Abstract
By combining the unique features of the quantum cutting luminescence and long persistent luminescence, we design a new concept called “near-infrared quantum cutting long persistent luminescence (NQPL)”, which makes it possible for us to obtain highly efficient (>100%) near-infrared long persistent luminescence in theory. Guided by the NQPL concept, we fabricate the first NQPL phosphor Ca2Ga2GeO7:Pr3+,Yb3+. It reveals that both the two-step energy transfer of model (I) and the one-step energy transfer of model (IV) occur in 3P0 levels of Pr3+. Although the actual efficiency is not sufficient for the practical application at this primitive stage, this discovery and the associated materials are still expected to have important implications for several fields such as crystalline Si solar cells and bio-medical imaging.
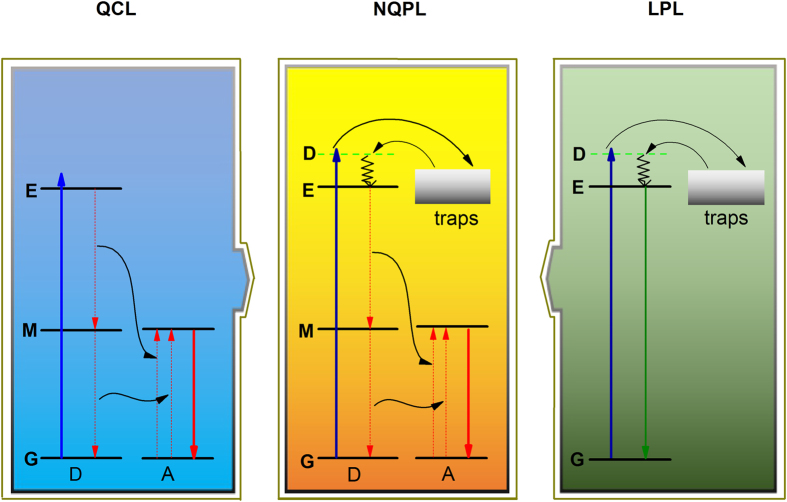
Quantum cutting luminescence (QCL) is an interesting and significant optical phenomenon occurring in materials that is able to convert one high-energy photon, generally at ultraviolet or visible wavelengths, into two low-energy photons, typically in near-infrared (NIR) spectral range1,2,3. As a schematic representation of the QCL illustrated in the left panel of Fig. 1, the donor ion (D) is excited to the emitting state (E) by absorbing one high-energy photon and then returns to the ground state (G) via an intermediate metastable state (M). The acceptor ion (A) is subsequently excited by the two-step energy transfer (ET) process, accompanied with two low-energy photons. In conventional QCL materials, many ions such as Pr3+ 2, Tb3+ 4, Tm3+ 5, Ho3+ 6, Er3+ 7, Nd3+ 8, Ce3+ 9, Eu3+ 10, Bi3+ 11 and Eu2+ 12 have been previously used as donor ions, owing to their ladderlike arranged energy levels that facilitate the photon absorption and subsequent ET steps. Yb3+ ion is generally used as acceptor ion due to its absorption and emission in NIR region, corresponding to 2F5/2 → 2F7/2 transition. Because the QCL process needs the low phonon frequency host to avoid the non-radiative losses, the present most efficient QCL has been achieved in some fluorides2,4,5.
Figure 1. Schematic diagram of quantum cutting luminescence (QCL), near-infrared quantum cutting long persistent luminescence (NQPL), and long persistent luminescence (LPL).
Long persistent luminescence (LPL) is a phenomenon whereby the light emission can last for hours after the stoppage of the excitation sources13,14. A typical LPL process can be also qualitatively shown by a simple schematic diagram, as shown in the right panel of Fig. 1. Under light excitation, the ion is excited to a delocalized state (D; i.e., an excited state associated with delocalization properties). The delocalized electrons can be captured by and stored in traps for an appropriately long time15. After ceasing the excitation sources, the trapped electrons can escape back to the D state due to the thermal or photon stimulation, followed by the non-radiative relaxation to the E state and then radiative return to the ground G state, accompanied with LPL. Nowadays, the representative LPL materials include SrAl2O4:Eu2+,Dy3+ (green)16, CaAl2O4:Eu2+,Nd3+ (blue)17, Y2O2S:Eu3+,Mg2+,Ti4+ (red)18, and Zn3Ga2Ge2O10:Cr3+ (near-infrared: NIR)19.
Although the QCL and LPL processes show the different luminescence forms and mechanisms, they share similar implications for a variety of technologies as well. In particular, both QCL and LPL phosphors have attracted enormous attention in recent years for many applications, particularly as the down-converting materials to enhance efficiency of crystalline silicon (c-Si) solar cells and as the optical nanoprobes to increase sensitivity and depth of biomedical imaging20,21,22. However, both the QCL and LPL processes separately suffer from their own drawbacks. In QCL-based materials, the real-time excitation sources are always needed. In LPL-based materials, the down-converting efficiency is not sufficient for practical application.
The QCL and LPL diagrams in Fig. 1 naturally suggest that the drawbacks of the QCL and LPL processes in c-Si solar cell and biomedical imaging could possibly be overcome by combining the unique features of these two processes. Therefore, we propose a new conceptual luminescence process called “near-infrared quantum cutting long persistent luminescence (NQPL)” by combining two processes, as illustrated in the middle panel of Fig. 1. According to this new NQPL concept, one high-energy incident photon can promote the ion system to the delocalized state, followed by filling of the traps. When the stored energy is thermally released, two low-energy photons can be created via a quantum cutting channel (two-step ET). The net effect of the NQPL process is that the theoretical quantum efficiency of LPL may reach 200% in maximum, a very interesting phenomenon that has not been reported previously.
To justify the NQPL concept, we designed a new phosphor by codoping the acceptor ion (Yb3+) into an efficient LPL phosphor: Ca2Ga2GeO7:Pr3+, obtaining the first NQPL phosphor Ca2Ga2GeO7:Pr3+,Yb3+. The Ca2Ga2GeO7 host was selected because of the lower low phonon frequency (800–975 cm−1) of germanate, close to fluorides (500–600 cm−1)23,24.
Results and Discussion
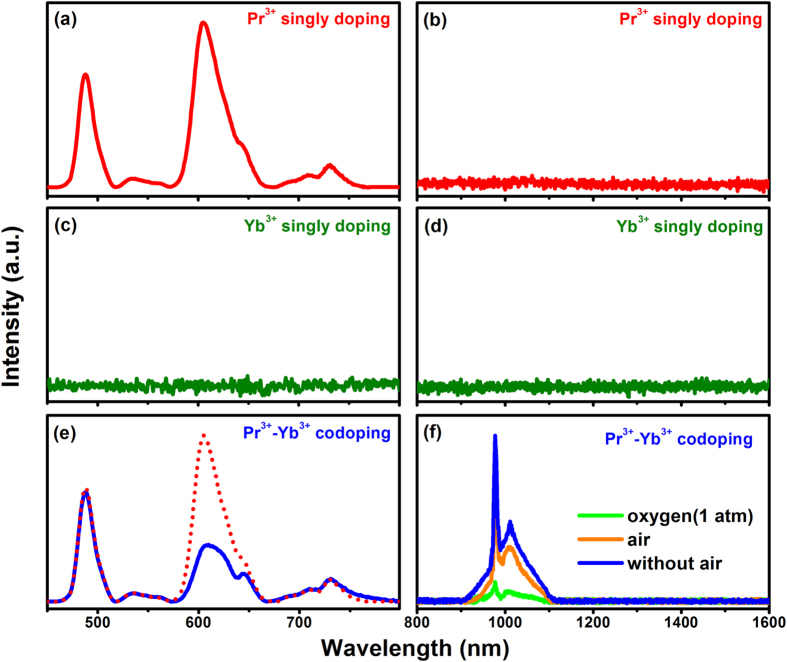
X-ray diffraction analyses show that the incorporation of the 0.1 mol% Pr3+ and 0.06–2 mol% Yb3+ ions into the Ca2Ga2GeO7 host does not induce obvious impurity, but the some impurity peaks arise when the content of Yb3+ is more than 2 mol% (see Fig. S1 in the supplemental material). Figure 2 shows the LPL spectra of the Pr3+ (a-b), Yb3+ (c-d) single doped and Pr3+-Yb3+ codoped (e-f) samples recorded after ultraviolet (254 nm) lamp irradiation for 10 min and again after a delay of 60 s. The LPL emissions due to 3P0 and 1D2 levels of Pr3+ in Ca2Ga2GeO7:Pr3+ sample can be clearly observed. The occurrence of 3P0 emissions of Pr3+ is very significant for the QCL of the Pr3+-Yb3+ pairs, and it should be associated with the low phonon energy of the Ca2Ga2GeO7 crystal, which partly prevents the multiphonon relaxation from 3P0 to 1D2. On the contrary, the NIR LPL of Yb3+ in the Ca2Ga2GeO7:Yb3+ sample is not recorded. Therefore, it can be clearly concluded that the characteristic NIR LPL (977 nm) of Yb3+ in the Ca2Ga2GeO7:Pr3+,Yb3+ sample which can last for more than 100 h must originate from the ET of Pr3+ → Yb3+. As shown in Fig. 2(e), the LPL band in range 560–660 nm due to 1D2 → 3H4, 3P0 → 3H6 and 3P0 → 3F2 transitions clearly reduces in intensity after codoping Yb3+, and it further suggests that the ET of Pr3+ → Yb3+ mainly originates from 3P0 and 1D2 levels of Pr3+. LPL in rare-earth ion doped crystals and glasses is a complex process and might be dependent on a combination of host-dopant defect state energy exchange. Thus, the samples were prepared in different atmospheric conditions including oxygen (1 atm), air and without air to gain the information of defect shown in Fig. 2(f)25,26. The intensity of LPL decreases with the partial pressure of oxygen increases. It is obvious that the LPL derives from the defect of vacancy oxygen, and the higher concentration of vacancy oxygen, the more traps in the bandgap which strengthen the intensity of LPL.
Figure 2.
The LPL spectra of the Pr3+ (a,b), Yb3+ (c,d) single doped and Pr3+-Yb3+ codoped (e,f) samples recorded at 30 s after ultraviolet (UV) lamp (254 nm) irradiation for 15 min, the red dots are the LPL spectra of Pr3+ single doped sample for comparison and and the (f) shows the LPL spectra in different atmospheric preparation condition.
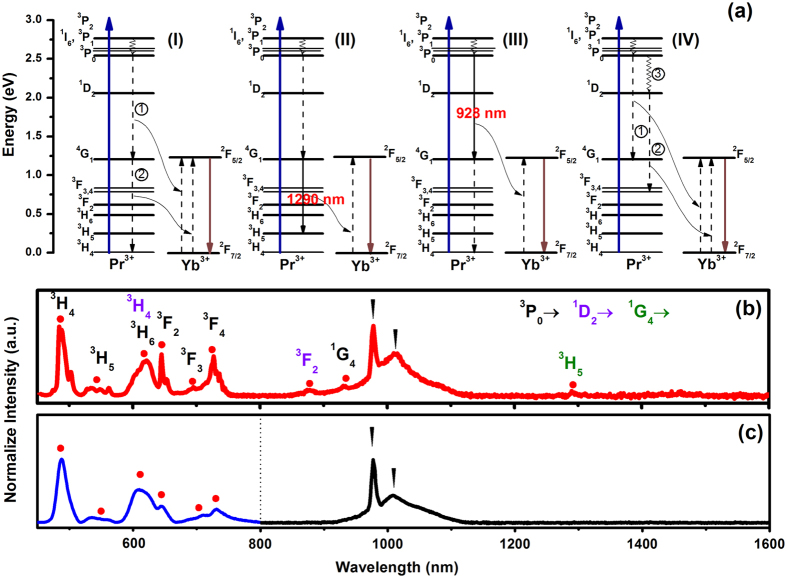
Generally, four possible models of ET mechanisms for the Pr3+ → Yb3+ pairs should be taken into consideration as shown in Fig. 3: Model (I) two-step ET from Pr3+ to Yb3+ as Pr3+ (3P0 → 1G4) → Yb3+ (2F5/2 → 2F7/2) (①) and Pr3+ (1G4 → 3H4) → Yb3+ (2F5/2 → 2F7/2) (②), which results in the generation of two NIR photons by absorbing one photon; Model (II) and (III) refers to one-step ET from Pr3+ to Yb3+, which results in generation of two photons via Pr3+ (3P0 → 1G4) → Yb3+ (2F5/2 → 2F7/2), accompanied with Pr3+ (1G4 → 3H5) or Pr3+ (1G4 → 3H5) → Yb3+ (2F5/2 → 2F7/2), accompanied with Pr3+ (3P0 → 1G4); Model (IV) is one-step ET from Pr3+ to Yb3+ as Pr3+ (3P0 → 1G4) → Yb3+ (2F5/2 → 2F7/2) (①) and one-step ET of Pr3+ (1D2 → 3G3,4) → Yb3+ (2F5/2 → 2F7/2) (②) after multiphonon relaxation (3P0 → 1D2) (③). To make clear understanding of the ET mechanisms in this case, a wide range spectral investigation including NIR region is indispensable. Figure 3(b,c) depict the photoluminescence (PL) and LPL spectra of the optimal Ca2Ga2GeO7:Pr3+,Yb3+ sample. It can be seen that the PL (λex = 254 nm) and LPL (after 254 nm irradiation) spectra are highly similar to each other, and the only difference is the observation of weak emission peaks at 928 nm (Pr3+:3P0 → 1G4) and 1290 nm (Pr3+:1G4 → 3H5), corresponding to model (II) and (III) in PL spectrum. However, because these emission peaks are very weak in PL spectrum and are completely not observed in LPL spectrum as shown in Fig. 3(b,c), the occurrence of the model (II) and (III) can be actually ignored in this discussion. Significantly, it is found that both the PL and LPL spectra consist of the characteristic emission from 1D2 level: 600 nm (1D2 → 3H4), indicating the existence of multiphonon relaxation form 3P0 to 1D2, i.e., model (IV). Because the NIR emission (977 nm) is the only characteristic of model (I), it can not be excluded or included at this stage. According to the mechanisms of models (I) and (IV), it is known that the ET to Yb3+ is efficient from both the 3P0 and the 1D2 levels of Pr3+. From the 3P0 level, a two-step ET or multiphonon relaxation may occur, while from the 1D2 level, resonant ET to Yb3+ is possible through a one-step ET process: Pr3+ (1D2 → 3F3,4) → Yb3+ (2F5/2 → 2F7/2). Note that in the proposed mechanisms, absorption of one photon to the 3PJ or higher 1I6 levels may be followed by the emission of two photons (977 nm); absorption to 1D2 would result in emission of only one 977 nm photon.
Figure 3.
Four possible models of energy transfer mechanisms for the Pr3+ → Yb3+ pairs (a), photoluminescence spectra (b) and LPL (c) spectra of Ca2Ga2GeO7:Pr3+,Yb3+ by UV lamp irradiation.
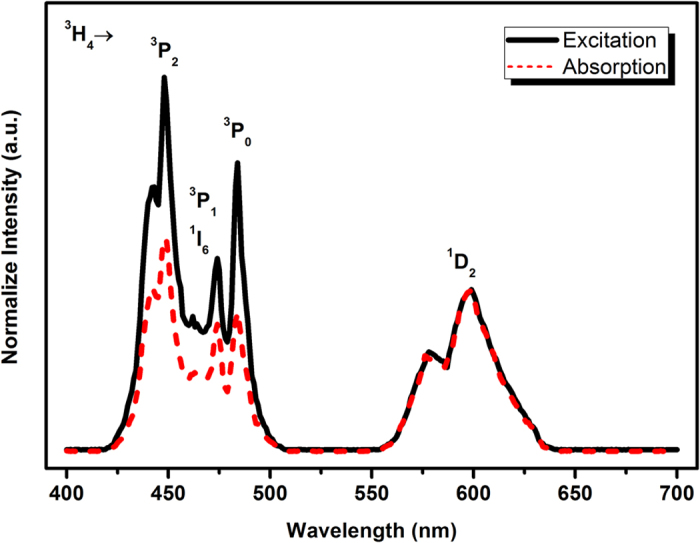
By comparing the relative absorption strengths of the 3H4 → 3PJ, 3H4 → 1I6, and 3H4 → 1D2 transitions with the corresponding relative photon fluxes in the excitation spectrum, the occurrence of the possible quantum cutting effect i.e., model (I) can be determined2. In Fig. 4, the normalized excitation (black line) and the diffuse reflectance (red line) spectra are shown for the Ca2Ga2GeO7:Pr3+,Yb3+ sample. The excitation spectrum is monitored by Yb3+ emission (977 nm). It can be seen that the area ratio (RE) of the 3PJ band to the 1D2 band in the excitation spectrum is 1.90, while that (RA) of the absorption spectrum is 1.15. When we assume that the quantum efficiencies to Yb3+ from 3PJ and that from 1D2 (100%) are equivalent, the emission intensity of Yb3+ ions by excited 3PJ levels should be also 1.15 times as strong as that of the 1D2 level. In fact, it is found in Fig. 4 that the excitation intensity by 3PJ is 1.90 times greater than that by 1D2, and this is direct evidence of quantum cutting as indicated in the model (I) of Fig. 3(a)2. However, if the model (I) is the only channel, the ratio of RE/RA should be 2 in theory. The roughly estimated value of 1.65 indicates that both the two-step ET of model (I) and the one-step ET of model (IV) occur in the Pr3+-Yb3+ codoped samples. As mentioned earlier, both the PL and LPL occurs through the direct recombination of the conduction electrons with the emission centers, and the only difference is that the electrons in the conduction band originate from direct excitation in PL or from traps in LPL. Both the processes are achieved through conduction band, and the electrons would finally reach the 3P0 level of Pr3+ via relaxation. Therefore, the electrons in the 3P0 level face the same choice in PL and LPL processes (also evidenced by the highly similar spectra profiles). At this stage, the occurrence of the QCL and NQPL at 3P0 levels of Pr3+ can be demonstrated. Note that the actual quantum efficiency should be lower than the theoretical value of 165% due to the quenching effect, which reduces the Yb3+ emission. An estimate of the overall ET efficiency, which is the fraction of 3P0 excited states that relax through ET rather than radiative decay, can be obtained from the integrals under the normalized fluorescent decay curves, as outlined in refe. 27. From the fluorescent decay curves in Fig. S2, it is determined that the roughly estimated ET efficiency from 3P0 level including the one-step and the two-step processes is only 17.7% for the optimal Ca2Ga2GeO7:Pr3+,Yb3+ sample, and thus the actual quantum efficiency should be less than 117.7%. The low ET efficiency may be due to the low quenching concentration of Yb3+ in this host. As previously mentioned, when the codoping content of Yb3+ is more than 2 mol%, some impurities clearly arise and thus badly quench the NIR emission of Yb3+ (Fig. S1). However, although the efficiency is not sufficient for the practical applications at this primitive stage, this study is of significance both in the theoretical research on NQPL and in the future developmental practices of the crystalline Si solar cells and the biomedical imaging.
Figure 4. Excitation spectra monitored at 977 nm (black line) and absorption spectra (red line) for for Ca2Ga2GeO7:Pr3+,Yb3+.
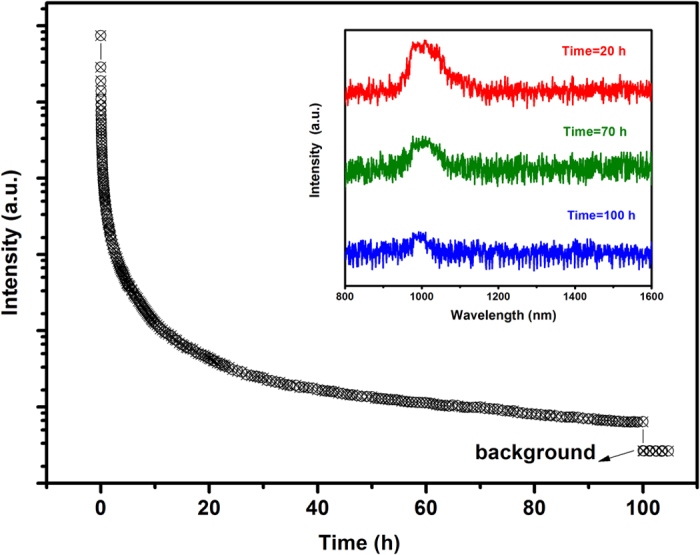
Additionally, the LPL duration time is also significant for the applications in the c-Si solar cells and biomedical imaging, and thus it is necessary to measure the LPL time of this material. Generally speaking, the duration time of visible LPL could be evaluated by the 0.32 mcd/m2, a value commonly used by the safety signage industry (about 100 times the sensitivity of the dark-adapted eye)28. However, NIR LPL is less efficiently sensed by the human eye. Instead, radiance is more appropriate than luminance for the evaluation of NIR LPL29. According to previous practices19,30, the NIR LPL around 977 nm of this materials could be recorded for more than 100 hours after irradiated for 15 min as shown in Fig. 5, although after such time from the end of the irradiation, the signal-to-noise ratio was strongly reduced making the Yb3+ emission barely detectable. The inset of Fig. 5 also gives the LPL spectrum acquired at different decay time. It is reasonable that the detectability of the NIR LPL at a given time strongly depends on the experimental conditions.
Figure 5. Near infrared (NIR) LPL decay curves of Ca2Ga2GeO7:Pr3+,Yb3+ monitored at 977 nm by UV lamp irradiation for 15 min and the NIR LPL spectra for different decay time (inset).
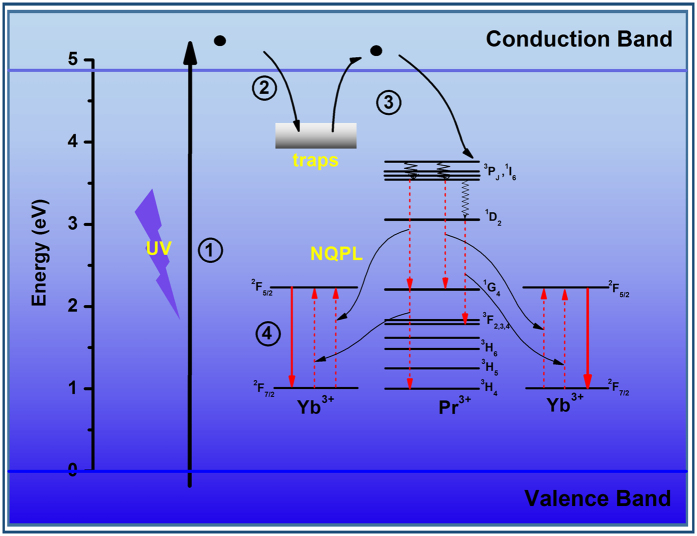
Accordingly, Fig. 6 exhibits a schematic representation of the NQPL mechanism. The trap levels continuously distribute over a wide range of energies and localize near the Pr3+ sites. Under ultraviolet light excitation, the electrons can be promoted to the conduction band (process ①). The electrons are subsequently captured by the traps below conduction band (process ②). The captured electrons are gradually released from the traps and are backtracked to the excited 3P0 level of Pr3+ via the conduction band (process ③). Finally, the energy is transferred from Pr3+ to Yb3+ via the one-step (model IV) and the two-step (model I) ET processes, and gives the NIR LPL of Yb3+ (④).
Figure 6. Schematic diagram of the NIR LPL mechanism for Ca2Ga2GeO7:Pr3+,Yb3+.
In summary, A new NQPL concept by combining the unique QCL and LPL processes is proposed for the first time. According to this idea, we designed the first NQPL phosphor Ca2Ga2GeO7:Pr3+,Yb3+ by incorporating acceptor Yb3+ ions into the LPL phosphor Ca2Ga2GeO7:Pr3+. It reveals that a two-step ET process from Pr3+ (3P0 → 1G4) → Yb3+ (2F5/2 → 2F7/2) and Pr3+ (1G4 → 3H4) → Yb3+ (2F5/2 → 2F7/2) occur in this phosphor, demonstrating the occurrence of the QCL and NQPL in 3P0 levels of Pr3+. Even though the actual QC efficiency still need to be improved, this interesting discovery enables the Ca2Ga2GeO7:Pr3+,Yb3+ phosphor to find potential applications in many important areas, particularly in c-Si solar cells and biomedical imaging that requires highly efficient, less environmental limitation, super-long and near-infrared LPL.
Methods
Synthesis
All phosphors were fabricated by a simple solid-state method. Stoichiometric amounts of CaCO3 (A.R.), Ga2O3 (A.R.), GeO2 (A.R.), Pr6O11 (4N) and Yb2O3 (4N) were used as starting materials. The ingredients were ground homogeneously in an agate mortar with anhydrous alcohol. Then the mixtures were sintered at 1573 K for 2 h in air (or oxygen (1 atm) and without air). After cooled down to room temperature, the final products were obtained.
Characterization
The X-ray diffraction patterns were obtained on a Rigaku D/max-2400 powder diffractometer by using Cu Kα radiation at 40 kV and 60 mA. The luminescence decay curves were measured by a FLS-920T fluorescence spectrophotometer with a nF900 microsecond flashlamp as the light source. The photoluminescence and the long persistent luminescence spectra were recorded by FLS-920 fluorescence spectrophotometer (Edinburgh Instruments). The absorption spectra were recorded by a PerkinElmer Lambda 950 spectrometer in the region of 400–700 nm, while BaSO4 was used as a reference.
Additional Information
How to cite this article: Zou, Z. et al. Near-Infrared Quantum Cutting Long Persistent Luminescence. Sci. Rep. 6, 24884; doi: 10.1038/srep24884 (2016).
Supplementary Material
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Nos 10904057, 51202099, 61106006 and 61376011), the Fundamental Research Funds for Central Universities (No. Lzjbky-2015-112), and the National Science Foundation for Fostering Talents in Basic Research of the National Natural Science Foundation of China (Nos 041105 and 041106).
Footnotes
Author Contributions Z.Z., L.F., C.C. and J.Z. designed the experiment. Z.Z., C.C. and L.F. conducted all experiments. Z.Z. wrote the paper. J.Z. and Y.W. commented on the manuscript at all stages. All authors reviewed the manuscript.
References
- Sommerdijk J. L., Bril A. & de Jager A. W. Two photon luminescence with ultraviolet excitation of trivalent praseodymium. J. Lumin. 8, 341–343 (1974). [Google Scholar]
- Van der Ende B. M., Aarts L. & Meijerink A. Near-Infrared Quantum Cutting for Photovoltaics. Adv. Mater. 21, 3073–3077 (2009). [Google Scholar]
- Chen D., Wang Y., Yu Y., Huang P. & Weng F. Near-infrared quantum cutting in transparent nanostructured glass ceramics. Opt. Lett. 33, 1884–1886 (2008). [DOI] [PubMed] [Google Scholar]
- Zheng B. et al. Plasmon enhanced near-infrared quantum cutting of KYF4:Tb3+,Yb3+ doped with Ag nanoparticles. Opt. Lett. 40, 2630–2633 (2015). [DOI] [PubMed] [Google Scholar]
- Fusari F. et al. Spectroscopic and lasing performance of Tm3+-doped bulk TZN and TZNG tellurite glasses operating around 1.9 μm. Opt. Express 16, 19146–19151 (2008). [DOI] [PubMed] [Google Scholar]
- Chen X. et al. Experiments and analysis of infrared quantum cutting luminescence phenomena of Ho3+/Yb3+ codoped nanophase oxyfluoride vitroceramics. Opt. Commun. 285, 5247–5253 (2012). [Google Scholar]
- Zhang J., Wang Y., Guo L. & Dong P. Up-conversion luminescence and near-infrared quantum cutting in Y6O5F8:RE3+ (RE = Yb, Er, and Ho) with controllable morphologies by hydrothermal synthesis. Dalton Trans. 42, 3542–3551 (2013). [DOI] [PubMed] [Google Scholar]
- Borrero-González L. J. & Nunes L. A. O. Near-infrared quantum cutting through a three-step energy transfer process in Nd3+-Yb3+co-doped fluoroindogallate glasses. J. Phys.: Condens. Matter. 24, 385501 (2012). [DOI] [PubMed] [Google Scholar]
- Liu Z. et al. Efficient near-infrared quantum cutting in Ce3+-Yb3+ codoped glass for solar photovoltaic. Sol. Enegy Mater. Sol. Cells 122, 46–50 (2014). [Google Scholar]
- Lau M. K. & Hao J.-H. Near-infrared Quantum Cutting in Eu3+-Yb3+ co-doped YAG through Downconversion for Silicon Solar Cell. Energy Proc. 15, 129–134 (2012). [Google Scholar]
- Huang X. Y. & Zhang Q. Y. Near-infrared quantum cutting via cooperative energy transfer in Gd2O3:Bi3+,Yb3+ phosphors. J. Appl. Phys. 107, 063505 (2010). [Google Scholar]
- Smedskjaer M. M., Qiu J., Wang J. & Yue Y. Near-infrared emission from Eu–Yb doped silicate glasses subjected to thermal reduction. Appl. Phys. Lett. 98, 071911 (2011). [Google Scholar]
- Hölsä J. Persistent Luminescence Beats the Afterglow: 400 Years of Persistent Luminescence. Electronchem. Soc. Interface 18, 42–45 (2009). [Google Scholar]
- Liu F., Liang Y. & Pan Z. Detection of Up-converted Persistent Luminescence in the Near Infrared Emitted by the Zn3Ga2GeO8:Cr3+,Yb3+,Er3+ Phosphor. Phys. Rev. Lett. 113, 117401 (2014). [DOI] [PubMed] [Google Scholar]
- Hölsä J. et al. Role of defect states in persistent luminescence materials. J. Alloys Compd. 374, 56–59 (2004). [Google Scholar]
- Matsuzawa T., Aoki Y., Takeuchi N. & Murayama Y. A New Long Phosphorescent Phosphor with High Brightness, SrAl2O4:Eu2+,Dy3+. J. Eletrochem. Soc. 143, 2670–2673 (1996). [Google Scholar]
- Hölsä J., Jungner H., Lastusaari M. & Niittykoski J. Persistent Luminescence of Eu2+ doped alkaline earth aluminates, MAl2O4 :Eu2 +. J. Alloys Compd. 323, 326–330 (2001). [Google Scholar]
- Wang X., Zhang Z., Tang Z. & Lin Y. Characterization and properties of a red and orange Y2O2S-based long afterglow phosphor. Mater. Chem. Phys. 80, 1–5 (2003). [Google Scholar]
- Pan Z., Lu Y.-Y. & Liu F. Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nat. Mater. 11, 58–63 (2012). [DOI] [PubMed] [Google Scholar]
- Wang C., Xuan T., Liu J., Li H. & Sun Z. Long Afterglow SrAl2O4:Eu2+,Dy3+ Phosphors as Luminescent Down-Shifting Layer for Crystalline Silicon Solar Cells. Int. J. Appl. Ceram. Technol. 12, 722–727 (2015). [Google Scholar]
- Wang W., Lei X., Gao H. & Mao Y. Near-infrared quantum cutting platform in transparent oxyfluoride glass–ceramics for solar sells. Opt. Mater. 47, 270–275 (2015). [Google Scholar]
- Maldiney T. et al. Gadolinium-Doped Persistent Nanophosphors as Versatile Tool for Multimodal In Vivo Imaging. Adv. Funct. Mater. 25, 331–338 (2015). [Google Scholar]
- Richards B. S. Luminescent layers for enhanced silicon solar cell performance: Down-conversion. Sol. Energy Mater. Sol. Cells 90, 1189–1207 (2006). [Google Scholar]
- Richards B. D. O., Teddy-Fernandez T., Jose G., Binks D. & Jha A. Mid-IR (3–4 μm) fluorescence and ASE studies in Dy3+ doped tellurite and germanate glasses and a fs laser inscribed waveguide. Laser Phys. Lett. 10, 085802 (2013). [Google Scholar]
- Naftaly M., Batchelor C. & Jha A. Pr3+-doped fluoride glass for a 589 nm fibre laser. J. Lumin. 91, 133–138 (2000). [Google Scholar]
- Jiang X. & Jha A. An investigation on the dependence of photoluminescence in Bi2O3-doped GeO2 glasses on controlled atmospheres during melting. Opt. Mater. 33, 14–18 (2010). [Google Scholar]
- Katayama Y. & Tanabe S. Mechanism of quantum cutting in Pr3+-Yb3+ codoped oxyfluoride glass ceramics. J. Lumin. 134, 825–829 (2013). [Google Scholar]
- Van den Eeckhout K., Poelman D. & Smet P. Persistent Luminescence in Non-Eu2+-Doped Compounds: A Review. Materials 6, 2789–2818 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Ueda J., Zhuang Y., Viana B. & Tanabe S. Y3Al5−xGaxO12:Cr3+: A novel red persistent phosphor with high brightness. Appl. Phys. Express 8, 042602 (2015). [Google Scholar]
- Caratto V. et al. NIR persistent luminescence of lanthanide ion-doped rare-earth oxycarbonates: the effect of dopants. ACS Appl. Mat. Interfaces 6, 17346–17351 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.