracts are considered rich in phenols, flavonoids, and tannins, which may exhibit antioxidant activity in different degree due to their hydroxyl [340]. Consequently, most bioactivities of Phyllanthus may be correlated with the hydroxyl rich compounds.
In recent years, the traditional uses of Phyllanthus had been partly confirmed, and more evidences such as pharmacological researches and clinical studies are urgently needed to be taken. Further studies of phytochemical discovery and subsequent screenings are necessary to be taken to extend the use of Phyllanthus and to develop leading compound.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (81274187 and 81274006) and Transformation and industrialization of Science and Technology Achievements, “New Drug AIKEXIN Development Research for the Treatment of AIDS.”
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Unander D. W., Webster G. L., Blumberg B. S. Usage and bioassays in Phyllanthus (Euphorbiaceae). IV. Clustering of antiviral uses and other effects. Journal of Ethnopharmacology. 1995;45(1):1–18. doi: 10.1016/0378-8741(94)01189-7. [DOI] [PubMed] [Google Scholar]
- 2.Xia Q. A pharmacognostic and ethnopharmacological studies of Chinese phyllanthus [Ph.D. thesis] Beijing, China: Peking Union Medical College; 1997. [Google Scholar]
- 3.Adil M. D., Kaiser P., Satti N. K., Zargar A. M., Vishwakarma R. A., Tasduq S. A. Effect of Emblica officinalis (fruit) against UVB-induced photo-aging in human skin fibroblasts. Journal of Ethnopharmacology. 2010;132(1):109–114. doi: 10.1016/j.jep.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 4.Nain P., Saini V., Sharma S., Nain J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. Journal of Ethnopharmacology. 2012;142(1):65–71. doi: 10.1016/j.jep.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Poompachee K., Chudapongse N. Comparison of the antioxidant and cytotoxic activities of Phyllanthus virgatus and Phyllanthus amarus extracts. Medical Principles and Practice. 2011;21(1):24–29. doi: 10.1159/000331596. [DOI] [PubMed] [Google Scholar]
- 6.Moreira J., Klein-Júnior L. C., Cechinel Filho V., de Campos Buzzi F. Anti-hyperalgesic activity of corilagin, a tannin isolated from Phyllanthus niruri L. (Euphorbiaceae) Journal of Ethnopharmacology. 2013;146(1):318–323. doi: 10.1016/j.jep.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 7.Omulokoli E., Khan B., Chhabra S. C. Antiplasmodial activity of four Kenyan medicinal plants. Journal of Ethnopharmacology. 1997;56(2):133–137. doi: 10.1016/S0378-8741(97)01521-3. [DOI] [PubMed] [Google Scholar]
- 8.Xia Q., Xiao P., Wang L., Kong J. Ethnopharmacology of Phyllanthus emblica L. Zhongguo Zhongyao Zazhi. 1997;22(9):515–525. [PubMed] [Google Scholar]
- 9.Ishtiaq M., Hanif W., Khan M. A., Ashraf M., Butt A. M. An ethnomedicinal survey and documentation of important medicinal folklore food phytonims of flora of Samahni valley, (Azad Kashmir) Pakistan. Pakistan Journal of Biological Sciences. 2007;10(13):2241–2256. doi: 10.3923/pjbs.2007.2241.2256. [DOI] [PubMed] [Google Scholar]
- 10.Kumar N., Rungseevijitprapa W., Narkkhong N.-A., Suttajit M., Chaiyasut C. 5α-reductase inhibition and hair growth promotion of some Thai plants traditionally used for hair treatment. Journal of Ethnopharmacology. 2012;139(3):765–771. doi: 10.1016/j.jep.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Mayachiew P., Devahastin S. Antimicrobial and antioxidant activities of Indian gooseberry and galangal extracts. LWT—Food Science and Technology. 2008;41(7):1153–1159. doi: 10.1016/j.lwt.2007.07.019. [DOI] [Google Scholar]
- 12.Rahmatullah M., Ghosh K. C., Mamun A. A., et al. A pharmacological study on antinociceptive and anti-hyperglycemic effects of methanol extract of leaves of Phyllanthus reticulatus Poir. In Swiss albino mice. Advances in Natural and Applied Sciences. 2010;4(3):229–232. [Google Scholar]
- 13.Ma J.-X., Lan M.-S., Qu S.-J., et al. Arylnaphthalene lignan glycosides and other constituents from Phyllanthus reticulatus . Journal of Asian Natural Products Research. 2012;14(11):1073–1077. doi: 10.1080/10286020.2012.712040. [DOI] [PubMed] [Google Scholar]
- 14.Maruthappan V., Shree K. S. Effects of Phyllanthus reticulatus on lipid profile and oxidative stress in hypercholesterolemic albino rats. Indian Journal of Pharmacology. 2010;42(6):388–391. doi: 10.4103/0253-7613.71923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma S., Kumar S. Phyllanthus reticulatus Poir.—an important medicinal plant: a review of its phytochemistry, traditional uses and pharmacological properties. International Journal of Pharmaceutical Sciences and Research. 2013;4(7):2528–2534. [Google Scholar]
- 16.Pojchaijongdee N., Sotanaphun U., Limsirichaikul S., Poobrasert O. Geraniinic acid derivative from the leaves of Phyllanthus reticulatus . Pharmaceutical Biology. 2010;48(7):740–744. doi: 10.3109/13880200903273898. [DOI] [PubMed] [Google Scholar]
- 17.Jamal A. K., Yaacob W. A., Din L. B. A chemical study on Phyllanthus reticulatus . Journal of Physical Science. 2008;19(2):45–50. [Google Scholar]
- 18.Ifeoma O., Samuel O., Itohan A. M., Adeola S. O. Isolation, fractionation and evaluation of the antiplasmodial properties of Phyllanthus niruri resident in its chloroform fraction. Asian Pacific Journal of Tropical Medicine. 2013;6(3):169–175. doi: 10.1016/S1995-7645(13)60018-8. [DOI] [PubMed] [Google Scholar]
- 19.Li X. R., Zhou W., Wei W. X. Chemical component and bioactivities of Phyllanthus niruri L. Tianran Chanwu Yanjiu Yu Kaifa. 2007;19(5):890–896. [Google Scholar]
- 20.Tona L., Ngimbi N. P., Tsakala M., et al. Antimalarial activity of 20 crude extracts from nine African medicinal plants used in Kinshasa, Congo. Journal of Ethnopharmacology. 1999;68(1–3):193–203. doi: 10.1016/s0378-8741(99)00090-2. [DOI] [PubMed] [Google Scholar]
- 21.Narendra K., Swathi J., Sowjanya K., Satya A. Phyllanthus niruri: a review on its ethno botanical, phytochemical and pharmacological profile. Journal of Pharmacy Research. 2012;5(9):4681–4691. [Google Scholar]
- 22.Bagalkotkar G., Sagineedu S. R., Saad M. S., Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. Journal of Pharmacy and Pharmacology. 2006;58(12):1559–1570. doi: 10.1211/jpp.58.12.0001. [DOI] [PubMed] [Google Scholar]
- 23.Shakil N. A., Pankaj, Kumar J., Pandey R. K., Saxena D. B. Nematicidal prenylated flavanones from Phyllanthus niruri . Phytochemistry. 2008;69(3):759–764. doi: 10.1016/j.phytochem.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Murugaiyah V., Chan K.-L. Mechanisms of antihyperuricemic effect of Phyllanthus niruri and its lignan constituents. Journal of Ethnopharmacology. 2009;124(2):233–239. doi: 10.1016/j.jep.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Zirihi G. N., Mambu L., Guédé-Guina F., Bodo B., Grellier P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. Journal of Ethnopharmacology. 2005;98(3):281–285. doi: 10.1016/j.jep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Das S., Das S., De B. In vitro inhibition of key enzymes related to diabetes by the aqueous extracts of some fruits of West Bengal, India. Current Nutrition and Food Science. 2012;8(1):19–24. doi: 10.2174/157340112800269614. [DOI] [Google Scholar]
- 27.Agyare C., Asase A., Lechtenberg M., Niehues M., Deters A., Hensel A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. Journal of Ethnopharmacology. 2009;125(3):393–403. doi: 10.1016/j.jep.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Eweka A. O., Enogieru A. Effects of oral administration of Phyllanthus amarus leaf extract on the kidneys of adult wistar rats- a histological study. African Journal of Traditional, Complementary and Alternative Medicines. 2011;8(3):307–311. doi: 10.4314/ajtcam.v8i3.65294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khatoon S., Rai V., Rawat A. K. S., Mehrotra S. Comparative pharmacognostic studies of three Phyllanthus species. Journal of Ethnopharmacology. 2006;104(1-2):79–86. doi: 10.1016/j.jep.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 30.Keluskar P., Ingle S. Ethnopharmacology guided screening of traditional Indian herbs for selective inhibition of Plasmodium specific lactate dehydrogenase. Journal of Ethnopharmacology. 2012;144(1):201–207. doi: 10.1016/j.jep.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Patel J. R., Tripathi P., Sharma V., Chauhan N. S., Dixit V. K. Phyllanthus amarus: ethnomedicinal uses, phytochemistry and pharmacology: a review. Journal of Ethnopharmacology. 2011;138(2):286–313. doi: 10.1016/j.jep.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 32.Xavier J. R., Gnanam R., Murugan M. P., Pappachan A. Clonal propagation of Phyllanthus amarus: a hepatoprotector. Pharmacognosy Magazine. 2012;8(29):78–82. doi: 10.4103/0973-1296.93332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamil I. G., Dineshkumar B., Nandhakumar M., Senthilkumar M., Mitra A. In vitro study on α-amylase inhibitory activity of an Indian medicinal plant, Phyllanthus amarus . Indian Journal of Pharmacology. 2010;42(5):280–282. doi: 10.4103/0253-7613.70107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komuraiah A., Bolla K., Rao K. N., Ragan A., Rajum V. S., Singara Charya M. A. Antibacterial studies and phytochemical constituents of South Indian Phyllanthus species. African Journal of Biotechnology. 2009;8(19):4991–4995. [Google Scholar]
- 35.Adeneye A. A. The leaf and seed aqueous extract of Phyllanthus amarus improves insulin resistance diabetes in experimental animal studies. Journal of Ethnopharmacology. 2012;144(3):705–711. doi: 10.1016/j.jep.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Ajaiyeoba E. O., Falade C. O., Fawole O. I., et al. Efficacy of herbal remedies used by herbalists in Oyo State Nigeria for treatment of Plasmodium falciparum infections—a survey and an observation. African Journal of Medicine and Medical Sciences. 2004;33(2):115–119. [PubMed] [Google Scholar]
- 37.Kloucek P., Polesny Z., Svobodova B., Vlkova E., Kokoska L. Antibacterial screening of some Peruvian medicinal plants used in Callería District. Journal of Ethnopharmacology. 2005;99(2):309–312. doi: 10.1016/j.jep.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 38.Chudapongse N., Kamkhunthod M., Poompachee K. Effects of Phyllanthus urinaria extract on HepG2 cell viability and oxidative phosphorylation by isolated rat liver mitochondria. Journal of Ethnopharmacology. 2010;130(2):315–319. doi: 10.1016/j.jep.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Huang S.-T., Yang R.-C., Yang L.-J., Lee P.-N., Pang J.-H. S. Phyllanthus urinaria triggers the apoptosis and Bcl-2 down-regulation in Lewis lung carcinoma cells. Life Sciences. 2003;72(15):1705–1716. doi: 10.1016/s0024-3205(03)00016-x. [DOI] [PubMed] [Google Scholar]
- 40.Xu M., Zha Z.-J., Qin X.-L., Zhang X.-L., Yang C.-R., Zhang Y.-J. Phenolic antioxidants from the whole plant of Phyllanthus urinaria . Chemistry & Biodiversity. 2007;4(9):2246–2252. doi: 10.1002/cbdv.200790183. [DOI] [PubMed] [Google Scholar]
- 41.Lin S.-Y., Wang C.-C., Lu Y.-L., Wu W.-C., Hou W.-C. Antioxidant, anti-semicarbazide-sensitive amine oxidase, and anti-hypertensive activities of geraniin isolated from Phyllanthus urinaria . Food and Chemical Toxicology. 2008;46(7):2485–2492. doi: 10.1016/j.fct.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Jain N. K., Singhai A. K. Protective effects of Phyllanthus acidus (L.) Skeels leaf extracts on acetaminophen and thioacetamide induced hepatic injuries in Wistar rats. Asian Pacific Journal of Tropical Medicine. 2011;4(6):470–474. doi: 10.1016/s1995-7645(11)60128-4. [DOI] [PubMed] [Google Scholar]
- 43.Chakraborty R., Biplab D., Devanna N., Sen S. Antiinflammatory, antinociceptive and antioxidant activities of Phyllanthus acidus L. extracts. Asian Pacific Journal of Tropical Biomedicine. 2012;2(2):S953–S961. [Google Scholar]
- 44.Leeya Y., Mulvany M. J., Queiroz E. F., Marston A., Hostettmann K., Jansakul C. Hypotensive activity of an n-butanol extract and their purified compounds from leaves of Phyllanthus acidus (L.) Skeels in rats. European Journal of Pharmacology. 2010;649(1–3):301–313. doi: 10.1016/j.ejphar.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 45.Durham D. G., Reid R. G., Wangboonskul J., Daodee S. Extraction of Phyllanthusols A and B from Phyllanthus acidus and analysis by capillary electrophoresis. Phytochemical Analysis. 2002;13(6):358–362. doi: 10.1002/pca.668. [DOI] [PubMed] [Google Scholar]
- 46.Vongvanich N., Kittakoop P., Kramyu J., Tanticharoen M., Thebtaranonth Y. Phyllanthusols A and B, cytotoxic norbisabolane glycosides from Phyllanthus acidus skeels. Journal of Organic Chemistry. 2000;65(17):5420–5423. doi: 10.1021/jo000439. [DOI] [PubMed] [Google Scholar]
- 47.Wanniarachchi K. K., Peiris L. D. C., Ratnasooriya W. D. Antihyperglycemic and hypoglycemic activities of Phyllanthus debilis aqueous plant extract in mice. Pharmaceutical Biology. 2009;47(3):260–265. doi: 10.1080/13880200802435754. [DOI] [Google Scholar]
- 48.Kumar S., Sachdeva N., Amir M., Kumar A., Singh S. K. Free radical scavenging effect of Phyllanthus simplex: in vitro and in vivo study. Saudi Pharmaceutical Journal. 2007;15(1):55–59. [Google Scholar]
- 49.Han S., Zhang Y., Wang J. R. Chemical composition in the leaves of Phyllanthus simplex Retz. Medicinal Plant. 2012;3(3):21–22. [Google Scholar]
- 50.Bum Ngo E., Pelanken M. M., Njikam N., et al. The decoction of leaves of Phyllanthus discoideus possesses anticonvulsant and sedative properties in mice. International Journal of Pharmacology. 2009;5(2):168–172. doi: 10.3923/ijp.2009.168.172. [DOI] [Google Scholar]
- 51.Ramakrishna V., Gopi S., Setty O. H. Protective effect of Phyllanthus fraternus against bromobenzene-induced mitochondrial dysfunction in rat kidney. Chinese Journal of Natural Medicines. 2012;10(5):328–333. doi: 10.1016/s1875-5364(12)60066-1. [DOI] [Google Scholar]
- 52.Kumaran A., Joel Karunakaran R. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Science and Technology. 2007;40(2):344–352. doi: 10.1016/j.lwt.2005.09.011. [DOI] [Google Scholar]
- 53.Álvarez Á. L., Dalton K. P., Nicieza I., et al. Bioactivity-guided fractionation of Phyllanthus orbicularis and identification of the principal anti HSV-2 compounds. Phytotherapy Research. 2012;26(10):1513–1520. doi: 10.1002/ptr.4608. [DOI] [PubMed] [Google Scholar]
- 54.Gaitén Y. I. G., Martínez M. M., Alarcón A. B., et al. Anti-inflammatory and antioxidant activity of a methanolic extract of Phyllanthus orbicularis and its derived flavonols. Journal of Essential Oil Research. 2011;23(5):50–53. doi: 10.1080/10412905.2011.9700482. [DOI] [Google Scholar]
- 55.Gertsch J., Niomawë, Gertsch-Roost K., Sticher O. Phyllanthus piscatorum, ethnopharmacological studies on a women's medicinal plant of the Yanomamï Amerindians. Journal of Ethnopharmacology. 2004;91(2-3):181–188. doi: 10.1016/j.jep.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 56.Ndlebe V. J., Crouch N. R., Mulholland D. A. Triterpenoids from the African tree Phyllanthus polyanthus . Phytochemistry Letters. 2008;1(1):11–17. doi: 10.1016/j.phytol.2007.12.002. [DOI] [Google Scholar]
- 57.Rajkapoor B., Sankari M., Sumithra M., et al. Antitumor and cytotoxic effects of Phyllanthus polyphyllus on ehrlich ascites carcinoma and human cancer cell lines. Bioscience, Biotechnology and Biochemistry. 2007;71(9):2177–2183. doi: 10.1271/bbb.70149. [DOI] [PubMed] [Google Scholar]
- 58.Sivajothi V., Dey A., Jayakar B., Rajkapoor B. Antihyperglycemic, antihyperlipidemic and antioxidant effect of Phyllanthus rheedii on streptozotocin induced diabetic rats. Iranian Journal of Pharmaceutical Research. 2008;7(1):53–59. [Google Scholar]
- 59.Hnatyszyn O., Miño J., Gorzalczany S., Ferraro G., Coussio J., Acevedo C. Antidiabetic activity of Phyllanthus sellowianus in streptozotocin-induced diabetic rats. Phytomedicine. 1997;4(3):251–253. doi: 10.1016/s0944-7113(97)80076-1. [DOI] [PubMed] [Google Scholar]
- 60.Sakkrom P., Pompimon W., Meepowpan P., Nuntasaen N., Loetchutinat C. The effect of Phyllanthus taxodiifolius beille extracts and its triterpenoids studying on cellular energetic stage of cancer cells. American Journal of Pharmacology and Toxicology. 2010;5(3):139–144. doi: 10.3844/ajptsp.2010.139.144. [DOI] [Google Scholar]
- 61.Santiago L. J. M., Louro R. P., De Oliveira D. E. Compartmentation of phenolic compounds and phenylalanine ammonia-lyase in leaves of Phyllanthus tenellus roxb. and their induction by copper sulphate. Annals of Botany. 2000;86(5):1023–1032. doi: 10.1006/anbo.2000.1271. [DOI] [Google Scholar]
- 62.Baliga M. S. Triphala, ayurvedic formulation for treating and preventing cancer: a review. Journal of Alternative and Complementary Medicine. 2010;16(12):1301–1308. doi: 10.1089/acm.2009.0633. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka R., Masuda K., Matsunaga S. Lup-20(29)-en-3β,15α-diol and ocotillol-II from the stem bark of Phyllanthus flexuosus . Phytochemistry. 1993;32(2):472–474. doi: 10.1016/s0031-9422(00)95021-0. [DOI] [Google Scholar]
- 64.Singh B., Agrawal P. K., Thakur R. S. Euphane triterpenoids from Phyllanthus niruri . Indian Journal of Chemistry Section B-Organic Chemistry Including Medicinal Chemistry. 1989;28(4):319–321. [Google Scholar]
- 65.Ren Y. L., Yuan C. H., Deng Y. C., et al. Cytotoxic and natural killer cell stimulatory constituents of Phyllanthus songboiensis . Phytochemistry. 2015;111:132–140. doi: 10.1016/j.phytochem.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tuchinda P., Kornsakulkarn J., Pohmakotr M., et al. Dichapetalin-type triterpenoids and lignans from the aerial parts of Phyllanthus acutissima . Journal of Natural Products. 2008;71(4):655–663. doi: 10.1021/np7007347. [DOI] [PubMed] [Google Scholar]
- 67.Zhao J., Wang Y., Zhu H., et al. Highly oxygenated limonoids and lignans from Phyllanthus flexuosus . Natural Products and Bioprospecting. 2014;4(4):233–242. doi: 10.1007/s13659-014-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagalkotkar G., Chuan T. S., Khalivulla S. I., et al. Isolation and cytotoxicity of triterpenes from the roots of Phyllanthus pulcher Wall. ex Müll. Arg. (Euphorbiaceae) African Journal of Pharmacy and Pharmacology. 2011;5(2):183–188. doi: 10.5897/ajmr10.331. [DOI] [Google Scholar]
- 69.Youkwan J., Srisomphot P., Sutthivaiyakit S. Bioactive constituents of the leaves of Phyllanthus polyphyllus var. siamensis. Journal of Natural Products. 2005;68(7):1006–1009. doi: 10.1021/np050001a. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka R., Tabuse M., Matsunaga S. Triterpenes from the stem bark of Phyllanthus flexuosus . Phytochemistry. 1988;27(11):3563–3567. doi: 10.1016/0031-9422(88)80769-6. [DOI] [Google Scholar]
- 71.Wada S.-I., Iida A., Tanaka R. Screening of triterpenoids isolated from Phyllanthus flexuosus for DNA topoisomerase inhibitory activity. Journal of Natural Products. 2001;64(12):1545–1547. doi: 10.1021/np010176u. [DOI] [PubMed] [Google Scholar]
- 72.Habib-Ur-Rehman, Atta-Ur-Rehman, Chodhary M. I., Raza A. R. Studies on the chemical constituents of Phyllanthus fraternus . Journal of the Chemical Society of Pakistan. 2004;26(1):77–81. [Google Scholar]
- 73.Hu Z., Lai Y., Zhang J., et al. Phytochemical and chemotaxonomic studies on Phyllanthus urinaria . Biochemical Systematics and Ecology. 2014;56:60–64. doi: 10.1016/j.bse.2014.04.016. [DOI] [Google Scholar]
- 74.Sun W. Chemical constituents of Phyllanthus amaus . Chinese Traditional and Herbal Drugs. 2012;43(1):23–26. [Google Scholar]
- 75.Lee S.-S., Kishore P. H., Chen C.-H. Three novel triterpenoid dienolides from Phyllanthus myrtifolius . Helvetica Chimica Acta. 2002;85(8):2403–2408. [Google Scholar]
- 76.Jamal A. K., Yaacob W. A., Din L. B. Triterpenes from the root bark of Phyllanthus columnaris . Australian Journal of Basic and Applied Sciences. 2009;3(2):1428–1431. [Google Scholar]
- 77.Joshi K. C., Singh P., Mehra A. Crystalline components of the roots of Phyllanthus reticulatus . Journal of the Indian Chemical Society. 1981;58(1):102–103. [Google Scholar]
- 78.Ramesh N., Viswanathan M. B., Selvi V. T., Lakshmanaperumalsamy P. Antimicrobial and phytochemical studies on the leaves of Phyllanthus singampattiana (Sebastine & A.N. Henry) Kumari & Chandrabose from India. Medicinal Chemistry Research. 2004;13(6-7):348–360. doi: 10.1007/s00044-004-0040-8. [DOI] [Google Scholar]
- 79.Agarwal T., Tiwari J. S. A note on the flavanoid and other constituents of Phyllanthus genus. Journal of the Indian Chemical Society. 1991;68(8):479–480. [Google Scholar]
- 80.Tanaka R., Matsunaga S. Triterpene dienols and other constituents from the bark of Phyllanthus flexuosus . Phytochemistry. 1988;27(7):2273–2277. doi: 10.1016/0031-9422(88)80141-9. [DOI] [Google Scholar]
- 81.Sengupta P., Mukhopad J. Terpenoids and related compounds 7. Triterpenoids of Phyllanthus acidus skeels. Phytochemistry. 1966;5(3):531–534. [Google Scholar]
- 82.Tanaka R., In Y., Ishida T., Matsunaga S. 11β-hydroxy-D: a-friedoolean-1-en-3-one from the stem bark of Phyllanthus flexuosus . Journal of Natural Products. 1994;57(11):1523–1528. doi: 10.1021/np50113a008. [DOI] [Google Scholar]
- 83.Adesida G. A., Girgis P., Taylor D. A. H. Friedelin derivatives from Phyllanthus muellerianus . Phytochemistry. 1972;11(2):851–852. doi: 10.1016/0031-9422(72)80070-0. [DOI] [Google Scholar]
- 84.Hui W.-H., Li M.-M., Wong K.-M. A new compound, 21α-hydroxyfriedel-4(23)-en-3-one and other triterpenoids from Phyllanthus reticulatus . Phytochemistry. 1976;15(5):797–798. doi: 10.1016/s0031-9422(00)94448-0. [DOI] [Google Scholar]
- 85.Matsunaga S., Tanaka R., Takaoka Y., et al. 26-Nor-D:A-friedooleanane triterpenes from Phyllanthus watsonii . Phytochemistry. 1992;32(1):165–170. doi: 10.1016/0031-9422(92)80125-x. [DOI] [Google Scholar]
- 86.Nasser J. A., Yaacob W. A., Din L. B. Studies on the chemical constituents of bark roots of Phyllanthus columnaris . Asian Journal of Chemistry. 2009;21(9):7067–7071. [Google Scholar]
- 87.Zhao Q., Liang R. J., Zhang Y. J., Hong A. H., Wang Y. F., Cen Y. Z. Chemical constituents in roots of Phyllanthus emblica . Zhongcaoyao. 2013;44(2):133–136. [Google Scholar]
- 88.Wei W. X., Pan Y. J., Zhang H., Chen Y. Z. Structures in crystal and in solution of friedelin isolated from Phyllanthus niruri linn. Tianran Chanwu Yanjiu Yu Kaifa. 2004;16(3):201–203. [Google Scholar]
- 89.Priya O. S., Viswanathan M. B. G., Balakrishna K., Venkatesan M. Chemical constituents and in vitro antioxidant activity of Phyllanthus wightianus . Natural Product Research. 2011;25(10):949–958. doi: 10.1080/14786419.2010.517203. [DOI] [PubMed] [Google Scholar]
- 90.Sutthivaiyakit S., Nakorn N. N., Kraus W., Sutthivaiyakit P. A novel 29-nor-3,4-seco-friedelane triterpene and a new guaiane sesquiterpene from the roots of Phyllanthus oxyphyllus . Tetrahedron. 2003;59(50):9991–9995. doi: 10.1016/j.tet.2003.10.023. [DOI] [Google Scholar]
- 91.Tanaka R., Matsunaga S., Ishida T. Revised structure of trichadenic acid B, a stem bark constituent of Phyllanthus flexuosus . Tetrahedron Letters. 1988;29(37):4751–4754. doi: 10.1016/s0040-4039(00)80598-5. [DOI] [Google Scholar]
- 92.Saleem M., Nazir M., Akhtar N., et al. New phthalates from Phyllanthus muellerianus (Euphorbiaceae) Journal of Asian Natural Products Research. 2009;11(11):974–977. doi: 10.1080/10286020903341388. [DOI] [PubMed] [Google Scholar]
- 93.Cechinel-Filho V., Santos A. R. S., Calixto J. B., Delle-Monache F., Miguel O. G., Yunes R. Triterpenes from Phyllanthus sellowianus roots. Planta Medica. 1998;64(2):p. 194. doi: 10.1055/s-2006-957406. [DOI] [PubMed] [Google Scholar]
- 94.Huang Y.-L., Chen C.-C., Hsu F.-L., Chen C.-F. Tannins, flavonol sulfonates, and a norlignan from Phyllanthus virgatus . Journal of Natural Products. 1998;61(10):1194–1197. doi: 10.1021/np970336v. [DOI] [PubMed] [Google Scholar]
- 95.Miguel O. G., Cechinel Filho V., Niero R., et al. Constituents of Phyllanthus sellowianus . Fitoterapia. 1995;66(3):p. 275. [Google Scholar]
- 96.Serra R. M. Investigation of quinine in Phyllanthus niruri L. Anales de la Universidad de Santo Domingo. 1944;8:295–297. [Google Scholar]
- 97.Li R. S., Wang S. Y., Zhang W. H. Studies on the chemical components of common leaf-flower (Phyllanthus urinaria) Zhongcaoyao. 1995;26(5):231–232. [Google Scholar]
- 98.Tanaka R., Kinouchi Y., Wada S.-I., Tokuda H. Potential anti-tumor promoting activity of lupane-type triterpenoids from the stem Bark of Glochidion zeylanicum and Phyllanthus flexuosus . Planta Medica. 2004;70(12):1234–1236. doi: 10.1055/s-2004-835858. [DOI] [PubMed] [Google Scholar]
- 99.Satyan K. S., Prakash A., Singh R. P., Srivastava R. S. Phthalic acid bis-ester and other phytoconstituents of Phyllanthus urinaria . Planta Medica. 1995;61(3):293–294. doi: 10.1055/s-2006-958083. [DOI] [PubMed] [Google Scholar]
- 100.Hui W. H., Sung M. An examination of the Euphorbiaceae of Hong Kong. II. The occurrence of epitaraxerol and other triterpenoids. Australian Journal of Chemistry. 1968;21(8):2137–2140. doi: 10.1071/ch9682137. [DOI] [Google Scholar]
- 101.Singh B., Agrawal P. K., Thakur R. S. An acyclic triterpene from Phyllanthus niruri . Phytochemistry. 1989;28(7):1980–1981. doi: 10.1016/s0031-9422(00)97901-9. [DOI] [Google Scholar]
- 102.Hnatyszyn O., Ferraro G. Phyllanthol from Phyllanthus sellowianus (Euphorbiaceae) Planta Medica. 1985;51(5):467–467. doi: 10.1055/s-2007-969560. [DOI] [PubMed] [Google Scholar]
- 103.Liu X., Zhao M., Luo W., Yang B., Jiang Y. Identification of volatile components in Phyllanthus emblica L. and their antimicrobial activity. Journal of Medicinal Food. 2009;12(2):423–428. doi: 10.1089/jmf.2007.0679. [DOI] [PubMed] [Google Scholar]
- 104.Pettit G. R., Schaufelberger D. E., Nieman R. A., Dufresne C., Saenz-Renauld J. A. Antineoplastic agents, 177. Isolation and structure of phyllanthostatin 6. Journal of Natural Products. 1990;53(6):1406–1413. doi: 10.1021/np50072a002. [DOI] [PubMed] [Google Scholar]
- 105.Lan M. S., Ma J. X., Tan C. H., et al. Chemical constituents from Phyllanthus reticulatus var. Glaber. Zhongcaoyao. 2011;42(9):1712–1714. [Google Scholar]
- 106.Ratnayake R., Covell D., Ransom T. T., Gustafson K. R., Beutler J. A. Englerin a, a selective inhibitor of renal cancer cell growth, from Phyllanthus engleri . Organic Letters. 2009;11(1):57–60. doi: 10.1021/ol802339w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lv J.-J., Wang Y.-F., Zhang J.-M., et al. Anti-hepatitis B virus activities and absolute configurations of sesquiterpenoid glycosides from Phyllanthus emblica . Organic and Biomolecular Chemistry. 2014;12(43):8764–8774. doi: 10.1039/c4ob01196a. [DOI] [PubMed] [Google Scholar]
- 108.Zhao J. Q., Wang Y. M., Wang D., Yang C. R., Xu M., Zhang Y. J. Five new sucrose esters from the whole plants of Phyllanthus cochinchinensis . Natural Products and Bioprospecting. 2013;3(2):61–65. doi: 10.1007/s13659-013-0026-7. [DOI] [Google Scholar]
- 109.Zhang Y.-J., Tanaka T., Iwamoto Y., Yang C.-R., Kouno I. Novel norsesquiterpenoids from the roots of Phyllanthus emblica . Journal of Natural Products. 2000;63(11):1507–1510. doi: 10.1021/np000135i. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y.-J., Tanaka T., Iwamoto Y., Yang C.-R., Kouno I. Novel sesquiterpenoids from the roots of Phyllanthus emblica . Journal of Natural Products. 2001;64(7):870–873. doi: 10.1021/np010059z. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Y.-J., Nagao T., Tanaka T., Yang C.-R., Okabe H., Kouno I. Antiproliferative activity of the main constituents from Phyllanthus emblica . Biological and Pharmaceutical Bulletin. 2004;27(2):251–255. doi: 10.1248/bpb.27.251. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y.-F., Wang X.-Y., Ren Z., et al. Phyllaemblicin B inhibits Coxsackie virus B3 induced apoptosis and myocarditis. Antiviral Research. 2009;84(2):150–158. doi: 10.1016/j.antiviral.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 113.Liu Q., Wang Y.-F., Chen R.-J., et al. Anti-coxsackie virus B3 norsesquiterpenoids from the roots of Phyllanthus emblica . Journal of Natural Products. 2009;72(5):969–972. doi: 10.1021/np800792d. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y., Zhao L., Guo X., et al. Chemical constituents from Phyllanthus emblica and the cytoprotective effects on H2O2-induced PC12 cell injuries. Archives of Pharmacal Research. 2014 doi: 10.1007/s12272-014-0433-2. [DOI] [PubMed] [Google Scholar]
- 115.Kupchan S. M., LaVoie E. J., Branfman A. R., Fei B. Y., Bright W. M., Bryan R. F. Tumor inhibitors. 120. Phyllanthocin, a novel bisabolane aglycone from the antileukemic glycoside, phyllanthoside. Journal of the American Chemical Society. 1977;99(9):3199–3201. doi: 10.1021/ja00451a074. [DOI] [PubMed] [Google Scholar]
- 116.Pettit G. R., Cragg G. M., Gust D., Brown P., Schmidt J. M. The structures of phyllanthostatin 1 and phyllanthoside from the central american tree Phyllanthus acuminatus vahl. Canadian Journal of Chemistry. 1982;60(7):939–941. doi: 10.1139/v82-141. [DOI] [Google Scholar]
- 117.Pettit G. R., Cragg G. M., Gust D., Brown P. The isolation and structure of phyllanthostatins 2 and 3. Canadian Journal of Chemistry. 1982;60(4):544–546. doi: 10.1139/v82-080. [DOI] [Google Scholar]
- 118.Lan M.-S., Ma J.-X., Tan C.-H., Wei S., Zhu D.-Y. Chemical constituents of Phyllanthus reticulatus . Helvetica Chimica Acta. 2010;93(11):2276–2280. doi: 10.1002/hlca.201000168. [DOI] [Google Scholar]
- 119.Zhao J.-Q., Lv J.-J., Wang Y.-M., et al. Phyllanflexoid C: first example of phenylacetylene-bearing 18-nor-diterpenoid glycoside from the roots of Phyllanthus flexuosus . Tetrahedron Letters. 2013;54(35):4670–4674. doi: 10.1016/j.tetlet.2013.06.082. [DOI] [Google Scholar]
- 120.Hossain M. A., Salehuddin S. M. Diterpenes from the leaves of Phyllanthus niruri . Indian Journal of Natural Products. 2006;22(2):18–20. [Google Scholar]
- 121.Gupta J., Ali M. Isolation of rare phytoconstituents from Phyllanthus fraternus roots. Journal of Medicinal and Aromatic Plant Sciences. 1999;21:352–357. [Google Scholar]
- 122.Singh B., Agrawal P. K., Thakur R. S. Studies on medicinal-plants 33. Isolation of trans-phytol from Phyllanthus niruri . Planta Medica. 1991;57(1):p. 98. doi: 10.1055/s-2006-960039. [DOI] [PubMed] [Google Scholar]
- 123.Ren Y. L., Lantvit D. D., Deng Y. C., et al. Potent cytotoxic arylnaphthalene lignan lactones from Phyllanthus poilanei . Journal of Natural Products. 2014;77(6):1494–1504. doi: 10.1021/np5002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tuchinda P., Kumkao A., Pohmakotr M., Sophasan S., Santisuk T., Reutrakul V. Cytotoxic arylnaphthalide lignan glycosides from the aerial parts of Phyllanthus taxodiifolius . Planta Medica. 2006;72(1):60–62. doi: 10.1055/s-2005-873141. [DOI] [PubMed] [Google Scholar]
- 125.Chang C.-C., Lien Y.-C., Liu K. C. S. C., Lee S.-S. Lignans from Phyllanthus urinaria . Phytochemistry. 2003;63(7):825–833. doi: 10.1016/s0031-9422(03)00371-6. [DOI] [PubMed] [Google Scholar]
- 126.Rao Y. K., Fang S.-H., Tzeng Y.-M. Anti-inflammatory activities of constituents isolated from Phyllanthus polyphyllus . Journal of Ethnopharmacology. 2006;103(2):181–186. doi: 10.1016/j.jep.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 127.Hussain R. A., Dickey J. K., Rosser M. P., et al. A novel class of non-peptidic endothelin antagonists isolated from the medicinal herb Phyllanthus niruri . Journal of Natural Products. 1995;58(10):1515–1520. doi: 10.1021/np50124a006. [DOI] [Google Scholar]
- 128.Ganeshpure P. A., Schneiders G. E., Stevenson R. Structure and synthesis of hypophyllanthin, nirtetralin, phyltetralin and lintetralin. Tetrahedron Letters. 1981;22(5):393–396. doi: 10.1016/0040-4039(81)80108-6. [DOI] [Google Scholar]
- 129.Syamasundar K. V., Singh B., Singh Thakur R., Husain A., Yoshinobu K., Hiroshi H. Antihepatotoxic principles of Phyllanthus niruri herbs. Journal of Ethnopharmacology. 1985;14(1):41–44. doi: 10.1016/0378-8741(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 130.Inchoo M., Chirdchupunseree H., Pramyothin P., Jianmongkol S. Endothelium-independent effects of phyllanthin and hypophyllanthin on vascular tension. Fitoterapia. 2011;82(8):1231–1236. doi: 10.1016/j.fitote.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 131.Huang Y.-L., Chen C.-C., Hsu F.-L., Chen C.-F. A new lignan from Phyllanthus virgatus . Journal of Natural Products. 1996;59(5):520–521. doi: 10.1021/np9603380. [DOI] [PubMed] [Google Scholar]
- 132.Singh M., Tiwari N., Shanker K., Verma R. K., Gupta A. K., Gupta M. M. Two new lignans from Phyllanthus amarus . Journal of Asian Natural Products Research. 2009;11(6):562–568. doi: 10.1080/10286020902939174. [DOI] [PubMed] [Google Scholar]
- 133.Islam A., Selvan T., Mazumder U. K., Gupta M., Ghosal S. Antitumour effect of phyllanthin and hypophyllanthin from Phyllanthus amarus against Ehrlich ascites carcinoma in mice. Pharmacologyonline. 2008;2:796–807. [Google Scholar]
- 134.Taesotikul T., Dumrongsakulchai W., Wattanachai N., et al. Inhibitory effects of Phyllanthus amarus and its major lignans on human microsomal cytochrome P450 activities: evidence for CYP3A4 mechanism-based Inhibition. Drug Metabolism and Pharmacokinetics. 2011;26(2):154–161. doi: 10.2133/dmpk.dmpk-10-rg-107. [DOI] [PubMed] [Google Scholar]
- 135.Chandrashekar K. S., Satyanarayana D., Joshi A. B., Subrahmanyam E. V. S. Phytochemical studies of Phyllanthus debilis . Natural Product Sciences. 2004;10(3):101–103. [Google Scholar]
- 136.Huang Y. L., Chen C. C., Ou J. C. Isolintetralin: a new lignan from Phyllanthus niruri . Planta Medica. 1992;58(5):473–474. doi: 10.1055/s-2006-961520. [DOI] [PubMed] [Google Scholar]
- 137.Lin M.-T., Lee S.-S., Chen Liu K. C. S. Phyllamyricins A-C, three novel lignans from Phyllanthus myrtifolius . Journal of Natural Products. 1995;58(2):244–249. doi: 10.1021/np50116a013. [DOI] [PubMed] [Google Scholar]
- 138.Bachmann T. L., Ghia F., Torssell K. B. G. Lignans and lactones from Phyllanthus anisolobus . Phytochemistry. 1993;33(1):189–191. doi: 10.1016/0031-9422(93)85420-v. [DOI] [Google Scholar]
- 139.Gertsch J., Tobler R. T., Brun R., Sticher O., Heilmann J. Antifungal, antiprotozoal, cytotoxic and piscicidal properties of justicidin B and a new arylnaphthalide lignan from Phyllanthus piscatorum . Planta Medica. 2003;69(5):420–424. doi: 10.1055/s-2003-39706. [DOI] [PubMed] [Google Scholar]
- 140.Wei W.-X., Gong X.-G., Ishrud O., Pan Y.-J. New lignan isolated from Phyllanthus niruri Linn. structure elucidation by NMR spectroscopy. Bulletin of the Korean Chemical Society. 2002;23(6):896–898. doi: 10.5012/bkcs.2002.23.6.896. [DOI] [Google Scholar]
- 141.Thanh N. V., Huong P. T. T., Nam N. H., et al. A new flavone sulfonic acid from Phyllanthus urinaria . Phytochemistry Letters. 2014;7(1):182–185. doi: 10.1016/j.phytol.2013.11.013. [DOI] [Google Scholar]
- 142.Wei W. X., Li X. R., Wang K. W., Zheng Z. W., Zhou M. Lignans with anti-hepatitis B virus activities from Phyllanthus niruri L. Phytotherapy Research. 2012;26(7):964–968. doi: 10.1002/ptr.3663. [DOI] [PubMed] [Google Scholar]
- 143.Huang R.-L., Huang Y.-L., Ou J.-C., Chen C.-C., Hsu F.-L., Chang C. Screening of 25 compounds isolated from Phyllanthus species for anti-human hepatitis B virus in vitro. Phytotherapy Research. 2003;17(5):449–453. doi: 10.1002/ptr.1167. [DOI] [PubMed] [Google Scholar]
- 144.Leite D. F. P., Kassuya C. A. L., Mazzuco T. L., et al. The cytotoxic effect and the multidrug resistance reversing action of lignans from Phyllanthus amarus . Planta Medica. 2006;72(15):1353–1358. doi: 10.1055/s-2006-951708. [DOI] [PubMed] [Google Scholar]
- 145.Kassuya C. A. L., Leite D. F. P., de Melo L. V., Rehder V. L. G., Calixto J. B. Anti-inflammatory properties of extracts, fractions and lignans isolated from Phyllanthus amarus . Planta Medica. 2005;71(8):721–726. doi: 10.1055/s-2005-871258. [DOI] [PubMed] [Google Scholar]
- 146.Liu S., Wei W. X., Li Y. B., et al. In vitro and in vivo anti-hepatitis B virus activities of the lignan nirtetralin B isolated from Phyllanthus niruri L. Journal of Ethnopharmacology. 2014;157:62–68. doi: 10.1016/j.jep.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 147.Lee S.-S., Lin M.-T., Liu C.-L., Lin Y.-Y., Liu K. C. S. C. Six lignans from Phyllanthus myrtifolius . Journal of Natural Products. 1996;59(11):1061–1065. doi: 10.1021/np960322. [DOI] [PubMed] [Google Scholar]
- 148.Pettit G. R., Schaufelberger D. E. Isolation and structure of the cytostatic lignan glycoside phyllanthostatin A. Journal of Natural Products. 1988;51(6):1104–1112. doi: 10.1021/np50060a009. [DOI] [PubMed] [Google Scholar]
- 149.Zhao J.-Q., Wang Y.-M., Lv J.-J., et al. New phenolic glycosides from Phyllanthus cochinchinensis . Journal of the Brazilian Chemical Society. 2014;25(8):1446–1454. doi: 10.5935/0103-5053.20140127. [DOI] [Google Scholar]
- 150.Wu S.-J., Wu T.-S. Cytotoxic arylnaphthalene lignans from Phyllanthus oligospermus . Chemical & Pharmaceutical Bulletin. 2006;54(8):1223–1225. doi: 10.1248/cpb.54.1223. [DOI] [PubMed] [Google Scholar]
- 151.Fang S.-H., Rao Y. K., Tzeng Y.-M. Anti-oxidant and inflammatory mediator's growth inhibitory effects of compounds isolated from Phyllanthus urinaria . Journal of Ethnopharmacology. 2008;116(2):333–340. doi: 10.1016/j.jep.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 152.Wang C.-Y., Sun S.-W., Lee S.-S. Pharmacokinetic and metabolic studies of retrojusticidin B, a potential anti-viral lignan, in rats. Planta Medica. 2004;70(12):1161–1165. doi: 10.1055/s-2004-835845. [DOI] [PubMed] [Google Scholar]
- 153.Satyanarayana P., Subrahmanyam P., Viswanatham K. N., Ward R. S. New seco- and hydroxy-lignans from Phyllanthus niruri . Journal of Natural Products. 1988;51(1):44–49. doi: 10.1021/np50055a004. [DOI] [Google Scholar]
- 154.Elfahmi E., Koulman A., Bos R., Woerdenbag H. J. Lignans from cell suspension cultures of Phyllanthus niruri, an Indonesian medicinal plant. Journal of Natural Products. 2006;69(1):55–58. doi: 10.1021/np050288b. [DOI] [PubMed] [Google Scholar]
- 155.Satyanarayana P., Venkateswarlu S. Isolation, structure and synthesis of new diarylbutane lignans from Phyllanthus niruri: synthesis of 5′-desmethoxy niranthin and an antitumour extractive. Tetrahedron. 1991;47(42):8931–8940. doi: 10.1016/s0040-4020(01)81005-x. [DOI] [Google Scholar]
- 156.Giridharan P., Somasundaram S. T., Perumal K., et al. Novel substituted methylenedioxy lignan suppresses proliferation of cancer cells by inhibiting telomerase and activation of c-myc and caspases leading to apoptosis. British Journal of Cancer. 2002;87(1):98–105. doi: 10.1038/sj.bjc.6600422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Singh B., Agrawal P. K., Thakur R. S. Chemical-constituents of Phyllanthus niruri Linn. Indian Journal of Chemistry, Section B—Organic Chemistry Including Medicinal Chemistry. 1986;25(6):600–602. [Google Scholar]
- 158.Kassuya C. A. L., Silvestre A., Menezes-de-Lima O., Jr., Marotta D. M., Rehder V. L. G., Calixto J. B. Antiinflammatory and antiallodynic actions of the lignan niranthin isolated from Phyllanthus amarus. Evidence for interaction with platelet activating factor receptor. European Journal of Pharmacology. 2006;546(1–3):182–188. doi: 10.1016/j.ejphar.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 159.Chowdhury S., Mukherjee T., Mukhopadhyay R., et al. The lignan niranthin poisons Leishmania donovani topoisomerase IB and favours a Th1 immune response in mice. EMBO Molecular Medicine. 2012;4(10):1126–1143. doi: 10.1002/emmm.201201316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Singh B., Agrawal P. K., Thakur R. S. A new lignan and a new neolignan from Phyllanthus niruri . Journal of Natural Products. 1989;52(1):48–51. doi: 10.1021/np50061a004. [DOI] [Google Scholar]
- 161.Ooi K. L., Loh S. I., Sattar M. A., Muhammad T. S. T., Sulaiman S. F. Cytotoxic, caspase-3 induction and in vivo hepatoprotective effects of phyllanthin, a major constituent of Phyllanthus niruri . Journal of Functional Foods. 2015;14:236–243. doi: 10.1016/j.jff.2015.01.032. [DOI] [Google Scholar]
- 162.Murugaiyah V., Chan K.-L. Antihyperuricemic lignans from the leaves of Phyllanthus niruri . Planta Medica. 2006;72(14):1262–1267. doi: 10.1055/s-2006-947224. [DOI] [PubMed] [Google Scholar]
- 163.Jantan I., Ilangkovan M., Yuandani, Mohamad H. F. Correlation between the major components of Phyllanthus amarus and Phyllanthus urinaria and their inhibitory effects on phagocytic activity of human neutrophils. BMC Complementary and Alternative Medicine. 2014;14, article 429 doi: 10.1186/1472-6882-14-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guha G., Mandal T., Rajkumar V., Ashok Kumar R. Antimycin A-induced mitochondrial apoptotic cascade is mitigated by phenolic constituents of Phyllanthus amarus aqueous extract in Hep3B cells. Food and Chemical Toxicology. 2010;48(12):3449–3457. doi: 10.1016/j.fct.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 165.Chirdchupunseree H., Pramyothin P. Protective activity of phyllanthin in ethanol-treated primary culture of rat hepatocytes. Journal of Ethnopharmacology. 2010;128(1):172–176. doi: 10.1016/j.jep.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 166.Kuo C.-H., Lee S.-S., Chang H.-Y., Sun S.-W. Analysis of lignans using micellar electrokinetic chromatography. Electrophoresis. 2003;24(6):1047–1053. doi: 10.1002/elps.200390121. [DOI] [PubMed] [Google Scholar]
- 167.Chen Y. W., Ren L. J., Li K. M., Zhang Y. W. Isolation and identification of novel polyphenolic compound from Phyllanthus urinaria . Acta Pharmaceutica Sinica. 1999;34(7):526–529. [Google Scholar]
- 168.Hnatyszyn O., Ferraro G., Coussio J. D. Constituents of Phyllanthus sellowianus . Fitoterapia. 1995;66(6):p. 543. [Google Scholar]
- 169.Agyare C., Lechtenberg M., Deters A., Petereit F., Hensel A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: Geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine. 2011;18(7):617–624. doi: 10.1016/j.phymed.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 170.Niu X., Qi L., Li W., Liu X. Simultaneous analysis of eight phenolic compounds in Phyllanthus simplex Retz by HPLC-DAD-ESI/MS. Journal of Medicinal Plants Research. 2012;6(9):1512–1518. [Google Scholar]
- 171.Luo W., Zhao M., Yang B., Shen G., Rao G. Identification of bioactive compounds in Phyllenthus emblica L. fruit and their free radical scavenging activities. Food Chemistry. 2009;114(2):499–504. doi: 10.1016/j.foodchem.2008.09.077. [DOI] [Google Scholar]
- 172.Wan Z. X., Zhou G. P., Yi Y. H. Chemical constituents of common leafflower (Phyllanthus urinaria) Zhongcaoyao. 1994;25(9):455–456. [Google Scholar]
- 173.Ahmed B., Khan S., Verma A. Antihepatotoxic activity of debelalactone, a new oxirano-furanocoumarin from Phyllanthus debilis . Journal of Asian Natural Products Research. 2009;11(8):687–692. doi: 10.1080/10286020802621864. [DOI] [PubMed] [Google Scholar]
- 174.Hnatyszyn O., Ferraro G., Coussio J. D. Coumarins of Phyllanthus sellowianus . Fitoterapia. 1993;64(6):556–556. [Google Scholar]
- 175.Xiang Y.-F., Ju H.-Q., Li S., Zhang Y.-J., Yang C.-R., Wang Y.-F. Effects of 1,2,4,6-tetra-O-galloyl-β-D-glucose from P. emblica on HBsAg and HBeAg secretion in HepG2.2.15 cell culture. Virologica Sinica. 2010;25(5):375–380. doi: 10.1007/s12250-010-3144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ishimaru K., Yoshimatsu K., Yamakawa T., Kamada H., Shimomura K. Phenolic constituents in tissue cultures of Phyllanthus niruri . Phytochemistry. 1992;31(6):2015–2018. doi: 10.1016/0031-9422(92)80352-f. [DOI] [Google Scholar]
- 177.Yang C.-M., Cheng H.-Y., Lin T.-C., Chiang L.-C., Lin C.-C. The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. Journal of Ethnopharmacology. 2007;110(3):555–558. doi: 10.1016/j.jep.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 178.Londhe J. S., Devasagayam T. P. A., Foo L. Y., Ghaskadbi S. S. Antioxidant activity of some polyphenol constituents of the medicinal plant Phyllanthus amarus Linn. Redox Report. 2008;13(5):199–207. doi: 10.1179/135100008x308984. [DOI] [PubMed] [Google Scholar]
- 179.Foo L. Y. Amariin, a di-dehydrohexahydroxydiphenoyl hydrolysable tannin from Phyllanthus amarus . Phytochemistry. 1993;33(2):487–491. doi: 10.1016/0031-9422(93)85545-3. [DOI] [Google Scholar]
- 180.Londhe J. S., Devasagayam T. P. A., Foo L. Y., Shastry P., Ghaskadbi S. S. Geraniin and amariin, ellagitannins from Phyllanthus amarus, protect liver cells against ethanol induced cytotoxicity. Fitoterapia. 2012;83(8):1562–1568. doi: 10.1016/j.fitote.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 181.Londhe J. S., Devasagayam T. P. A., Foo L. Y., Ghaskadbi S. S. Radioprotective properties of polyphenols from Phyllanthus amarus Linn. Journal of Radiation Research. 2009;50(4):303–309. doi: 10.1269/jrr.08096. [DOI] [PubMed] [Google Scholar]
- 182.Yeap Foo L. Amariinic acid and related ellagitannins from Phyllanthus amarus . Phytochemistry. 1995;39(1):217–224. doi: 10.1016/0031-9422(94)00836-i. [DOI] [Google Scholar]
- 183.Foo L. Y. Amarulone, a novel cyclic hydrolysable tannin from Phyllanthus amarus . Natural Product Letters. 1993;3(1):45–52. doi: 10.1080/10575639308043836. [DOI] [Google Scholar]
- 184.Zhang Y.-J., Abe T., Tanaka T., Yang C.-R., Kouno I. Phyllanemblinins A-F, new ellagitannins from Phyllanthus emblica . Journal of Natural Products. 2001;64(12):1527–1532. doi: 10.1021/np010370g. [DOI] [PubMed] [Google Scholar]
- 185.Luo W., Wen L., Zhao M., et al. Structural identification of isomallotusinin and other phenolics in Phyllanthus emblica L. fruit hull. Food Chemistry. 2012;132(3):1527–1533. doi: 10.1016/j.foodchem.2011.11.146. [DOI] [PubMed] [Google Scholar]
- 186.Lin M.-T., Lee S.-S., Chen Liu K. C. S. C. Polar constituents from Phyllanthus myrtifolius . Chinese Pharmaceutical Journal. 1998;50(6):327–336. [Google Scholar]
- 187.Kumaran A., Karunakaran R. J. Nitric oxide radical scavenging active components from Phyllanthus emblica L. Plant Foods for Human Nutrition. 2006;61(1):1–5. doi: 10.1007/s11130-006-0001-0. [DOI] [PubMed] [Google Scholar]
- 188.Gambari R., Borgatti M., Lampronti I., et al. Corilagin is a potent inhibitor of NF-κB activity and downregulates TNF-α induced expression of IL-8 gene in cystic fibrosis IB3-1 cells. International Immunopharmacology. 2012;13(3):308–315. doi: 10.1016/j.intimp.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 189.Sha D. X., Liu Y. H., Wang L. S., et al. Study on chemical constituents of Phyllanthus urinaria . Shenyang Yaoke Daxue Xuebao. 2000;17(3):176–178. [Google Scholar]
- 190.Yeo S.-G., Song J. H., Hong E.-H., et al. Antiviral effects of Phyllanthus urinaria containing corilagin against human enterovirus 71 and Coxsackievirus A16 in vitro. Archives of Pharmacal Research. 2015;38(2):193–202. doi: 10.1007/s12272-014-0390-9. [DOI] [PubMed] [Google Scholar]
- 191.Shen Z.-Q., Dong Z.-J., Peng H., Liu J.-K. Modulation of PAI-1 and tPA activity and thrombolytic effects of corilagin. Planta Medica. 2003;69(12):1109–1112. doi: 10.1055/s-2003-45191. [DOI] [PubMed] [Google Scholar]
- 192.Lam S.-H., Wang C.-Y., Chen C.-K., Lee S.-S. Chemical investigation of Phyllanthus reticulatus by HPLC-SPE-NMR and conventional methods. Phytochemical Analysis. 2007;18(3):251–255. doi: 10.1002/pca.979. [DOI] [PubMed] [Google Scholar]
- 193.Joshi C. S., Sanmuga Priya E. β-Glucuronidase inhibitory effect of phenolic constituents from Phyllanthus amarus . Pharmaceutical Biology. 2007;45(5):363–365. doi: 10.1080/13880200701214706. [DOI] [Google Scholar]
- 194.Notka F., Meier G. R., Wagner R. Inhibition of wild-type human immunodeficiency virus and reverse transcriptase inhibitor-resistant variants by Phyllanthus amarus . Antiviral Research. 2003;58(2):175–186. doi: 10.1016/s0166-3542(02)00213-9. [DOI] [PubMed] [Google Scholar]
- 195.Kumaran A., Karunakaran R. J. Anti-oxidant activity of polyphenols from Phyllanthus debilis Klein ex Willd. Journal of Natural Remedies. 2006;6(2):141–146. [Google Scholar]
- 196.Chen Y. W., Ren L. J. Studies on the anti-cancer active constituents of Matsumura leafflower (Phyllanthus matsumurae). II. Isolation and identification of polyphenolic compounds. Chinese Traditional & Herbal Drugs. 1997;28(4):198–202. [Google Scholar]
- 197.Ham I., Wang T., Cho E. S., Cho H. K., Whang W. K. Phenolic compounds from Phyllanthus ussuriensis . Yakhak Hoechi. 2001;45(3):237–244. [Google Scholar]
- 198.Chung S.-K., Nam J.-A., Jeon S.-Y., et al. A prolyl endopeptidase-inhibiting antioxidant from Phyllanthus ussurensis . Archives of Pharmacal Research. 2003;26(12):1024–1028. doi: 10.1007/bf02994753. [DOI] [PubMed] [Google Scholar]
- 199.Cheng H. Y., Yang C. M., Lin T. C., Lin L. T., Chiang L. C., Lin C. C. Excoecarianin, isolated from Phyllanthus urinaria Linnea, inhibits herpes simplex virus type 2 infection through inactivation of viral particles. Evidence-Based Complementary and Alternative Medicine. 2011;2011:10. doi: 10.1093/ecam/nep157.259103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Miguel O. G., Calixto J. B., Santos A. R. S., et al. Chemical and preliminary analgesic evaluation of geraniin and furosin isolated from Phyllanthus sellowianus . Planta Medica. 1996;62(2):146–149. doi: 10.1055/s-2006-957838. [DOI] [PubMed] [Google Scholar]
- 201.Liu X. L., Cui C., Zhao M. M., et al. Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chemistry. 2008;109(4):909–915. doi: 10.1016/j.foodchem.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 202.Ndjonka D., Bergmann B., Agyare C., et al. In vitro activity of extracts and isolated polyphenols from West African medicinal plants against Plasmodium falciparum . Parasitology Research. 2012;111(2):827–834. doi: 10.1007/s00436-012-2905-y. [DOI] [PubMed] [Google Scholar]
- 203.Cechinel Filho V., Santos A. R. S., De Campos R. O. P., et al. Chemical and pharmacological studies of Phyllanthus caroliniensis in mice. Journal of Pharmacy and Pharmacology. 1996;48(12):1231–1236. doi: 10.1111/j.2042-7158.1996.tb03928.x. [DOI] [PubMed] [Google Scholar]
- 204.Yang C.-M., Cheng H.-Y., Lin T.-C., Chiang L.-C., Lin C.-C. Hippomanin A from acetone extract of Phyllanthus urinaria inhibited HSV-2 but not HSV-1 infection in vitro. Phytotherapy Research. 2007;21(12):1182–1186. doi: 10.1002/ptr.2232. [DOI] [PubMed] [Google Scholar]
- 205.Liu X., Zhao M., Wu K., et al. Immunomodulatory and anticancer activities of phenolics from emblica fruit (Phyllanthus emblica L.) Food Chemistry. 2012;131(2):685–690. doi: 10.1016/j.foodchem.2011.09.063. [DOI] [Google Scholar]
- 206.Than N. N., Fotso S., Poeggeler B., Hardeland R., Laatsch H. Niruriflavone, a new antioxidant flavone sulfonic acid from Phyllanthus niruri . Zeitschrift fur Naturforschung-Section B: Journal of Chemical Sciences. 2006;61(1):57–60. [Google Scholar]
- 207.Hossain M. A., Mizanur Rahman S. M. Structure characterization and quantification of a new isoflavone from the arial parts of Phyllanthus niruri . Arabian Journal of Chemistry. 2015 doi: 10.1016/j.arabjc.2014.12.041. [DOI] [Google Scholar]
- 208.Zhang L.-Z., Zhao W.-H., Guo Y.-J., Tu G.-Z., Lin S., Xin L.-G. Studies on chemical constituents in fruits of Tibetan medicine Phyllanthus emblica . Zhongguo Zhongyao Zazhi. 2003;28(10):942–943. [PubMed] [Google Scholar]
- 209.Zhang L.-Z., Guo Y.-J., Tu G.-Z., Guo W.-B., Miao F. Studies on chemical constituents of Phyllanthus urinaria L. Zhongguo Zhongyao Zazhi. 2000;25(10):616–617. [PubMed] [Google Scholar]
- 210.Luo W., Zhao M., Yang B., Ren J., Shen G., Rao G. Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit. Food Chemistry. 2011;126(1):277–282. doi: 10.1016/j.foodchem.2010.11.018. [DOI] [Google Scholar]
- 211.Yoshida T., Itoh H., Matsunaga S., Tanaka R., Okuda T. Tannins and related polyphenols of Euphorbiaceous plants. IX. Hydrolyzable tannins with 1C4 glucose core form Phyllanthus flexuosus MUELL. ARG. Chemical & Pharmaceutical Bulletin. 1992;40(1):53–60. doi: 10.1248/cpb.40.53. [DOI] [Google Scholar]
- 212.Yang C.-B., Zhang F., Deng M.-C., He G.-Y., Yue J.-M., Lu R.-H. A new ellagitannin from the fruit of Phyllanthus emblica L. Journal of the Chinese Chemical Society. 2007;54(6):1615–1618. doi: 10.1002/jccs.200700228. [DOI] [Google Scholar]
- 213.Zhang L.-Z., Guo Y.-J., Tu G.-Z., Guo W.-B., Miao F. Isolation and identification of a novel ellagitannin from Phyllanthus urinaria L. Yaoxue Xuebao. 2004;39(2):119–122. [PubMed] [Google Scholar]
- 214.Huang Y.-L., Chen C.-C., Hsu F.-L., Chen C.-F. Two tannins from Phyllanthus tenellus . Journal of Natural Products. 1998;61(4):523–524. doi: 10.1021/np970428k. [DOI] [PubMed] [Google Scholar]
- 215.Nara T. K., Gleye J., Lavergne de Cerval E., Stanislas E. Flavonoids of Phyllanthus niruri L., Phyllanthus urinaria L., and Phyllanthus orbiculatus L. C. Rich. Plantes Medicinales et Phytotherapie. 1977;11(2):82–86. [Google Scholar]
- 216.Miguel O. G., Filho V. C., Pizzolatti M. G., et al. A triterpene and phenolic compounds from leaves and stems of Phyllanthus sellowianus . Planta Medica. 1995;61(4, article 391) doi: 10.1055/s-2006-958120. [DOI] [PubMed] [Google Scholar]
- 217.Wu D. M. Determination of quercetin content in the herba of Phyllanthus matsumurae Hayata by HPLC. Zhongcaoyao. 2007;38(8):1259–1261. [Google Scholar]
- 218.Chen Y. W., Ren L. J. Studies on the anticancer constituents of matsumura leafflower (Phyllanthus matsumurae). I. Isolation and identification of flavonoid compounds. Chinese Traditional & Herbal Drugs. 1997;28(1):5–7. [Google Scholar]
- 219.Liang C. Y., Zhen H. S., Tong X. L., et al. Studies on the chemical constituents from leaves of Phyllanthus emblica . Zhongchengyao. 2009;31(5):761–763. [Google Scholar]
- 220.Yao Q. Q., Zuo C. X. Chemical studies on the constituents of Phyllanthus urinaria L. Acta Pharmaceutica Sinica. 1993;28(11):829–835. [PubMed] [Google Scholar]
- 221.Habib-Ur-Rehman, Yasin K. A., Choudhary M. A., et al. Studies on the chemical constituents of Phyllanthus emblica . Natural Product Research. 2007;21(9):775–781. doi: 10.1080/14786410601124664. [DOI] [PubMed] [Google Scholar]
- 222.Wu C., Wei C.-S., Yu S.-F., et al. Two new acetylated flavonoid glycosides from Phyllanthus urinaria . Journal of Asian Natural Products Research. 2013;15(7):703–707. doi: 10.1080/10286020.2013.794792. [DOI] [PubMed] [Google Scholar]
- 223.Subeki, Matsuura H., Takahashi K., et al. Anti-babesial and anti-plasmodial compounds from Phyllanthus niruri . Journal of Natural Products. 2005;68(4):537–539. doi: 10.1021/np0497245. [DOI] [PubMed] [Google Scholar]
- 224.Boeira V. T., Leite C. E., Santos A. A., Jr., et al. Effects of the hydroalcoholic extract of Phyllanthus niruri and its isolated compounds on cyclophosphamide-induced hemorrhagic cystitis in mouse. Naunyn-Schmiedeberg's Archives of Pharmacology. 2011;384(3):265–275. doi: 10.1007/s00210-011-0668-0. [DOI] [PubMed] [Google Scholar]
- 225.Whang W. K., Oh I. S., Ham I. H., Hahn D. R. The phenolic constituents of Phyllanthus ussuriensis leaves. Saengyak Hakhoechi. 1994;25(2):113–116. [Google Scholar]
- 226.Tran D. T., Bui Q. C., Hoang V. L., Nguyen X. D. Isolation and structural elucidation of some phenolic compounds from Phyllanthus urinaria L. in Vietnam. Tap Chi Duoc Hoc. 2007;47(3):14–17. [Google Scholar]
- 227.El-Desouky S. K., Young R. S., Young-Kyoon K. A new cytotoxic acylated apigenin glucoside from Phyllanthus emblica L. Natural Product Research. 2008;22(1):91–95. doi: 10.1080/14786410701590236. [DOI] [PubMed] [Google Scholar]
- 228.Sarg T., Abdel-Ghani A., Zayed R., El-Sayed M. Bioactive compounds from Phyllanthus atropurpureus . Journal of Natural Products. 2012;5:10–20. [Google Scholar]
- 229.Gupta D. R., Ahmed B. Nirurin: a new prenylated flavanone glycoside from Phyllanthus nirurii . Journal of Natural Products. 1984;47(6):958–963. doi: 10.1021/np50036a008. [DOI] [PubMed] [Google Scholar]
- 230.Zhang Y.-J., Abe T., Tanaka T., Yang C.-R., Kouno I. Two new acylated flavanone glycosides from the leaves and branches of Phyllanthus emblica . Chemical & Pharmaceutical Bulletin. 2002;50(6):841–843. doi: 10.1248/cpb.50.841. [DOI] [PubMed] [Google Scholar]
- 231.Hossain M. A. A new prenylated flavanol from the arial part of Phyllanthus niruri and confirmed by GC-MS/MS. Nigerian Journal of Natural Products and Medicine. 2007;11:85–86. doi: 10.4314/njnpm.v11i1.11891. [DOI] [Google Scholar]
- 232.Qi W.-Y., Li Y., Hua L., Wang K., Gao K. Cytotoxicity and structure activity relationships of phytosterol from Phyllanthus emblica . Fitoterapia. 2013;84(1):252–256. doi: 10.1016/j.fitote.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 233.Ahmad B., Alam T. Components from whole plant of Phyllanthus amarus Linn. Indian Journal of Chemistry. 2003;42(7):1786–1790. [Google Scholar]
- 234.Gupta J., Ali M. Four new seco-sterols of Phyllanthus fraternus roots. Indian Journal of Pharmaceutical Sciences. 1999;61(1–6):90–96. [Google Scholar]
- 235.Hassarajani S. A., Mulchandani N. B. Securinine type of alkaloids from Phyllanthus niruri . Indian Journal of Chemistry Section B: Organic Chemistry Including Medicinal Chemistry. 1990;29(9):801–803. [Google Scholar]
- 236.Mulchandani N., Hassarajani S. 4-Methoxy-nor-securinine, a new alkaloid from Phyllanthus niruri . Planta Medica. 1984;50(1):104–105. doi: 10.1055/s-2007-969635. [DOI] [PubMed] [Google Scholar]
- 237.Sparzak B., Dybowski F., Krauze-Baranowska M. Analysis of Securinega-type alkaloids from Phyllanthus glaucus biomass. Phytochemistry Letters. 2015;11:353–357. doi: 10.1016/j.phytol.2014.10.013. [DOI] [Google Scholar]
- 238.Joshi B. S., Gawad D. H., Pelletier S. W., Kartha G., Bhandary K. Isolation and structure (X-ray analysis) of ent-norsecurinine, an alkaloid from Phyllanthus niruri . Journal of Natural Products. 1986;49(4):614–620. doi: 10.1021/np50046a009. [DOI] [PubMed] [Google Scholar]
- 239.Zhou M., Zhu H. L., Wang K. W., Wei W. X., Zhang Y. Isolation and X-ray crystal structure of a securinega-type alkaloid from Phyllanthus niruri Linn. Natural Product Research. 2012;26(8):762–764. doi: 10.1080/14786419.2010.546795. [DOI] [PubMed] [Google Scholar]
- 240.Houghton P. J., Woldemariam T. Z., O'Shea S., Thyagarajan S. P. Two securinega-type alkaloids from Phyllanthus amarus . Phytochemistry. 1996;43(3):715–717. doi: 10.1016/0031-9422(96)00345-7. [DOI] [Google Scholar]
- 241.Petchnaree P., Bunyapraphatsara N., Cordell G. A., et al. X-ray crystal and molecular structure of nirurine, a novel alkaloid related to the securinega alkaloid skeleton, from Phyllanthus niruri (Euphorbiaceae) Journal of the Chemical Society, Perkin Transactions. 1986;1(9):1551–1556. [Google Scholar]
- 242.Babady-Bila, Gedris T. E., Herz W. Niruroidine, a norsecurinine-type alkaloid from Phyllanthus niruroides . Phytochemistry. 1996;41(5):1441–1443. doi: 10.1016/0031-9422(95)00738-5. [DOI] [Google Scholar]
- 243.Cesari I., Grisoli P., Paolillo M., Milanese C., Massolini G., Brusotti G. Isolation and characterization of the alkaloid Nitidine responsible for the traditional use of Phyllanthus muellerianus (Kuntze) Excell stem bark against bacterial infections. Journal of Pharmaceutical and Biomedical Analysis. 2015;105:115–120. doi: 10.1016/j.jpba.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 244.Sahni S., Maurya S., Singh U. P., Singh A. K., Singh V. P., Pandey V. B. Antifungal activity of nor-securinine against some Phytopathogenic fungi. Mycobiology. 2005;33(2):97–103. doi: 10.4489/myco.2005.33.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Negi R. S., Fakhir T. M. Simplexine (14-hydroxy-4-methoxy-13,14-dihydronorsecurinine): an alkaloid from Phyllanthus simplex . Phytochemistry. 1988;27(9):3027–3028. doi: 10.1016/0031-9422(88)80724-6. [DOI] [Google Scholar]
- 246.Horii Z., Imanishi T., Yamauchi M., Hanaoka M., Parello J., Munavalli S. Structure of phyllantidine. Tetrahedron Letters. 1972;13(19):1877–1880. doi: 10.1016/s0040-4039(01)84740-7. [DOI] [Google Scholar]
- 247.Parello J., Melera A., Goutarel R. Phyllochrysine and securinine, alkaloids of Phyllanthus discoides . Bulletin de la Societe Chimique de France. 1963;(4):898–910. [Google Scholar]
- 248.Wei W.-X., Pan Y.-J. The crystal structure of one natural compound cyclo-(1,10-docandiamino-11,20-docanedioic) amide (1,12-diazacyclodocosane-2,11-dione) Bulletin of the Korean Chemical Society. 2002;23(11):1527–1530. doi: 10.5012/bkcs.2002.23.11.1527. [DOI] [Google Scholar]
- 249.Nicolis E., Lampronti I., Dechecchi M. C., et al. Pyrogallol, an active compound from the medicinal plant Emblica officinalis, regulates expression of pro-inflammatory genes in bronchial epithelial cells. International Immunopharmacology. 2008;8(12):1672–1680. doi: 10.1016/j.intimp.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 250.Sittie A. A., Lemmich E., Olsen C. E., Hviid L., Christensen S. B. Alkamides from Phyllanthus fraternus . Planta Medica. 1998;64(2):192–193. doi: 10.1055/s-2006-957405. [DOI] [PubMed] [Google Scholar]
- 251.Tempesta M. S., Corley D. G., Beutler J. A., et al. Phyllanthimide, a new alkaloid from Phyllanthus sellowianus . Journal of Natural Products. 1988;51(3):617–618. doi: 10.1021/np50057a036. [DOI] [PubMed] [Google Scholar]
- 252.Miyoshi E., Shizuri Y., Yamamura S. Isolation of potassium chelidonate as a bioactive substance concerning with circadian rhythm in nyctinastic plants. Chemistry Letters. 1987;16(3):511–514. doi: 10.1246/cl.1987.511. [DOI] [Google Scholar]
- 253.Zhang Y.-J., Tanaka T., Yang C.-R., Kouno I. New phenolic constituents from the fruit juice of Phyllanthus emblica . Chemical and Pharmaceutical Bulletin. 2001;49(5):537–540. doi: 10.1248/cpb.49.537. [DOI] [PubMed] [Google Scholar]
- 254.Puppala M., Ponder J., Suryanarayana P., Reddy G. B., Petrash M., LaBarbera D. V. The isolation and characterization of β-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis . PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0031399.e31399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wei W. X., Pan Y. J., Chen Y. Z., Lin C. W., Wei T. Y., Zhao S. K. Carboxylic acids from Phyllanthus urinaria . Chemistry of Natural Compounds. 2005;41(1):17–21. doi: 10.1007/s10600-005-0064-4. [DOI] [Google Scholar]
- 256.Wei W.-X., Pan Y.-J., Zhang H., Lin C.-W., Wei T.-Y. Two new compounds from Phyllanthus niruri . Chemistry of Natural Compounds. 2004;40(5):460–464. doi: 10.1007/s10600-005-0011-4. [DOI] [Google Scholar]
- 257.Amin Z. A., Alshawsh M. A., Kassim M., Ali H. M., Abdulla M. A. Gene expression profiling reveals underlying molecular mechanism of hepatoprotective effect of Phyllanthus niruri on thioacetamide-induced hepatotoxicity in Sprague Dawley rats. BMC Complementary and Alternative Medicine. 2013;13(1, article 160) doi: 10.1186/1472-6882-13-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Niu X., Li W., He L. Pharmacokinetics and tissue distribution of 8,9-epoxy brevifolin in rats, a hepatoprotective constituent isolated from Phyllanthus simplex Retz by liquid chromatography coupled with mass spectrometry method. Biopharmaceutics & Drug Disposition. 2008;29(5):251–258. doi: 10.1002/bdd.608. [DOI] [PubMed] [Google Scholar]
- 259.Luo H. P., Chen L. R., Li Z. Q., Ding Z. S., Xu X. J. Frontal immunoaffinity chromatography with mass spectrometric detection: a method for finding active compounds from traditional Chinese herbs. Analytical Chemistry. 2003;75(16):3994–3998. doi: 10.1021/ac034190i. [DOI] [PubMed] [Google Scholar]
- 260.Niu X.-F., He L.-C., Fan T., Li Y. Protecting effect of brevifolin and 8,9-single-epoxy brevifolin of Phyllanthus simplex on rat liver injury. Zhongguo Zhongyao Zazhi. 2006;31(18):1529–1532. [PubMed] [Google Scholar]
- 261.Shimizu M., Horie S., Terashima S., et al. Studies on aldose reductase inhibitors from natural products. II. Active components of a Paraguayan crude drug “Para-parai mí,” Phyllanthus niruri . Chemical & Pharmaceutical Bulletin. 1989;37(9):2531–2532. doi: 10.1248/cpb.37.2531. [DOI] [PubMed] [Google Scholar]
- 262.Pang J.-H. S., Huang S.-T., Wang C.-Y., et al. Ellagic acid, the active compound of Phyllanthus urinaria, exerts in vivo anti-angiogenic effect and inhibits MMP-2 activity. Evidence-Based Complementary and Alternative Medicine. 2011;2011:10. doi: 10.1093/ecam/nep207.215035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Paulino N., Pizollatti M. G., Yunes R. A., Creczynski-Pasa T. B., Calixto J. B. The mechanisms underlying the relaxant effect of methyl and ethyl gallates in the guinea pig trachea in vitro: contribution of potassium channels. Naunyn-Schmiedeberg's Archives of Pharmacology. 1999;360(3):331–336. doi: 10.1007/s002109900081. [DOI] [PubMed] [Google Scholar]
- 264.Bhattacharya S., Chatterjee S., Bauri A., et al. Immunopharmacological basis of the healing of indomethacin-induced gastric mucosal damage in rats by the constituents of Phyllanthus emblica . Phytochemistry. 2007;93(1):47–53. [Google Scholar]
- 265.Maity S., Nag N., Chatterjee S., Adhikari S., Mazumder S. Bilirubin clearance and antioxidant activities of ethanol extract of Phyllanthus amarus root in phenylhydrazine-induced neonatal jaundice in mice. Journal of Physiology and Biochemistry. 2013;69(3):467–476. doi: 10.1007/s13105-013-0234-y. [DOI] [PubMed] [Google Scholar]
- 266.Santos A. R., De Campos R. O., Miguel O. G., Cechinel-Filho V., Yunes R. A., Calixto J. B. The involvement of K+ channels and Gi/o protein in the antinociceptive action of the gallic acid ethyl ester. European Journal of Pharmacology. 1999;379(1):7–17. doi: 10.1016/s0014-2999(99)00490-2. [DOI] [PubMed] [Google Scholar]
- 267.Iizuka T., Moriyama H., Nagai M. Vasorelaxant effects of methyl brevifolincarboxylate from the leaves of Phyllanthus niruri . Biological and Pharmaceutical Bulletin. 2006;29(1):177–179. doi: 10.1248/bpb.29.177. [DOI] [PubMed] [Google Scholar]
- 268.Iizuka T., Nagai M., Taniguchi A., Moriyama H., Hoshi K. Inhibitory effects of methyl brevifolincarboxylate isolated from Phyllanthus niruri L. on platelet aggregation. Biological and Pharmaceutical Bulletin. 2007;30(2):382–384. doi: 10.1248/bpb.30.382. [DOI] [PubMed] [Google Scholar]
- 269.Zhorig Y., Zuo C. X., Li F., et al. Studies on chemical constituents of Phyllanthus urinaria L. and its antiviral activity against hepatitis B virus. Zhongguo Zhongyao Zazhi. 1998;23(6):363–364. [PubMed] [Google Scholar]
- 270.She G., Cheng R., Sha L., et al. A novel phenolic compound from Phyllanthus emblica . Natural Product Communications. 2013;8(4):461–462. [PubMed] [Google Scholar]
- 271.Zhang L.-Z., Guo Y.-J., Tu G.-Z., Miao F., Guo W.-B. Isolation and identification of a noval polyphenolic compound from Phyllanthus urinaria L. Zhongguo Zhongyao Zazhi. 2000;25(12):725–725. [PubMed] [Google Scholar]
- 272.Khan M. T. H., Lampronti I., Martello D., et al. Identification of pyrogallol as an antiproliferative compound present in extracts from the medicinal plant Emblica officinalis: effects on in vitro cell growth of human tumor cell lines. International journal of oncology. 2002;21(1):187–192. [PubMed] [Google Scholar]
- 273.Ajaiyeoba E., Kingston D. Cytotoxicity evaluation and isolation of a chroman derivative from Phyllanthus amarus aerial part extract. Pharmaceutical Biology. 2006;44(9):668–671. doi: 10.1080/13880200601006905. [DOI] [Google Scholar]
- 274.Quader M. A., Khatun M., Mosihuzzaman M. Isolation of 4-hydroxysesamin and ent-norsecurinine from Phyllanthus niruri and their chemotaxonomic significance. Journal of Bangladesh Academy of Sciences. 1994;18(2):229–234. [Google Scholar]
- 275.Kuster R. M., Mors W. B., Wagner H. Cyclohexenyl butenolides from Phyllanthus klotzschianus . Biochemical Systematics and Ecology. 1997;25(7):p. 675. doi: 10.1016/s0305-1978(97)00061-6. [DOI] [Google Scholar]
- 276.Huang Y. L. Phytochemical pharmacological studies on Phyllanthus multiflorus, Phyllanthus tenellus, and Phyllanthus virgatus [Ph.D. thesis] Taipei, Taiwan: Taipei Medical College; 1999. [Google Scholar]
- 277.Soman R., Pillay P. P. Isolation of mucic acid from the fruits of Emblica officinalis [Phyllanthus emblica] Current Science. 1962;31:13–14. [Google Scholar]
- 278.Zhao J.-Q., Wang Y.-M., He H.-P., et al. Two new highly oxygenated and rearranged limonoids from Phyllanthus cochinchinensis . Organic Letters. 2013;15(10):2414–2417. doi: 10.1021/ol400875n. [DOI] [PubMed] [Google Scholar]
- 279.Ueda M., Shigemori-Suzuki T., Yamamura S. Phyllanthurinolactone, a leaf-closing factor of nyctinastic plant, Phyllanthus urinaria L. Tetrahedron Letters. 1995;36(35):6267–6270. doi: 10.1016/0040-4039(95)01256-h. [DOI] [Google Scholar]
- 280.Zhu H. L., Wei W. X., Zhou M., Yang D., Fan X. W., Liu J. X. Chemical constituents of Phyllanthus niruri L. Tianran Chanwu Yanjiu Yu Kaifa. 2011;23(2):401–403. [Google Scholar]
- 281.Lima E. O., Morais V. M. F., Gomes S. T. A., Filho V. C., Miguel O. G., Yunes R. A. Preliminary evaluation of antifungal activity of xanthoxyline. Acta Farmaceutica Bonaerense. 1995;14(3):213–216. [Google Scholar]
- 282.Bothiraja C., Shinde M. B., Rajalakshmi S., Pawar A. P. In vitro anti-HIV-type 1 and antioxidant activity of Emblica officinalis . Research Journal of Pharmacy and Technology. 2011;2(3):556–558. [Google Scholar]
- 283.Hnatyszyn O., Broussalis A., Herrera G., et al. Argentine plant extracts active against polymerase and ribonuclease H activities of HIV-1 reverse transcriptase. Phytotherapy Research. 1999;13(3):206–209. doi: 10.1002/(SICI)1099-1573(199905)13:3<206::AID-PTR409>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 284.Tai B. H., Nhut N. D., Nhiem N. X., et al. An evaluation of the RNase H inhibitory effects of Vietnamese medicinal plant extracts and natural compounds. Pharmaceutical Biology. 2011;49(10):1046–1051. doi: 10.3109/13880209.2011.563316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ravikumar Y. S., Ray U., Nandhitha M., et al. Inhibition of hepatitis C virus replication by herbal extract: Phyllanthus amarus as potent natural source. Virus Research. 2011;158(1-2):89–97. doi: 10.1016/j.virusres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 286.Álvarez Á. L., del Barrio G., Kourí V., Martínez P. A., Suárez B., Parra F. In vitro anti-herpetic activity of an aqueous extract from the plant Phyllanthus orbicularis . Phytomedicine. 2009;16(10):960–966. doi: 10.1016/j.phymed.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 287.Del Barrio G., Parra F. Evaluation of the antiviral activity of an aqueous extract from Phyllanthus orbicularis . Journal of Ethnopharmacology. 2000;72(1-2):317–322. doi: 10.1016/s0378-8741(00)00228-2. [DOI] [PubMed] [Google Scholar]
- 288.Harikumar K. B., Kuttan G., Kuttan R. Inhibition of viral carcinogenesis by Phyllanthus amarus . Integrative Cancer Therapies. 2009;8(3):254–260. doi: 10.1177/1534735409340162. [DOI] [PubMed] [Google Scholar]
- 289.Jain N. K., Lodhi S., Jain A., Nahata A., Singhai A. K. Effects of Phyllanthus acidus (L.) Skeels fruit on carbon tetrachloride-induced acute oxidative damage in livers of rats and mice. Journal of Chinese Integrative Medicine. 2011;9(1):49–56. doi: 10.3736/jcim20110109. [DOI] [PubMed] [Google Scholar]
- 290.Rajkapoor B., Venugopal Y., Anbu J., Harikrishnan N., Gobinath M., Ravichandran V. Protective effect of Phyllanthus polyphyllus on acetaminophen induced hepatotoxicity in rats. Pakistan Journal of Pharmaceutical Sciences. 2008;21(1):57–62. [PubMed] [Google Scholar]
- 291.Gopi S., Setty O. H. Protective effect of Phyllanthus fraternus against bromobenzene induced mitochondrial dysfunction in rat liver mitochondria. Food and Chemical Toxicology. 2010;48(8-9):2170–2175. doi: 10.1016/j.fct.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 292.Ferrer M., Cristófol C., Sánchez-Lamar A., Fuentes J. L., Barbé J., Llagostera M. Modulation of rat and human cytochromes P450 involved in PhIP and 4-ABP activation by an aqueous extract of Phyllanthus orbicularis . Journal of Ethnopharmacology. 2004;90(2-3):273–277. doi: 10.1016/j.jep.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 293.Fuentes J. L., Alonso A. E., Cuétara E., et al. Usefulness of the SOS Chromotest in the study of medicinal plants as radioprotectors. International Journal of Radiation Biology. 2006;82(5):323–329. doi: 10.1080/09553000600733168. [DOI] [PubMed] [Google Scholar]
- 294.Vernhes M., González-Pumariega M., Andrade L., et al. Protective effect of a Phyllanthus orbicularis aqueous extract against UVB light in human cells. Pharmaceutical Biology. 2013;51(1):1–7. doi: 10.3109/13880209.2012.695800. [DOI] [PubMed] [Google Scholar]
- 295.Giribabu N., Rao P. V., Kumar K. P., Muniandy S., Swapna Rekha S., Salleh N. Aqueous extract of Phyllanthus niruri leaves displays in vitro antioxidant activity and prevents the elevation of oxidative stress in the kidney of streptozotocin-induced diabetic male rats. Evidence-Based Complementary and Alternative Medicine. 2014;2014:10. doi: 10.1155/2014/834815.834815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kumar S., Kumar D., Deshmukh R. R., Lokhande P. D., More S. N., Rangari V. D. Antidiabetic potential of Phyllanthus reticulatus in alloxan-induced diabetic mice. Fitoterapia. 2008;79(1):21–23. doi: 10.1016/j.fitote.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 297.Hnatyszyn O., Miño J., Ferraro G., Acevedo C. The hypoglycemic effect of Phyllanthus sellowianus fractions in streptozotocin-induced diabetic mice. Phytomedicine. 2002;9(6):556–559. doi: 10.1078/09447110260573209. [DOI] [PubMed] [Google Scholar]
- 298.Hashim A., Salman Khan M., Ahmad S. Alleviation of hyperglycemia and hyperlipidemia by Phyllanthus virgatus forst extract and its partially purified fraction in streptozotocin induced diabetic rats. EXCLI Journal. 2014;13:809–824. [PMC free article] [PubMed] [Google Scholar]
- 299.Shabeer J., Srivastava R. S., Singh S. K. Antidiabetic and antioxidant effect of various fractions of Phyllanthus simplex in alloxan diabetic rats. Journal of Ethnopharmacology. 2009;124(1):34–38. doi: 10.1016/j.jep.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 300.Hashim A., Khan M. S., Khan M. S., Baig M. H., Ahmad S. Antioxidant and α-amylase inhibitory property of Phyllanthus virgatus L.: an in vitro and molecular interaction study. BioMed Research International. 2013;2013:12. doi: 10.1155/2013/729393.729393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Buszniez P., Di Sapio O., Riquelme B. Effects of phyllanthus sellowianus Müll Arg. extracts on the rheological properties of human erythrocytes. Cell Biochemistry and Biophysics. 2014;70(2):1407–1416. doi: 10.1007/s12013-014-0072-8. [DOI] [PubMed] [Google Scholar]
- 302.Ngamkitidechakul C., Jaijoy K., Hansakul P., Soonthornchareonnon N., Sireeratawong S. Antitumour effects of Phyllanthus emblica L.: induction of cancer cell apoptosis and inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells. Phytotherapy Research. 2010;24(9):1405–1413. doi: 10.1002/ptr.3127. [DOI] [PubMed] [Google Scholar]
- 303.Zhao H.-J., Liu T., Mao X., et al. Fructus phyllanthi tannin fraction induces apoptosis and inhibits migration and invasion of human lung squamous carcinoma cells in vitro via MAPK/MMP pathways. Acta Pharmacologica Sinica. 2015;36(6):758–768. doi: 10.1038/aps.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Huang S.-T., Bi K.-W., Kuo H.-M., et al. Phyllanthus urinaria induces mitochondrial dysfunction in human osteosarcoma 143B cells associated with modulation of mitochondrial fission/fusion proteins. Mitochondrion. 2014;17:22–33. doi: 10.1016/j.mito.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 305.Lee S. H., Jaganath I. B., Wang S. M., Sekaran S. D. Antimetastatic effects of Phyllanthus on human lung (A549) and breast (MCF-7) cancer cell lines. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0020994.e20994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tang Y.-Q., Jaganath I. B., Sekaran S. D. Phyllanthus spp. induces selective growth inhibition of PC-3 and mewo human cancer cells through modulation of cell cycle and induction of apoptosis. PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012644.e12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tang Y.-Q., Jaganath I., Manikam R., Sekaran S. D. Phyllanthus suppresses prostate cancer cell, PC-3, proliferation and induces apoptosis through multiple signalling pathways (MAPKs, PI3K/Akt, NF B, and Hypoxia) Evidence-Based Complementary and Alternative Medicine. 2013;2013:13. doi: 10.1155/2013/609581.609581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ramasamy S., Wahab N., Zainal Abidin N., Manickam S., Zakaria Z. Growth inhibition of human gynecologic and colon cancer cells by Phyllanthus watsonii through apoptosis induction. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0034793.e34793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ismail M., Bagalkotkar G., Iqbal S., Adamu H. A. Anticancer properties and phenolic contents of sequentially prepared extracts from different parts of selected medicinal plants indigenous to malaysia. Molecules. 2012;17(5):5745–5756. doi: 10.3390/molecules17055745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Kumar S., Sharma S., Kumar D., Kumar K., Arya R. Immunostimulant activity of Phyllanthus reticulatus Poir: a useful plant for infectious tropical diseases. Asian Pacific Journal of Tropical Disease. 2014;4(supplement 1):S491–S495. doi: 10.1016/s2222-1808(14)60496-5. [DOI] [Google Scholar]
- 311.Santos A. R. S., Filho V. C., Niero R., et al. Analgesic effects of callus culture extracts from selected species of Phyllanthus in mice. Journal of Pharmacy & Pharmacology. 1994;46(9):755–759. doi: 10.1111/j.2042-7158.1994.tb03897.x. [DOI] [PubMed] [Google Scholar]
- 312.Santos A. R. S., De Campos R. O. P., Miguel O. G., et al. Antinociceptive properties of extracts of new species of plants of the genus Phyllanthus (Euphorbiaceae) Journal of Ethnopharmacology. 2000;72(1-2):229–238. doi: 10.1016/s0378-8741(00)00256-7. [DOI] [PubMed] [Google Scholar]
- 313.Perianayagam J. B., Sharma S. K., Joseph A., Christina A. J. M. Evaluation of anti-pyretic and analgesic activity of Emblica officinalis Gaertn. Journal of Ethnopharmacology. 2004;95(1):83–85. doi: 10.1016/j.jep.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 314.Mali S. M., Sinnathambi A., Kapase C. U., Bodhankar S. L., Mahadik K. R. Anti-arthritic activity of standardised extract of Phyllanthus amarus in Freund's complete adjuvant induced arthritis. Biomedicine & Aging Pathology. 2011;1(3):185–190. doi: 10.1016/j.biomag.2011.09.004. [DOI] [Google Scholar]
- 315.Chouhan H. S., Singh S. K. Phytochemical analysis, antioxidant and anti-inflammatory activities of Phyllanthus simplex . Journal of Ethnopharmacology. 2011;137(3):1337–1344. doi: 10.1016/j.jep.2011.07.069. [DOI] [PubMed] [Google Scholar]
- 316.Mehmood M. H., Siddiqi H. S., Gilani A. H. The antidiarrheal and spasmolytic activities of Phyllanthus emblica are mediated through dual blockade of muscarinic receptors and Ca2+ channels. Journal of Ethnopharmacology. 2011;133(2):856–865. doi: 10.1016/j.jep.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 317.Amaechina F. C., Omogbai E. K. Hypotensive effect of aqueous extract of the leaves of Phyllanthus amarus Schum and Thonn (Euphorbiaceae) Acta Poloniae Pharmaceutica. 2007;64(6):547–552. [PubMed] [Google Scholar]
- 318.Sumitra M., Manikandan P., Gayathri V. S., Mahendran P., Suguna L. Emblica officinalis exerts wound healing action through up-regulation of collagen and extracellular signal-regulated kinases (ERK1/2) Wound Repair and Regeneration. 2009;17(1):99–107. doi: 10.1111/j.1524-475X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- 319.Bandyopadhyay S. K., Chatterjee A., Chattopadhyay S. Biphasic effect of Phyllanthus emblica L. extract on NSAID-induced ulcer: An antioxidative trail weaved with immunomodulatory effect. Evidence-Based Complementary and Alternative Medicine. 2011;2011:13. doi: 10.1155/2011/146808.146808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Venkateshwarlu G., Veliyath S. K., Vijayabhaskar K., Harishbabu K., Malothu R., Sahoo S. Wound healing activity of Phyllanthus niruri in albino wistar rats. Asian Journal of Chemistry. 2012;24(9):3929–3930. [Google Scholar]
- 321.Bagavan A., Rahuman A. A., Kamaraj C., Kaushik N. K., Mohanakrishnan D., Sahal D. Antiplasmodial activity of botanical extracts against Plasmodium falciparum . Parasitology Research. 2011;108(5):1099–1109. doi: 10.1007/s00436-010-2151-0. [DOI] [PubMed] [Google Scholar]
- 322.Appiah-Opong R., Nyarko A. K., Dodoo D., Gyang F. N., Koram K. A., Ayisi N. K. Antiplasmodial activity of extracts of Tridax procumbens and Phyllanthus amarus in in vitro Plasmodium falciparum culture systems. Ghana Medical Journal. 2011;45(4):143–150. [PMC free article] [PubMed] [Google Scholar]
- 323.Dhingra D., Joshi P., Gupta A., Chhillar R. Possible involvement of monoaminergic neurotransmission in antidepressant-like activity of Emblica officinalis fruits in mice. CNS Neuroscience and Therapeutics. 2012;18(5):419–425. doi: 10.1111/j.1755-5949.2011.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Vasudevan M., Parle M. Effect of Anwala churna (Emblica offcinalis GAERTN.): an ayurvedic preparation on memory deficit rats. Journal of the Pharmaceutical Society of Japan. 2007;127(10):1701–1707. doi: 10.1248/yakushi.127.1701. [DOI] [PubMed] [Google Scholar]
- 325.Vasudevan M., Parle M. Memory enhancing activity of Anwala churna (Emblica officinalis Gaertn.): an Ayurvedic preparation. Physiology and Behavior. 2007;91(1):46–54. doi: 10.1016/j.physbeh.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 326.Brusotti G., Cesari I., Gilardoni G., et al. Chemical composition and antimicrobial activity of Phyllanthus muellerianus (Kuntze) Excel essential oil. Journal of Ethnopharmacology. 2012;142(3):657–662. doi: 10.1016/j.jep.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 327.Goun E., Cunningham G., Chu D., Nguyen C., Miles D. Antibacterial and antifungal activity of Indonesian ethnomedical plants. Fitoterapia. 2003;74(6):592–596. doi: 10.1016/s0367-326x(03)00117-5. [DOI] [PubMed] [Google Scholar]
- 328.Sousa M., Ousingsawat J., Seitz R., et al. An extract from the medicinal plant Phyllanthus acidus and its isolated compounds induce airway chloride secretion: a potential treatment for cystic fibrosis. Molecular Pharmacology. 2007;71(1):366–376. doi: 10.1124/mol.106.025262. [DOI] [PubMed] [Google Scholar]
- 329.Hnatyszyn O., Miño J., Gorzalczany S., et al. Diuretic activity of an aqueous extract of Phyllanthus sellowianus . Phytomedicine. 1999;6(3):177–179. doi: 10.1016/s0944-7113(99)80006-3. [DOI] [PubMed] [Google Scholar]
- 330.Kuttan R., Harikumar K. B. Phyllanthus Species: Scientific Evaluation and Medicinal Applications. CRC Press; 2011. [Google Scholar]
- 331.Boim M. A., Heilberg I. P., Schor N. Phyllanthus niruri as a promising alternative treatment for nephrolithiasis. International Brazilian Journal of Urology. 2010;36(6):657–664. doi: 10.1590/s1677-55382010000600002. [DOI] [PubMed] [Google Scholar]
- 332.Cheng Y. A., Wang S. D., Dang S. S. Clinical study of Phyllanthus pill on treating chronic hepatitis B. Zhongxiyi Jiehe Ganbing Zazhi. 2009;19(17):195–197. [Google Scholar]
- 333.Asare G. A., Addo P., Bugyei K., et al. Acute toxicity studies of aqueous leaf extract of Phyllanthus niruri . Interdisciplinary Toxicology. 2011;4(4):206–210. doi: 10.2478/v10102-011-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Asare G. A., Bugyei K., Sittie A., et al. Genotoxicity, cytotoxicity and toxicological evaluation of whole plant extracts of the medicinal plant Phyllanthus niruri (Phyllanthaceae) Genetics & Molecular Research. 2012;11(1):100–111. doi: 10.4238/2012.january.13.3. [DOI] [PubMed] [Google Scholar]
- 335.Asare G. A., Bugyei K., Fiawoyi I., et al. Male rat hormone imbalance, testicular changes and toxicity associated with aqueous leaf extract of an antimalarial plant: Phyllanthus niruri . Pharmaceutical Biology. 2013;51(6):691–699. doi: 10.3109/13880209.2013.764325. [DOI] [PubMed] [Google Scholar]
- 336.Josiah Obaghwarhieywo A., Ezekiel Uba N. Histological effects of chronic administration of Phyllanthus amarus on the kidney of adult Wistar rat. North American Journal of Medical Sciences. 2010;2(4):193–195. doi: 10.4297/najms.2010.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Bakare A. A., Oguntolu G. O., Adedokun L. A., et al. In vivo evaluation of genetic and systemic toxicity of aqueous extracts of Phyllanthus amarus in mice and rats. International Journal of Toxicological & Pharmacological Research. 2015;7(4):1–9. [Google Scholar]
- 338.Chopade A. R., Sayyad F. Toxicity studies and evaluation of Phyllanthus amarus and Phyllanthus fraternus extracts on the central nervous system and musculoskeletal function. International Journal of Chemical and Pharmaceutical Sciences. 2013;2(3):1333–1338. [Google Scholar]
- 339.Singh S. K., Prakash V. Toxicity assessment of Oxalis corniculata and phyllanthus fraternus plants. International Journal of Pharmacy & Pharmaceutical Sciences. 2014;6(4):388–392. [Google Scholar]
- 340.Fukumoto L. R., Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. Journal of Agricultural & Food Chemistry. 2000;48(8):3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]


