Abstract
Bone mineral density, muscle mass and physical function reach their peak between the second and fourth decade of life and then decline steadily with aging. The crucial question is: what factors contribute to or modulate this decline? The aim of this mini-review is to propose a theoretical framework for the potential role of emerging biomarkers such as klotho, fibroblast growth factors (FGF)21 and FGF23 on musculoskeletal health, with a particular focus on decline in muscle mass and function, and calls for future research to examine this proposed link. The identification of new physiological mechanisms underlying these declines may open a potentially important avenue for the development of novel intervention strategies aimed at preventing or reducing their potentially detrimental consequences.
Keywords: Klotho, fibroblast growth factors, aging, skeletal muscle
Musculoskeletal Changes with Aging and their Implications
Bone loss and the decline in muscle mass, strength and physical function that occur with aging are major risk factors for the development of adverse outcomes, including falls (1, 2), mobility limitation (3) and recurrent hospitalization (4), and often represent the early stage of a continuum leading to disability and dependency (3, 5, 6). Considering the projected demographic transition, with an estimate of 19% of Americans being 65 or older in 2030 (7), these aging-related conditions will dramatically increase in the next years, as well as their medical and health care costs (8, 9). Therefore, the identification of factors contributing to the exacerbation and progression of bone/muscle loss and functional decline represent an important public health concern, and a crucial step for the development of primary and secondary prevention strategies.
Bone mineral density, muscle mass and physical function reach their peak between the second and fourth decade of life and then decline steadily with aging (10–12). The crucial question is: what factors contribute to or modulate this decline? It is well-known that the level of physical activity is reduced with advanced age and that muscle disuse plays an important role in bone loss and the decline in muscle mass and physical function (13, 14). However, it has been shown that even highly active older persons, including master swimmers and athletes, still have significantly lower muscle mass and strength than their younger counterparts (15), suggesting that other factors contribute to this aging-related process.
The aim of this mini-review is to propose a theoretical framework for the potential role of emerging biomarkers such as klotho, fibroblast growth factors (FGF)21 and FGF23, on musculoskeletal health, with a particular focus on decline in muscle mass and function, and calls for future research to examine this proposed link. The identification of new physiological mechanisms underlying these declines may open a potentially important avenue for the development of novel intervention strategies aimed at preventing or reducing their potentially detrimental consequences.
Klotho, FGF21 and FGF23: Metabolism and Clinical Phenotypes
Klotho is a recently discovered protein (16) that was named after the Greek goddess, Klotho, who spins the thread of life. It is mainly expressed in the distal renal tubule and the choroidplexus in the brain (16) and is composed of a very short (10 amino acids) intracellular domain, a transmembrane, and a large extracellular domain which can act as a circulating hormone (17). It is released into the extracellular space and can be detected in sera (18). There are two forms of klotho protein: membrane klotho and secreted klotho.
Membrane klotho functions as a receptor for FGF21 and FGF23 and is required for their metabolic activity (19, 20). Because of the lack of a heparin-binding domain, these FGFs can leave the tissues of origin and serve as circulating hormones (21).
Secreted klotho functions as a humoral factor with a number of activities, including lowering intracellular oxidative stress and regulation of ion channel and transport (22, 23). Experimental studies have shown that klotho extends lifespan by 19–31% when overexpressed (24) and causes a phenotype of premature aging, including muscle atrophy and muscle weakness (16, 25), when its expression is disrupted. Furthermore, klotho deficient mice are osteopenic (16, 26) with low bone turnover, resulting in a decreased cortical bone thickness of femur, tibia and vertebrae by 20–40% when compared with wild-type mice (27). Although the underlying factors contributing to this premature aging phenomenon are unclear, putative mechanisms are its role in repressing insulin/IGF1 signaling through FGF21 (24), lowering intracellular oxidative stress (22) and regulating phosphate and calcium homeostasis through FGF23 (24, 28).
FGF21 is a recently discovered endocrine factor that is emerging as a regulator of glucose and lipid metabolism. It is mostly expressed in the liver but also in the pancreas, white adipose tissue and muscle (29, 30). FGF21 expression in the liver is primarily induced by prolonged fasting through peroxisome proliferator-activated receptor (PPAR)-α activation and in white adipose tissue by feeding through activation of PPAR-y, a master transcriptional regulator of adipogenesis (31). The preferred receptor for FGF21 (FGFR1c) is abundantly expressed in adipose tissue (19, 20, 32) where FGF21-regulated genes are involved in lipogenesis, lipolysis, and fatty acid oxidation (33, 34). When administered to rodents and monkeys with obesity and diabetes, recombinant FGF21 causes weight loss, and reduces plasma glucose, triglycerides, insulin resistance, and hepatic steatosis (33, 35, 36). Experimental studies suggest that FGF21 also regulates skeletal homeostasis, by potentiating PPAR-y activity and inhibiting osteoblastogenesis (37). However, little is known on the functional role of FGF21 in humans, where its role in glucose metabolism is controversial (38–43).
FGF23 was first identified in the ventrolateral thalamic nucleus of the brain in mice (44) and its importance was discovered when its mutation lead to the development of autosomal dominant hypophosphatemic rickets (ADHR) (45). FGF23 is a bone-derived hormone that acts on the kidney to modulate bone mineralization by regulating phosphate excretion, and the synthesis of 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] and parathyroid hormone (PTH)(46). In particular, when phosphate is in excess, FGF23 acts on kidney to promote phosphate excretion into urine. FGF23 also reduces serum 1,25-(OH)2D3 levels to suppress phosphate absorption from intestine. Thus, FGF23 functions as a hormone that induces negative phosphate balance (47, 48) and it has been shown to play a causative role in the development of several hypophosphatemic rickets/osteomalacia. Furthermore, FGF23 functions as an inhibiting factor of PTH synthesis (49, 50) and has been associated with PTH levels in humans as well (51). Mice knock-out for FGF23 show a clinical phenotype resembling aging, including growth retardation, skin atrophy, decreased bone density and decreased longevity (46). Patients with hypophosphatemic rickets/osteomalacia often report muscle weakness and bone pain that severely affects their daily life activities (52).
Novel Pathways to Musculoskeletal Health?
FGF21 and FGF23 share common structural and biological features (53) and both require klotho to bind their cognate FGF receptors and exert their biological activities (19, 54). Therefore, they likely act systemically and synergistically and may affect musculoskeletal health through different pathways. For example, klotho contributes to phosphate and calcium homeostasis (28) by affecting FGF23 (54), which is considered a putative cornerstone for bone mineralization, and also FGF21 might contribute to skeletal homeostasis not only in mice (37) but also in humans.
The main functions of FGF23 signaling in the kidney are the reduction of 1,25(OH)2D3 synthesis and of renal tubular phosphate reabsorption (55). Consequently, FGF23 is directly involved in the regulation of the active form of vitamin D and of serum phosphate levels. Extracellular phosphate is necessary to allow mineralization of bone matrix, while intracellular phosphate plays an important role in energy stores and production (e.g. in the form of phosphocreatine and ATP) (77), which are needed for muscles to function. Consequently, klotho and FGF23 may play an important role not only in bone mineralization but also in the maintenance of muscle mass and function given their interplay with vitamin D and the critical role of phosphate in energy (ATP) and protein production. In line with this hypothesis, a number of studies have shown that low levels of vitamin D are associated with decline in muscle mass (56), muscle strength (57) and physical function (58, 59). Furthermore, skeletal muscle is a major user of ATP (60) to power the movement of the myosin heads and to allow muscle contractions. Interestingly, experimental studies have shown that injections of FGF23 antibodies increased serum phosphate and 1,25-(OH)2D3 levels as well as grip strength and spontaneous movements in hypophosphatemic mice (61).
FGF21 may play a role in muscle mass and function with its involvement in energy metabolism as well. Indeed, during starvation and intense physical activity the levels of FGF21 increase through the PPAR-α pathway in order to enhance energy production (ketogenesis) and utilization (oxidation) of free fatty acids. Chau et al. (62) demonstrated that FGF21 regulates energy homeostasis in adipocytes through activation of adenosine monophosphate (AMP)-activated protein kinase, sirtuin 1, and PPAR g co-activator-1a leading to enhanced mitochondrial function and oxidative capacity. FGF21 also causes growth hormone resistance, and therefore, plays a key role in orchestrating the adaptive starvation response (21). Finally, circulating levels of FGF21 are positively associated with insulin resistance (43, 63) and with type II diabetes (64), conditions associated with musculoskeletal-related outcomes (65, 66).
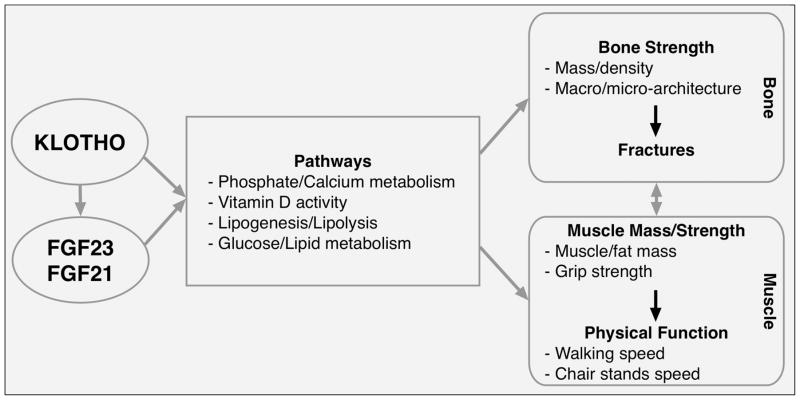
Additional potential pathways that may link these novel biomarkers with musculoskeletal health are their involvement in the regulation of systemic inflammation and oxidative stress, as these conditions are associated with decline in muscle mass and strength (67–70). Indeed, Lee et al. recently showed that FGF21 plays a role in inhibiting the activation of the transcription factor nuclear factor-kappa B (NF-kB) (71), the master regulator of inflammation that can be activated in skeletal muscle cells under inflammatory conditions (72). Finally, NF-kB activation is tightly linked to increased oxidative stress, which alters the balance between protein synthesis and degradation and, consequently, may affect the rate of protein degradation in skeletal muscle (73, 74). Interestingly, klotho has been shown to be a cytoprotective protein that defends against oxidative stress (75) and, in turn, may contribute to reduce protein degradation and muscle loss. Therefore, as simplified in the conceptual framework (figure), these novel biomarkers may play a role in musculoskeletal health through different mechanisms, and are likely to function in an interaction network rather than in an additive fashion. However, despite this strong theoretical basis, there is a gap in the current scientific knowledge on the effect of klotho, FGF21 and FGF23 on muscle mass and function in humans. Indeed, there are only two studies in humans published to date on this topic, and they show that one standard deviation increase in plasma klotho was significantly associated with muscle strength (β=1.20, standard error=0.35, p=0.0009)(76) and with reduced risk of developing Activities of Daily Living disability (odds ratio=0.57, 95% confidence interval=0.35–0.93)(77) in Italian older persons. In conclusion, this mini-review provides a theoretical basis for the potential role of these emerging biomarkers on musculoskeletal health, with a particular focus on muscle mass and function. This could represent an interesting opportunity for the development of novel intervention strategies aimed at reducing muscle and functional decline with aging and their detrimental consequences.
Figure 1.
Simplified conceptual framework
Acknowledgments
This work was supported by NIH awards R01AG020727 and R01AG027012 from the National Institute on Aging, and R01HL111271 from the National Heart, Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIA, NHLBI or NIH.
References
- 1.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. New Engl J Med. 1988;319(26):1701–7. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 2.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398(3):513–8. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 4.Manton KG. The global impact of noncommunicable diseases: estimates and projections. World Health Stat Q. 1988;41(3–4):255–66. [PubMed] [Google Scholar]
- 5.Marsh AP, Rejeski WJ, Espeland MA, Miller ME, Church TS, Fielding RA, et al. Muscle strength and BMI as predictors of major mobility disability in the Lifestyle Interventions and Independence for Elders pilot (LIFE-P) J Gerontol A Biol Sci Med Sci. 2011;66(12):1376–83. doi: 10.1093/gerona/glr158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45(1):92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 7.Deaprtment of Health & Human Services - Administration on Aging. Projections of future growth of the older population. http://www.aoa.gov/AoARoot/Aging_Statistics/future_growth/future_growth.aspx#age.
- 8.Guralnik JM, Alecxih L, Branch LG, Wiener JM. Medical and long-term care costs when older persons become more dependent. Am J Publ Health. 2002;92(8):1244–5. doi: 10.2105/ajph.92.8.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manton KG, Gu X, Lamb VL. Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the U.S. elderly population. Proc Natl Acad Sci U S A. 2006;103(48):18374–9. doi: 10.1073/pnas.0608483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95(5):1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 11.Clement FJ. Longitudinal and cross-sectional assessments of age changes in physical strength as related to sex, social class, and mental ability. J Gerontol. 1974;29(4):423–9. doi: 10.1093/geronj/29.4.423. [DOI] [PubMed] [Google Scholar]
- 12.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71(2):644–50. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 13.Bloomfield SA. Disuse osteopenia. Curr Osteoporos Rep. 2010;8(2):91–7. doi: 10.1007/s11914-010-0013-4. [DOI] [PubMed] [Google Scholar]
- 14.Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev. 2013;71(4):195–208. doi: 10.1111/nure.12019. [DOI] [PubMed] [Google Scholar]
- 15.Arthur ST, Cooley ID. The effect of physiological stimuli on sarcopenia; impact of notch and wnt signaling on impaired aged skeletal muscle repair. Int J Biol Sci. 2012;8(5):731–60. doi: 10.7150/ijbs.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 17.Kuro-o M. Klotho and the aging process. Korean J Intern Med. 2011;26(2):113–22. doi: 10.3904/kjim.2011.26.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565(1–3):143–7. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 19.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282(37):26687–95. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A. 2007;104(18):7432–7. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kliewer SA, Mangelsdorf DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr. 2010;91(1):254S–7S. doi: 10.3945/ajcn.2009.28449B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280(45):38029–34. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang CL. Regulation of ion channels by secreted Klotho: mechanisms and implications. Kidney Int. 2010;77(10):855–60. doi: 10.1038/ki.2010.73. [DOI] [PubMed] [Google Scholar]
- 24.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–33. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23(2):433–41. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest. 1999;104(3):229–37. doi: 10.1172/JCI5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuro-o M. Klotho and aging. Biochim Biophys Acta. 2009;1790(10):1049–58. doi: 10.1016/j.bbagen.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, et al. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316(5831):1615–8. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 29.Kharitonenkov A, Shanafelt AB. FGF21: a novel prospect for the treatment of metabolic diseases. Curr Opin Investig Drugs. 2009;10(4):359–64. [PubMed] [Google Scholar]
- 30.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492(1):203–6. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 31.Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J Clin Endocrinol Metab. 2009;94(9):3594–601. doi: 10.1210/jc.2009-0111. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, et al. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol. 2008;22(4):1006–14. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018–27. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models--association with liver and adipose tissue effects. Am J Physiol. 2009;297(5):E1105–14. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 35.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148(2):774–81. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 37.Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A. 2012;109(8):3143–8. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, et al. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with Type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116(1):65–8. doi: 10.1055/s-2007-985148. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57(5):1246–53. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 40.Mai K, Andres J, Biedasek K, Weicht J, Bobbert T, Sabath M, et al. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes. 2009;58(7):1532–8. doi: 10.2337/db08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Bao Y, Xu A, Pan X, Lu J, Wu H, et al. Serum fibroblast growth factor 21 is associated with adverse lipid profiles and gamma-glutamyltransferase but not insulin sensitivity in Chinese subjects. J Clin Endocrinol Metab. 2009;94(6):2151–6. doi: 10.1210/jc.2008-2331. [DOI] [PubMed] [Google Scholar]
- 42.Hojman P, Pedersen M, Nielsen AR, Krogh-Madsen R, Yfanti C, Akerstrom T, et al. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes. 2009;58(12):2797–801. doi: 10.2337/db09-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semba RD, Sun K, Egan JM, Crasto C, Carlson OD, Ferrucci L. Relationship of serum fibroblast growth factor 21 with abnormal glucose metabolism and insulin resistance: the Baltimore longitudinal study of aging. J Clin Endocrinol Metab. 2012;97(4):1375–82. doi: 10.1210/jc.2011-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277(2):494–8. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 45.ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–8. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 46.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–8. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukumoto S. Physiological regulation and disorders of phosphate metabolism--pivotal role of fibroblast growth factor 23. Intern Med. 2008;47(5):337–43. doi: 10.2169/internalmedicine.47.0730. [DOI] [PubMed] [Google Scholar]
- 48.Razzaque MS. FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am J Physiol. 2009;296(3):F470–6. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117(12):4003–8. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silver J, Naveh-Many T. FGF23 and the parathyroid. Adv Exp Med Biol. 2012;728:92–9. doi: 10.1007/978-1-4614-0887-1_6. [DOI] [PubMed] [Google Scholar]
- 51.Marsell R, Grundberg E, Krajisnik T, Mallmin H, Karlsson M, Mellstrom D, et al. Fibroblast growth factor-23 is associated with parathyroid hormone and renal function in a population-based cohort of elderly men. Eur J Endocrinol. 2008;158(1):125–9. doi: 10.1530/EJE-07-0534. [DOI] [PubMed] [Google Scholar]
- 52.Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet. 1976;1(7960):626–9. doi: 10.1016/s0140-6736(76)90428-1. [DOI] [PubMed] [Google Scholar]
- 53.Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237(1):18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- 54.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–4. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 55.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98(11):6500–5. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott D, Blizzard L, Fell J, Ding C, Winzenberg T, Jones G. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin Endocrinol. 2010;73(5):581–7. doi: 10.1111/j.1365-2265.2010.03858.x. [DOI] [PubMed] [Google Scholar]
- 57.Diamond T, Wong YK, Golombick T. Effect of oral cholecalciferol 2,000 versus 5,000 IU on serum vitamin D, PTH, bone and muscle strength in patients with vitamin D deficiency. Osteoporos Int. 2013;24(3):1101–5. doi: 10.1007/s00198-012-1944-7. [DOI] [PubMed] [Google Scholar]
- 58.Houston DK, Tooze JA, Hausman DB, Johnson MA, Nicklas BJ, Miller ME, et al. Change in 25-hydroxyvitamin D and physical performance in older adults. J Gerontol A Biol Sci Med Sci. 2011;66(4):430–6. doi: 10.1093/gerona/glq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Houston DK, Tooze JA, Davis CC, Chaves PH, Hirsch CH, Robbins JA, et al. Serum 25-hydroxyvitamin D and physical function in older adults: the Cardiovascular Health Study All Stars. J Am Geriatr Soc. 2011;59(10):1793–801. doi: 10.1111/j.1532-5415.2011.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tyynismaa H, Carroll CJ, Raimundo N, Ahola-Erkkila S, Wenz T, Ruhanen H, et al. Mitochondrial myopathy induces a starvation-like response. Hum Mol Genet. 2010;19(20):3948–58. doi: 10.1093/hmg/ddq310. [DOI] [PubMed] [Google Scholar]
- 61.Aono Y, Hasegawa H, Yamazaki Y, Shimada T, Fujita T, Yamashita T, et al. Anti-FGF-23 neutralizing antibodies ameliorate muscle weakness and decreased spontaneous movement of Hyp mice. J Bone Miner Res. 2011;26(4):803–10. doi: 10.1002/jbmr.275. [DOI] [PubMed] [Google Scholar]
- 62.Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci U S A. 2010;107(28):12553–8. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32(8):1542–6. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev. 2011;27(3):286–97. doi: 10.1002/dmrr.1177. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305(21):2184–92. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volpato S, Bianchi L, Lauretani F, Lauretani F, Bandinelli S, Guralnik JM, et al. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35(8):1672–9. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287(4):C834–43. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 68.Fano G, Mecocci P, Vecchiet J, Belia S, Fulle S, Polidori MC, et al. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil. 2001;22(4):345–51. doi: 10.1023/a:1013122805060. [DOI] [PubMed] [Google Scholar]
- 69.Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50(12):1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 70.Mecocci P, Fano G, Fulle S, MacGarvey U, Shinobu L, Polidori MC, et al. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26(3–4):303–8. doi: 10.1016/s0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 71.Lee MS, Choi SE, Ha ES, An SY, Kim TH, Han SJ, et al. Fibroblast growth factor-21 protects human skeletal muscle myotubes from palmitate-induced insulin resistance by inhibiting stress kinase and NF-kappaB. Metab Clin Exp. 2012;61(8):1142–51. doi: 10.1016/j.metabol.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 72.Henriques-Pons A, Nagaraju K. Nonimmune mechanisms of muscle damage in myositis: role of the endoplasmic reticulum stress response and autophagy in the disease pathogenesis. Curr Opin Rheumatol. 2009;21(6):581–7. doi: 10.1097/BOR.0b013e3283319265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pellegrino MA, Desaphy JF, Brocca L, Pierno S, Camerino DC, Bottinelli R. Redox homeostasis, oxidative stress and disuse muscle atrophy. J Physiol. 2011;589(Pt 9):2147–60. doi: 10.1113/jphysiol.2010.203232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Musaro A, Fulle S, Fano G. Oxidative stress and muscle homeostasis. Curr Opin Clin Nutr Metab Care. 2010;13(3):236–42. doi: 10.1097/MCO.0b013e3283368188. [DOI] [PubMed] [Google Scholar]
- 75.Rakugi H, Matsukawa N, Ishikawa K, Yang J, Imai M, Ikushima M, et al. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31(1):82–7. doi: 10.1007/s12020-007-0016-9. [DOI] [PubMed] [Google Scholar]
- 76.Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: the InCHIANTI study. Eur J Appl Physiol. 2012;112(4):1215–20. doi: 10.1007/s00421-011-2072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crasto CL, Semba RD, Sun K, Cappola AR, Bandinelli S, Ferrucci L. Relationship of low-circulating “anti-aging” klotho hormone with disability in activities of daily living among older community-dwelling adults. Rejuvenation Res. 2012;15(3):295–301. doi: 10.1089/rej.2011.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]