Abstract
Background/Aims:
Healthcare-associated pneumonia (HCAP) was proposed asa new pneumonia category in 2005, and treatment recommendations includebroad-spectrum antibiotics directed at multidrug-resistant (MDR) pathogens.However, this concept continues to be controversial, and microbiological data arelacking for HCAP patients in the intensive care unit (ICU). This study was conductedto determine the rate and type of antibiotic-resistant organisms and theclinical outcomes in patients with HCAP in the ICU, compared to patients withcommunity-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP).
Methods:
We conducted a retrospective cohort analysis of patients with pneumonia(n = 195) who admitted to medical ICU in tertiary teaching hospital fromMarch 2011 to February 2013. Clinical characteristics, microbiological distributions,treatment outcomes, and prognosis of HCAP (n = 74) were compared tothose of CAP (n = 75) and HAP (n = 46).
Results:
MDR pathogens were significantly higher in HCAP patients (39.1%) thanin CAP (13.5%) and lower than in HAP (79.3%, p < 0.001). The initial use of inappropriateantibiotic treatment occurred more frequently in the HCAP (32.6%) andHAP (51.7%) groups than in the CAP group (11.8%, p = 0.006). There were no differencesin clinical outcomes. The significant prognostic factors were pneumoniaseverity and treatment response.
Conclusions:
MDR pathogens were isolated in HCAP patients requiring ICU admissionat intermediate rates between those of CAP and HAP.
Keywords: Community-acquired pneumonia, Healthcare-associated pneumonia, Hospital-acquired pneumonia, Intensive care units, Multidrug-resistant pathogens
INTRODUCTION
Pneumonia is one of the most common infectious diseases requiring admission to the intensive care unit (ICU) for medical treatment. With an aging population, the number of patients who receive care at facilities other than hospitals, such as long-term healthcare facilities, assisted-living environments, or rehabilitation facilities are increasing. Therefore, the traditional classifications for pneumonia based on the patient’s location before admission such as community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP) needed to be updated [1,2], consequently, a new term, healthcare-associated pneumonia (HCAP) was introduced by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) in 2005 [3].
Patients who develop HCAP are more similar to hospitalised patients than to independently living community-based patients, in that they have a greater burden of comorbidities, including cancer, chronic kidney disease, heart disease, chronic obstructive lung disease, immunosuppression, dementia, and impaired mobility [1,3,4]. These diverse spectra of HCAP patients may result in varied epidemiology and patient-specific risks for antibiotic-resistant pathogens [5-7].
To address this, the IDSA/ATS guidelines recommend broad empirical antibiotic therapy followed by culture-guided de-escalation for patients with HCAP [3]. However, despite an excellent negative predictive value (96%), the IDSA/ATS criteria have a low positive predictive value (18%) for differentiating a true infection or colonization with multidrug-resistant (MDR) bacteria in patients with HCAP who admitted to the ICU [8]. Therefore, the adherence to these guidelines is not required in all cases and is able to result in the overuse of antibiotics [9]. Moreover, the current approach to HCAP treatment is also in the need of revision [10-12].
Herein, we tried to determine the differences in the presence of antibiotic-resistant organisms and clinical outcomes in HCAP patients who need ICU care, compared with CAP and HAP patients.
METHODS
Study subjects and design
From March 2011 to February 2013, we conducted a prospective cohort in a 16-bed medical ICU and a retrospective analysis of patients who required an ICU admission for pneumonia. A clinical diagnosis of pneumonia required the presence of new radiographic infiltrates and at least 2 of the following clinical criteria: fever (> 38°C) or hypothermia (≤ 35°C), new cough with or without sputum production, pleuritic chest pain, dyspnoea, or altered breath sounds on auscultation. We excluded patients with a documented do-not resuscitate order. The decision of admission to ICU was done in the case of who was required for close monitoring with septic shock under vasopressor or acute respiratory failure requiring intubation and mechanical ventilation [3].
We define HAP as pneumonia that developed after being hospitalised for > 48 to 72 hours and HCAP as pneumonia that also met at least 1 of the following criteria: (1) recent history of hospitalisation for ≥ 2 days within 90 days of the infection; (2) residence in a nursing home or long-term care facility; (3) recent intravenous antibiotic therapy, chemotherapy, or wound care within 30 days prior to the current infection; or (4) attendance at a haemodialysis clinic [3]. Patients with pneumonia who did not meet any of the criteria for HCAP or HAP were identified as having CAP. We compared clinical characteristics, pneumonia severity, the distribution of pathogens, and outcomes between the three groups (CAP, HCAP, and HAP). If patients admitted to the ICU for pneumonia ≥ 2 times during one hospital admission, only the first event of pneumonia was included. The Institutional Review Board Committee of Seoul National University Bundang Hospital waived the informed consent in this study (No. B-1105/127-001).
Microbiological studies
At the day of ICU admission, microbiological studies were conducted using two sets of blood culture samples, gram staining and culture using the transendotracheal aspirate or sputum from patients without intubation, and when available, a bronchoscopic lower respiratory tract culture that was obtained by bronchoscopy at the bedside of ICU. Obtained samples were cultured in a semi-quantitative manner.
An etiological diagnosis was made when a respiratory pathogen was isolated from a sterile specimen, a pneumococcal antigen was detected in urine, the antibody titers for an atypical pathogen increased 4-fold or converted to positive, or a predominant micro-organism was isolated from adequate sputa (> 25 neutrophils and < 10 squamous epithelial cells per low-power field) or bronchial washing or alveolar lavage fluids with compatible gram staining results. Methicillin-resistant Staphylococcus aureus (MRSA), drug-resistant strains of Pseudomonas aeruginosa, Acinetobacter species, Stenotrophomonas maltophilia, and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae were considered to be MDR pathogens, as previously reported [13].
Antibiotic therapy
Empirical antibiotic therapy was defined as the use of any antibiotics for > 48 hours during the first 3 days of admission. Broad-spectrum antibiotics were defined as the use of any antibiotics that included anti-pseudomonal β-lactamase, vancomycin, or carbapenem.
Antibiotics therapy was initiated after at least blood culture samples were done because of severe condition requiring admission to ICU in basic accordance with the ATS/IDSA guideline [3]. However, the detailed antibiotic regimen complied with the attending physician’s choice taking into consideration patient’s risk factors and the severity of the disease. The appropriateness of antibiotic therapy was analysed for all cases with an etiological diagnosis according to susceptibility test criteria for lower respiratory tract pathogens. Antibiotic therapy was classified as being inappropriate if the initially prescribed antibiotics were not directed at the identified pathogens, and treatment failure was defined as death during the initial treatment or poor treatment response. Poor treatment response defined as a change in the empirical antibiotics from the initial agents within the 7th day of the ICU admission.
Statistical analysis
To compare the differences between the groups, Fisher exact tests were used for categorical variables, and the two-tailed t test, analysis of variance, or Mann-Whitney test was used for continuous variables, as appropriate. Statistical significance was established at a two-tailed p = 0.05. All analysis was conducted using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics
During the study period, 195 patients that required ICU care for pneumonia were eligible for the study: 75 with CAP (38.1%), 74 with HCAP (37.6%), and 46 with HAP (24.4%) (Table 1). Distribution of HCAP were described in Table 2, Supplementary Table 1, and HAP in Supplementary Table 2. Patients with HCAP were significantly more likely to have comorbidities, particularly cerebrovascular disease (55.4% vs. 30.7%, p = 0.009) and chronic kidney disease (16.2% vs. 1.3%, p = 0.002), than CAP patients. Leukopenia was also significantly more common in patients with HCAP than in those with CAP (23.0% vs. 5.3%, p = 0.005). There were no significant differences in pneumonia severity measured using the confusion, urea, respiratory rate, age ≥ 65 (CURB-65) criteria (≥ 3) and pneumonia severity index (PSI; high-risk class). Disease severity according to the Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment scores was similar across the three groups.
Table 1.
Baselines characteristics of the study groups
| Characteristic | CAP (n = 75) | HCAP (n = 74) | HAP (n = 46) | p value |
|---|---|---|---|---|
| Age, yr | 72 (19–90) | 73 (32–99) | 75 (46–91) | 0.409 |
| Male sex | 59 (78.7) | 56 (75.7) | 38 (79.2) | 0.872 |
| Comorbidities | ||||
| Chronic lung diseasea | 29 (38.7) | 17 (23.0) | 15 (31.3) | 0.117 |
| Chronic heart disease | 29 (38.7) | 32 (43.2) | 24 (50.0) | 0.350 |
| Diabetes mellitus | 19 (31.7) | 24 (32.4) | 17 (35.4) | 0.571 |
| Chronic liver disease | 5 (6.7) | 5 (6.8) | 5 (10.4) | 0.655 |
| Chronic kidney disease | 1 (1.3)a | 12 (16.5) | 9 (18.8)b | 0.002 |
| Cerebrovascular disease | 23 (30.7)c | 41 (55.4) | 21 (43.8) | 0.009 |
| Rheumatoid disease | 1 (1.3) | 2 (2.7) | 0 | 0.485 |
| Current malignancy | 7 (9.3) | 24 (32.4) | 17 (37.0) | 0.095 |
| Radiographic finding | ||||
| Bilateral lung involvement | 54 (72.0) | 51 (71.8) | 27 (56.3) | 0.130 |
| Pleural effusion | 16 (21.3) | 18 (25.0) | 18 (37.5) | 0.130 |
| Clinical parameters | ||||
| Leukopenia | 4 (5.3) | 17 (23.0) | 5 (10.6) | 0.005 |
| C-reactive protein, mg/dL | 16.3 ± 9.6 | 15.6 ± 9.7 | 14.1 ± 8.1 | 0.440 |
| Procalcitonin, ng/mL | 12.5 ± 24.1 | 13.7 ± 28.0 | 33.5 ± 57.0 | 0.080 |
| ARDS | 19 (25.7) | 11 (14.9) | 12 (25.0) | 0.229 |
| Sepsis | 58 (77.3) | 66 (89.2) | 42 (91.3) | 0.800 |
| Mechanical ventilation | 66 (88.0) | 70 (94.3) | 48 (100) | 0.613 |
| CRRT | 15 (20.0) | 18 (24.3) | 14 (29.2) | 0.132 |
| Severity | ||||
| APACHE II | 25.1 ± 8.2 | 27.1 ± 10.4 | 24.0 ± 8.0 | 0.152 |
| SOFA (day 1) | 8.8 ± 4.0 | 9.89 ± 4.3 | 8.6 ± 4.1 | 0.153 |
| PSI risk class ≥ IV | 67 (89.3) | 71 (95.9) | 45 (97.8) | 0.108 |
| CURB-65 ≥ 3 | 35 (49.0) | 41 (58.5) | 24 (50) | 0.691 |
Values are presented as median (range), number (%), or mean ± SD.
CAP, community-acquired pneumonia; HCAP, healthcare-acquired pneumonia; HAP, hospital-acquired pneumonia; ARDS, acute respiratory distress syndrome; CRRT, continuous renal replacement therapy; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; PSI, pneumonia severity index; CURB-65, confusion, urea, respiratory rate, age ≥ 65.
p < 0.05 when compared with HCAP.
p < 0.05 when compared with CAP.
Chronic lung disease includes chronic obstructive lung disease and structural lung diseases, such as bronchiectasis.
Table 2.
Distribution of HCAP (n = 117)
The numbers add up to more than the total, as many patients presented more than one HCAP criteria.
HCAP, healthcare-acquired pneumonia.
Hospitalization in an acute care hospital for 2 or more days within 90 days of the infection.
Infusion therapy, such as intravenous antibiotic therapy, chemotherapy, or wound care, within 30 days of a current infection.
Residence in a nursing home or long-term care facility.
Regular attendance at a dialysis clinic, including hemodialysis and peritoneal dialysis.
Pathogen distribution
All of the patients had results of the gram staining and cultures of their blood and sputum. The number of bronchoscopic lower respiratory tract specimens was 44 in HCAP patients (61.1%), 50 in CAP patients (66.7%), and 23 in HAP patients (60.9%, p = 0.730). Bacterial pathogens were identified in 46 HCAP patients (62.2%), 37 CAP patients (46.3%), and 29 HAP patients (63.0%). Table 3 lists the frequency of each of the etiologic micro-organisms-for each group. The most common pathogens were methicillin-susceptible Staphylococcus aureus (MSSA) (29.7%), Streptococcus pneumoniae (13.5%), and P. aeruginosa (10.8%) in the CAP patients; Klebsiella pneumonia (45.6%), MRSA (19.6%), and Escherichia coli (15.3%) in the HCAP patients; and MRSA (44.8%), P. aeruginosa (24.1%), and K. pneumonia (24.1%) in the HAP patients.
Table 3.
Distribution of the isolated pathogens in CAP, HCAP, and HAP patients
| Pathogen identifieda | CAP (n = 37) | HCAP (n = 46) | HAP (n = 29) | p value |
|---|---|---|---|---|
| Gram-positive pathogen | 21 (56.8) | 17 (37.0) | 14 (48.3) | 0.193 |
| Streptococcus pneumoniae | 5 (13.5) | 3 (6.5) | 0 | 0.249 |
| Streptococci other than S. pneumoniae | 3 (8.1) | 2 (4.3) | 0 | 0.285 |
| Staphylococcus aureus | 14 (37.8) | 12 (26,1) | 13 (44.8) | 0.226 |
| MSSA | 11 (29.7) | 4 (8.7) | 0 | 0.001 |
| MRSA | 3 (8.1) | 9 (19.6) | 13 (44.8) | 0.002 |
| Gram-negative pathogen | 18 (48.6) | 35 (76.1) | 23 (79.3) | 0.009 |
| Pseudomonas aeruginosa | 4 (10.8) | 5 (10.9) | 7 (24.1) | 0.212 |
| Klebsiella pneumoniae | 9 (23.3) | 21 (45.6) | 7 (24.1) | 0.060 |
| Escherichia coli | 2 (5.4) | 7 (15.3) | 4 (13.7) | 0.349 |
| Enterobacter spp. | 2 (5.4) | 3 (6.5) | 3 (10.3) | 0.725 |
| MDR | 5 (13.5) | 18 (39.1) | 23 (79.3) | < 0.001 |
| MRSA | 3 (8.1) | 9 (19.6) | 13 (44.8) | 0.002 |
| ESBL producing Enterobacteriaeb | 1 (2.7) | 10 (21.7) | 8 (27.6) | 0.015 |
| MDR-Pseudomonas spp.c | 0 | 1 (2.2) | 2 (6.9) | 0.221 |
| CRAB | 1 (2.7) | 3 (6.5) | 4 (13.8) | 0.219 |
| Stenotrophomonas maltophilia | 1 (2.7) | 0 | 2 (6.9) | 0.200 |
| Inappropriate antibiotics treatment | 4 (11.8) | 15 (32.6)d | 14 (51.7)d | 0.006 |
Values are presented as number (%; no/patients with pathogen identified).
CAP, community-acquired pneumonia; HCAP, healthcare-acquired pneumonia; HAP, hospital-acquired pneumonia; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; MDR, multidrug-resistant; ESBL, extended-spectrum β-lactamase; CRAB, carbapenem-resistant Acinetobacter baumannii.
Numbers include mixed population of pathogens (4 in CAP, 7 in HCAP, and 9 in HAP).
ESBL producing Enterobacteriae include Klebsiella pneumoniae, Escherichia coli, Enterobacter spp.
MDR Pseudomonas spp. means resistant Pseudomonas aeruginosa.
p < 0.05 when compared with CAP.
In all three groups, S. aureus was the most common gram-positive pathogen. Of the S. aureus pathogens, MSSA was detected significantly more often in the CAP group than in the HCAP and HAP groups (p = 0.001). MRSA was detected at comparable rates in the CAP and HCAP groups and significantly more often in the HAP group (p = 0.002). Of the gram-negative pathogens, HCAP and HAP patients had significantly higher rates of ESBL-producing Enterobacteriaceae than the CAP patients (p = 0.015).
The prevalence of MDR pathogens in the HCAP group (39.1%) was significantly higher than in the CAP group (p < 0.005) and lower than that in the HAP group (p = 0.001) (Fig. 1). Inappropriate initial antibiotic treatment was administered significantly less often in the CAP group (p = 0.034) than in the HCAP and HAP groups (p = 0.146).
Figure 1.
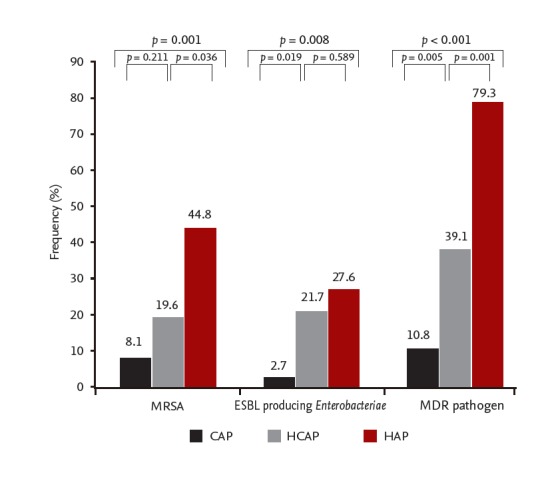
The distribution of multidrug-resistant (MDR) pneumonia pathogens in patients admitted to the intensive care unit for pneumonia, compared between three groups. CAP, community-acquired pneumonia; HCAP, healthcare-acquired pneumonia; HAP, hospital-acquired pneumonia; MRSA, methicillin-resistant Staphylococcus aureus; ESBL, extended-spectrum β-lactamase.
Antimicrobial treatment and clinical outcomes
In all three groups, the majority of the patients received combination antibiotic therapy as the initial treatment (CAP 86.7%, HCAP 78.4%, and HAP 71.7%) (Table 4). Among the combination therapies, antipseudomonal β-lactamase in combination with fluoroquinolone was the most frequently used in HCAP and HAP (39.2% and 34.8%). β-Lactamase in combination with fluoroquinolone was the most common in CAP (34.6%). Among the monotherapies, antipseudomonal β-lactamase was the most frequently used in three groups (CAP 6.7%, HCAP 12.2%, and HAP 17.4%). Broad-spectrum antibiotics were administered to the CAP patients significantly less often than to the HCAP and HAP patients (p < 0.05).
Table 4.
Initial antibiotic treatment
| Empiric antibiotic | CAP (n = 75) | HCAP (n = 74) | HAP (n = 46) | p value |
|---|---|---|---|---|
| Monotherapy | 10 (13.3) | 16 (21.6) | 13 (26.7) | 0.175 |
| β-Lactamase | 4 (5.3) | 2 (2.7) | 0 | 0.254 |
| Antipseudomonal β-lactamase | 5 (6.7) | 9 (12.2) | 8 (17.4) | 0.187 |
| Vancomycin | 1 (1.3) | 1 (1.4) | 0 | 0.733 |
| Carbapenem | 1 (1.3) | 4 (5.4) | 4 (8.7) | 0.051 |
| Combination therapy | 65 (86.7) | 58 (78.4) | 33 (71.7) | 0.175 |
| β-Lactamase + Quinolonea | 26 (34.6) | 7 (9.5) | 1 (2.2) | < 0.001 |
| β-Lactamase + Macrolide | 1 (1.3) | 0 | 0 | 0.449 |
| β-Lactamase + Clindamycin | 6 (8.0) | 8 (11.0) | 3 (6.5) | 0.568 |
| Antipseudomonal β-lactamase + Quinolonea | 25 (33.3) | 29 (39.2) | 16 (34.8) | 0.747 |
| Antipseudomonal β-lactamase + Vancomycin | 0 | 1 (1.4) | 3 (6.5) | 0.043 |
| Carbapenem + Quinolonea | 0 | 1 (1.4) | 0 | |
| Vancomycin + Carbapenem | 3 (4.0) | 7 (9.5) | 8 (17.4) | 0.048 |
| Antipseudomonal β-lactamase + Quinolone + Vancomycin | 1 (1.3) | 4 (5.4) | 2 (4.3) | 0.392 |
| Others | 2 (2.6) | 1 (1.4) | 1 (2.2) | |
| Broad spectrum antibioticsb | 32 (42.7) | 47 (63.5)c | 33 (71.7)c | 0.003 |
| Treatment failured | 15 (20.0) | 26 (35.1) | 19 (41.3) | 0.076 |
Values are presented as number (%).
CAP, community-acquired pneumonia; HCAP, healthcare-acquired pneumonia; HAP, hospital-acquired pneumonia.
Quinolone was levofloxacin.
Broad spectrum antibiotic use was defined as the use of any antibiotics including antipseudomonal β-lactamase or vancomycin or carbapenem.
p < 0.05 when compared with CAP.
Treatment failure means death during initial treatment or change of empirical antibiotics from the initial agents to others on the 7th day from medical intensive care unit admission.
There were also no significant differences in clinical outcomes, including ICU mortality, 28-day mortality, length of ICU stay, and the duration of mechanical ventilation (Table 5). The multiple logistic regression analysis resulted in a significantly increased odds of mortality associated with the acute physiologic PSI score and treatment response (Table 6).
Table 5.
Clinical outcomes of study populations
| Variable | CAP (n = 75) | HCAP (n = 74) | HAP (n = 46) | p value |
|---|---|---|---|---|
| Duration, day | ||||
| ICU | 10.48 ± 11.9 | 11.0 ± 10.39 | 12.65 ± 11.20 | 0.321 |
| MV | 10.15 ± 12.54 | 10.48 ± 10.89 | 12.44 ± 11.29 | 0.575 |
| Ventilator free daysa | 2.0 ± 1.90 | 2.8 ± 5.87 | 2.06 ± 2.69 | 0.662 |
| ICU free daysb | 14.0 ± 29.9 | 27.9 ± 29.0 | 39.7 ± 27.7 | 0.831 |
| Mortality | ||||
| ICU mortality | 21 (28.0) | 20 (27.0) | 20 (43.5) | 0.124 |
| 28-Day mortality | 26 (38.2) | 22 (32.8) | 14 (31.1) | 0.694 |
Values are presented as mean ± SD or number (%).
CAP, community-acquired pneumonia; HCAP, healthcare-acquired pneumonia; HAP, hospital-acquired pneumonia; ICU, intensive care unit; MV, mechanical ventilation.
A total of 92 patients were successfully weaned from mechanical ventilation in the ICU.
ICU free days refers to the period from ICU discharge to hospital discharge.
Table 6.
Results of the logistic regression analysis to determine the factors associated with mortality
| Predictor | OR | 95% CI | p value |
|---|---|---|---|
| Male sex | 1.88 | 0.64–5.49 | 0.249 |
| Age, yr | 1.03 | 0.99–1.08 | 0.151 |
| CAPa | 1.41 | 0.54–3.67 | 0.477 |
| HAP | 1.80 | 0.63–5.15 | 0.270 |
| PSI | 1.01 | 1.00–1.03 | 0.036 |
| MDR pathogens | 0.45 | 0.15–1.10 | 0.142 |
| Poor treatment responseb | 3.51 | 1.57–9.24 | 0.003 |
OR, odds ratio; CI, confidence interval; CAP, community-acquired pneumonia; HAP, hospital-acquired pneumonia; PSI, pneumonia severity index; MDR, multidrug-resistant.
Compared with healthcare-acquired pneumonia.
Change of empirical antibiotics from initial agents to others within the 7th day.
DISCUSSION
Previous studies have compared bacteriological differences and clinical outcomes between HCAP and CAP, or between HCAP and HAP [1,14-18]. A study compared HCAP with CAP and HAP at the same time without mention of ICU admission [19]. To our knowledge, this is the first report to compare the microbiologic epidemiology and clinical outcomes in patients admitted to the ICU with HCAP, to those with CAP and HAP. Three groups of pneumonia had similar baseline characteristics and pneumonia severity.
We identified the rate of MDR pathogens in the patients with HCAP was less than that in the patients with HAP and greater than that in patients with CAP as per the IDSA/ATS guidelines. However, the distribution of pathogens in the patients with HCAP was different from previous studies. Most common pathogen in HCAP reported previous studies was S. aureus or S. pneumonia [1,9,14,20]. In our study, K. pneumoniae (45.6%) was the most common pathogen. Consequently, ESBL-producing K. pneumoniae was also the most common MDR pathogen. The incidence of MRSA in the HCAP group (19.6%) was similar to that in the CAP group (8.1%, p = 0.221) and lower than that in the HAP group (44.8%, p = 0.036). Similarly, one other study of microbial characteristics of HCAP and HAP in Korea showed similar microbial distribution. K. pneumoniae was the most common pathogen in HCAP group. The incidence of MRSA was lower than that of HAP group [21]. The explanation for these differences is not clear. A study in residents of long-term care facilities reported that the most common pneumonia pathogens were gram-negative bacilli (18%) [22]. Pop-Vicas and D’Agata [23] noted the factors that were independently associated with the isolation of MDR gram-negative bacilli in these patients were an age > 65 years, prior antibiotic therapy for > 2 weeks, and residence in a long-term care facility. These are similar to the definition for HCAP.
The rate of initial administration of broad-spectrum antibiotics in the patients with HCAP and HAP were higher than those in patients with CAP as per the IDSA/ATS guidelines [3]. Despite the more common use of broad spectrum antibiotics in the HCAP and HAP groups, the initial antibiotic treatment was inappropriate more frequently in the HCAP and HAP groups than in the CAP group. This difference may be explained by the differing prevalence of MDR pathogens between the groups. ESBL-producing K. pneumoniae was common in HCAP, whereas Pseudomonas spp. were less common in our study. According to our study, regional antimicrobial prescribing guidelines should contain the diversity in regional trends in microbial drug resistance.
Generally, the clinical course is poorer and the length of hospital stay is prolonged in patients with HCAP, compared to patients with CAP [1,6,8]. Our study failed to show a significant difference in the clinical outcomes among the three groups because of the disease severity who were requiring ICU care by itself. Our study population was characterized by high severity of disease, approaching PSI stage IV and V disease. Overall mortality at 28 days was more than 20% in all of three groups. In one previous study that reported poorer clinical outcomes in patients with HCAP than that in those with CAP for low-risk patients, the mortality rates were not different for the high-risk patients [24]. Especially, we did demonstrate that ICU mortality was associated with pneumonia severity. With similar disease severity, patients with CAP may demonstrate similar mortality as patients with HCAP or HAP, regardless of the presence of MDR pathogens.
Treatment response was another important factor for ICU mortality. Despite significant gradual differences among the groups in the rate of MDR pathogens and the presence of a high rate of broad-spectrum antibiotic use and inappropriate treatment in our study, there were no differences in the clinical outcomes including hospital length and mortality. Physician choice the initial antibiotics considering the risk factor of MDR pathogen or disease severity at the time of admission. There were no definite criteria for evaluating treatment response under treatments. It is critical to identify patients at risk for non-response pneumonia using defined criteria to institute early appropriate therapy. El Solh et al. [17] evaluated treatment failure of severe pneumonia including nursing home residents. However, no specific definition of treatment failure was used. The parameters such as the PSI score, CURB-65, or APACHE II evaluate the severity of pneumonia at the time of admission and not response to treatment. We evaluated treatment response with definite criteria; a change in the empirical antibiotics from the initial agents within the 7th day of the ICU admission. The appropriate stewardship of antibiotics considering the treatment response could be more important factor influencing better clinical outcomes in this population.
The present study analysed data retrospectively within a single institution, which is a limitation. However, data were collected from a prospective cohort of patients who required ICU admission, and uniform methods were used to detect pathogens. Sputum and blood samples were evaluated for all of the patients, and > 60% of the patients underwent a bronchoscopy to obtain specimens. Our successful pathogen identification rate of 57% (112/195) was high compared to the 20% to 50% reported in other prospectively designed studies [1,4,23,24]. Second, prior antibiotic use in the HCAP group could not be accurately estimated due to insufficient information in the medical records from other clinics. In Korea, there are a wide variety of long-term health care facilities including assisted-living, rehabilitation, haemodialysis, and convalescent hospital facilities where antibiotics could be administered. Therefore, the number of patients in the HCAP subgroup (Supplementary Table 1) that were identified by the receipt of intravenous antibiotic therapy within 30 days of a current infection could have been underestimated. Finally, we excluded subsequent pneumonia events from patients who experienced ≥ 2 events in the same admission, potentially underestimating the number of HAP patients.
In conclusion, MDR pathogens were isolated in HCAP patients requiring ICU admission at intermediate rates between those of CAP and HAP. However, there were no significant differences among type of pneumonia in the clinical outcomes, including mortality.
KEY MESSAGE
1. Multidrug-resistant pathogens were isolated in healthcare-associated pneumonia patients requiring intensive care unit admission at intermediate rates between those of community-acquired pneumonia and hospital-acquired pneumonia.
2. There were no significant differences among type of pneumonia in the clinical outcomes, including mortality.
3. The mortality was associated with the acute physiologic pneumonia severity index score and treatment response.
Footnotes
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
Subgroups of healthcare-acquired pneumonia
Subgroups of hospital-acquired pneumonia (n = 46)
REFERENCES
- 1.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3754–3862.2. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 2.Hiramatsu K, Niederman MS. Health-care-associated pneumonia: a new therapeutic paradigm. Chest. 2005;128:3784–3787. doi: 10.1378/chest.128.6.3784. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society. Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcareassociated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 4.Shindo Y, Sato S, Maruyama E, et al. Health-care-associated pneumonia among hospitalized patients in a Japanese community hospital. Chest. 2009;135:633–640. doi: 10.1378/chest.08-1357. [DOI] [PubMed] [Google Scholar]
- 5.Sopena N, Sabria M, Neunos 2000 Study Group Multicenter study of hospital-acquired pneumonia in non-ICU patients. Chest. 2005;127:213–219. doi: 10.1378/chest.127.1.213. [DOI] [PubMed] [Google Scholar]
- 6.Abrahamian FM, Deblieux PM, Emerman CL, et al. Health care-associated pneumonia: identification and initial management in the ED. Am J Emerg Med. 2008;26(6 Suppl):1–11. doi: 10.1016/j.ajem.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Valles J, Calbo E, Anoro E, et al. Bloodstream infections in adults: importance of healthcare-associated infections. J Infect. 2008;56:27–34. doi: 10.1016/j.jinf.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect. 2010;16:902–908. doi: 10.1111/j.1469-0691.2009.03027.x. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Vidal C, Viasus D, Roset A, et al. Low incidence of multidrug-resistant organisms in patients with healthcare-associated pneumonia requiring hospitalization. Clin Microbiol Infect. 2011;17:1659–1665. doi: 10.1111/j.1469-0691.2011.03484.x. [DOI] [PubMed] [Google Scholar]
- 10.Ewig S, Welte T, Chastre J, Torres A. Rethinking the concepts of community-acquired and health-care-associated pneumonia. Lancet Infect Dis. 2010;10:279–287. doi: 10.1016/S1473-3099(10)70032-3. [DOI] [PubMed] [Google Scholar]
- 11.Brito V, Niederman MS. Healthcare-associated pneumonia is a heterogeneous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr Opin Infect Dis. 2009;22:316–325. doi: 10.1097/QCO.0b013e328329fa4e. [DOI] [PubMed] [Google Scholar]
- 12.Webb BJ, Dangerfield BS, Pasha JS, Agrwal N, Vikram HR. Guideline-concordant antibiotic therapy and clinical outcomes in healthcare-associated pneumonia. Respir Med. 2012;106:1606–1612. doi: 10.1016/j.rmed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Nseir S, Blazejewski C, Lubret R, Wallet F, Courcol R, Durocher A. Risk of acquiring multidrug-resistant Gramnegative bacilli from prior room occupants in the intensive care unit. Clin Microbiol Infect. 2011;17:1201–1208. doi: 10.1111/j.1469-0691.2010.03420.x. [DOI] [PubMed] [Google Scholar]
- 14.Carratala J, Mykietiuk A, Fernandez-Sabe N, et al. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med. 2007;167:1393–1399. doi: 10.1001/archinte.167.13.1393. [DOI] [PubMed] [Google Scholar]
- 15.Yakovlev SV, Stratchounski LS, Woods GL, et al. Ertapenem versus cefepime for initial empirical treatment of pneumonia acquired in skilled-care facilities or in hospitals outside the intensive care unit. Eur J Clin Microbiol Infect Dis. 2006;25:633–641. doi: 10.1007/s10096-006-0193-0. [DOI] [PubMed] [Google Scholar]
- 16.Craven DE, Palladino R, McQuillen DP. Healthcare-associated pneumonia in adults: management principles to improve outcomes. Infect Dis Clin North Am. 2004;18:939–962. doi: 10.1016/j.idc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 17.El Solh AA, Pietrantoni C, Bhat A, Bhora M, Berbary E. Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis. 2004;39:474–480. doi: 10.1086/422317. [DOI] [PubMed] [Google Scholar]
- 18.Depuydt P, Putman B, Benoit D, Buylaert W, De Paepe P. Nursing home residence is the main risk factor for increased mortality in healthcare-associated pneumonia. J Hosp Infect. 2011;77:138–142. doi: 10.1016/j.jhin.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Giannella M, Pinilla B, Capdevila JA, et al. Pneumonia treated in the internal medicine department: focus on healthcare-associated pneumonia. Clin Microbiol Infect. 2012;18:786–794. doi: 10.1111/j.1469-0691.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 20.Ewig S, Klapdor B, Pletz MW, et al. Nursing-home-acquired pneumonia in Germany: an 8-year prospective multicentre study. Thorax. 2012;67:132–138. doi: 10.1136/thoraxjnl-2011-200630. [DOI] [PubMed] [Google Scholar]
- 21.Yoon WK, Kim M, Kim YY, et al. The clinical and microbial characteristics of healthcare-associated pneumonia. Korean J Med. 2010;78:709–716. [Google Scholar]
- 22.Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med. 1998;105:319–330. doi: 10.1016/s0002-9343(98)00262-9. [DOI] [PubMed] [Google Scholar]
- 23.Pop-Vicas AE, D'Agata EM. The rising influx of multidrug-resistant gram-negative bacilli into a tertiary care hospital. Clin Infect Dis. 2005;40:1792–1798. doi: 10.1086/430314. [DOI] [PubMed] [Google Scholar]
- 24.Jung JY, Park MS, Kim YS, et al. Healthcare-associated pneumonia among hospitalized patients in a Korean tertiary hospital. BMC Infect Dis. 2011;11:61. doi: 10.1186/1471-2334-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroups of healthcare-acquired pneumonia
Subgroups of hospital-acquired pneumonia (n = 46)


